

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00545 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Evaluated for inhibition of dihydrofolate reductase (DHFR) isolated from L1210 cells | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50022738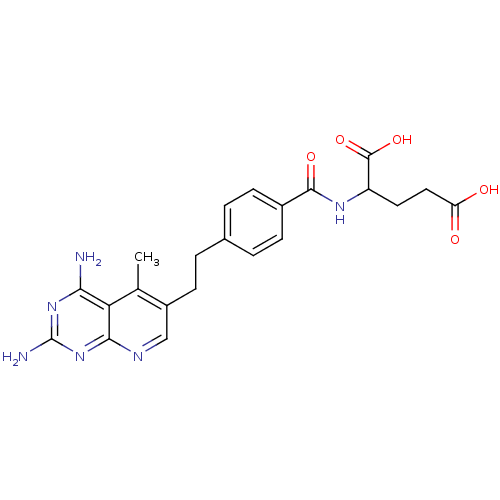 (2-{4-[2-(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0283 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Evaluated for inhibition of dihydrofolate reductase (DHFR) isolated from L1210 cells | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50022741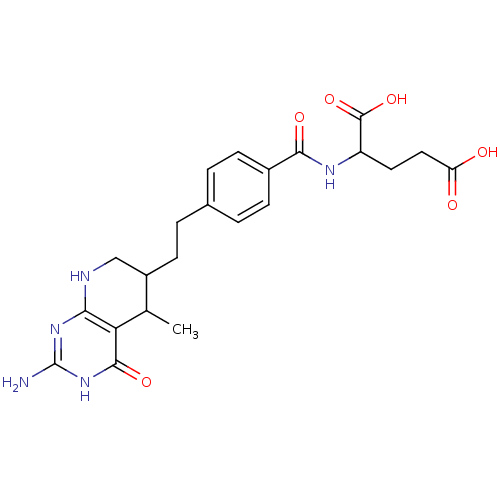 ((5RS, 6RS) 2-{4-[2-(2-Amino-5-methyl-4-oxo-3,4,5,6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Evaluated for inhibition of dihydrofolate reductase (DHFR) isolated from L1210 cells | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50022740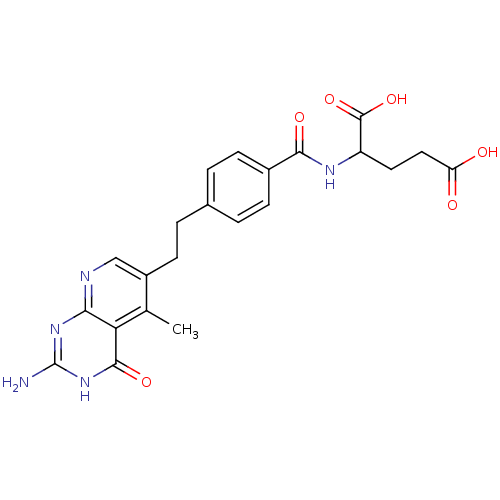 (2-{4-[2-(2-Amino-5-methyl-4-oxo-3,4-dihydro-pyrido...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Evaluated for inhibition of dihydrofolate reductase (DHFR) isolated from L1210 cells | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50022739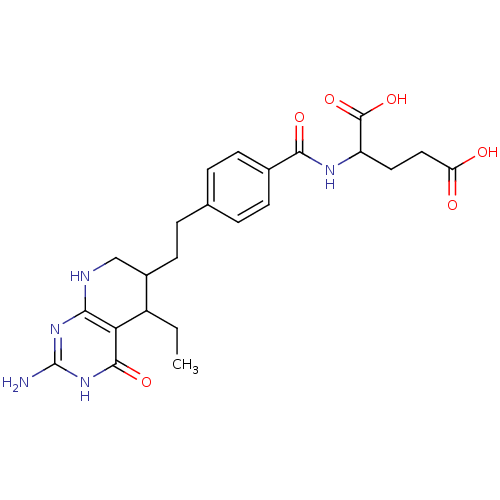 ((6RS) 2-{4-[2-(2-Amino-5-ethyl-4-oxo-3,4,5,6,7,8-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Evaluated for inhibition of dihydrofolate reductase (DHFR) isolated from L1210 cells | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50016658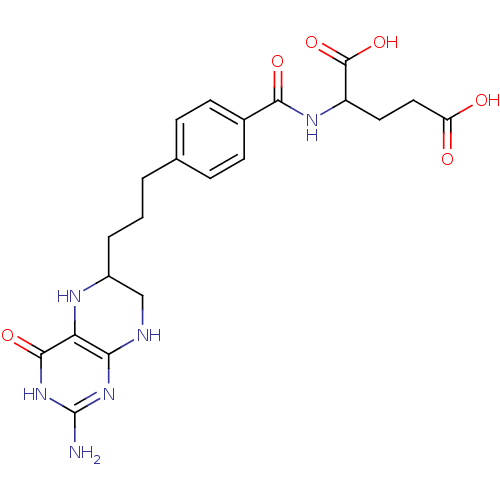 (2-{4-[3-(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pteri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Alabama Curated by ChEMBL | Assay Description Inhibition of the GAR transformylase in lactobacillus casei | J Med Chem 32: 1277-83 (1989) BindingDB Entry DOI: 10.7270/Q20K27J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50016659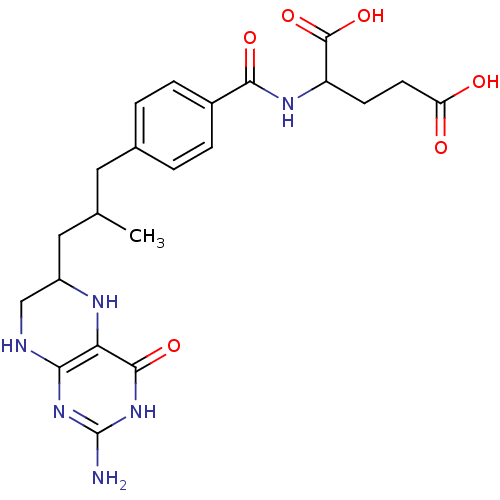 (2-{4-[3-(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pteri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Alabama Curated by ChEMBL | Assay Description Inhibition of the GAR transformylase in lactobacillus casei | J Med Chem 32: 1277-83 (1989) BindingDB Entry DOI: 10.7270/Q20K27J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50016660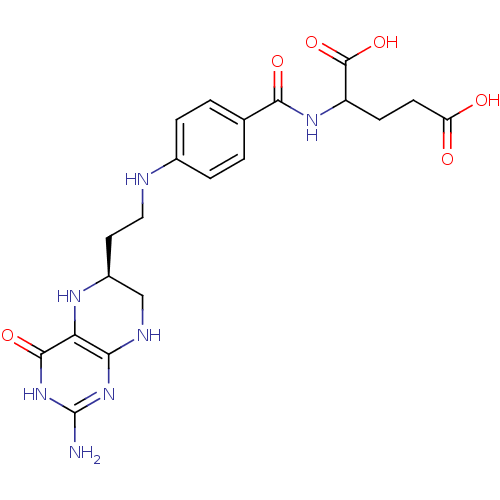 (2-{4-[2-(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pteri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Alabama Curated by ChEMBL | Assay Description Inhibition of the GAR transformylase in lactobacillus casei | J Med Chem 32: 1277-83 (1989) BindingDB Entry DOI: 10.7270/Q20K27J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50016662 (2-{4-[3-(2-Amino-4-oxo-3,4,7,8-tetrahydro-pteridin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Alabama