

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50059171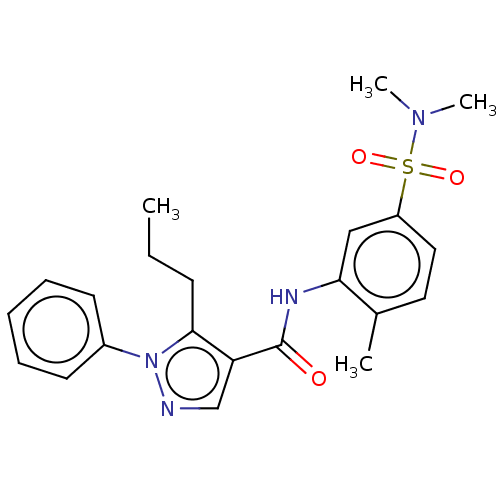 (CHEMBL3393296) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant 5'-c-myc, His6-tagged p38-alpha using MK2 (46-400) as substrate | J Med Chem 58: 278-93 (2015) Article DOI: 10.1021/jm501038s BindingDB Entry DOI: 10.7270/Q269758W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50418818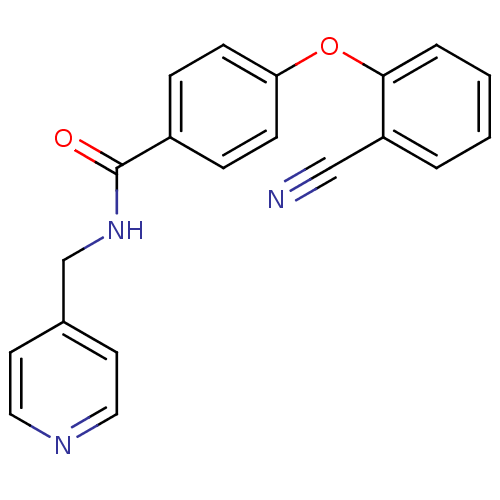 (CHEMBL1796293) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant 5'-c-myc, His6-tagged p38-alpha using MK2 (46-400) as substrate | J Med Chem 58: 278-93 (2015) Article DOI: 10.1021/jm501038s BindingDB Entry DOI: 10.7270/Q269758W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50026750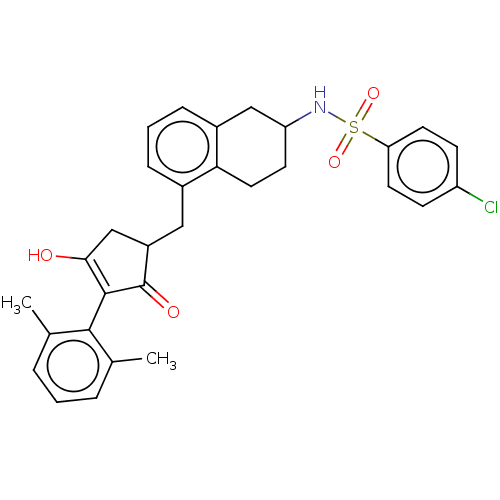 (CHEMBL3354618) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Antagonist activity against human thromboxane A2 receptor alpha expressed in QBI-HEK293A cells assessed as reduction in I-BOP-induced inositol monoph... | ACS Med Chem Lett 5: 1015-20 (2014) Article DOI: 10.1021/ml5002085 BindingDB Entry DOI: 10.7270/Q27M09HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50026748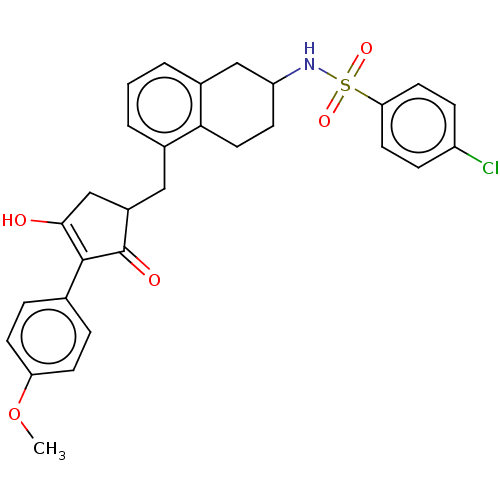 (CHEMBL3354616) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Antagonist activity against human thromboxane A2 receptor alpha expressed in QBI-HEK293A cells assessed as reduction in I-BOP-induced inositol monoph... | ACS Med Chem Lett 5: 1015-20 (2014) Article DOI: 10.1021/ml5002085 BindingDB Entry DOI: 10.7270/Q27M09HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50058027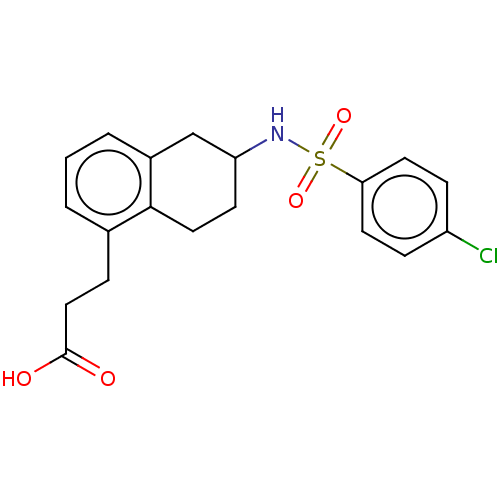 (CHEMBL29374) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human TP receptor expressed in QBI-HEK 293A cells assessed as IP3 metabolite level after 1 hr by HTRF assay | Bioorg Med Chem Lett 24: 4171-5 (2014) Article DOI: 10.1016/j.bmcl.2014.07.047 BindingDB Entry DOI: 10.7270/Q2QF8VJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50026760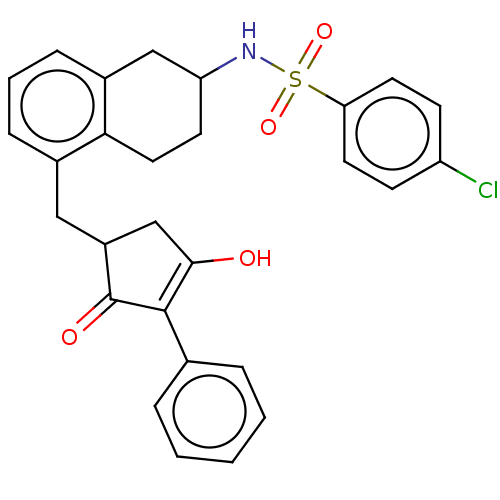 (CHEMBL3354614) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Antagonist activity against human thromboxane A2 receptor alpha expressed in QBI-HEK293A cells assessed as reduction in I-BOP-induced inositol monoph... | ACS Med Chem Lett 5: 1015-20 (2014) Article DOI: 10.1021/ml5002085 BindingDB Entry DOI: 10.7270/Q27M09HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50026761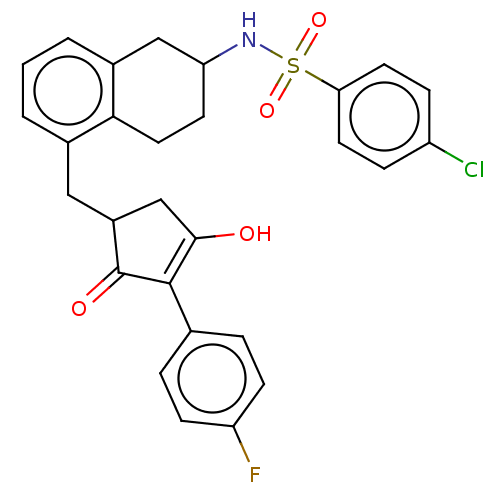 (CHEMBL3354615) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Antagonist activity against human thromboxane A2 receptor alpha expressed in QBI-HEK293A cells assessed as reduction in I-BOP-induced inositol monoph... | ACS Med Chem Lett 5: 1015-20 (2014) Article DOI: 10.1021/ml5002085 BindingDB Entry DOI: 10.7270/Q27M09HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50026758 (CHEMBL3335473) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Antagonist activity against human thromboxane A2 receptor alpha expressed in QBI-HEK293A cells assessed as reduction in I-BOP-induced inositol monoph... | ACS Med Chem Lett 5: 1015-20 (2014) Article DOI: 10.1021/ml5002085 BindingDB Entry DOI: 10.7270/Q27M09HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50026759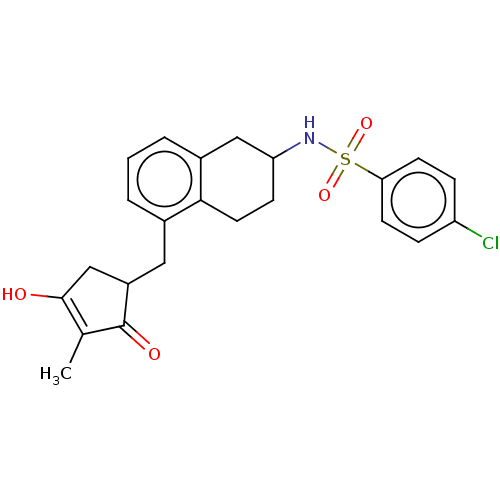 (CHEMBL3354613) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Antagonist activity against human thromboxane A2 receptor alpha expressed in QBI-HEK293A cells assessed as reduction in I-BOP-induced inositol monoph... | ACS Med Chem Lett 5: 1015-20 (2014) Article DOI: 10.1021/ml5002085 BindingDB Entry DOI: 10.7270/Q27M09HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L [114-333,T223A] (Homo sapiens (Human)) | BDBM31992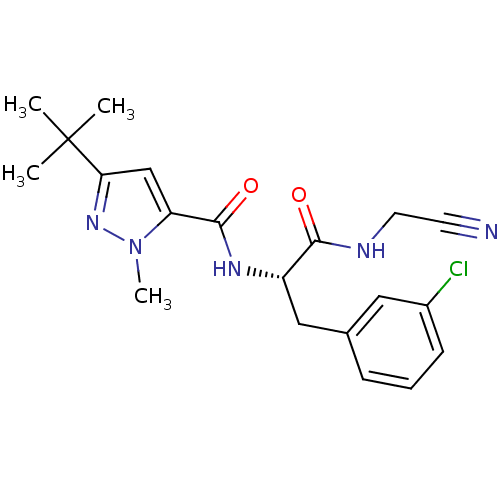 (Dipeptidyl nitrile inhibitor, 25) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca | Assay Description IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. | Bioorg Med Chem Lett 19: 4280-3 (2009) Article DOI: 10.1016/j.bmcl.2009.05.071 BindingDB Entry DOI: 