

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398598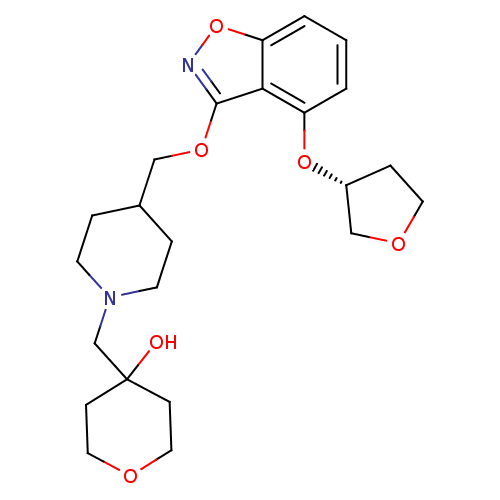 (CHEMBL2152922) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398597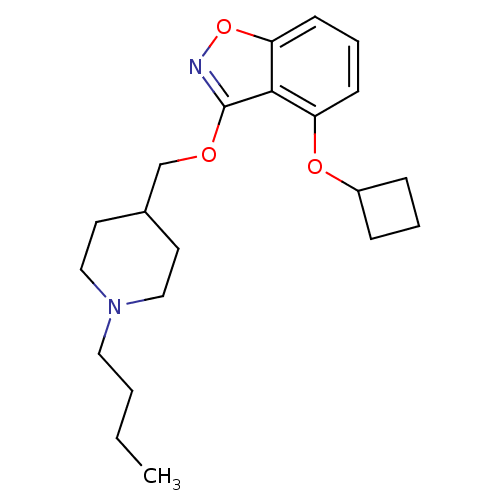 (CHEMBL2179584) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398593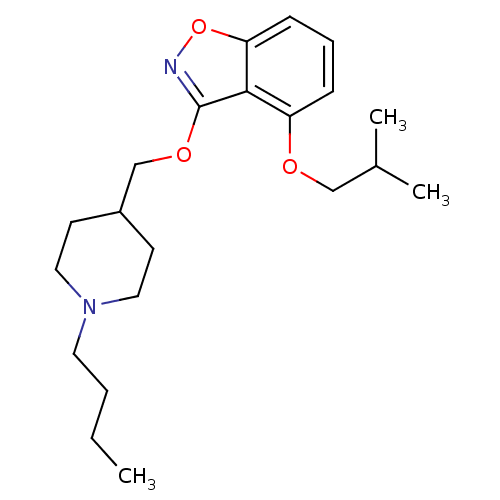 (CHEMBL2179587) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (RAT) | BDBM50398598 (CHEMBL2152922) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5HT4 receptor in rat striatal membrane after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398598 (CHEMBL2152922) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4E receptor expressed in CHO cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398596 (CHEMBL2179589) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398598 (CHEMBL2152922) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4A receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398598 (CHEMBL2152922) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4B receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398599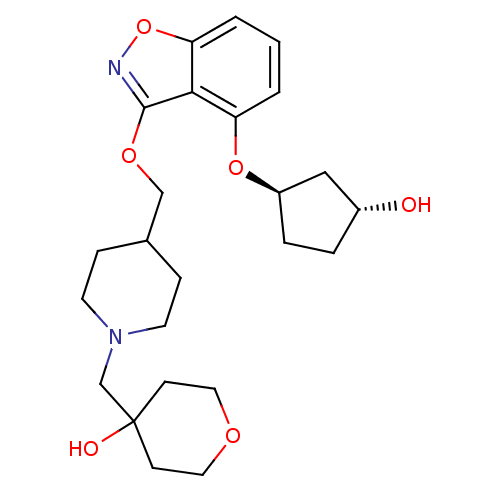 (CHEMBL2179580) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM160878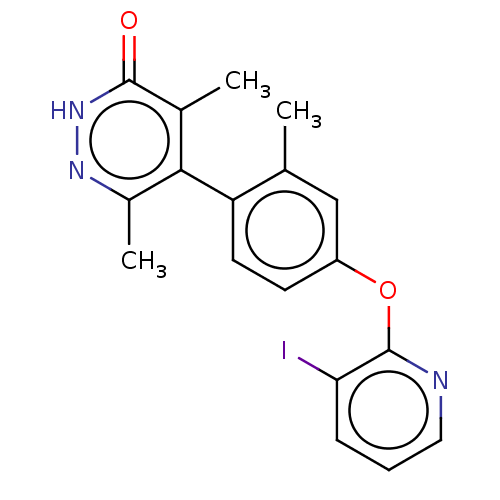 (US10093655, Example 48 | US11014909, Example 48 | ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.571 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description The affinity of the compounds described herein was determined by competition binding assays similar to those described in Ryman-Rasmussen et al., "Di... | US Patent US10093655 (2018) BindingDB Entry DOI: 10.7270/Q2SQ92F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM160878 (US10093655, Example 48 | US11014909, Example 48 | ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.571 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description D1 binding assays were performed using over-expressing LTK human cell lines. To determine basic assay parameters, ligand concentrations were determin... | US Patent US11014909 (2021) BindingDB Entry DOI: 10.7270/Q27D2Z7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM160878 (US10093655, Example 48 | US11014909, Example 48 | ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.571 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The affinity of the compounds described herein was determined by competition binding assays similar to those described in Ryman-Rasmussen et