

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (MOUSE) | BDBM50177898 (2-Fluoro-N-[4-methoxymethyl-1-(2-thiophen-2-yl-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description Binding constant for Opioid receptor mu 1 in mouse | Bioorg Med Chem Lett 15: 1773-7 (2005) Article DOI: 10.1016/j.bmcl.2005.02.049 BindingDB Entry DOI: 10.7270/Q28916M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50300442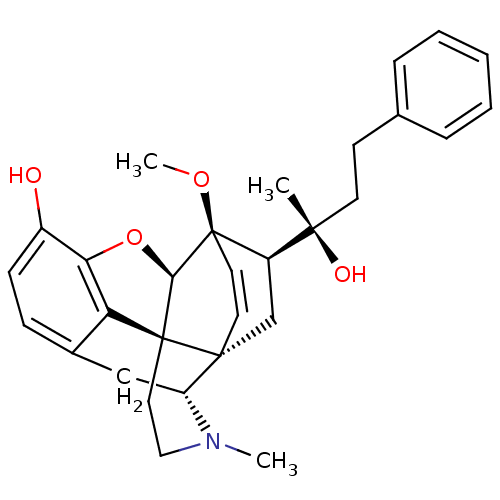 ((20R)-4,5-alpha-Epoxy-17-methyl-3-hydroxy-6-methox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biomedizinische Forschungsreagenzien GmbH Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human cloned kappa opioid receptor expressed in CHO cells after 2 hrs by liquid scintillation counting | J Med Chem 52: 5586-9 (2009) Article DOI: 10.1021/jm900892x BindingDB Entry DOI: 10.7270/Q2SQ90F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50177898 (2-Fluoro-N-[4-methoxymethyl-1-(2-thiophen-2-yl-eth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from recombinant human mu opioid receptor | J Med Chem 48: 7720-32 (2005) Article DOI: 10.1021/jm0507274 BindingDB Entry DOI: 10.7270/Q2G44PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50177895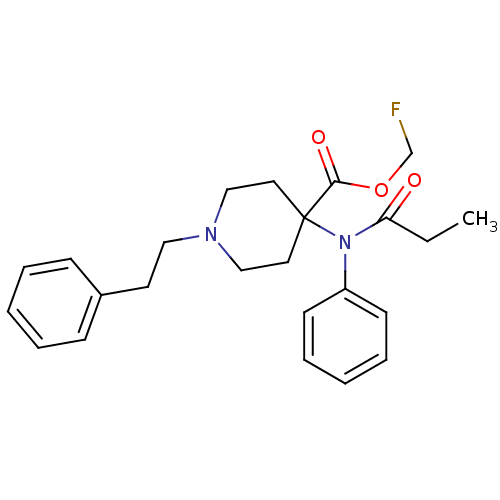 (CHEMBL200456 | fluoromethyl 1-(2-phenylethyl)-4-(N...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from recombinant human mu opioid receptor | J Med Chem 48: 7720-32 (2005) Article DOI: 10.1021/jm0507274 BindingDB Entry DOI: 10.7270/Q2G44PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50331552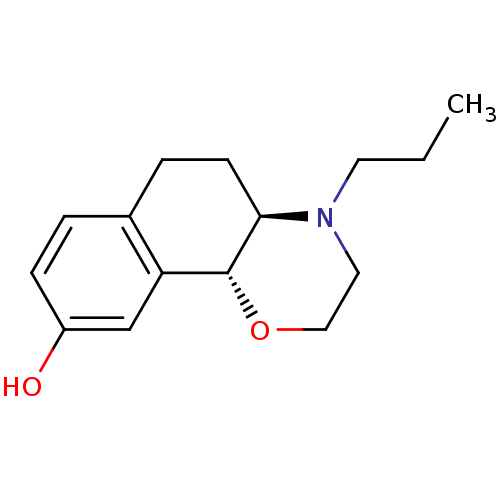 (CHEMBL1288585 | [11C]-(+)-(4aR,10bR)-4-propyl-3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universität Erlangen-Nürnberg Curated by ChEMBL | Assay Description Binding affinity to human dopamine D3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6933-7 (2010) Article DOI: 10.1016/j.bmcl.2010.09.142 BindingDB Entry DOI: 10.7270/Q2474B38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50300442 ((20R)-4,5-alpha-Epoxy-17-methyl-3-hydroxy-6-methox...