Curated by ChEMBL | Assay Description Inhibition of the GAR transformylase in lactobacillus casei | J Med Chem 32: 1277-83 (1989) BindingDB Entry DOI: 10.7270/Q20K27J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50016658 (2-{4-[3-(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pteri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Alabama Curated by ChEMBL | Assay Description Inhibition of the GAR transformylase in MOLT-4 human leukemia cells | J Med Chem 32: 1277-83 (1989) BindingDB Entry DOI: 10.7270/Q20K27J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50016658 (2-{4-[3-(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pteri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Alabama Curated by ChEMBL | Assay Description Inhibition of the GAR transformylase in L1210 | J Med Chem 32: 1277-83 (1989) BindingDB Entry DOI: 10.7270/Q20K27J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50016660 (2-{4-[2-(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pteri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Alabama Curated by ChEMBL | Assay Description Inhibition of the GAR transformylase in L1210 | J Med Chem 32: 1277-83 (1989) BindingDB Entry DOI: 10.7270/Q20K27J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50022739 ((6RS) 2-{4-[2-(2-Amino-5-ethyl-4-oxo-3,4,5,6,7,8-h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of GAR transformylase from Lactobacillus casei | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase of Lactobacillus casei | J Med Chem 31: 150-3 (1988) BindingDB Entry DOI: 10.7270/Q2GX4C44 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Evaluated for inhibition of dihydrofolate reductase (DHFR) from Lactobacillus casei | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50022738 (2-{4-[2-(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Evaluated for inhibition of dihydrofolate reductase (DHFR) from Lactobacillus casei | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50022739 ((6RS) 2-{4-[2-(2-Amino-5-ethyl-4-oxo-3,4,5,6,7,8-h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of GAR transformylase from mammalian Manca | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50022739 ((6RS) 2-{4-[2-(2-Amino-5-ethyl-4-oxo-3,4,5,6,7,8-h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of GAR transformylase from mammalian L1210 cells | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50022741 ((5RS, 6RS) 2-{4-[2-(2-Amino-5-methyl-4-oxo-3,4,5,6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of GAR transformylase from mammalian Manca | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50022390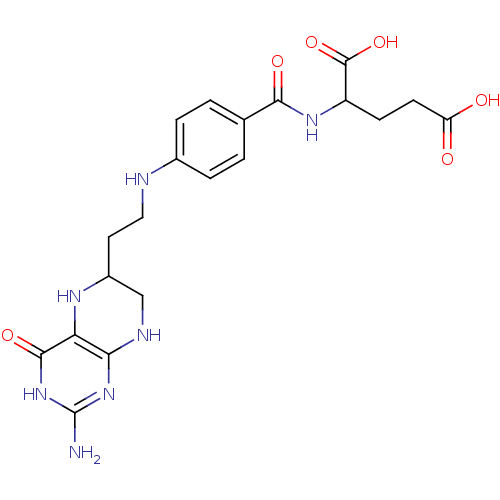 (2-{4-[2-(2-Amino-4-hydroxy-5,6,7,8-tetrahydro-pter...