al., Dif... | US Patent US9107923 (2015) BindingDB Entry DOI: 10.7270/Q2C24V5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398594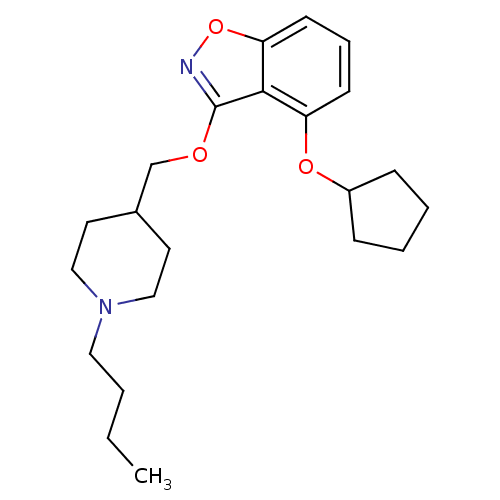 (CHEMBL2179585) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398595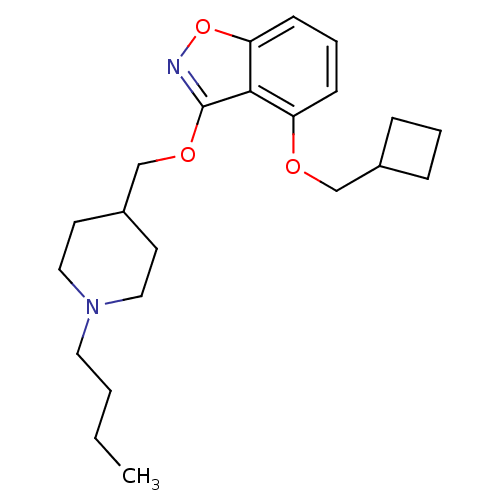 (CHEMBL2179586) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (RAT) | BDBM50398590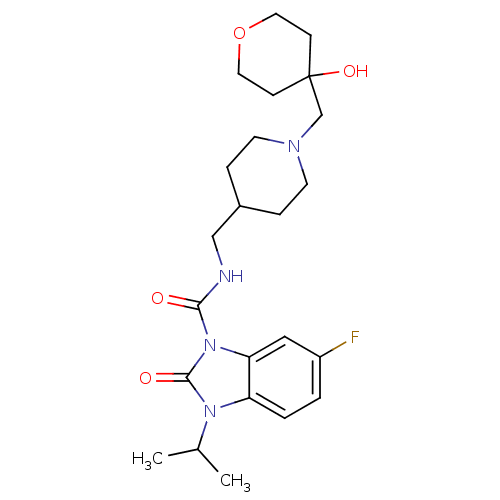 (CHEMBL2179583) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5HT4 receptor in rat striatal membrane after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398590 (CHEMBL2179583) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM200951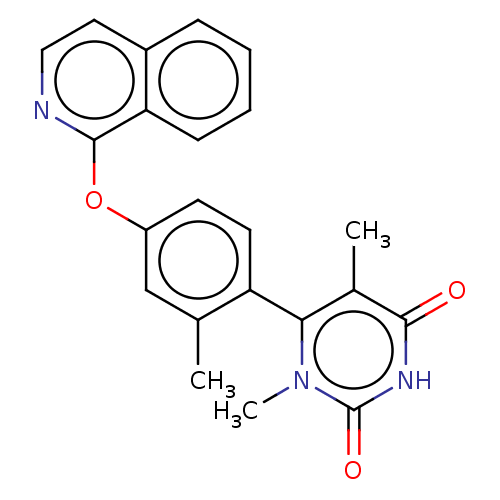 (6-[4-(isoquinolin-1-yloxy)-2-methylphenyl]-1,5-dim...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description The affinity of the compounds described herein was determined by competition binding assays similar to those described in Ryman-Rasmussen et al., Dif... | US Patent US9540352 (2017) BindingDB Entry DOI: 10.7270/Q2X34VND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398588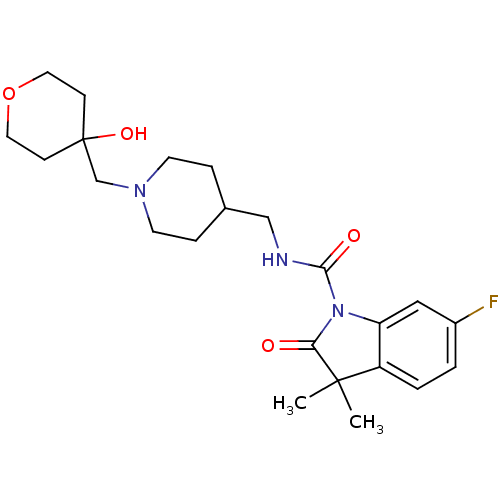 (CHEMBL2179582) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM160912 (US10093655, Example 2 | US11014909, Example 2 | US...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description D1 binding assays were performed using over-expressing LTK human cell lines. To determine basic assay parameters, ligand concentrations were determin... | US Patent US11014909 (2021) BindingDB Entry DOI: 10.7270/Q27D2Z7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM160912 (US10093655, Example 2 | US11014909, Example 2 | US...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.11 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description The affinity of the compounds described herein was determined by competition binding assays similar to those described in Ryman-Rasmussen et al., "Di... | US Patent US10093655 (2018) BindingDB Entry DOI: 10.7270/Q2SQ92F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM160912 (US10093655, Example 2 | US11014909, Example 2 | US...