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biomedizinische Forschungsreagenzien GmbH Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human cloned mu opioid receptor expressed in CHO cells after 2 hrs by liquid scintillation counting | J Med Chem 52: 5586-9 (2009) Article DOI: 10.1021/jm900892x BindingDB Entry DOI: 10.7270/Q2SQ90F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol lipase-alpha (Mus musculus) | BDBM50519345 (CHEMBL4447844) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Inhibition of mouse DAGL-alpha expressed in HEK293T cell membranes assessed as inhibition constant pre-incubated for 20 mins before PNP-butyrate subs... | J Med Chem 62: 7910-7922 (2019) Article DOI: 10.1021/acs.jmedchem.9b00686 BindingDB Entry DOI: 10.7270/Q2GQ724G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol lipase-alpha (Mus musculus) | BDBM50211257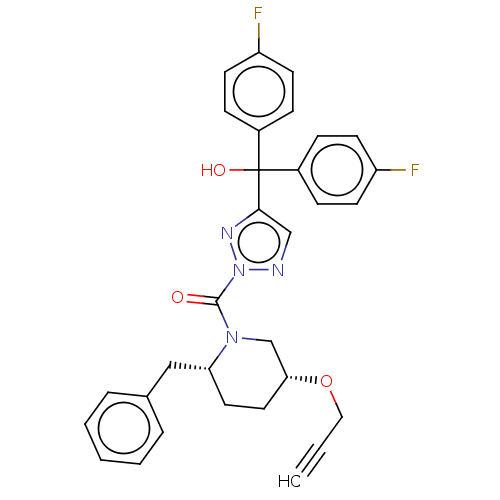 (CHEMBL3895863 | US10583137, Compound 14) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Inhibition of mouse DAGL-alpha expressed in HEK293T cell membranes assessed as inhibition constant pre-incubated for 20 mins before PNP-butyrate subs... | J Med Chem 62: 7910-7922 (2019) Article DOI: 10.1021/acs.jmedchem.9b00686 BindingDB Entry DOI: 10.7270/Q2GQ724G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062443 (5-(4-Chloro-phenyl)-9-fluoro-2,2,4-trimethyl-2,5-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity measured using baculovirus-expressed hPR-A in sf21 cells. | J Med Chem 41: 303-10 (1998) Article DOI: 10.1021/jm9705770 BindingDB Entry DOI: 10.7270/Q2RV0PC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062432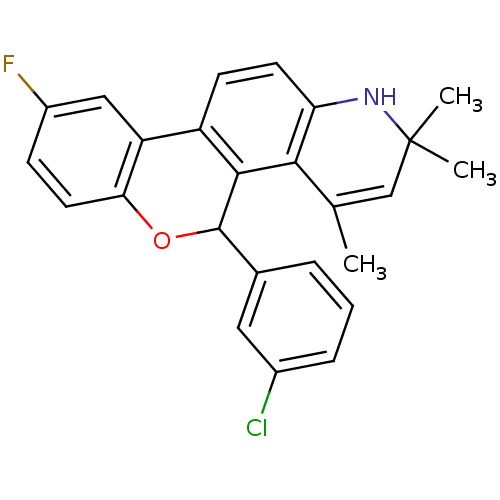 (5-(3-Chloro-phenyl)-9-fluoro-2,2,4-trimethyl-2,5-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity measured using baculovirus-expressed hPR-A in sf21 cells. | J Med Chem 41: 303-10 (1998) Article DOI: 10.1021/jm9705770 BindingDB Entry DOI: 10.7270/Q2RV0PC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132057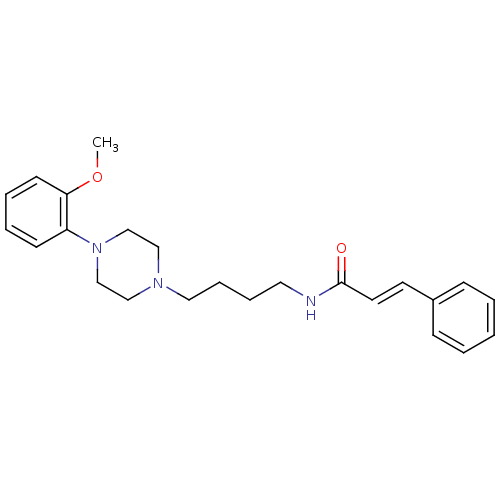 (CHEMBL1180430 | CHEMBL126128 | N-{4-[4-(2-Methoxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universität Erlangen-Nürnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6933-7 (2010) Article DOI: 10.1016/j.bmcl.2010.09.142 BindingDB Entry DOI: 10.7270/Q2474B38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062444 (9-Chloro-5-(3-fluoro-phenyl)-2,2,4-trimethyl-2,5-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity measured using