 Found 183 hits with Last Name = 'blevitt' and Initial = 'jm'
Found 183 hits with Last Name = 'blevitt' and Initial = 'jm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50402386
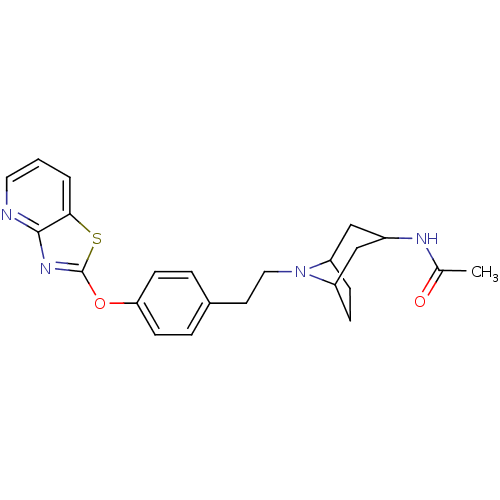
(CHEMBL2207747)Show SMILES CC(=O)NC1CC2CCC(C1)N2CCc1ccc(Oc2nc3ncccc3s2)cc1 |TLB:3:4:11:7.8,THB:12:11:4.5.10:7.8| Show InChI InChI=1S/C23H26N4O2S/c1-15(28)25-17-13-18-6-7-19(14-17)27(18)12-10-16-4-8-20(9-5-16)29-23-26-22-21(30-23)3-2-11-24-22/h2-5,8-9,11,17-19H,6-7,10,12-14H2,1H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... |
Bioorg Med Chem Lett 22: 7504-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.036
BindingDB Entry DOI: 10.7270/Q2ZG6TDQ |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50377765
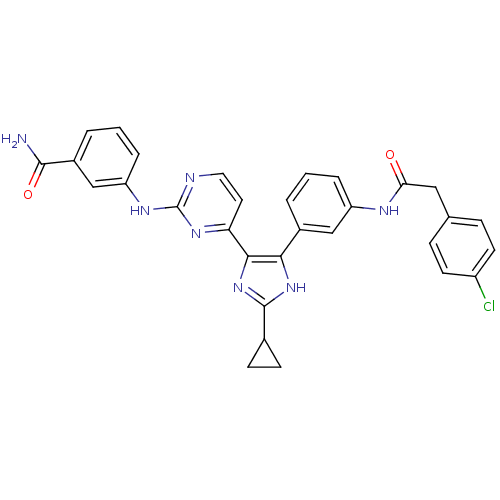
(CHEMBL254966)Show SMILES NC(=O)c1cccc(Nc2nccc(n2)-c2nc([nH]c2-c2cccc(NC(=O)Cc3ccc(Cl)cc3)c2)C2CC2)c1 Show InChI InChI=1S/C31H26ClN7O2/c32-22-11-7-18(8-12-22)15-26(40)35-23-5-1-3-20(16-23)27-28(39-30(38-27)19-9-10-19)25-13-14-34-31(37-25)36-24-6-2-4-21(17-24)29(33)41/h1-8,11-14,16-17,19H,9-10,15H2,(H2,33,41)(H,35,40)(H,38,39)(H,34,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of C-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50402382
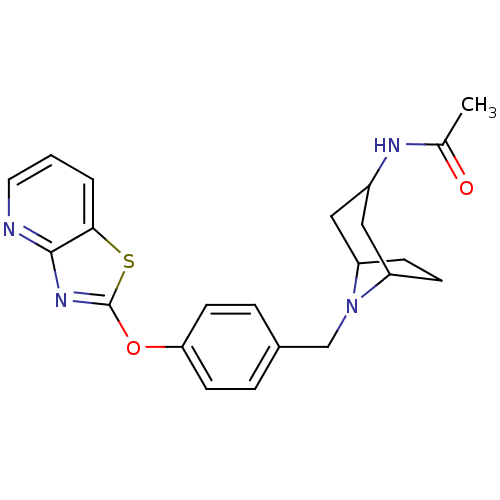
(CHEMBL2207751)Show SMILES CC(=O)NC1CC2CCC(C1)N2Cc1ccc(Oc2nc3ncccc3s2)cc1 |TLB:3:4:11:7.8| Show InChI InChI=1S/C22H24N4O2S/c1-14(27)24-16-11-17-6-7-18(12-16)26(17)13-15-4-8-19(9-5-15)28-22-25-21-20(29-22)3-2-10-23-21/h2-5,8-10,16-18H,6-7,11-13H2,1H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... |
Bioorg Med Chem Lett 22: 7504-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.036
BindingDB Entry DOI: 10.7270/Q2ZG6TDQ |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50377767

(CHEMBL429498)Show SMILES Clc1ccc(CC(=O)Nc2cccc(c2)-c2[nH]c(nc2-c2ccnc(NCCN3CCNC3=O)n2)C2CC2)cc1 Show InChI InChI=1S/C29H29ClN8O2/c30-21-8-4-18(5-9-21)16-24(39)34-22-3-1-2-20(17-22)25-26(37-27(36-25)19-6-7-19)23-10-11-31-28(35-23)32-12-14-38-15-13-33-29(38)40/h1-5,8-11,17,19H,6-7,12-16H2,(H,33,40)(H,34,39)(H,36,37)(H,31,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of C-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50377766
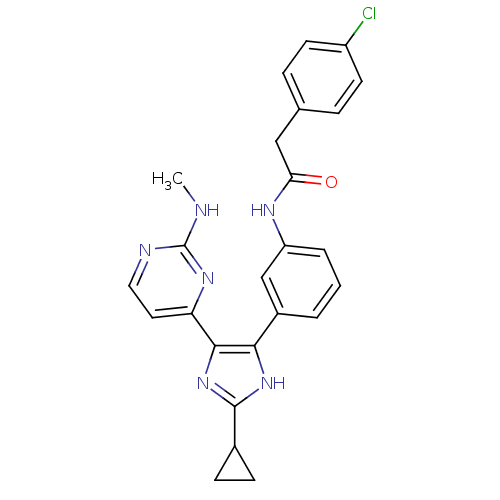
(CHEMBL403854)Show SMILES CNc1nccc(n1)-c1nc([nH]c1-c1cccc(NC(=O)Cc2ccc(Cl)cc2)c1)C1CC1 Show InChI InChI=1S/C25H23ClN6O/c1-27-25-28-12-11-20(30-25)23-22(31-24(32-23)16-7-8-16)17-3-2-4-19(14-17)29-21(33)13-15-5-9-18(26)10-6-15/h2-6,9-12,14,16H,7-8,13H2,1H3,(H,29,33)(H,31,32)(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of C-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50425166

(CHEMBL2313573)Show SMILES CC(=O)N[C@@H]1C[C@@H]2CC[C@H](C1)N2Cc1coc2cc(Oc3nc4ncccc4s3)ccc12 |r,THB:12:11:10.4.5:7.8| Show InChI InChI=1S/C24H24N4O3S/c1-14(29)26-16-9-17-4-5-18(10-16)28(17)12-15-13-30-21-11-19(6-7-20(15)21)31-24-27-23-22(32-24)3-2-8-25-23/h2-3,6-8,11,13,16-18H,4-5,9-10,12H2,1H3,(H,26,29)/t16-,17+,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human LTA4H expressed in Sf9 cells using LTA4 as substrate incubated for 10 mins prior to substrate addition measured after... |
Bioorg Med Chem Lett 23: 811-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.074