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The affinity of the compounds described herein was determined by competition binding assays similar to those described in Ryman-Rasmussen et al., Dif... | US Patent US9107923 (2015) BindingDB Entry DOI: 10.7270/Q2C24V5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398592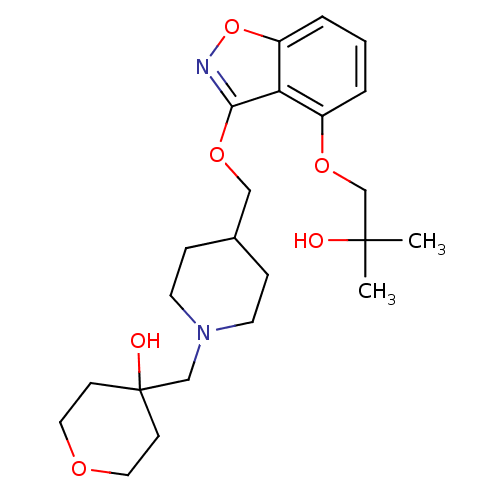 (CHEMBL2179590) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM160861 (US10093655, Example 31 | US11014909, Example 31 | ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description D1 binding assays were performed using over-expressing LTK human cell lines. To determine basic assay parameters, ligand concentrations were determin... | US Patent US11014909 (2021) BindingDB Entry DOI: 10.7270/Q27D2Z7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM160861 (US10093655, Example 31 | US11014909, Example 31 | ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The affinity of the compounds described herein was determined by competition binding assays similar to those described in Ryman-Rasmussen et al., Dif... | US Patent US9107923 (2015) BindingDB Entry DOI: 10.7270/Q2C24V5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM160861 (US10093655, Example 31 | US11014909, Example 31 | ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.61 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description The affinity of the compounds described herein was determined by competition binding assays similar to those described in Ryman-Rasmussen et al., "Di... | US Patent US10093655 (2018) BindingDB Entry DOI: 10.7270/Q2SQ92F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (RAT) | BDBM50398588 (CHEMBL2179582) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5HT4 receptor in rat striatal membrane after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM160894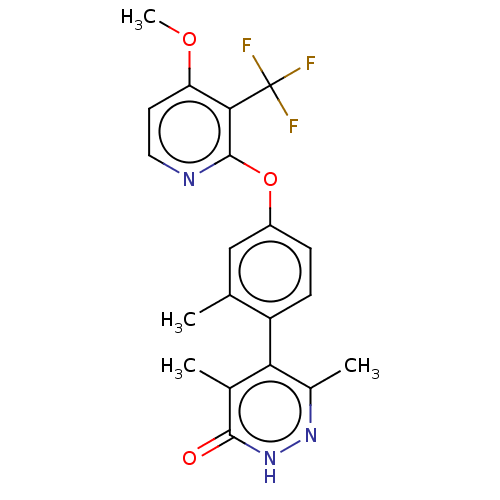 (US10093655, Example 64 | US11014909, Example 64 | ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The affinity of the compounds described herein was determined by competition binding assays similar to those described in Ryman-Rasmussen et al., Dif... | US Patent US9107923 (2015) BindingDB Entry DOI: 10.7270/Q2C24V5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM160894 (US10093655, Example 64 | US11014909, Example 64 | ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description D1 binding assays were performed using over-expressing LTK human cell lines. To determine basic assay parameters, ligand concentrations were determin... | US Patent US11014909 (2021) BindingDB Entry DOI: 10.7270/Q27D2Z7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM160894 (US10093655, Example 64 | US11014909, Example 64 | ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.17 