baculovirus-expressed hPR-A in sf21 cells. | J Med Chem 41: 303-10 (1998) Article DOI: 10.1021/jm9705770 BindingDB Entry DOI: 10.7270/Q2RV0PC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity measured using baculovirus-expressed hPR-A in sf21 cells. | J Med Chem 41: 303-10 (1998) Article DOI: 10.1021/jm9705770 BindingDB Entry DOI: 10.7270/Q2RV0PC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity was determined against hPR-A (human progesterone receptor) using progesterone radioligand in competitive binding assay | Bioorg Med Chem Lett 8: 3365-70 (1999) BindingDB Entry DOI: 10.7270/Q2BC402X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132069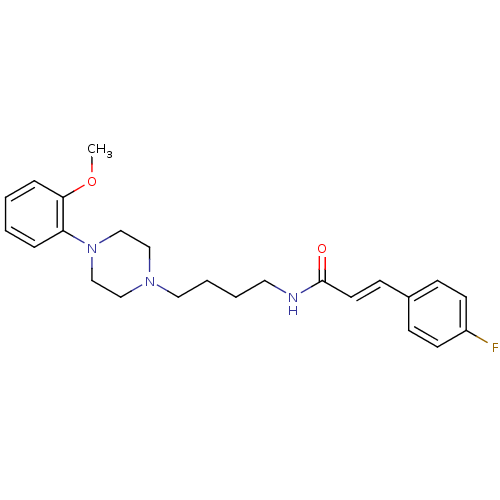 (3-(4-Fluoro-phenyl)-N-{4-[4-(2-methoxy-phenyl)-pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universität Erlangen-Nürnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6933-7 (2010) Article DOI: 10.1016/j.bmcl.2010.09.142 BindingDB Entry DOI: 10.7270/Q2474B38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol lipase-alpha (Mus musculus) | BDBM50211236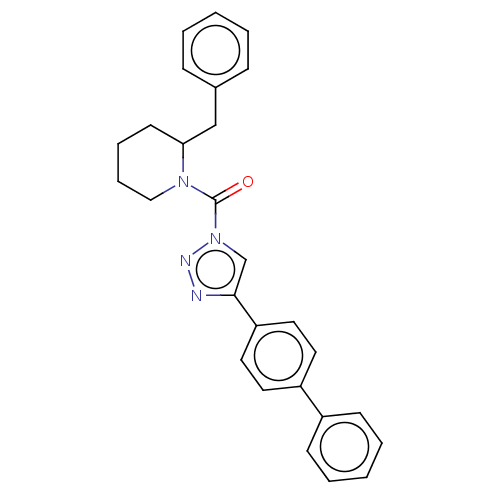 (CHEMBL2144065) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Inhibition of mouse DAGL-alpha expressed in HEK293T cell membranes assessed as inhibition constant pre-incubated for 20 mins before PNP-butyrate subs... | J Med Chem 62: 7910-7922 (2019) Article DOI: 10.1021/acs.jmedchem.9b00686 BindingDB Entry DOI: 10.7270/Q2GQ724G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062427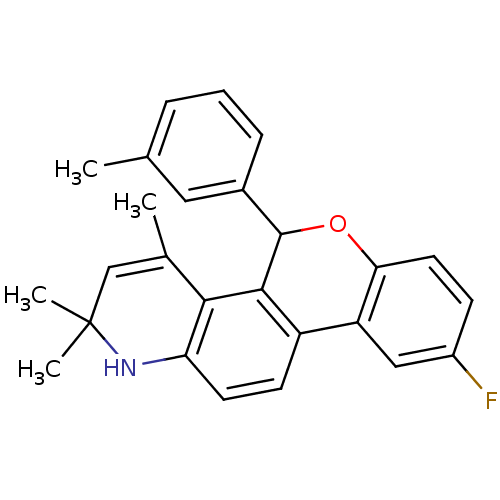 (9-Fluoro-2,2,4-trimethyl-5-m-tolyl-2,5-dihydro-1H-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity measured using baculovirus-expressed hPR-A in sf21 cells. | J Med Chem 41: 303-10 (1998) Article DOI: 10.1021/jm9705770 BindingDB Entry DOI: 10.7270/Q2RV0PC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50065585 (5-(4-Chloro-3-methyl-phenyl)-9-fluoro-2,2,4-trimet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity for human progesterone receptor isoform A expressed in CV-1 cells | J Med Chem 41: 2779-85 (1998) Article DOI: 10.1021/jm980190c BindingDB Entry DOI: 10.7270/Q2CV4JFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50065585 (5-(4-Chloro-3-methyl-phenyl)-9-fluoro-2,2,4-trimet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity for human progesterone receptor isoform A expressed in CV-1 cells | J Med Chem 41: 2779-85 (1998) Article DOI: 10.1021/jm980190c BindingDB Entry DOI: 10.7270/Q2CV4JFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50072701 (9-Chloro-2,2,4,5-tetramethyl-2,5-dihydro-1H-6-oxa-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity was determined against hPR-A (human progesterone receptor) using progesterone radioligand in competitive binding assay | Bioorg Med Chem Lett 8: 3365-70 (1999) BindingDB Entry DOI: 10.7270/Q2BC402X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132047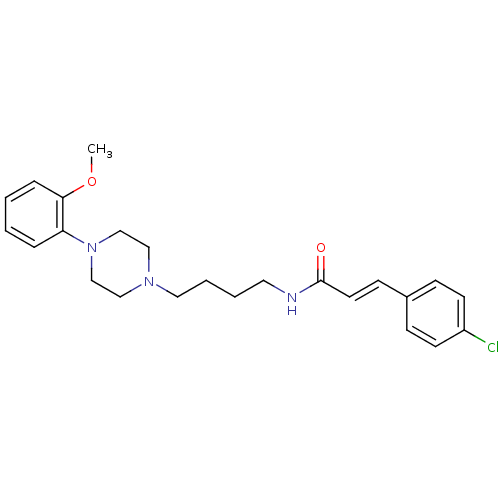 (3-(4-Chloro-phenyl)-N-{4-[4-(2-methoxy-phenyl)-pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universität Erlangen-Nürnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6933-7 (2010) Article DOI: 10.1016/j.bmcl.2010.09.142 BindingDB Entry DOI: 10.7270/Q2474B38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062430 (9-Chloro-5-(3-chloro-phenyl)-2,2,4-trimethyl-2,5-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity for human progesterone receptor isoform A expressed in CV-1 cells | J Med Chem 41: 2779-85 (1998) Article DOI: 10.1021/jm980190c BindingDB Entry DOI: 10.7270/Q2CV4JFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062430 (9-Chloro-5-(3-chloro-phenyl)-2,2,4-trimethyl-2,5-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity measured using baculovirus-expressed hPR-A in sf21 cells. | J Med Chem 41: 303-10 (1998) Article DOI: 10.1021/jm9705770 BindingDB Entry DOI: 10.7270/Q2RV0PC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062429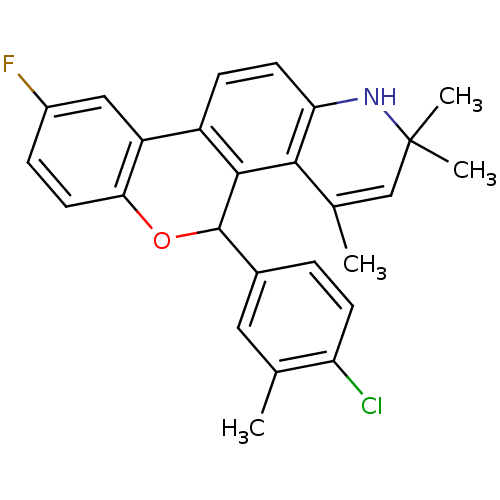 (5-(4-Chloro-3-methyl-phenyl)-9-fluoro-2,2,4-trimet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity for human progesterone receptor isoform A expressed in CV-1 cells | J Med Chem 41: 2779-85 (1998) Article DOI: 10.1021/jm980190c BindingDB Entry DOI: 10.7270/Q2CV4JFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062429 (5-(4-Chloro-3-methyl-phenyl)-9-fluoro-2,2,4-trimet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity measured using baculovirus-expressed hPR-A in sf21 cells. | J Med Chem 41: 303-10 (1998) Article DOI: 10.1021/jm9705770 BindingDB Entry DOI: 