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibitory activity against thymidylate synthase of Escherichia coli | J Med Chem 31: 150-3 (1988) BindingDB Entry DOI: 10.7270/Q2GX4C44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50022738 (2-{4-[2-(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Evaluated for inhibition of thymidylate synthase (TS) from Lactobacillus casei | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50022388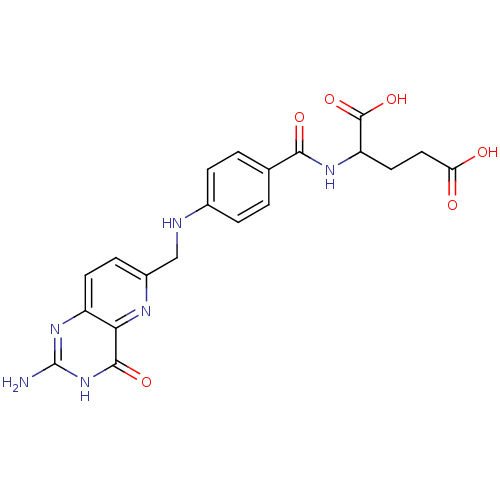 (2-{4-[(2-Amino-4-hydroxy-pyrido[3,2-d]pyrimidin-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibitory activity against AICAR formyltransferase of Lactobacillus casei | J Med Chem 31: 150-3 (1988) BindingDB Entry DOI: 10.7270/Q2GX4C44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50022388 (2-{4-[(2-Amino-4-hydroxy-pyrido[3,2-d]pyrimidin-6-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase of Lactobacillus casei | J Med Chem 31: 150-3 (1988) BindingDB Entry DOI: 10.7270/Q2GX4C44 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50022741 ((5RS, 6RS) 2-{4-[2-(2-Amino-5-methyl-4-oxo-3,4,5,6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of GAR transformylase from Lactobacillus casei | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50022388 (2-{4-[(2-Amino-4-hydroxy-pyrido[3,2-d]pyrimidin-6-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibitory activity against thymidylate synthase of Lactobacillus casei | J Med Chem 31: 150-3 (1988) BindingDB Entry DOI: 10.7270/Q2GX4C44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50022740 (2-{4-[2-(2-Amino-5-methyl-4-oxo-3,4-dihydro-pyrido...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Evaluated for inhibition of dihydrofolate reductase (DHFR) from Lactobacillus casei | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50022739 ((6RS) 2-{4-[2-(2-Amino-5-ethyl-4-oxo-3,4,5,6,7,8-h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Evaluated for inhibition of dihydrofolate reductase (DHFR) from Lactobacillus casei | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50022740 (2-{4-[2-(2-Amino-5-methyl-4-oxo-3,4-dihydro-pyrido...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of GAR transformylase from Lactobacillus casei | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50022739 ((6RS) 2-{4-[2-(2-Amino-5-ethyl-4-oxo-3,4,5,6,7,8-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibition of AICAR formyltransferase | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50011325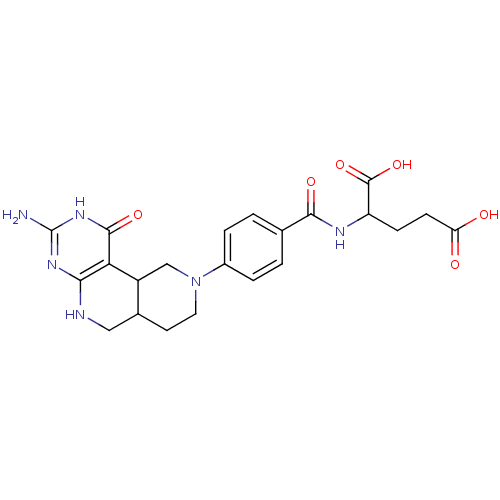 (2-[4-(2-Amino-4-oxo-4,4b,5,7,8,8a,9,10-octahydro-3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Compound was evaluated for the ability to inhibit Glycinamide ribonucleotide formyltransferase from