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description The affinity of the compounds described herein was determined by competition binding assays similar to those described in Ryman-Rasmussen et al., "Di... | US Patent US10093655 (2018) BindingDB Entry DOI: 10.7270/Q2SQ92F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398590 (CHEMBL2179583) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4A receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398591 (CHEMBL2179581) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398588 (CHEMBL2179582) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4A receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM337378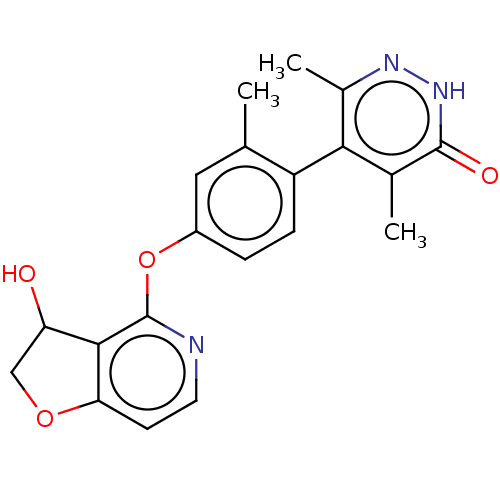 (5-{4-[(3-Hydroxy-2,3-dihydrofuro[3,2-c]pyridin-4-y...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description D1 binding assays were performed using over-expressing LTK human cell lines. To determine basic assay parameters, ligand concentrations were determin... | US Patent US9745317 (2017) BindingDB Entry DOI: 10.7270/Q2W95CBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398590 (CHEMBL2179583) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4E receptor expressed in CHO cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398589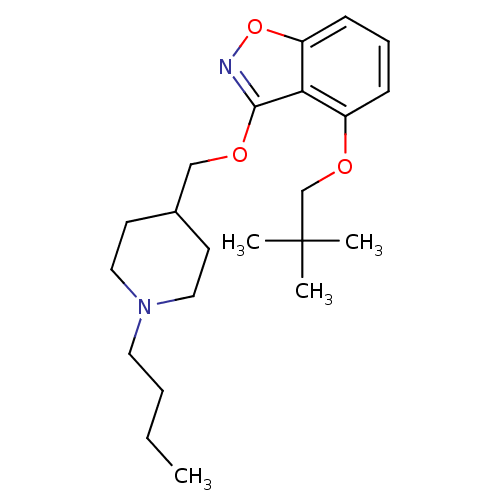 (CHEMBL2179588) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM160871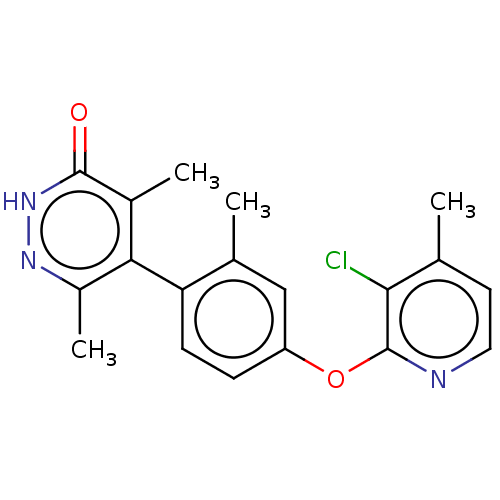 (US10093655, Example 41 | US11014909, Example 41 | ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5.41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The affinity of the compounds described herein was determined by competition binding assays similar to those described in Ryman-Rasmussen et al., Dif... | US Patent US9107923 (2015) BindingDB Entry DOI: 10.7270/Q2C24V5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM160871 (US10093655, Example 41 | US11014909, Example 41 | ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5.41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description D1 binding assays were performed using over-expressing LTK human cell lines. To determine basic assay parameters, ligand concentrations were determin... | US Patent US11014909 (2021) BindingDB Entry DOI: 10.7270/Q27D2Z7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM160871 (US10093655, Example 41 | US11014909, Example 41 | ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5.41 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description