10.7270/Q2RV0PC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062439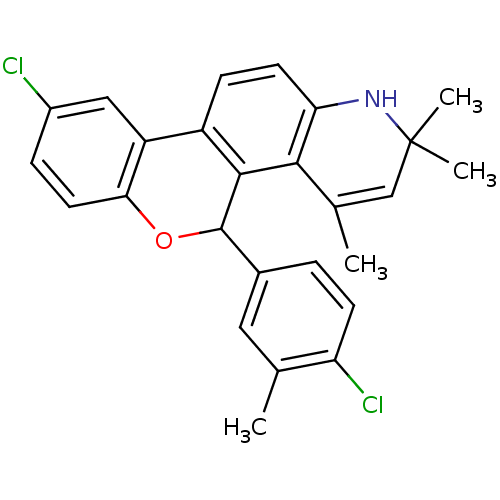 (9-Chloro-5-(4-chloro-3-methyl-phenyl)-2,2,4-trimet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity measured using baculovirus-expressed hPR-A in sf21 cells. | J Med Chem 41: 303-10 (1998) Article DOI: 10.1021/jm9705770 BindingDB Entry DOI: 10.7270/Q2RV0PC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062439 (9-Chloro-5-(4-chloro-3-methyl-phenyl)-2,2,4-trimet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity for human progesterone receptor isoform A expressed in CV-1 cells | J Med Chem 41: 2779-85 (1998) Article DOI: 10.1021/jm980190c BindingDB Entry DOI: 10.7270/Q2CV4JFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50331549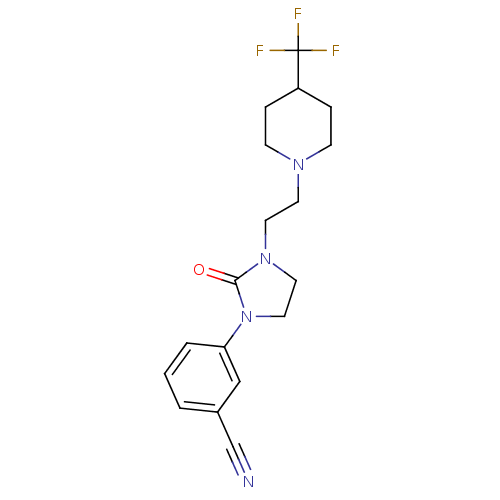 (CHEMBL567286 | [11C]-3-(2-oxo-3-(2-(4-(trifluorome...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universität Erlangen-Nürnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6933-7 (2010) Article DOI: 10.1016/j.bmcl.2010.09.142 BindingDB Entry DOI: 10.7270/Q2474B38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062438 (9-Chloro-2,2,4-trimethyl-5-m-tolyl-2,5-dihydro-1H-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity measured using baculovirus-expressed hPR-A in sf21 cells. | J Med Chem 41: 303-10 (1998) Article DOI: 10.1021/jm9705770 BindingDB Entry DOI: 10.7270/Q2RV0PC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50205280 (2,6-diphenyl-8-isobutyl-1-deazapurine | CHEMBL2214...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden/Amsterdam Center for Drug Research Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cells | J Med Chem 50: 828-34 (2007) Article DOI: 10.1021/jm0607956 BindingDB Entry DOI: 10.7270/Q2VX0G65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062442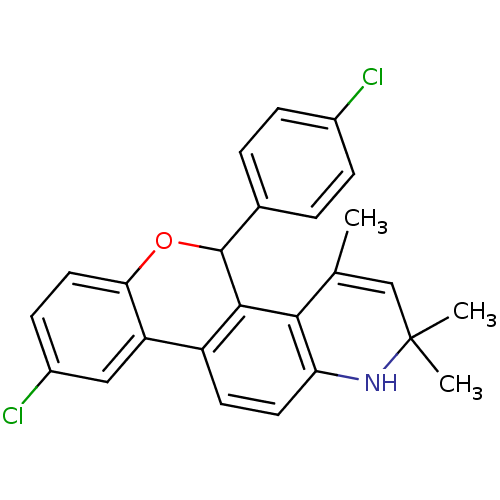 (9-Chloro-5-(4-chloro-phenyl)-2,2,4-trimethyl-2,5-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity measured using baculovirus-expressed hPR-A in sf21 cells. | J Med Chem 41: 303-10 (1998) Article DOI: 10.1021/jm9705770 BindingDB Entry DOI: 10.7270/Q2RV0PC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062445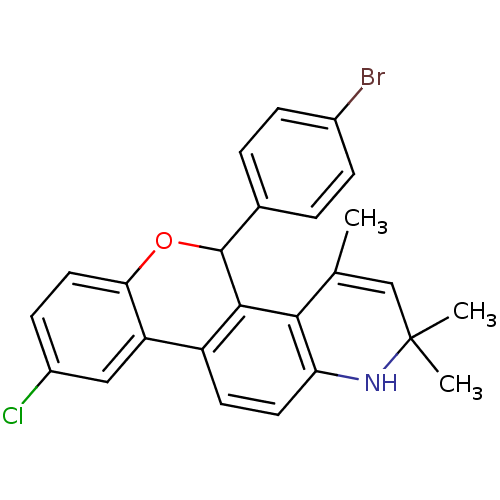 (5-(4-Bromo-phenyl)-9-chloro-2,2,4-trimethyl-2,5-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity measured using baculovirus-expressed hPR-A in sf21 cells. | J Med Chem 41: 303-10 (1998) Article DOI: 10.1021/jm9705770 BindingDB Entry DOI: 10.7270/Q2RV0PC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50004774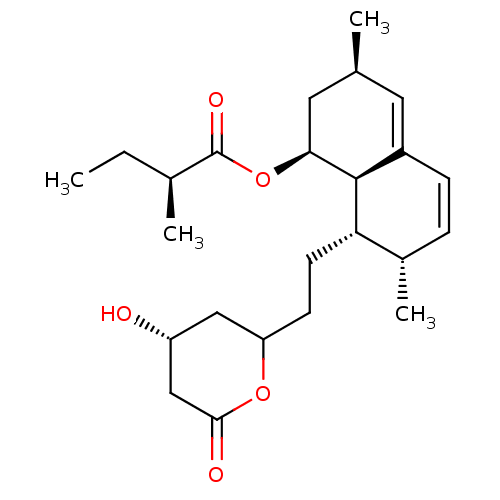 ((S)-2-Methyl-butyric acid (1S,3R,7S,8S,8aR)-8-[2-(...