

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50262295 (4-[(4R,7S,10S,13R)-10-(3-{[amino(iminiumyl)methyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity to human NOP receptor | J Med Chem 51: 4385-7 (2008) Article DOI: 10.1021/jm800394v BindingDB Entry DOI: 10.7270/Q2B56KN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50262291 (4-[(3S,6S,9S,17S)-6-(3-{[amino(iminiumyl)methyl]am...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Displacement of [3H]N/OFQ from human NOP receptor expressed in CHO cells | J Med Chem 51: 4385-7 (2008) Article DOI: 10.1021/jm800394v BindingDB Entry DOI: 10.7270/Q2B56KN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50262290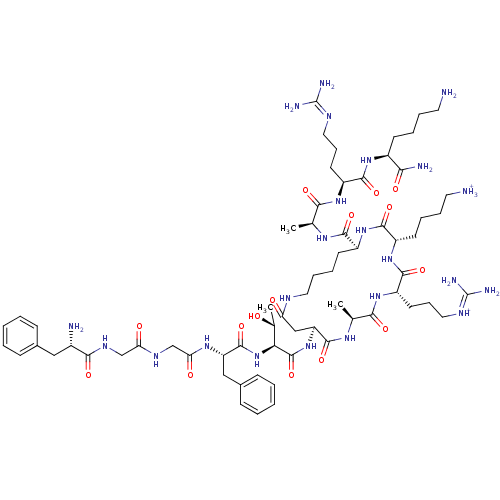 (4-[(3S,6S,9S,12S,20S)-6-(3-{[amino(iminiumyl)methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Displacement of [3H]N/OFQ from human NOP receptor expressed in CHO cells | J Med Chem 51: 4385-7 (2008) Article DOI: 10.1021/jm800394v BindingDB Entry DOI: 10.7270/Q2B56KN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50106479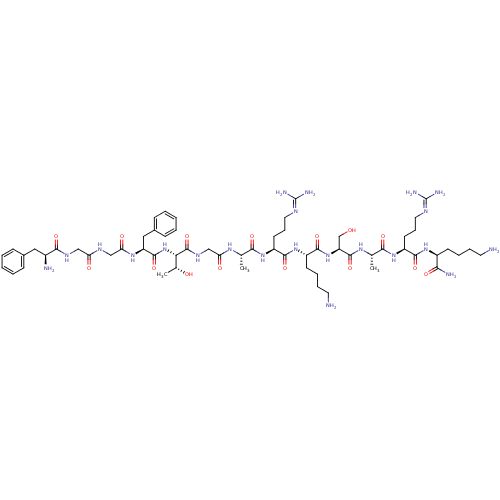 (CHEMBL384755 | FGGFTGARKSARK | H-FGGFTGARKSARK-NH2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity to human NOP receptor | J Med Chem 51: 4385-7 (2008) Article DOI: 10.1021/jm800394v BindingDB Entry DOI: 10.7270/Q2B56KN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50262292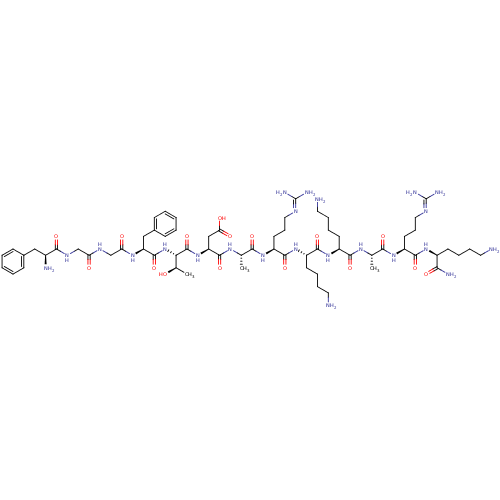 (CHEMBL507269 | [Asp6,Lys10]N/OFQ(1-13)NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Displacement of [3H]N/OFQ from human NOP receptor expressed in CHO cells | J Med Chem 51: 4385-7 (2008) Article DOI: 10.1021/jm800394v BindingDB Entry DOI: 10.7270/Q2B56KN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50106479 (CHEMBL384755 | FGGFTGARKSARK | H-FGGFTGARKSARK-NH2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Displacement of [3H]N/OFQ from human NOP receptor expressed in CHO cells | J Med Chem 51: 4385-7 (2008) Article DOI: 10.1021/jm800394v BindingDB Entry DOI: 10.7270/Q2B56KN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50106469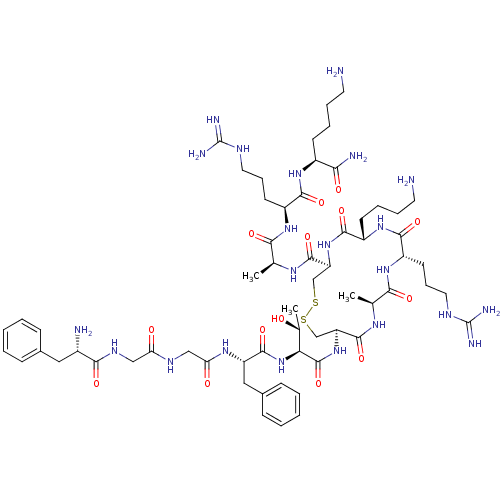 (CHEMBL269029 | FGGFTCARKCARK | cyclo[Cys6,Cys10]N/...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity to human NOP receptor | J Med Chem 51: 4385-7 (2008) Article DOI: 10.1021/jm800394v BindingDB Entry DOI: 10.7270/Q2B56KN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50086880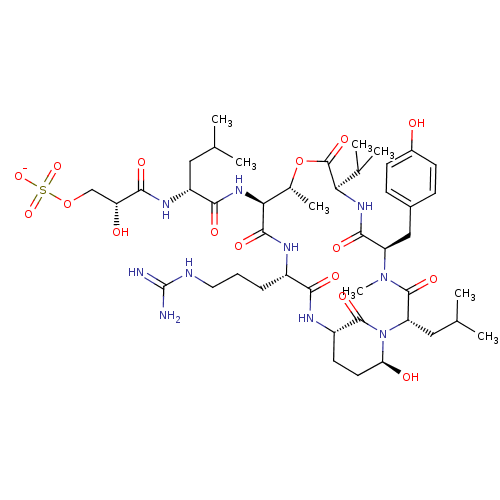 (Proteolytic Enzyme inhibitor) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description binding affinity towards HIV-1 Protease enzyme | J Med Chem 43: 1271-81 (2001) BindingDB Entry DOI: 10.7270/Q23779F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50086884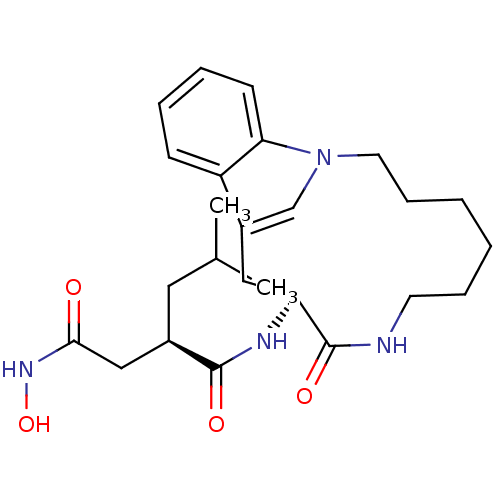 ((R)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-9-oxo-1,8-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description binding affinity towards HIV-1 Protease enzyme | J Med Chem 43: 1271-81 (2001) BindingDB Entry DOI: 10.7270/Q23779F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50262293 (CHEMBL507653 | [D-Asp7,Lys10]N/OFQ(1-13)NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Displacement of [3H]N/OFQ from human NOP receptor expressed in CHO cells | J Med Chem 51: 4385-7 (2008) Article DOI: 10.1021/jm800394v BindingDB Entry DOI: 10.7270/Q2B56KN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50369797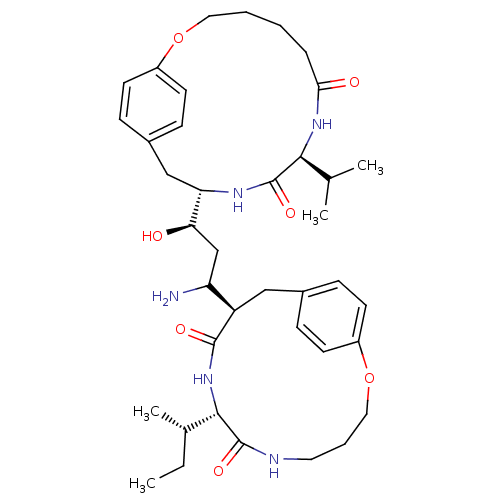 (CHEMBL1794029) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description binding affinity towards HIV-1 Protease enzyme | J Med Chem 43: 1271-81 (2001) BindingDB