The affinity of the compounds described herein was determined by competition binding assays similar to those described in Ryman-Rasmussen et al., "Di... | US Patent US10093655 (2018) BindingDB Entry DOI: 10.7270/Q2SQ92F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398590 (CHEMBL2179583) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4B receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398588 (CHEMBL2179582) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4E receptor expressed in CHO cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398588 (CHEMBL2179582) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5HT4B receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting | J Med Chem 55: 9240-54 (2012) Article DOI: 10.1021/jm300953p BindingDB Entry DOI: 10.7270/Q2FQ9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM289103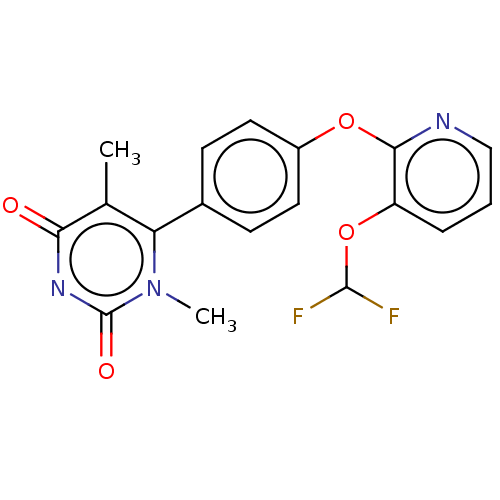 ((−)-6-(4-{[3-(Difluoromethoxy)pyridin-2-yl]o...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.91 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description The affinity of the compounds described herein was determined by competition binding assays similar to those described in Ryman-Rasmussen et al., "Di... | US Patent US10093655 (2018) BindingDB Entry DOI: 10.7270/Q2SQ92F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM160875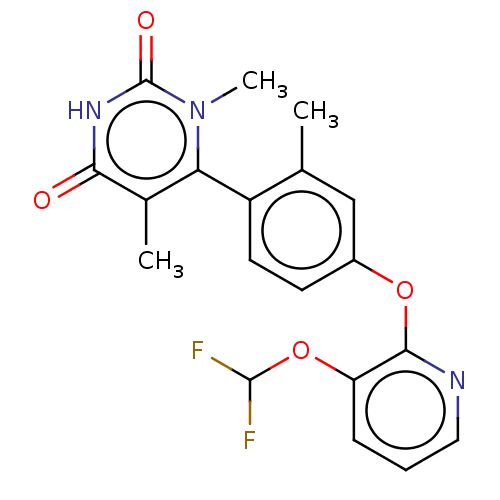 (US10093655, Example 45 | US11014909, Example 45 | ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | 6.91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description D1 binding assays were performed using over-expressing LTK human cell lines. To determine basic assay parameters, ligand concentrations were determin... | US Patent US11014909 (2021) BindingDB Entry DOI: 10.7270/Q27D2Z7D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM160924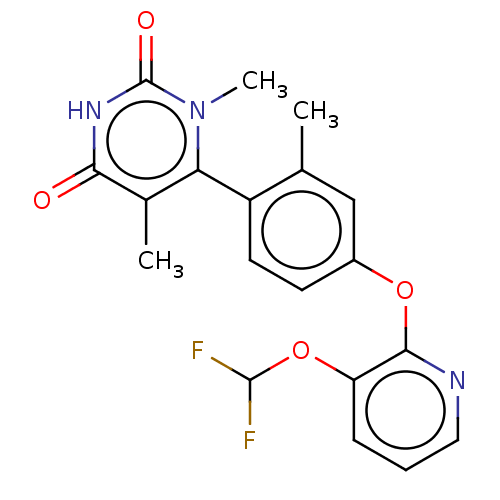 (US9107923, 13) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | 6.91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The affinity of the compounds described herein was determined by competition binding assays similar to those described in Ryman-Rasmussen et al., Dif... | US Patent US9107923 (2015) BindingDB Entry DOI: 10.7270/Q2C24V5T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM160876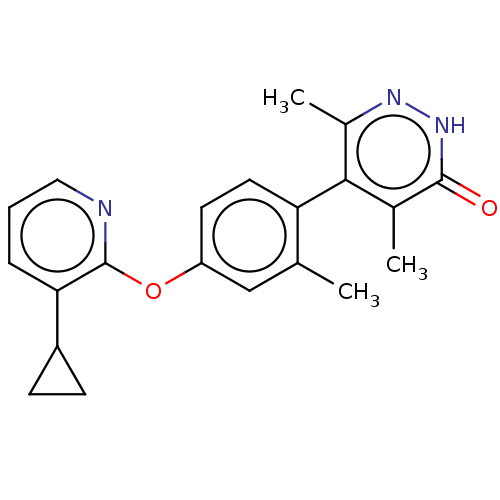 (US10093655, Example 46 | US11014909, Example 46 | ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 7.