BindingDB Entry DOI: 10.7270/Q27H1KW1 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50377763
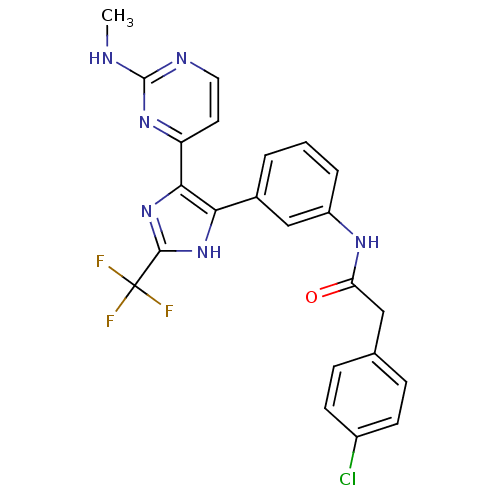
(CHEMBL258454)Show SMILES CNc1nccc(n1)-c1nc([nH]c1-c1cccc(NC(=O)Cc2ccc(Cl)cc2)c1)C(F)(F)F Show InChI InChI=1S/C23H18ClF3N6O/c1-28-22-29-10-9-17(31-22)20-19(32-21(33-20)23(25,26)27)14-3-2-4-16(12-14)30-18(34)11-13-5-7-15(24)8-6-13/h2-10,12H,11H2,1H3,(H,30,34)(H,32,33)(H,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of C-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50163251
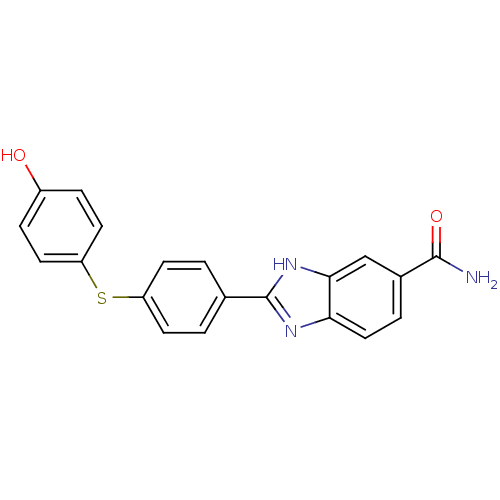
(2-(4-(4-hydroxyphenylthio)phenyl)-1H-benzo[d]imida...)Show SMILES NC(=O)c1ccc2nc([nH]c2c1)-c1ccc(Sc2ccc(O)cc2)cc1 Show InChI InChI=1S/C20H15N3O2S/c21-19(25)13-3-10-17-18(11-13)23-20(22-17)12-1-6-15(7-2-12)26-16-8-4-14(24)5-9-16/h1-11,24H,(H2,21,25)(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Chk2 |
Bioorg Med Chem Lett 17: 6467-71 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.098
BindingDB Entry DOI: 10.7270/Q2KP81XB |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50425168
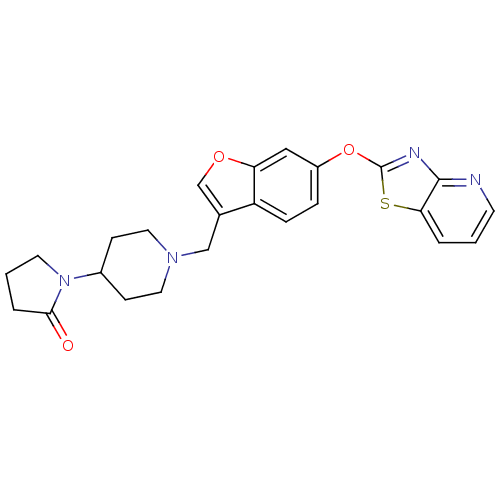
(CHEMBL2313571)Show SMILES O=C1CCCN1C1CCN(Cc2coc3cc(Oc4nc5ncccc5s4)ccc23)CC1 Show InChI InChI=1S/C24H24N4O3S/c29-22-4-2-10-28(22)17-7-11-27(12-8-17)14-16-15-30-20-13-18(5-6-19(16)20)31-24-26-23-21(32-24)3-1-9-25-23/h1,3,5-6,9,13,15,17H,2,4,7-8,10-12,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human LTA4H expressed in Sf9 cells using LTA4 as substrate incubated for 10 mins prior to substrate addition measured after... |
Bioorg Med Chem Lett 23: 811-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.074
BindingDB Entry DOI: 10.7270/Q27H1KW1 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50402403
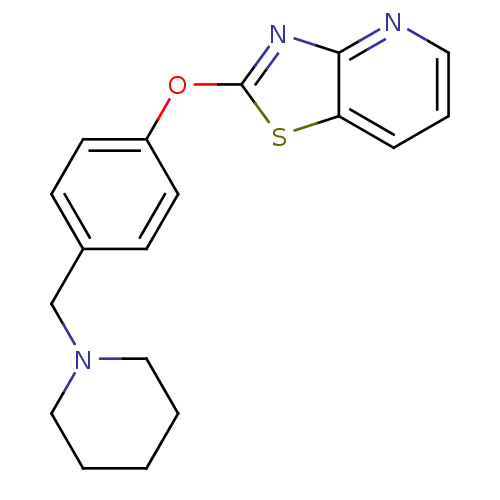
(CHEMBL2207730)Show InChI InChI=1S/C18H19N3OS/c1-2-11-21(12-3-1)13-14-6-8-15(9-7-14)22-18-20-17-16(23-18)5-4-10-19-17/h4-10H,1-3,11-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... |
Bioorg Med Chem Lett 22: 7504-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.036
BindingDB Entry DOI: 10.7270/Q2ZG6TDQ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50402391
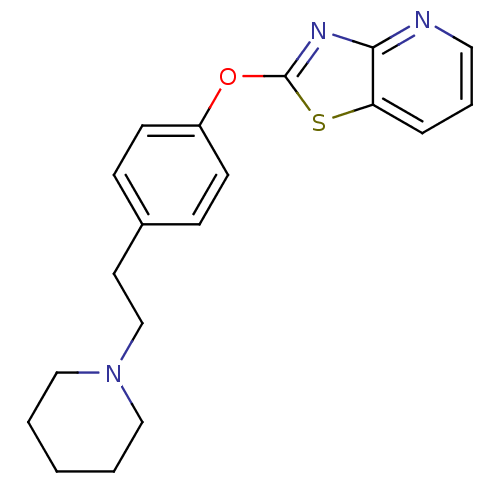
(CHEMBL2207742)Show InChI InChI=1S/C19H21N3OS/c1-2-12-22(13-3-1)14-10-15-6-8-16(9-7-15)23-19-21-18-17(24-19)5-4-11-20-18/h4-9,11H,1-3,10,12-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... |
Bioorg Med Chem Lett 22: 7504-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.036
BindingDB Entry DOI: 10.7270/Q2ZG6TDQ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50402384
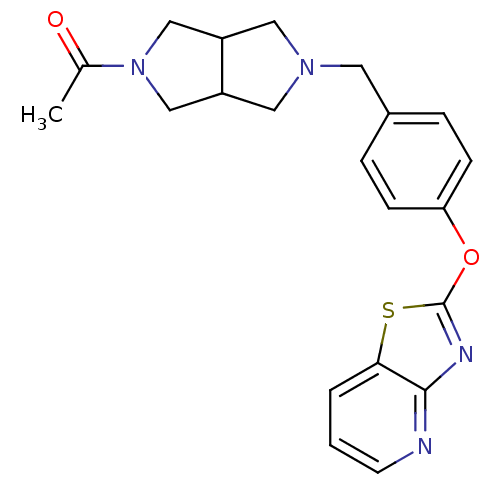
(CHEMBL2207749)Show SMILES CC(=O)N1CC2CN(Cc3ccc(Oc4nc5ncccc5s4)cc3)CC2C1 Show InChI InChI=1S/C21H22N4O2S/c1-14(26)25-12-16-10-24(11-17(16)13-25)9-15-4-6-18(7-5-15)27-21-23-20-19(28-21)3-2-8-22-20/h2-8,16-17H,9-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... |
Bioorg Med Chem Lett 22: 7504-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.036
BindingDB Entry DOI: 10.7270/Q2ZG6TDQ |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of C-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50425170
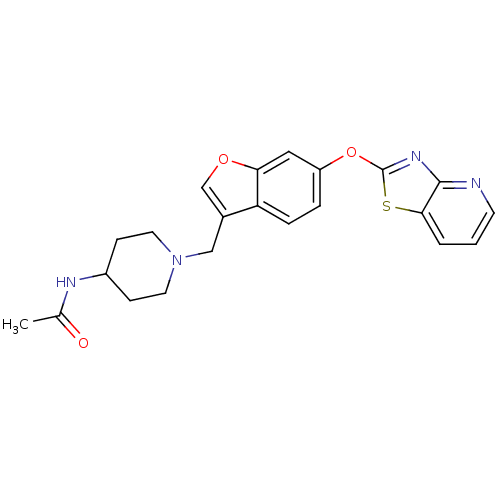