Entry DOI: 10.7270/Q23779F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50369799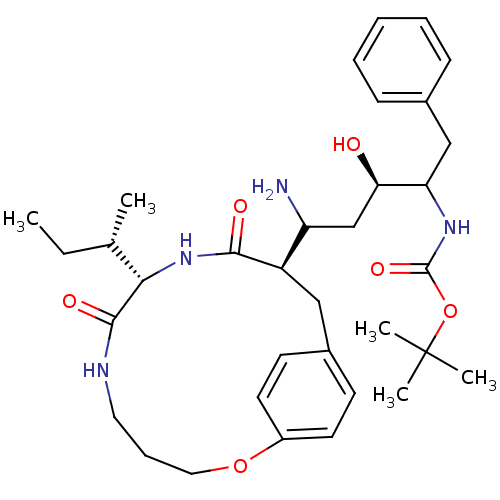 (CHEMBL1794024) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description binding affinity towards HIV-1 Protease enzyme | J Med Chem 43: 1271-81 (2001) BindingDB Entry DOI: 10.7270/Q23779F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50086886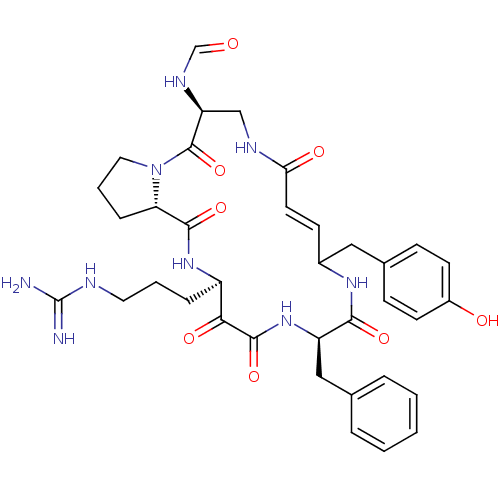 (CHEMBL436149 | N-[14-Benzyl-18-(3-guanidino-propyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description binding affinity towards HIV-1 Protease enzyme | J Med Chem 43: 1271-81 (2001) BindingDB Entry DOI: 10.7270/Q23779F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50086883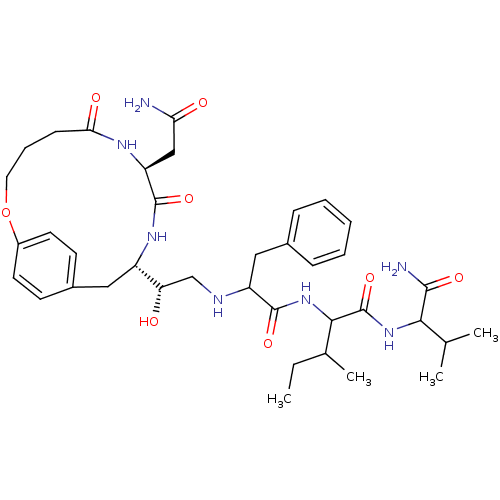 (2-{2-[2-(8-Carbamoylmethyl-6,9-dioxo-2-oxa-7,10-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description binding affinity towards HIV-1 Protease enzyme | J Med Chem 43: 1271-81 (2001) BindingDB Entry DOI: 10.7270/Q23779F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50075951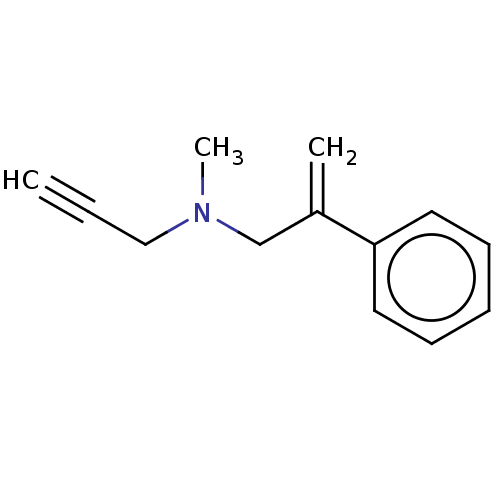 (CHEMBL3415810) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
A* STAR (Agency of Science, Technology and Research) Curated by ChEMBL | Assay Description Binding affinity to human recombinant MAO-B measured after longer preincubation | J Med Chem 58: 1400-19 (2015) Article DOI: 10.1021/jm501722s BindingDB Entry DOI: 10.7270/Q2V989R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50369800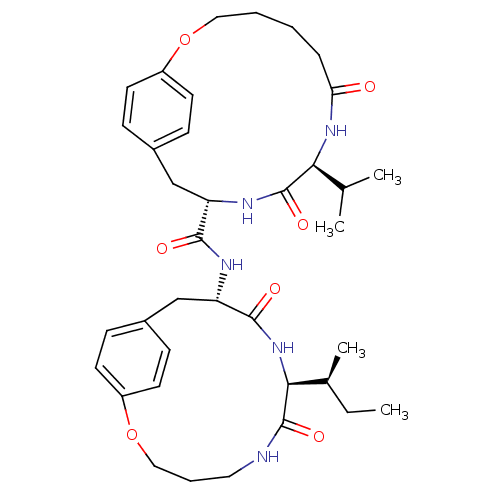 (CHEMBL1232357) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description binding affinity towards HIV-1 Protease enzyme | J Med Chem 43: 1271-81 (2001) BindingDB Entry DOI: 10.7270/Q23779F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50308110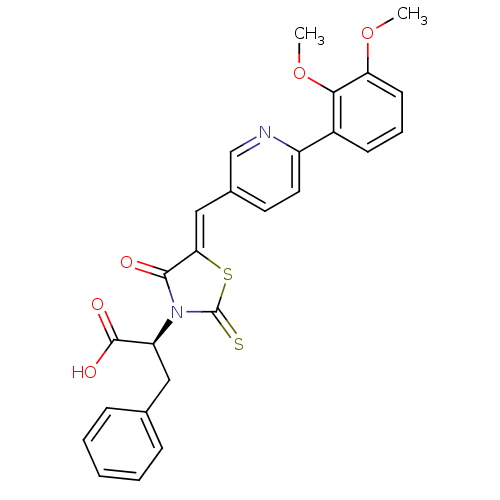 ((S,Z)-2-(5-((6-(2,3-dimethoxyphenyl)pyridin-3-yl)m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical and Engineering Sciences Curated by ChEMBL | Assay Description Binding affinity to human Bcl-XL by isothermal titration calorimetry | J Med Chem 53: 2314-8 (2010) Article DOI: 10.1021/jm901469p BindingDB Entry DOI: 10.7270/Q2K937NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50308110 ((S,Z)-2-(5-((6-(2,3-dimethoxyphenyl)pyridin-3-yl)m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical and Engineering Sciences Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled Bak-BH3 peptide from human Bcl-XL by fluorescence polarization assay | J Med Chem 53: 2314-8 (2010) Article DOI: 10.1021/jm901469p BindingDB Entry DOI: 10.7270/Q2K937NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50107130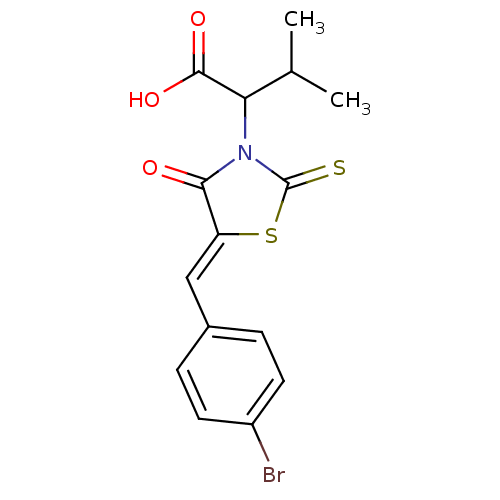 ((Z)-2-(5-(4-bromobenzylidene)-4-oxo-2-thioxothiazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical and Engineering Sciences Curated by ChEMBL | Assay Description Binding affinity to human Bcl-XL by isothermal titration calorimetry | J Med Chem 53: 2314-8 (2010) Article DOI: 10.1021/jm901469p BindingDB Entry DOI: 10.7270/Q2K937NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50107130 ((Z)-2-(5-(4-bromobenzylidene)-4-oxo-2-thioxothiazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical and Engineering Sciences Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled Bak-BH3 peptide from human Bcl-XL by fluorescence polarization assay | J Med Chem 53: 2314-8 (2010) Article DOI: 10.1021/jm901469p BindingDB Entry DOI: 10.7270/Q2K937NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50369798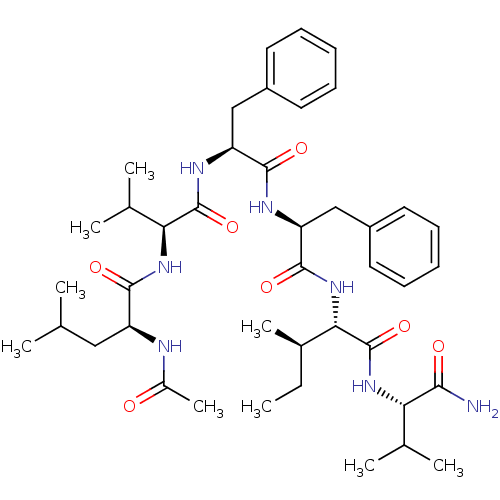 (CHEMBL1794028) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description binding