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human HMGCoA reductase | J Nat Prod 52: 153-161 (1989) Article DOI: 10.1021/np50061a020 BindingDB Entry DOI: 10.7270/Q28052MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd Curated by ChEMBL | Assay Description Inhibition of Aurora-A by coupled assay | Bioorg Med Chem Lett 19: 3586-92 (2009) Article DOI: 10.1016/j.bmcl.2009.04.136 BindingDB Entry DOI: 10.7270/Q28K7944 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50205281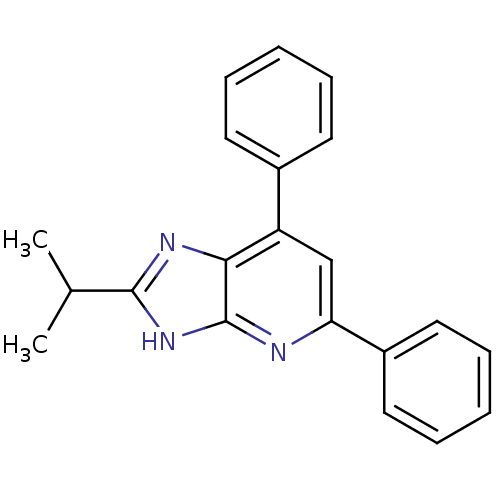 (2,6-diphenyl-8-isopropyl-1-deazapurine | CHEMBL221...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden/Amsterdam Center for Drug Research Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cells | J Med Chem 50: 828-34 (2007) Article DOI: 10.1021/jm0607956 BindingDB Entry DOI: 10.7270/Q2VX0G65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50205286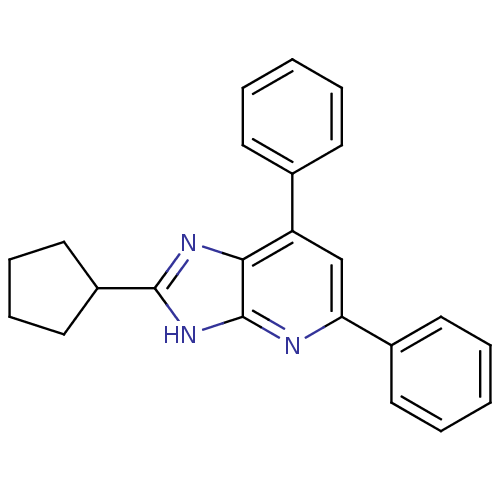 (8-cyclopentyl-2,6-diphenyl-1-deazapurin | CHEMBL21...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden/Amsterdam Center for Drug Research Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cells | J Med Chem 50: 828-34 (2007) Article DOI: 10.1021/jm0607956 BindingDB Entry DOI: 10.7270/Q2VX0G65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062417 (5-(4-Chloro-phenyl)-2,2,4-trimethyl-2,5-dihydro-1H...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity measured using baculovirus-expressed hPR-A in sf21 cells. | J Med Chem 41: 303-10 (1998) Article DOI: 10.1021/jm9705770 BindingDB Entry DOI: 10.7270/Q2RV0PC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062433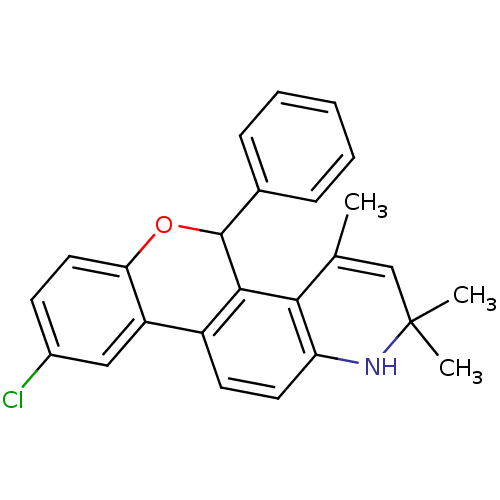 (9-Chloro-2,2,4-trimethyl-5-phenyl-2,5-dihydro-1H-6...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity measured using baculovirus-expressed hPR-A in sf21 cells. | J Med Chem 41: 303-10 (1998) Article DOI: 10.1021/jm9705770 BindingDB Entry DOI: 10.7270/Q2RV0PC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50177899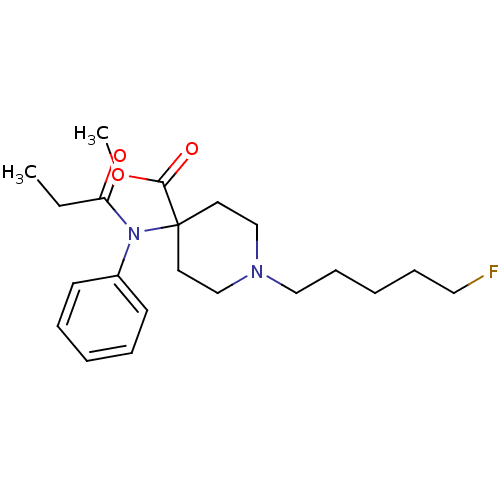 (CHEMBL371710 | methyl 4-[(N-1-oxopropyl)-N-phenyla...