(CHEMBL2313569)Show SMILES CC(=O)NC1CCN(Cc2coc3cc(Oc4nc5ncccc5s4)ccc23)CC1 Show InChI InChI=1S/C22H22N4O3S/c1-14(27)24-16-6-9-26(10-7-16)12-15-13-28-19-11-17(4-5-18(15)19)29-22-25-21-20(30-22)3-2-8-23-21/h2-5,8,11,13,16H,6-7,9-10,12H2,1H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human LTA4H expressed in Sf9 cells using LTA4 as substrate incubated for 10 mins prior to substrate addition measured after... |
Bioorg Med Chem Lett 23: 811-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.074
BindingDB Entry DOI: 10.7270/Q27H1KW1 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50402392
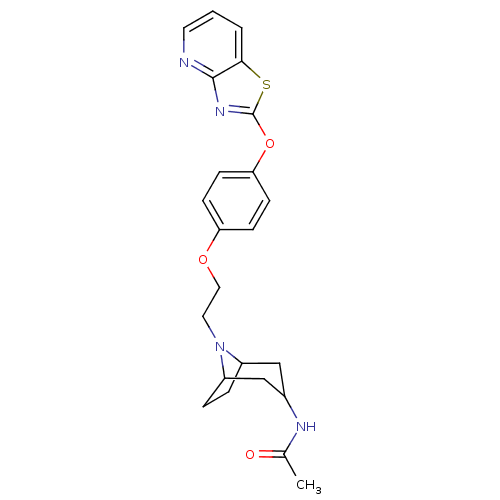
(CHEMBL2207741)Show SMILES CC(=O)NC1CC2CCC(C1)N2CCOc1ccc(Oc2nc3ncccc3s2)cc1 |TLB:3:4:11:7.8| Show InChI InChI=1S/C23H26N4O3S/c1-15(28)25-16-13-17-4-5-18(14-16)27(17)11-12-29-19-6-8-20(9-7-19)30-23-26-22-21(31-23)3-2-10-24-22/h2-3,6-10,16-18H,4-5,11-14H2,1H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... |
Bioorg Med Chem Lett 22: 7504-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.036
BindingDB Entry DOI: 10.7270/Q2ZG6TDQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50377765

(CHEMBL254966)Show SMILES NC(=O)c1cccc(Nc2nccc(n2)-c2nc([nH]c2-c2cccc(NC(=O)Cc3ccc(Cl)cc3)c2)C2CC2)c1 Show InChI InChI=1S/C31H26ClN7O2/c32-22-11-7-18(8-12-22)15-26(40)35-23-5-1-3-20(16-23)27-28(39-30(38-27)19-9-10-19)25-13-14-34-31(37-25)36-24-6-2-4-21(17-24)29(33)41/h1-8,11-14,16-17,19H,9-10,15H2,(H2,33,41)(H,35,40)(H,38,39)(H,34,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of B-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50425151
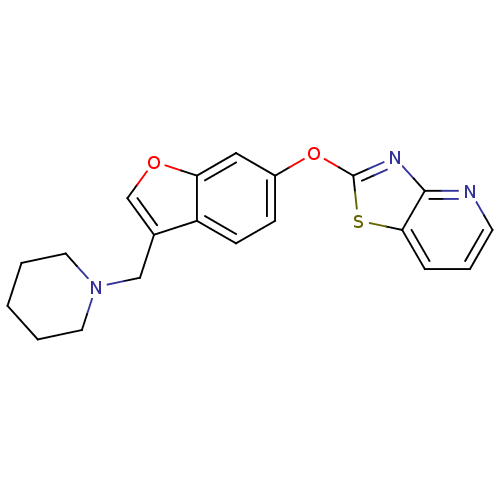
(CHEMBL2313563)Show InChI InChI=1S/C20H19N3O2S/c1-2-9-23(10-3-1)12-14-13-24-17-11-15(6-7-16(14)17)25-20-22-19-18(26-20)5-4-8-21-19/h4-8,11,13H,1-3,9-10,12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human LTA4H expressed in Sf9 cells using LTA4 as substrate incubated for 10 mins prior to substrate addition measured after... |
Bioorg Med Chem Lett 23: 811-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.074
BindingDB Entry DOI: 10.7270/Q27H1KW1 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50377761

(CHEMBL430413)Show SMILES CNc1nccc(n1)-c1nc([nH]c1-c1cccc(NC(=O)Nc2ccc(Cl)c(c2)C(F)(F)F)c1)C1CC1 Show InChI InChI=1S/C25H21ClF3N7O/c1-30-23-31-10-9-19(34-23)21-20(35-22(36-21)13-5-6-13)14-3-2-4-15(11-14)32-24(37)33-16-7-8-18(26)17(12-16)25(27,28)29/h2-4,7-13H,5-6H2,1H3,(H,35,36)(H,30,31,34)(H2,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of C-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50402383
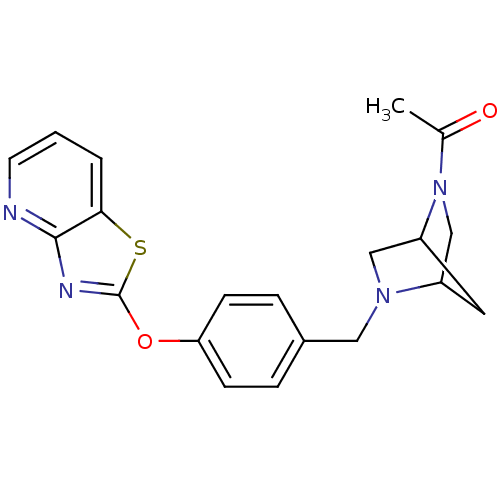
(CHEMBL2207750)Show SMILES CC(=O)N1CC2CC1CN2Cc1ccc(Oc2nc3ncccc3s2)cc1 |THB:10:9:6:4.3,1:3:8.9:6| Show InChI InChI=1S/C20H20N4O2S/c1-13(25)24-12-15-9-16(24)11-23(15)10-14-4-6-17(7-5-14)26-20-22-19-18(27-20)3-2-8-21-19/h2-8,15-16H,9-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... |
Bioorg Med Chem Lett 22: 7504-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.036
BindingDB Entry DOI: 10.7270/Q2ZG6TDQ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50402405
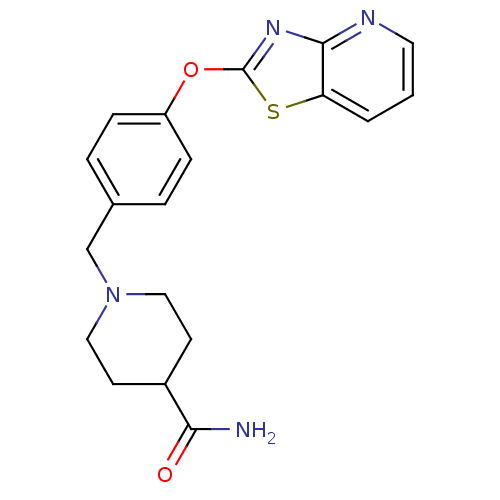
(CHEMBL2207752)Show InChI InChI=1S/C19H20N4O2S/c20-17(24)14-7-10-23(11-8-14)12-13-3-5-15(6-4-13)25-19-22-18-16(26-19)2-1-9-21-18/h1-6,9,14H,7-8,10-12H2,(H2,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... |