affinity towards HIV-1 Protease enzyme | J Med Chem 43: 1271-81 (2001) BindingDB Entry DOI: 10.7270/Q23779F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50308111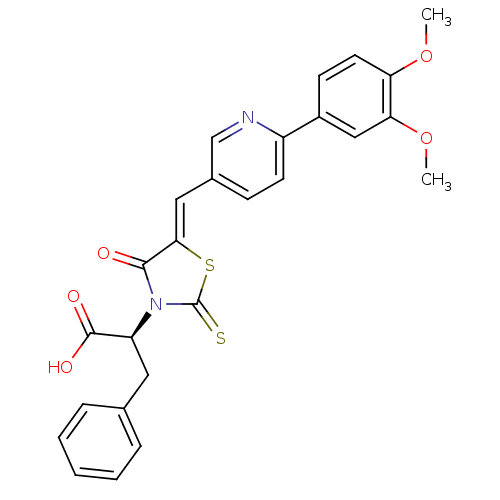 ((S,Z)-2-(5-((6-(3,4-dimethoxyphenyl)pyridin-3-yl)m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical and Engineering Sciences Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled Bak-BH3 peptide from human Bcl-XL by fluorescence polarization assay | J Med Chem 53: 2314-8 (2010) Article DOI: 10.1021/jm901469p BindingDB Entry DOI: 10.7270/Q2K937NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50308111 ((S,Z)-2-(5-((6-(3,4-dimethoxyphenyl)pyridin-3-yl)m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >7.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical and Engineering Sciences Curated by ChEMBL | Assay Description Binding affinity to human Bcl-XL by isothermal titration calorimetry | J Med Chem 53: 2314-8 (2010) Article DOI: 10.1021/jm901469p BindingDB Entry DOI: 10.7270/Q2K937NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194684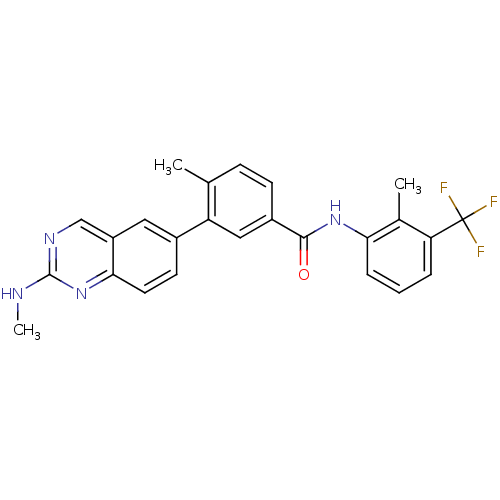 (4-methyl-N-(2-methyl-3-4-methyl-N-(2-methyl-3-(2-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194688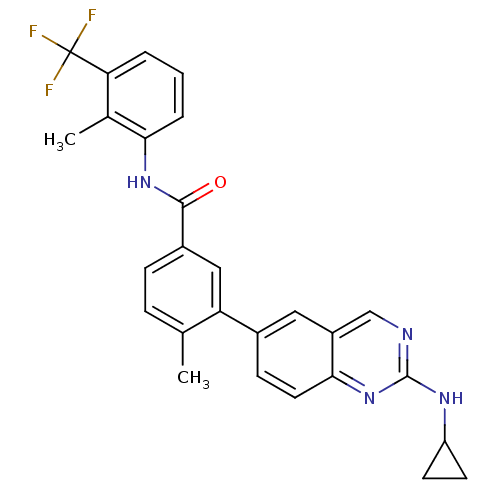 (4-methyl-N-(2-methyl-3-(trifluoromethyl)phenyl)-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194668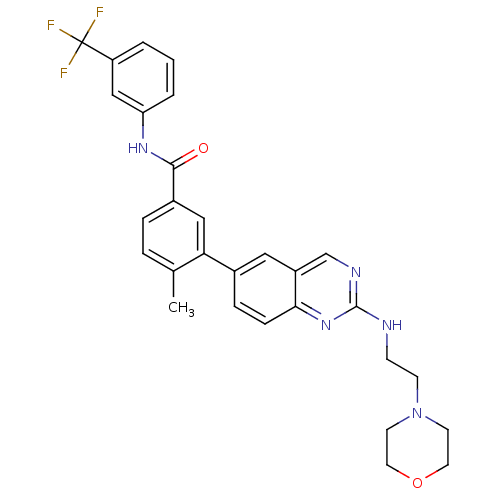 (4-methyl-3-(2-(2-morpholinoethylamino)quinazolin-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM14949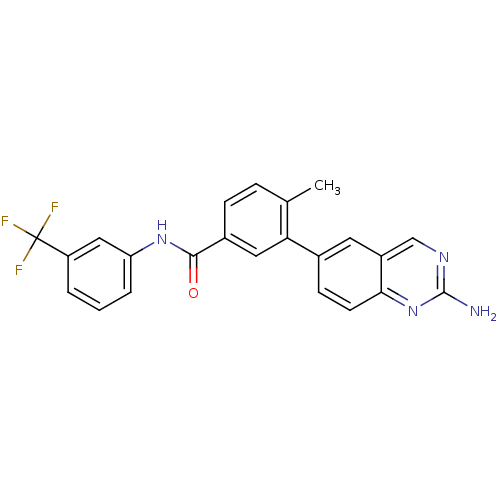 (2-aminoquinazoline 5 | 3-(2-aminoquinazolin-6-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 51: 1681-94 (2008) Article DOI: 10.1021/jm7010996 BindingDB Entry DOI: 10.7270/Q2Q81BCB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM14949 (2-aminoquinazoline 5 | 3-(2-aminoquinazolin-6-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194694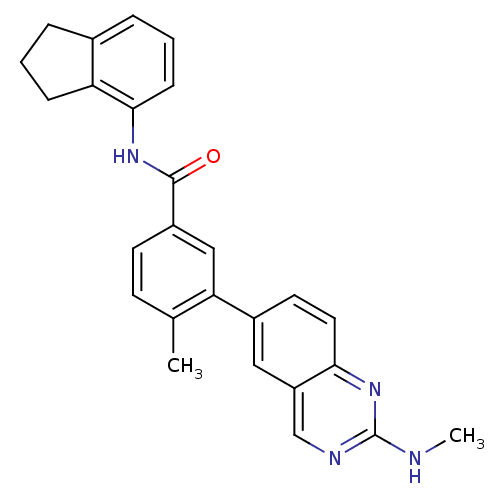 (CHEMBL427233 | N-(2,3-dihydro-1H-inden-4-yl)-4-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM35317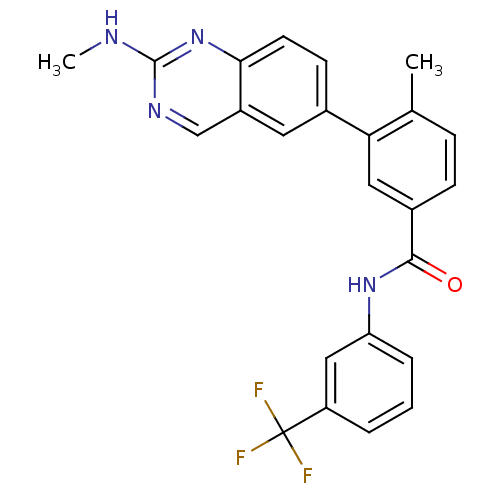 (4-Methyl-3-(2-(methylamino)quinazolin-6-yl)-N-(3-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM26381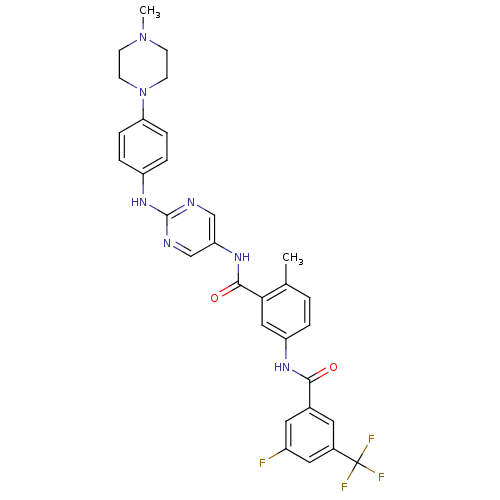 (1-N-[3-fluoro-5-(trifluoromethyl)benzene]-4-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 51: 1681-94 (2008) Article DOI: 10.1021/jm7010996 BindingDB Entry DOI: 10.7270/Q2Q81BCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194691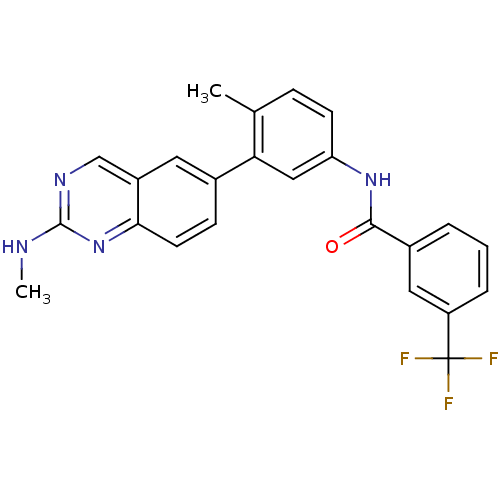 (CHEMBL212128 | N-(4-methyl-3-(2-(methylamino)quina...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194686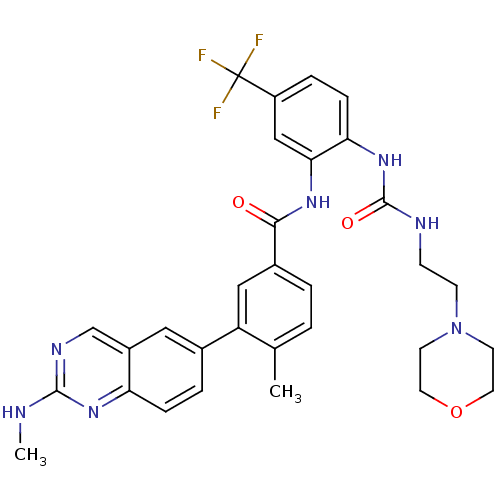 (1-(2-(4-methyl-3-(2-(methylamino)quinazolin-6-yl)b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194678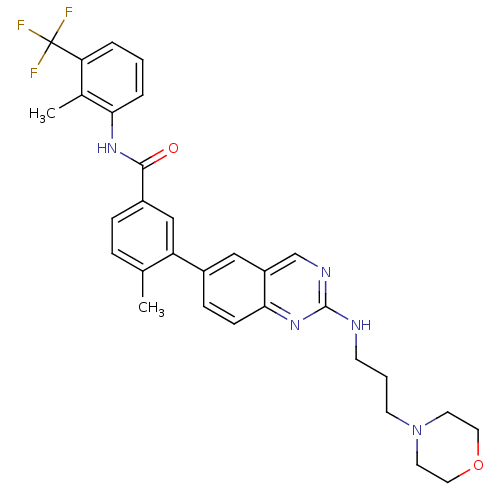 (4-methyl-N-(2-methyl-3-(trifluoromethyl)phenyl)-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM26367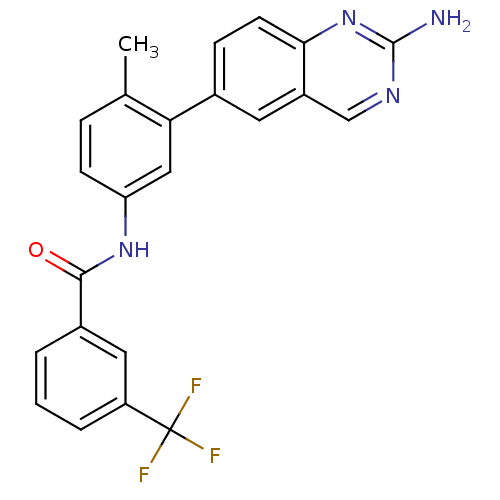 (Aminoquinazoline amide, 35 | N-[3-(2-aminoquinazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 51: 1681-94 (2008) Article DOI: 10.1021/jm7010996 BindingDB Entry DOI: 10.7270/Q2Q81BCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194670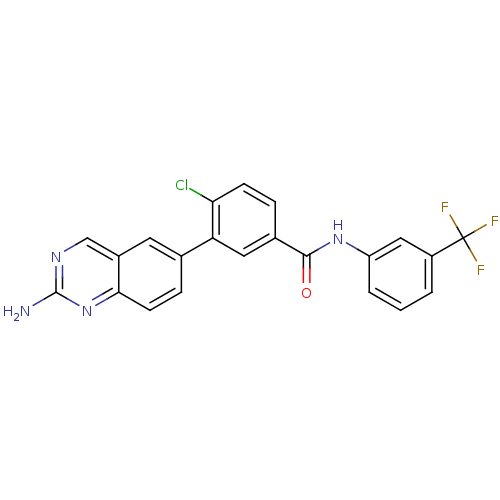 (3-(2-aminoquinazolin-6-yl)-4-chloro-N-(3-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194683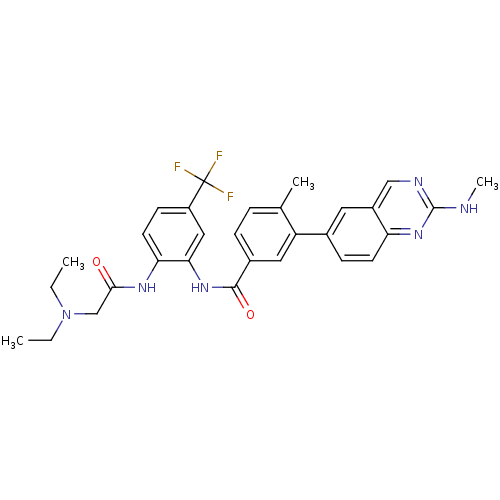 (CHEMBL212953 | N-(2-(2-(diethylamino)acetamido)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194671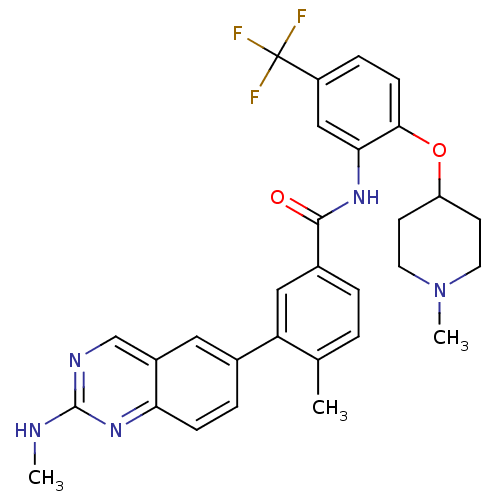 (4-methyl-3-(2-(methylamino)quinazolin-6-yl)-N-(2-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194681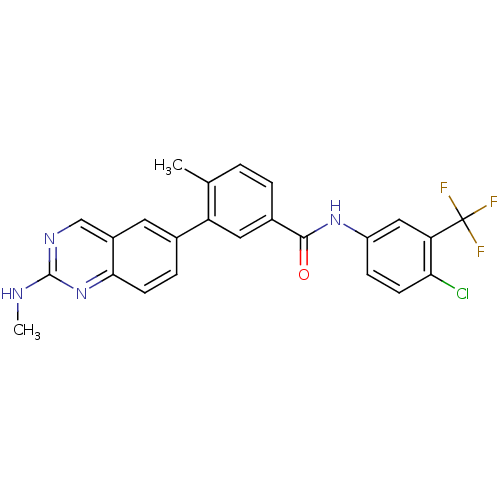 (CHEMBL215019 | N-(4-chloro-3-(trifluoromethyl)phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194690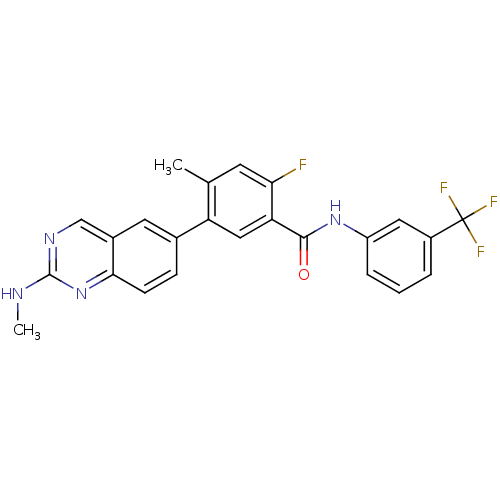 (2-fluoro-4-methyl-5-(2-(methylamino)quinazolin-6-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM26366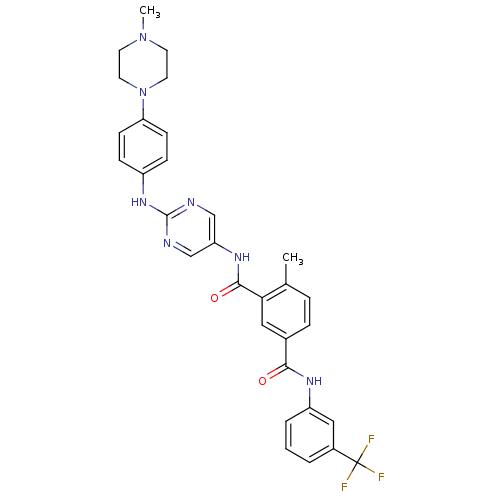 (4-methyl-3-N-(2-{[4-(4-methylpiperazin-1-yl)phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 51: 1681-94 (2008) Article DOI: 10.1021/jm7010996 BindingDB Entry DOI: 10.7270/Q2Q81BCB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM17744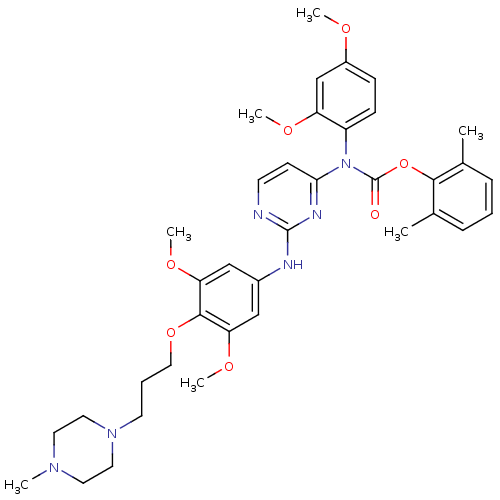 (2,6-dimethylphenyl N-[2-({3,5-dimethoxy-4-[3-(4-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 49: 4981-91 (2006) Article DOI: 10.1021/jm060435i BindingDB Entry DOI: 10.7270/Q2SB440C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194675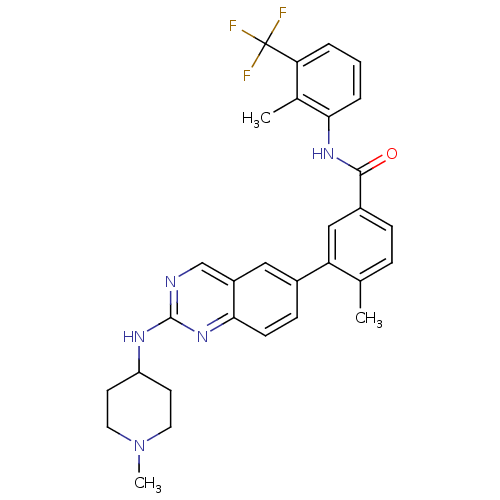 (4-methyl-N-(2-methyl-3-(trifluoromethyl)phenyl)-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50194679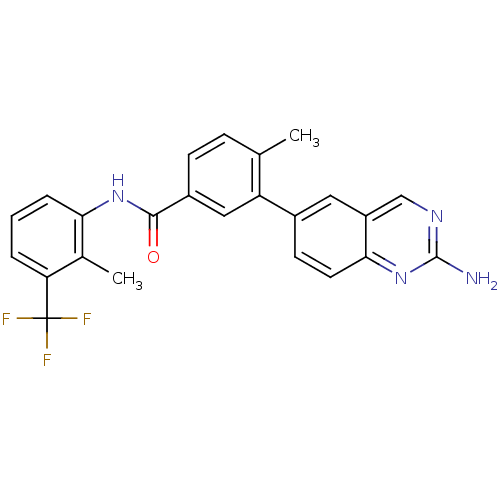 (3-(2-aminoquinazolin-6-yl)-4-methyl-N-(2-methyl-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of Lck by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM26369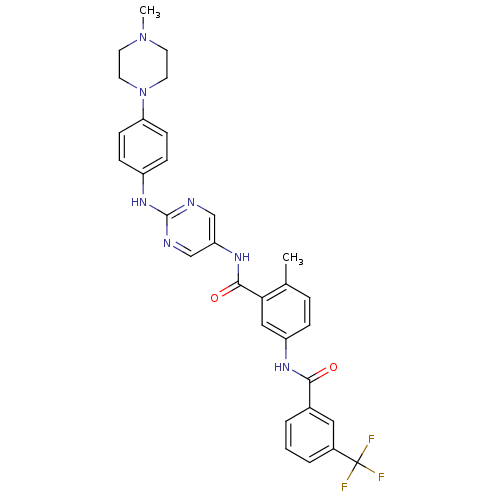 (4-methyl-3-N-(2-{[4-(4-methylpiperazin-1-yl)phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 51: 1681-94 (2008) Article DOI: 10.1021/jm7010996 BindingDB Entry DOI: 10.7270/Q2Q81BCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM26372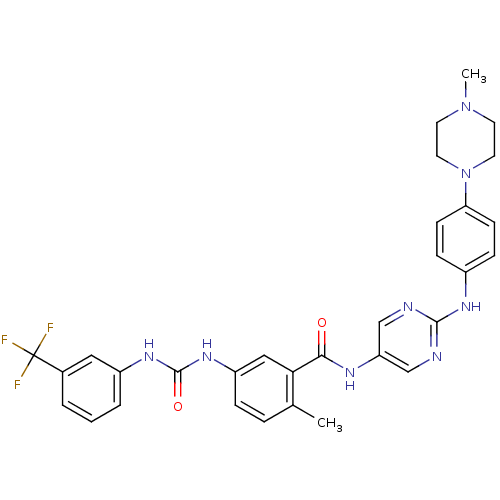 (2-methyl-N-(2-{[4-(4-methylpiperazin-1-yl)phenyl]a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 51: 1681-94 (2008) Article DOI: 10.1021/jm7010996 BindingDB Entry DOI: 10.7270/Q2Q81BCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50075952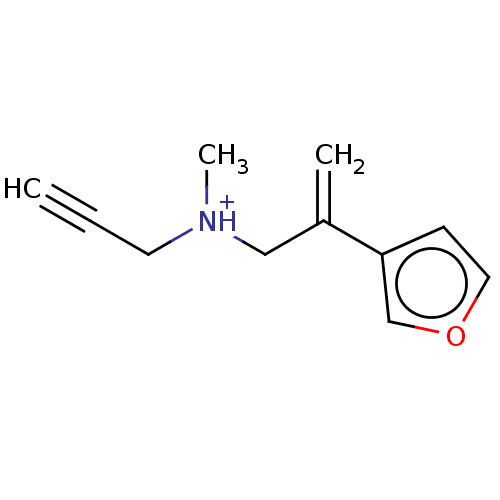 (CHEMBL3415804) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