10.7270/Q2TT4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L [114-333,T223A] (Homo sapiens (Human)) | BDBM31993 (Dipeptidyl nitrile inhibitor, 26) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca | Assay Description IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. | Bioorg Med Chem Lett 19: 4280-3 (2009) Article DOI: 10.1016/j.bmcl.2009.05.071 BindingDB Entry DOI: 10.7270/Q2TT4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50059175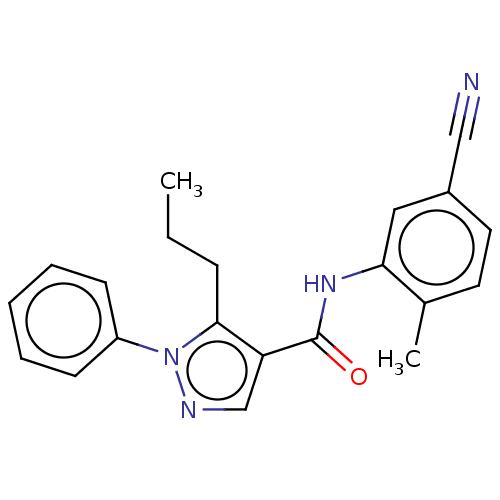 (CHEMBL3393298) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant 5'-c-myc, His6-tagged p38-alpha using MK2 (46 to 400) as substrate assessed as inhibition of phosphorylation after 60... | J Med Chem 58: 278-93 (2015) Article DOI: 10.1021/jm501038s BindingDB Entry DOI: 10.7270/Q269758W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50026753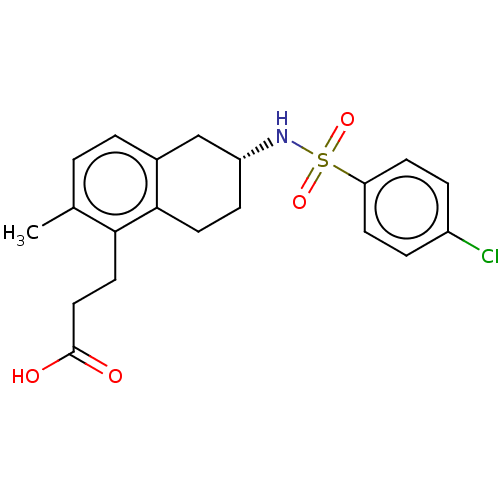 (S-18886 | Terutroban) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Antagonist activity against human thromboxane A2 receptor alpha expressed in QBI-HEK293A cells assessed as reduction in I-BOP-induced inositol monoph... | ACS Med Chem Lett 5: 1015-20 (2014) Article DOI: 10.1021/ml5002085 BindingDB Entry DOI: 10.7270/Q27M09HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L [114-333,T223A] (Homo sapiens (Human)) | BDBM31984 (Dipeptidyl nitrile inhibitor, 17) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca | Assay Description IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. | Bioorg Med Chem Lett 19: 4280-3 (2009) Article DOI: 10.1016/j.bmcl.2009.05.071 BindingDB Entry DOI: 10.7270/Q2TT4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50026751 (CHEMBL3354619) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Antagonist activity against human thromboxane A2 receptor alpha expressed in QBI-HEK293A cells assessed as reduction in I-BOP-induced inositol monoph... | ACS Med Chem Lett 5: 1015-20 (2014) Article DOI: 10.1021/ml5002085 BindingDB Entry DOI: 