Bioorg Med Chem Lett 22: 7504-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.036
BindingDB Entry DOI: 10.7270/Q2ZG6TDQ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50402395
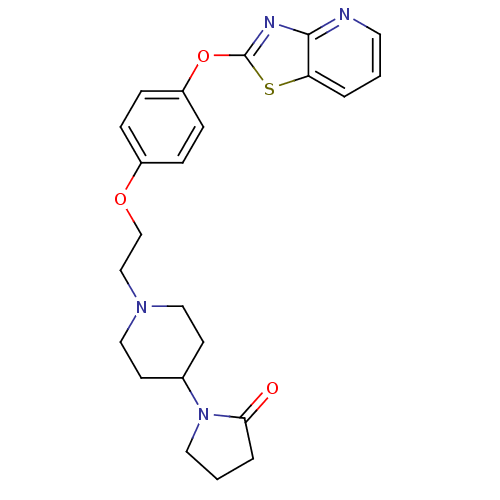
(CHEMBL2207738)Show SMILES O=C1CCCN1C1CCN(CCOc2ccc(Oc3nc4ncccc4s3)cc2)CC1 Show InChI InChI=1S/C23H26N4O3S/c28-21-4-2-12-27(21)17-9-13-26(14-10-17)15-16-29-18-5-7-19(8-6-18)30-23-25-22-20(31-23)3-1-11-24-22/h1,3,5-8,11,17H,2,4,9-10,12-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... |
Bioorg Med Chem Lett 22: 7504-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.036
BindingDB Entry DOI: 10.7270/Q2ZG6TDQ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50402385
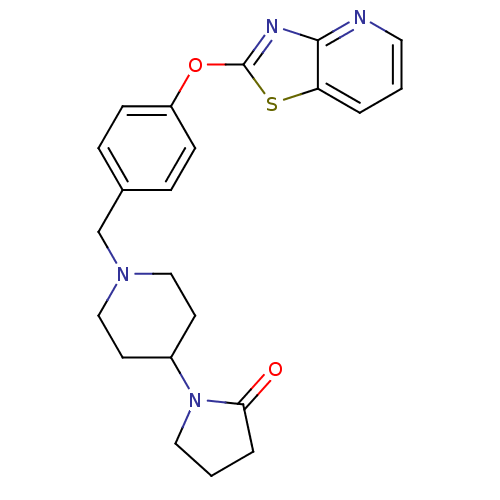
(CHEMBL2207748)Show SMILES O=C1CCCN1C1CCN(Cc2ccc(Oc3nc4ncccc4s3)cc2)CC1 Show InChI InChI=1S/C22H24N4O2S/c27-20-4-2-12-26(20)17-9-13-25(14-10-17)15-16-5-7-18(8-6-16)28-22-24-21-19(29-22)3-1-11-23-21/h1,3,5-8,11,17H,2,4,9-10,12-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... |
Bioorg Med Chem Lett 22: 7504-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.036
BindingDB Entry DOI: 10.7270/Q2ZG6TDQ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50402388
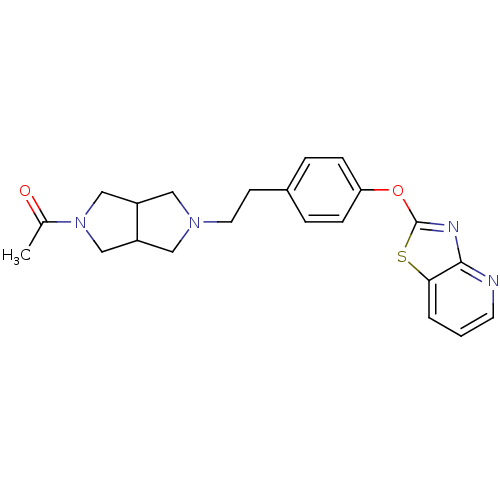
(CHEMBL2207745)Show SMILES CC(=O)N1CC2CN(CCc3ccc(Oc4nc5ncccc5s4)cc3)CC2C1 Show InChI InChI=1S/C22H24N4O2S/c1-15(27)26-13-17-11-25(12-18(17)14-26)10-8-16-4-6-19(7-5-16)28-22-24-21-20(29-22)3-2-9-23-21/h2-7,9,17-18H,8,10-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... |
Bioorg Med Chem Lett 22: 7504-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.036
BindingDB Entry DOI: 10.7270/Q2ZG6TDQ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50425163

(CHEMBL2313266)Show SMILES CC(=O)N1CC2CC1CN2Cc1cc2cc(Oc3nc4ncccc4s3)ccc2o1 Show InChI InChI=1S/C22H20N4O3S/c1-13(27)26-11-15-9-16(26)10-25(15)12-18-8-14-7-17(4-5-19(14)28-18)29-22-24-21-20(30-22)3-2-6-23-21/h2-8,15-16H,9-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human LTA4H expressed in Sf9 cells using LTA4 as substrate incubated for 10 mins prior to substrate addition measured after... |
Bioorg Med Chem Lett 23: 811-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.074
BindingDB Entry DOI: 10.7270/Q27H1KW1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50377767

(CHEMBL429498)Show SMILES Clc1ccc(CC(=O)Nc2cccc(c2)-c2[nH]c(nc2-c2ccnc(NCCN3CCNC3=O)n2)C2CC2)cc1 Show InChI InChI=1S/C29H29ClN8O2/c30-21-8-4-18(5-9-21)16-24(39)34-22-3-1-2-20(17-22)25-26(37-27(36-25)19-6-7-19)23-10-11-31-28(35-23)32-12-14-38-15-13-33-29(38)40/h1-5,8-11,17,19H,6-7,12-16H2,(H,33,40)(H,34,39)(H,36,37)(H,31,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of B-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50402393
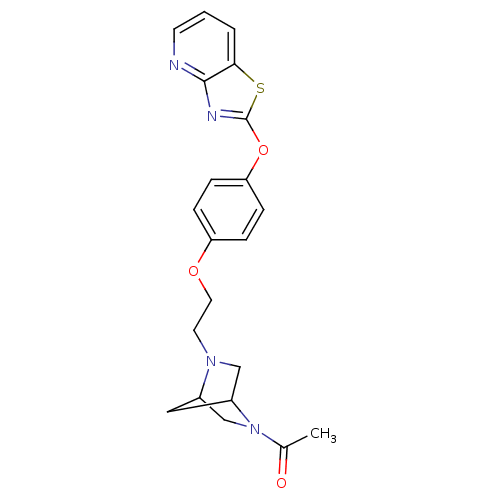
(CHEMBL2207740)Show SMILES CC(=O)N1CC2CC1CN2CCOc1ccc(Oc2nc3ncccc3s2)cc1 |THB:10:9:6:4.3,1:3:8.9:6| Show InChI InChI=1S/C21H22N4O3S/c1-14(26)25-13-15-11-16(25)12-24(15)9-10-27-17-4-6-18(7-5-17)28-21-23-20-19(29-21)3-2-8-22-20/h2-8,15-16H,9-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... |
Bioorg Med Chem Lett 22: 7504-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.036
BindingDB Entry DOI: 10.7270/Q2ZG6TDQ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50402394
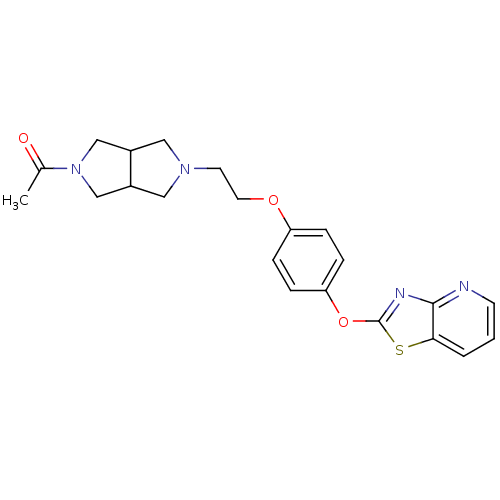