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The affinity of the compounds described herein was determined by competition binding assays similar to those described in Ryman-Rasmussen et al., Dif... | US Patent US9107923 (2015) BindingDB Entry DOI: 10.7270/Q2C24V5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM160876 (US10093655, Example 46 | US11014909, Example 46 | ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 7.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description D1 binding assays were performed using over-expressing LTK human cell lines. To determine basic assay parameters, ligand concentrations were determin... | US Patent US11014909 (2021) BindingDB Entry DOI: 10.7270/Q27D2Z7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM160876 (US10093655, Example 46 | US11014909, Example 46 | ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 7.66 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description The affinity of the compounds described herein was determined by competition binding assays similar to those described in Ryman-Rasmussen et al., "Di... | US Patent US10093655 (2018) BindingDB Entry DOI: 10.7270/Q2SQ92F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM200985 (5-[4-(isoquinolin-1-yloxy)-2-methylphenyl]- 4,6-di...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description The affinity of the compounds described herein was determined by competition binding assays similar to those described in Ryman-Rasmussen et al., Dif... | US Patent US9540352 (2017) BindingDB Entry DOI: 10.7270/Q2X34VND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50240692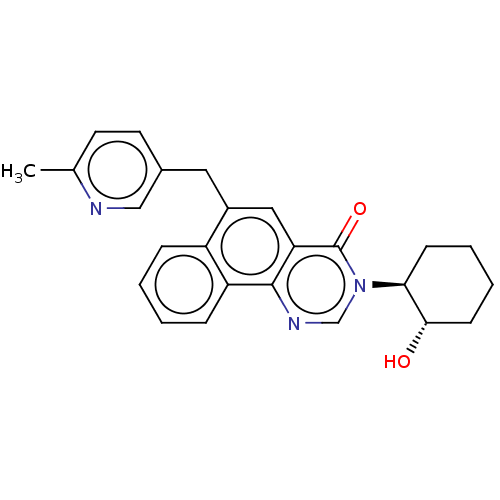 (CHEMBL4078588) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PT-1284 human muscarinic acetylcholine receptor M1 expressed in CHO cell membranes after 90 mins scintillation counting method | J Med Chem 60: 6649-6663 (2017) Article DOI: 10.1021/acs.jmedchem.7b00597 BindingDB Entry DOI: 10.7270/Q2ZK5JS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM337370 (5-[4-(2,3-dihydro-1H-pyrrolo[3,2-c]pyridin-4-yloxy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description D1 binding assays were performed using over-expressing LTK human cell lines. To determine basic assay parameters, ligand concentrations were determin... | US Patent US9745317 (2017) BindingDB Entry DOI: 10.7270/Q2W95CBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2030 total ) | Next | Last >> |