Lactobacillus casei cells. | J Med Chem 34: 611-6 (1991) BindingDB Entry DOI: 10.7270/Q2MW2G32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of GAR transformylase from mammalian L1210 cells | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50022386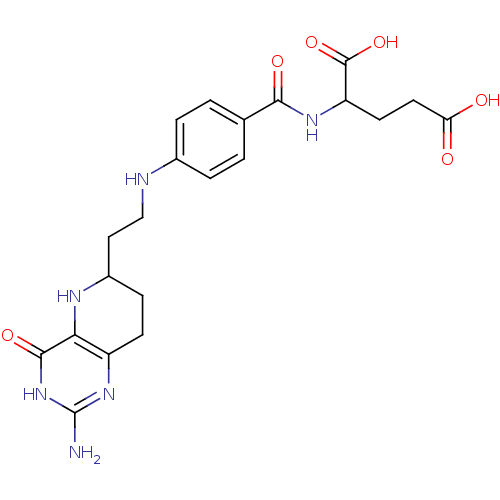 (2-{4-[2-(2-Amino-4-hydroxy-5,6,7,8-tetrahydro-pyri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibitory activity against GAR transformylase of Lactobacillus casei | J Med Chem 31: 150-3 (1988) BindingDB Entry DOI: 10.7270/Q2GX4C44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50022740 (2-{4-[2-(2-Amino-5-methyl-4-oxo-3,4-dihydro-pyrido...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of GAR transformylase from mammalian L1210 cells | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibitory activity against AICAR formyltransferase of Lactobacillus casei | J Med Chem 31: 150-3 (1988) BindingDB Entry DOI: 10.7270/Q2GX4C44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50022740 (2-{4-[2-(2-Amino-5-methyl-4-oxo-3,4-dihydro-pyrido...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of GAR transformylase from mammalian Manca | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50016661 (2-{4-[3-(2-Amino-4-oxo-3,4-dihydro-pteridin-6-yl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Alabama Curated by ChEMBL | Assay Description Inhibition of the GAR formyl transferase in lactobacillus casei | J Med Chem 32: 1277-83 (1989) BindingDB Entry DOI: 10.7270/Q20K27J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibitory activity against GAR transformylase of Lactobacillus casei | J Med Chem 31: 150-3 (1988) BindingDB Entry DOI: 10.7270/Q2GX4C44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50011325 (2-[4-(2-Amino-4-oxo-4,4b,5,7,8,8a,9,10-octahydro-3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Compound was evaluated for the ability to inhibit thymidylate synthase from Lactobacillus casei cells. | J Med Chem 34: 611-6 (1991) BindingDB Entry DOI: 10.7270/Q2MW2G32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50022387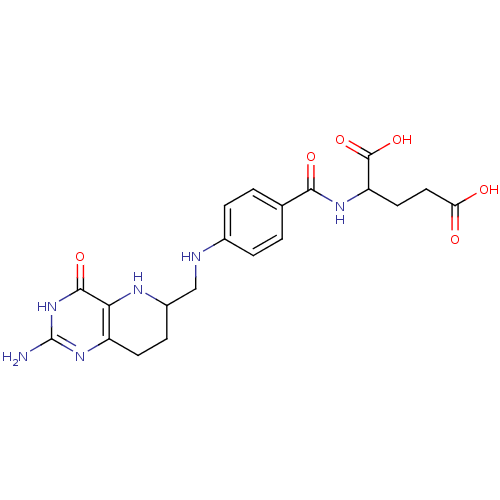 (2-{4-[(2-Amino-4-hydroxy-5,6,7,8-tetrahydro-pyrido...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibitory activity against AICAR formyltransferase of Lactobacillus casei | J Med Chem 31: 150-3 (1988) BindingDB Entry DOI: 10.7270/Q2GX4C44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50022386 (2-{4-[2-(2-Amino-4-hydroxy-5,6,7,8-tetrahydro-pyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibitory activity against AICAR formyltransferase of Lactobacillus casei | J Med Chem 31: 150-3 (1988) BindingDB Entry DOI: 10.7270/Q2GX4C44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50022387 (2-{4-[(2-Amino-4-hydroxy-5,6,7,8-tetrahydro-pyrido...