(CHEMBL2207739)Show SMILES CC(=O)N1CC2CN(CCOc3ccc(Oc4nc5ncccc5s4)cc3)CC2C1 Show InChI InChI=1S/C22H24N4O3S/c1-15(27)26-13-16-11-25(12-17(16)14-26)9-10-28-18-4-6-19(7-5-18)29-22-24-21-20(30-22)3-2-8-23-21/h2-8,16-17H,9-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... |
Bioorg Med Chem Lett 22: 7504-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.036
BindingDB Entry DOI: 10.7270/Q2ZG6TDQ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50425157
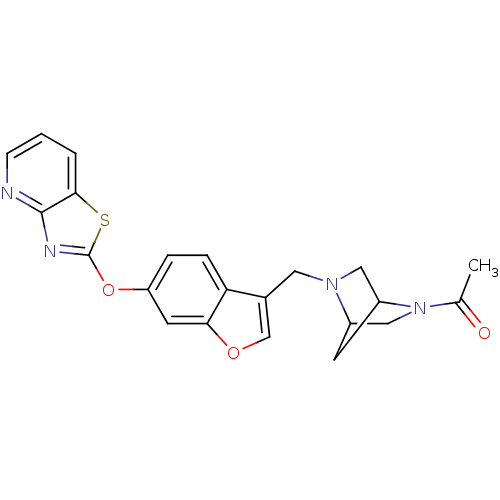
(CHEMBL2313272)Show SMILES CC(=O)N1CC2CC1CN2Cc1coc2cc(Oc3nc4ncccc4s3)ccc12 Show InChI InChI=1S/C22H20N4O3S/c1-13(27)26-11-15-7-16(26)10-25(15)9-14-12-28-19-8-17(4-5-18(14)19)29-22-24-21-20(30-22)3-2-6-23-21/h2-6,8,12,15-16H,7,9-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human LTA4H expressed in Sf9 cells using LTA4 as substrate incubated for 10 mins prior to substrate addition measured after... |
Bioorg Med Chem Lett 23: 811-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.074
BindingDB Entry DOI: 10.7270/Q27H1KW1 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50425167
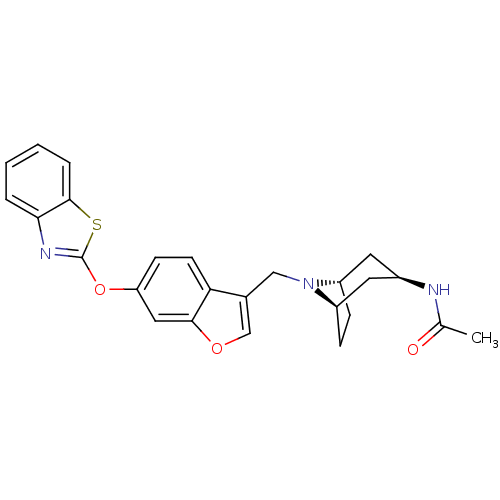
(CHEMBL2313572)Show SMILES CC(=O)N[C@@H]1C[C@@H]2CC[C@H](C1)N2Cc1coc2cc(Oc3nc4ccccc4s3)ccc12 |r,THB:12:11:10.4.5:7.8| Show InChI InChI=1S/C25H25N3O3S/c1-15(29)26-17-10-18-6-7-19(11-17)28(18)13-16-14-30-23-12-20(8-9-21(16)23)31-25-27-22-4-2-3-5-24(22)32-25/h2-5,8-9,12,14,17-19H,6-7,10-11,13H2,1H3,(H,26,29)/t17-,18+,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human LTA4H expressed in Sf9 cells using LTA4 as substrate incubated for 10 mins prior to substrate addition measured after... |
Bioorg Med Chem Lett 23: 811-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.074
BindingDB Entry DOI: 10.7270/Q27H1KW1 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50425164

(CHEMBL2313265)Show SMILES CC(=O)N1C[C@@H]2C[C@H]1CN2Cc1coc2cc(Oc3nc4ncccc4s3)ccc12 |r| Show InChI InChI=1S/C22H20N4O3S/c1-13(27)26-11-15-7-16(26)10-25(15)9-14-12-28-19-8-17(4-5-18(14)19)29-22-24-21-20(30-22)3-2-6-23-21/h2-6,8,12,15-16H,7,9-11H2,1H3/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human LTA4H expressed in Sf9 cells using LTA4 as substrate incubated for 10 mins prior to substrate addition measured after... |
Bioorg Med Chem Lett 23: 811-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.074
BindingDB Entry DOI: 10.7270/Q27H1KW1 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50402397
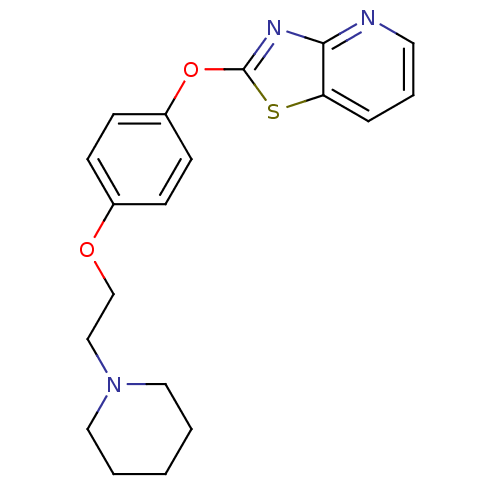
(CHEMBL2207736)Show InChI InChI=1S/C19H21N3O2S/c1-2-11-22(12-3-1)13-14-23-15-6-8-16(9-7-15)24-19-21-18-17(25-19)5-4-10-20-18/h4-10H,1-3,11-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... |
Bioorg Med Chem Lett 22: 7504-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.036
BindingDB Entry DOI: 10.7270/Q2ZG6TDQ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50425147
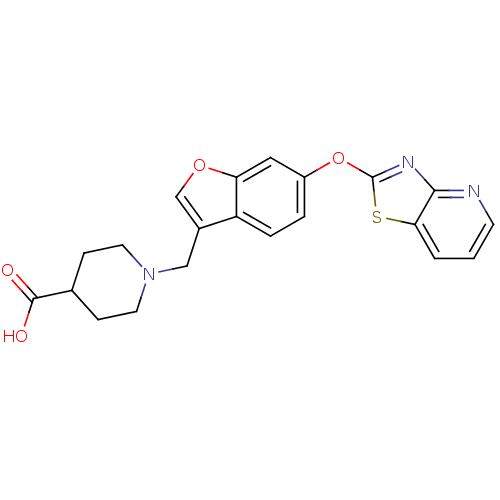
(CHEMBL2313567)Show SMILES OC(=O)C1CCN(Cc2coc3cc(Oc4nc5ncccc5s4)ccc23)CC1 Show InChI InChI=1S/C21H19N3O4S/c25-20(26)13-5-8-24(9-6-13)11-14-12-27-17-10-15(3-4-16(14)17)28-21-23-19-18(29-21)2-1-7-22-19/h1-4,7,10,12-13H,5-6,8-9,11H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human LTA4H expressed in Sf9 cells using LTA4 as substrate incubated for 10 mins prior to substrate addition measured after... |