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from recombinant human mu opioid receptor | J Med Chem 48: 7720-32 (2005) Article DOI: 10.1021/jm0507274 BindingDB Entry DOI: 10.7270/Q2G44PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062436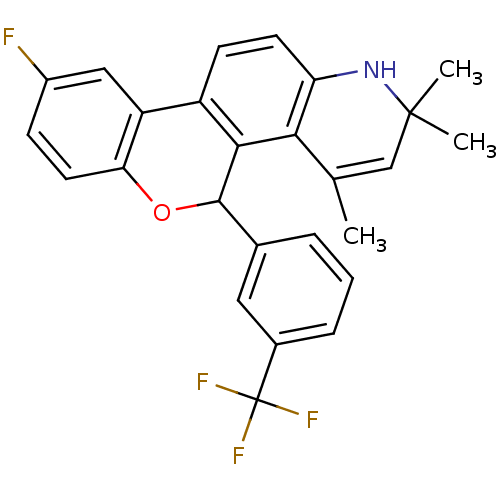 (9-Fluoro-2,2,4-trimethyl-5-(3-trifluoromethyl-phen...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity measured using baculovirus-expressed hPR-A in sf21 cells. | J Med Chem 41: 303-10 (1998) Article DOI: 10.1021/jm9705770 BindingDB Entry DOI: 10.7270/Q2RV0PC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50277679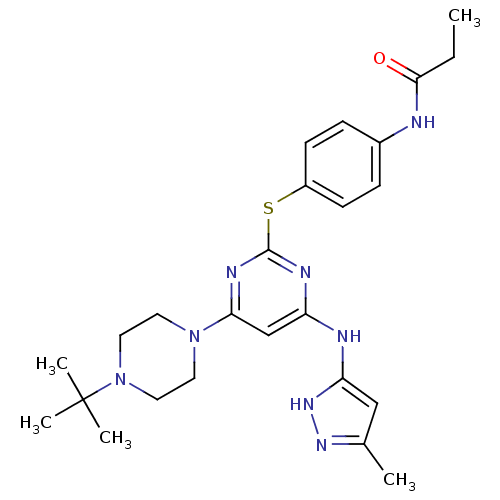 (CHEMBL484006 | N-(4-(4-(4-tert-butylpiperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd Curated by ChEMBL | Assay Description Inhibition of Aurora-B by time dependent kinetic study | Bioorg Med Chem Lett 19: 3586-92 (2009) Article DOI: 10.1016/j.bmcl.2009.04.136 BindingDB Entry DOI: 10.7270/Q28K7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50205285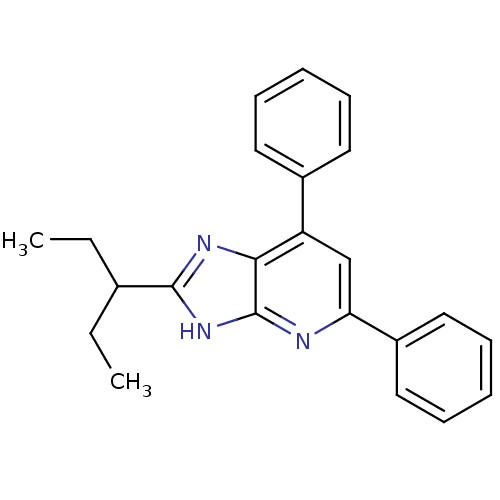 (2,6-diphenyl-8-(1-ethylpropyl)-1-deazapurine | CHE...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden/Amsterdam Center for Drug Research Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cells | J Med Chem 50: 828-34 (2007) Article DOI: 10.1021/jm0607956 BindingDB Entry DOI: 10.7270/Q2VX0G65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50072704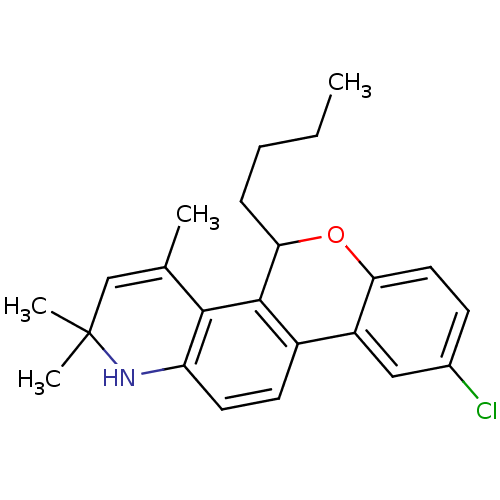 (5-Butyl-9-chloro-2,2,4-trimethyl-2,5-dihydro-1H-6-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity was determined against hPR-A (human progesterone receptor) using progesterone radioligand in competitive binding assay | Bioorg Med Chem Lett 8: 3365-70 (1999) BindingDB Entry DOI: 10.7270/Q2BC402X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50205283 (2,6-diphenyl-8-cyclohexyl-1-deazapurine | CHEMBL21...