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibitory activity against GAR transformylase of Lactobacillus casei | J Med Chem 31: 150-3 (1988) BindingDB Entry DOI: 10.7270/Q2GX4C44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of GAR transformylase from Lactobacillus casei | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50022388 (2-{4-[(2-Amino-4-hydroxy-pyrido[3,2-d]pyrimidin-6-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibitory activity against GAR transformylase of Lactobacillus casei | J Med Chem 31: 150-3 (1988) BindingDB Entry DOI: 10.7270/Q2GX4C44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50022741 ((5RS, 6RS) 2-{4-[2-(2-Amino-5-methyl-4-oxo-3,4,5,6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibition of AICAR formyltransferase | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50016657 (2-{4-[3-(2-Amino-4-oxo-3,4-dihydro-pteridin-6-yl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Alabama Curated by ChEMBL | Assay Description Inhibition of the GAR formyl transferase in L1210 | J Med Chem 32: 1277-83 (1989) BindingDB Entry DOI: 10.7270/Q20K27J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50016657 (2-{4-[3-(2-Amino-4-oxo-3,4-dihydro-pteridin-6-yl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Alabama Curated by ChEMBL | Assay Description Inhibition of the GAR formyl transferase in lactobacillus casei | J Med Chem 32: 1277-83 (1989) BindingDB Entry DOI: 10.7270/Q20K27J9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50022385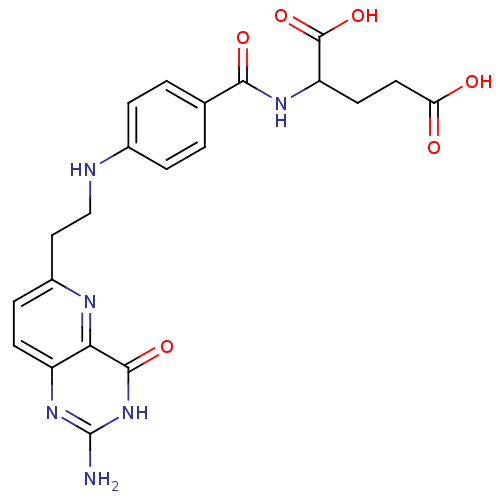 (2-{4-[2-(2-Amino-4-hydroxy-pyrido[3,2-d]pyrimidin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibitory activity against GAR transformylase of Lactobacillus casei | J Med Chem 31: 150-3 (1988) BindingDB Entry DOI: 10.7270/Q2GX4C44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50022385 (2-{4-[2-(2-Amino-4-hydroxy-pyrido[3,2-d]pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibitory activity against AICAR formyltransferase of Lactobacillus casei | J Med Chem 31: 150-3 (1988) BindingDB Entry DOI: 10.7270/Q2GX4C44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50022740 (2-{4-[2-(2-Amino-5-methyl-4-oxo-3,4-dihydro-pyrido...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Evaluated for inhibition of thymidylate synthase (TS) from Lactobacillus casei | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50022387 (2-{4-[(2-Amino-4-hydroxy-5,6,7,8-tetrahydro-pyrido...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase of Lactobacillus casei | J Med Chem 31: 150-3 (1988) BindingDB Entry DOI: 10.7270/Q2GX4C44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 63 total ) | Next | Last >> |