Bioorg Med Chem Lett 23: 811-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.074
BindingDB Entry DOI: 10.7270/Q27H1KW1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50377766

(CHEMBL403854)Show SMILES CNc1nccc(n1)-c1nc([nH]c1-c1cccc(NC(=O)Cc2ccc(Cl)cc2)c1)C1CC1 Show InChI InChI=1S/C25H23ClN6O/c1-27-25-28-12-11-20(30-25)23-22(31-24(32-23)16-7-8-16)17-3-2-4-19(14-17)29-21(33)13-15-5-9-18(26)10-6-15/h2-6,9-12,14,16H,7-8,13H2,1H3,(H,29,33)(H,31,32)(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of B-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24220
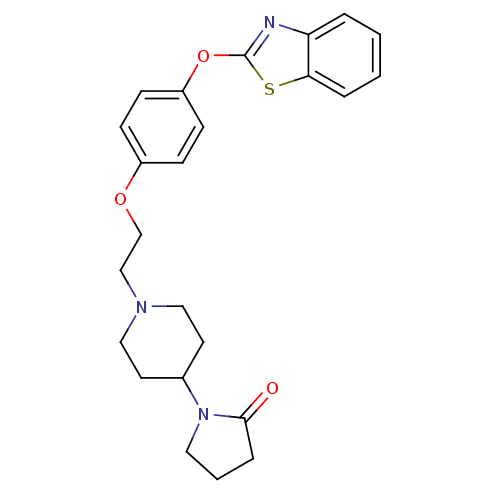
(1-(1-{2-[4-(1,3-benzothiazol-2-yloxy)phenoxy]ethyl...)Show SMILES O=C1CCCN1C1CCN(CCOc2ccc(Oc3nc4ccccc4s3)cc2)CC1 Show InChI InChI=1S/C24H27N3O3S/c28-23-6-3-13-27(23)18-11-14-26(15-12-18)16-17-29-19-7-9-20(10-8-19)30-24-25-21-4-1-2-5-22(21)31-24/h1-2,4-5,7-10,18H,3,6,11-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human LTA4H expressed in Sf9 cells using LTA4 as substrate incubated for 10 mins prior to substrate addition measured after... |
Bioorg Med Chem Lett 23: 811-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.074
BindingDB Entry DOI: 10.7270/Q27H1KW1 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Mus musculus) | BDBM50425166

(CHEMBL2313573)Show SMILES CC(=O)N[C@@H]1C[C@@H]2CC[C@H](C1)N2Cc1coc2cc(Oc3nc4ncccc4s3)ccc12 |r,THB:12:11:10.4.5:7.8| Show InChI InChI=1S/C24H24N4O3S/c1-14(29)26-16-9-17-4-5-18(10-16)28(17)12-15-13-30-21-11-19(6-7-20(15)21)31-24-27-23-22(32-24)3-2-8-25-23/h2-3,6-8,11,13,16-18H,4-5,9-10,12H2,1H3,(H,26,29)/t16-,17+,18- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC
Curated by ChEMBL
| Assay Description
Inhibition of LTA4H in CD1 mouse whole blood assessed as decrease in calcium ionophore-stimulated LTB4 production incubated for 15 mins prior to calc... |
Bioorg Med Chem Lett 23: 811-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.074
BindingDB Entry DOI: 10.7270/Q27H1KW1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50377771
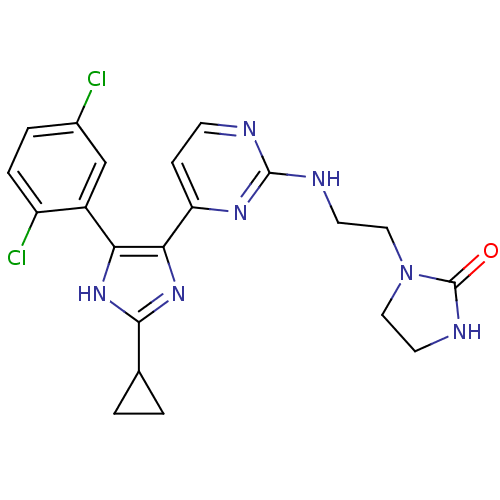
(CHEMBL401710)Show SMILES Clc1ccc(Cl)c(c1)-c1[nH]c(nc1-c1ccnc(NCCN2CCNC2=O)n1)C1CC1 Show InChI InChI=1S/C21H21Cl2N7O/c22-13-3-4-15(23)14(11-13)17-18(29-19(28-17)12-1-2-12)16-5-6-24-20(27-16)25-7-9-30-10-8-26-21(30)31/h3-6,11-12H,1-2,7-10H2,(H,26,31)(H,28,29)(H,24,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of B-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50402390
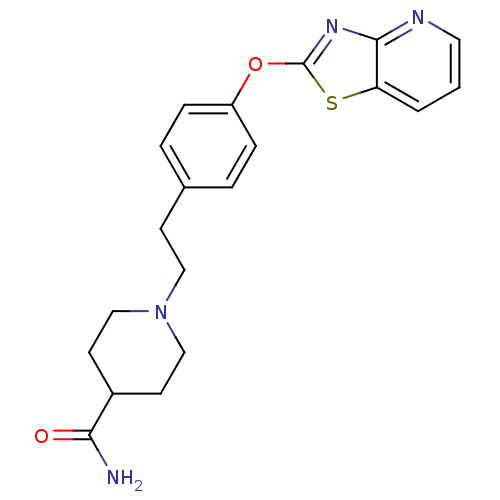
(CHEMBL2207743)Show InChI InChI=1S/C20H22N4O2S/c21-18(25)15-8-12-24(13-9-15)11-7-14-3-5-16(6-4-14)26-20-23-19-17(27-20)2-1-10-22-19/h1-6,10,15H,7-9,11-13H2,(H2,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... |
Bioorg Med Chem Lett 22: 7504-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.036
BindingDB Entry DOI: 10.7270/Q2ZG6TDQ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50425148
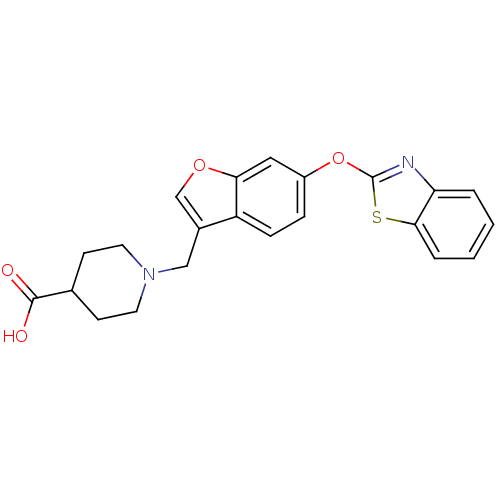
(CHEMBL2313566)Show SMILES OC(=O)C1CCN(Cc2coc3cc(Oc4nc5ccccc5s4)ccc23)CC1 Show InChI InChI=1S/C22H20N2O4S/c25-21(26)14-7-9-24(10-8-14)12-15-13-27-19-11-16(5-6-17(15)19)28-22-23-18-3-1-2-4-20(18)29-22/h1-6,11,13-14H,7-10,12H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human LTA4H expressed in Sf9 cells using LTA4 as substrate incubated for 10 mins prior to substrate addition measured after... |