A* STAR (Agency of Science, Technology and Research) Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A expressed in Sf9 cells using 5-hydroxytryptamine substrate assessed as hydrogen peroxide production after 1 hr ... | J Med Chem 58: 1400-19 (2015) Article DOI: 10.1021/jm501722s BindingDB Entry DOI: 10.7270/Q2V989R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM26382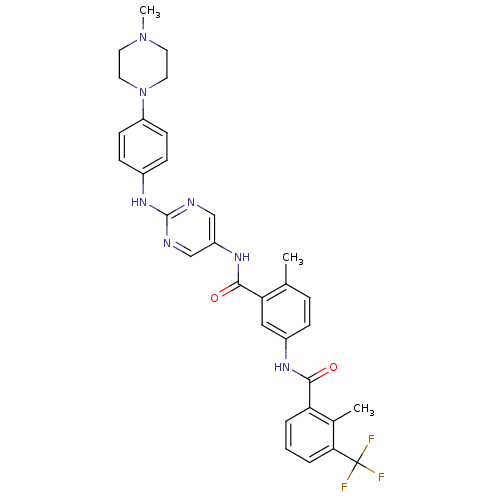 (4-methyl-1-N-[2-methyl-3-(trifluoromethyl)benzene]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay involves the phosphorylation of a biotinylated substrate and the detection of this phosphorylation after the addition of a streptavidin-all... | J Med Chem 51: 1681-94 (2008) Article DOI: 10.1021/jm7010996 BindingDB Entry DOI: 10.7270/Q2Q81BCB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50075959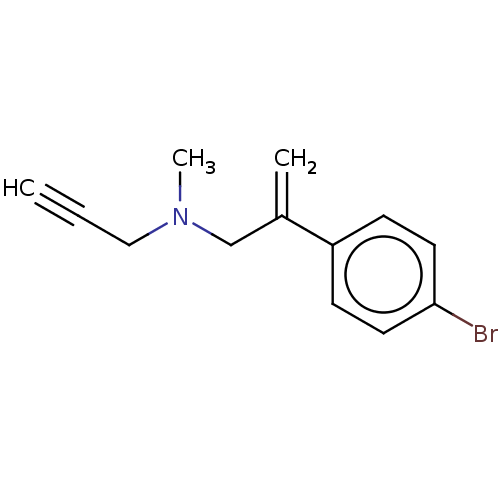 (CHEMBL3415795) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
A* STAR (Agency of Science, Technology and Research) Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A expressed in Sf9 cells using 5-hydroxytryptamine substrate assessed as hydrogen peroxide production after 1 hr ... | J Med Chem 58: 1400-19 (2015) Article DOI: 10.1021/jm501722s BindingDB Entry DOI: 10.7270/Q2V989R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50194691 (CHEMBL212128 | N-(4-methyl-3-(2-(methylamino)quina...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of p38-alpha by HTRF kinase assay | J Med Chem 49: 5671-86 (2006) Article DOI: 10.1021/jm0605482 BindingDB Entry DOI: 10.7270/Q29G5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 956 total ) | Next | Last >> |