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden/Amsterdam Center for Drug Research Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cells | J Med Chem 50: 828-34 (2007) Article DOI: 10.1021/jm0607956 BindingDB Entry DOI: 10.7270/Q2VX0G65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062437 (9-Chloro-5-(4-methoxy-phenyl)-2,2,4-trimethyl-2,5-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity measured using baculovirus-expressed hPR-A in sf21 cells. | J Med Chem 41: 303-10 (1998) Article DOI: 10.1021/jm9705770 BindingDB Entry DOI: 10.7270/Q2RV0PC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50072714 (5-Butyl-2,2,4-trimethyl-2,5-dihydro-1H-6-oxa-1-aza...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity was determined against hPR-A (human progesterone receptor) using progesterone radioligand in competitive binding assay | Bioorg Med Chem Lett 8: 3365-70 (1999) BindingDB Entry DOI: 10.7270/Q2BC402X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50277640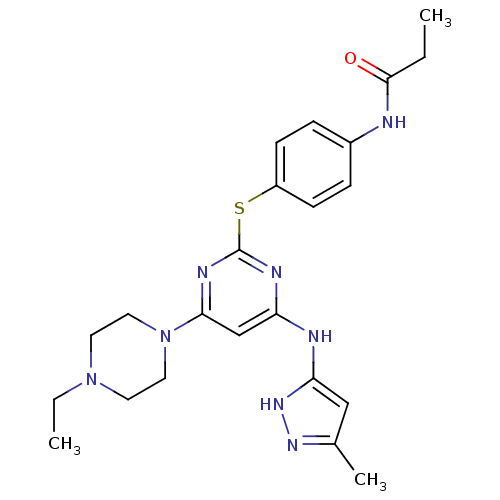 (CHEMBL485351 | N-(4-(4-(4-ethylpiperazin-1-yl)-6-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd Curated by ChEMBL | Assay Description Inhibition of Aurora-A by coupled assay | Bioorg Med Chem Lett 19: 3586-92 (2009) Article DOI: 10.1016/j.bmcl.2009.04.136 BindingDB Entry DOI: 10.7270/Q28K7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50277678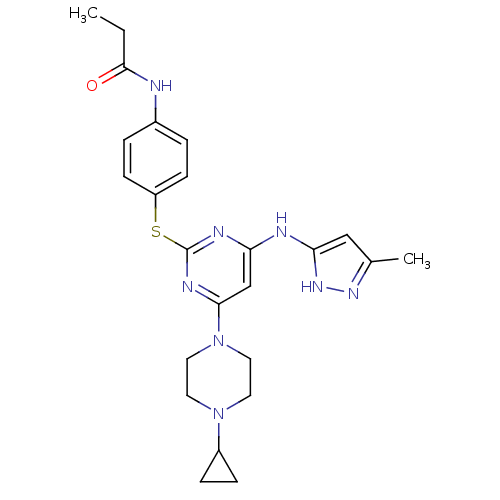 (CHEMBL485186 | N-(4-(4-(4-cyclopropylpiperazin-1-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd Curated by ChEMBL | Assay Description Inhibition of Aurora-A by coupled assay | Bioorg Med Chem Lett 19: 3586-92 (2009) Article DOI: 10.1016/j.bmcl.2009.04.136 BindingDB Entry DOI: 10.7270/Q28K7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50293689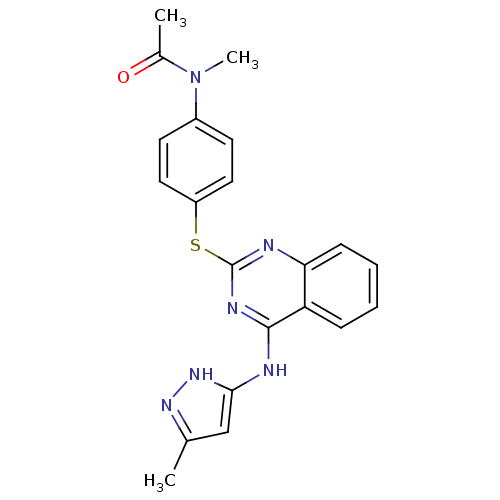 (CHEMBL549668 | N-methyl-N-(4-(4-(5-methyl-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd Curated by ChEMBL | Assay Description Inhibition of Aurora-A by coupled assay | Bioorg Med Chem Lett 19: 3586-92 (2009) Article DOI: 10.1016/j.bmcl.2009.04.136 BindingDB Entry DOI: 10.7270/Q28K7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50293686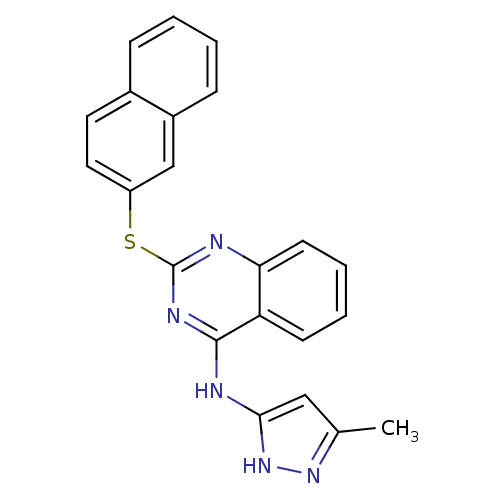 (CHEMBL559900 | N-(5-methyl-1H-pyrazol-3-yl)-2-(nap...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd Curated by ChEMBL | Assay Description Inhibition of Aurora-A by coupled assay | Bioorg Med Chem Lett 19: 3586-92 (2009) Article DOI: 10.1016/j.bmcl.2009.04.136 BindingDB Entry DOI: 10.7270/Q28K7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4035 total ) | Next | Last >> |