Bioorg Med Chem Lett 23: 811-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.074
BindingDB Entry DOI: 10.7270/Q27H1KW1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50225014
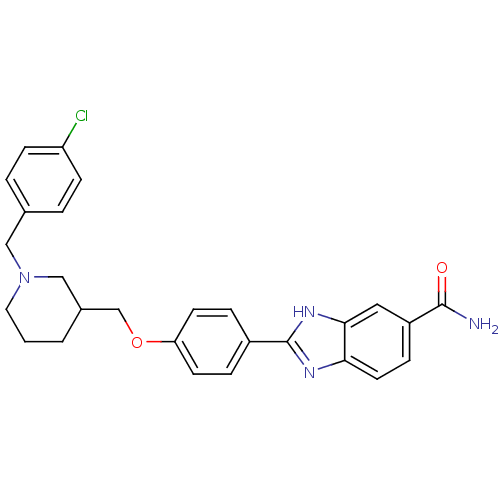
(2-(4-((1-(4-chlorobenzyl)piperidin-3-yl)methoxy)ph...)Show SMILES NC(=O)c1ccc2nc([nH]c2c1)-c1ccc(OCC2CCCN(Cc3ccc(Cl)cc3)C2)cc1 Show InChI InChI=1S/C27H27ClN4O2/c28-22-8-3-18(4-9-22)15-32-13-1-2-19(16-32)17-34-23-10-5-20(6-11-23)27-30-24-12-7-21(26(29)33)14-25(24)31-27/h3-12,14,19H,1-2,13,15-17H2,(H2,29,33)(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Chk2 |
Bioorg Med Chem Lett 17: 6467-71 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.098
BindingDB Entry DOI: 10.7270/Q2KP81XB |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50402389
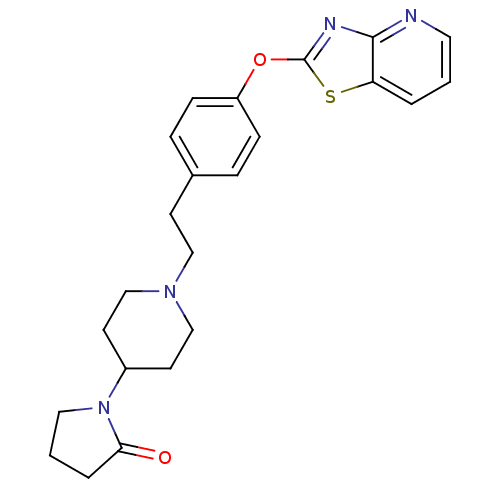
(CHEMBL2207744)Show SMILES O=C1CCCN1C1CCN(CCc2ccc(Oc3nc4ncccc4s3)cc2)CC1 Show InChI InChI=1S/C23H26N4O2S/c28-21-4-2-13-27(21)18-10-15-26(16-11-18)14-9-17-5-7-19(8-6-17)29-23-25-22-20(30-23)3-1-12-24-22/h1,3,5-8,12,18H,2,4,9-11,13-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... |
Bioorg Med Chem Lett 22: 7504-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.036
BindingDB Entry DOI: 10.7270/Q2ZG6TDQ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50402387
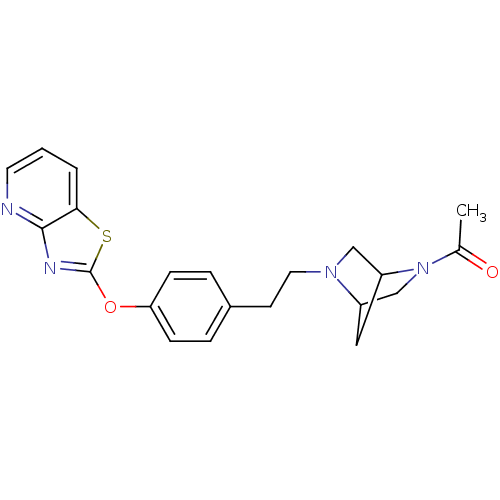
(CHEMBL2207746)Show SMILES CC(=O)N1CC2CC1CN2CCc1ccc(Oc2nc3ncccc3s2)cc1 |THB:1:3:8.9:6,10:9:6:4.3| Show InChI InChI=1S/C21H22N4O2S/c1-14(26)25-13-16-11-17(25)12-24(16)10-8-15-4-6-18(7-5-15)27-21-23-20-19(28-21)3-2-9-22-20/h2-7,9,16-17H,8,10-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... |
Bioorg Med Chem Lett 22: 7504-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.036
BindingDB Entry DOI: 10.7270/Q2ZG6TDQ |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50377758
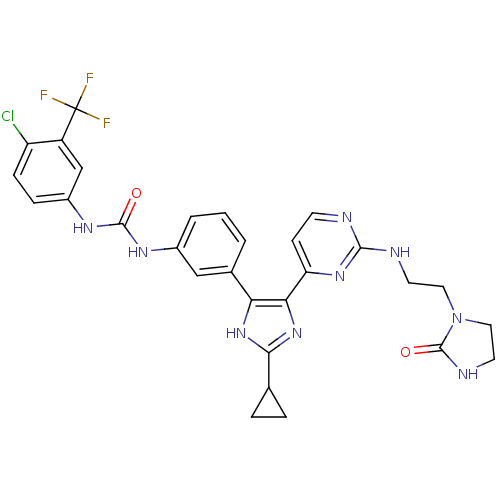
(CHEMBL256921)Show SMILES FC(F)(F)c1cc(NC(=O)Nc2cccc(c2)-c2[nH]c(nc2-c2ccnc(NCCN3CCNC3=O)n2)C2CC2)ccc1Cl Show InChI InChI=1S/C29H27ClF3N9O2/c30-21-7-6-19(15-20(21)29(31,32)33)38-27(43)37-18-3-1-2-17(14-18)23-24(41-25(40-23)16-4-5-16)22-8-9-34-26(39-22)35-10-12-42-13-11-36-28(42)44/h1-3,6-9,14-16H,4-5,10-13H2,(H,36,44)(H,40,41)(H,34,35,39)(H2,37,38,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of C-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of B-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM34064
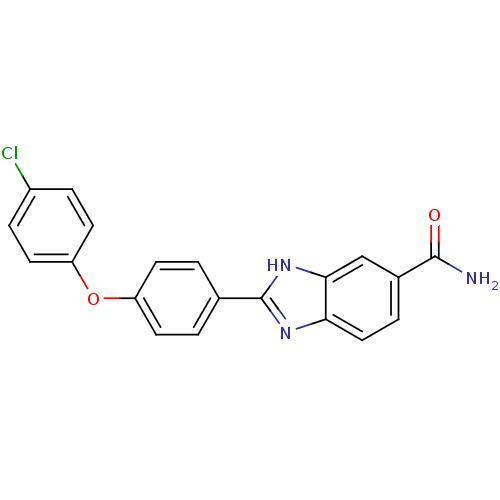
(2-arylbenzimidazole | CHEMBL179583)Show SMILES NC(=O)c1ccc2nc([nH]c2c1)-c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C20H14ClN3O2/c21-14-4-8-16(9-5-14)26-15-6-1-12(2-7-15)20-23-17-10-3-13(19(22)25)11-18(17)24-20/h1-11H,(H2,22,25)(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Chk2 |
Bioorg Med Chem Lett 17: 6467-71 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.098
BindingDB Entry DOI: 10.7270/Q2KP81XB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50402396
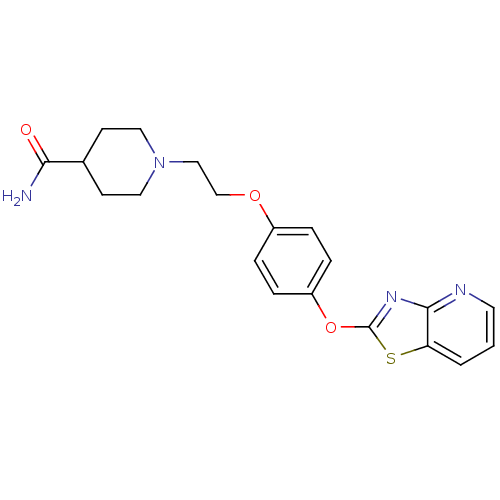
(CHEMBL2207737)Show SMILES NC(=O)C1CCN(CCOc2ccc(Oc3nc4ncccc4s3)cc2)CC1 Show InChI InChI=1S/C20H22N4O3S/c21-18(25)14-7-10-24(11-8-14)12-13-26-15-3-5-16(6-4-15)27-20-23-19-17(28-20)2-1-9-22-19/h1-6,9,14H,7-8,10-13H2,(H2,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... |
Bioorg Med Chem Lett 22: 7504-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.036
BindingDB Entry DOI: 10.7270/Q2ZG6TDQ |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50377762
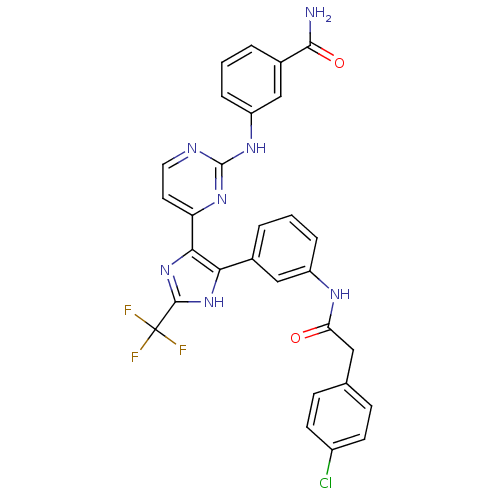
(CHEMBL256775)Show SMILES NC(=O)c1cccc(Nc2nccc(n2)-c2nc([nH]c2-c2cccc(NC(=O)Cc3ccc(Cl)cc3)c2)C(F)(F)F)c1 Show InChI InChI=1S/C29H21ClF3N7O2/c30-19-9-7-16(8-10-19)13-23(41)36-20-5-1-3-17(14-20)24-25(40-27(39-24)29(31,32)33)22-11-12-35-28(38-22)37-21-6-2-4-18(15-21)26(34)42/h1-12,14-15H,13H2,(H2,34,42)(H,36,41)(H,39,40)(H,35,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of C-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Mus musculus) | BDBM50425170

(CHEMBL2313569)Show SMILES CC(=O)NC1CCN(Cc2coc3cc(Oc4nc5ncccc5s4)ccc23)CC1 Show InChI InChI=1S/C22H22N4O3S/c1-14(27)24-16-6-9-26(10-7-16)12-15-13-28-19-11-17(4-5-18(15)19)29-22-25-21-20(30-22)3-2-8-23-21/h2-5,8,11,13,16H,6-7,9-10,12H2,1H3,(H,24,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC
Curated by ChEMBL
| Assay Description
Inhibition of LTA4H in CD1 mouse whole blood assessed as decrease in calcium ionophore-stimulated LTB4 production incubated for 15 mins prior to calc... |
Bioorg Med Chem Lett 23: 811-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.074
BindingDB Entry DOI: 10.7270/Q27H1KW1 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Mus musculus) | BDBM50425148

(CHEMBL2313566)Show SMILES OC(=O)C1CCN(Cc2coc3cc(Oc4nc5ccccc5s4)ccc23)CC1 Show InChI InChI=1S/C22H20N2O4S/c25-21(26)14-7-9-24(10-8-14)12-15-13-27-19-11-16(5-6-17(15)19)28-22-23-18-3-1-2-4-20(18)29-22/h1-6,11,13-14H,7-10,12H2,(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC
Curated by ChEMBL
| Assay Description
Inhibition of LTA4H in CD1 mouse whole blood assessed as decrease in calcium ionophore-stimulated LTB4 production incubated for 15 mins prior to calc... |
Bioorg Med Chem Lett 23: 811-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.074
BindingDB Entry DOI: 10.7270/Q27H1KW1 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50377764
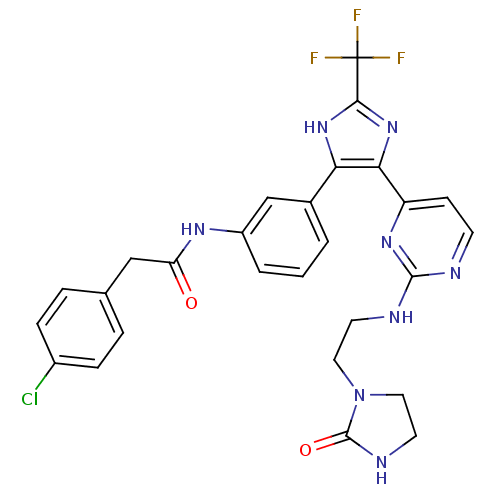
(CHEMBL254965)Show SMILES FC(F)(F)c1nc(c([nH]1)-c1cccc(NC(=O)Cc2ccc(Cl)cc2)c1)-c1ccnc(NCCN2CCNC2=O)n1 Show InChI InChI=1S/C27H24ClF3N8O2/c28-18-6-4-16(5-7-18)14-21(40)35-19-3-1-2-17(15-19)22-23(38-24(37-22)27(29,30)31)20-8-9-32-25(36-20)33-10-12-39-13-11-34-26(39)41/h1-9,15H,10-14H2,(H,34,41)(H,35,40)(H,37,38)(H,32,33,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of C-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Mus musculus) | BDBM50425151

(CHEMBL2313563)Show InChI InChI=1S/C20H19N3O2S/c1-2-9-23(10-3-1)12-14-13-24-17-11-15(6-7-16(14)17)25-20-22-19-18(26-20)5-4-8-21-19/h4-8,11,13H,1-3,9-10,12H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC
Curated by ChEMBL
| Assay Description
Inhibition of LTA4H in CD1 mouse whole blood assessed as decrease in calcium ionophore-stimulated LTB4 production incubated for 15 mins prior to calc... |
Bioorg Med Chem Lett 23: 811-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.074
BindingDB Entry DOI: 10.7270/Q27H1KW1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data