10.7270/Q27M09HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50059180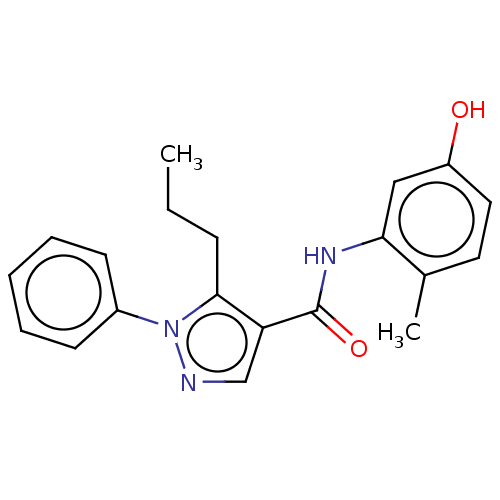 (CHEMBL3393299) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <46 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant 5'-c-myc, His6-tagged p38-alpha using MK2 (46 to 400) as substrate assessed as inhibition of phosphorylation after 60... | J Med Chem 58: 278-93 (2015) Article DOI: 10.1021/jm501038s BindingDB Entry DOI: 10.7270/Q269758W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50059180 (CHEMBL3393299) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <46 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant 5'-c-myc, His6-tagged p38-alpha using MSK1 as substrate assessed as inhibition of phosphorylation after 60 mins by EL... | J Med Chem 58: 278-93 (2015) Article DOI: 10.1021/jm501038s BindingDB Entry DOI: 10.7270/Q269758W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L [114-333,T223A] (Homo sapiens (Human)) | BDBM31980 (Dipeptidyl nitrile inhibitor, 13) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca | Assay Description IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. | Bioorg Med Chem Lett 19: 4280-3 (2009) Article DOI: 10.1016/j.bmcl.2009.05.071 BindingDB Entry DOI: 10.7270/Q2TT4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50059187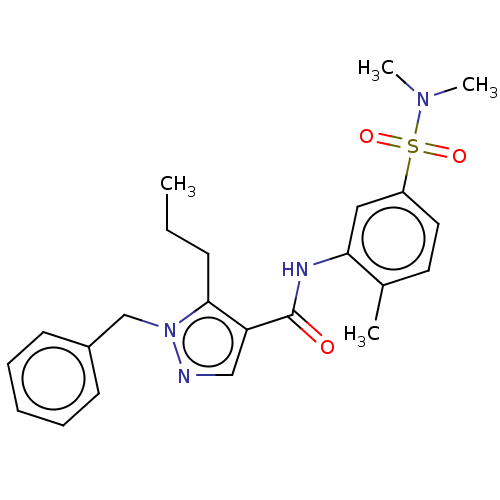 (CHEMBL3393306) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant 5'-c-myc, His6-tagged p38-alpha using MK2 (46 to 400) as substrate assessed as inhibition of phosphorylation after 60... | J Med Chem 58: 278-93 (2015) Article DOI: 10.1021/jm501038s BindingDB Entry DOI: 10.7270/Q269758W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50058026 (CHEMBL3326602) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human TP receptor expressed in QBI-HEK 293A cells assessed as IP3 metabolite level after 1 hr by HTRF assay | Bioorg Med Chem Lett 24: 4171-5 (2014) Article DOI: 10.1016/j.bmcl.2014.07.047 BindingDB Entry DOI: 10.7270/Q2QF8VJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50026755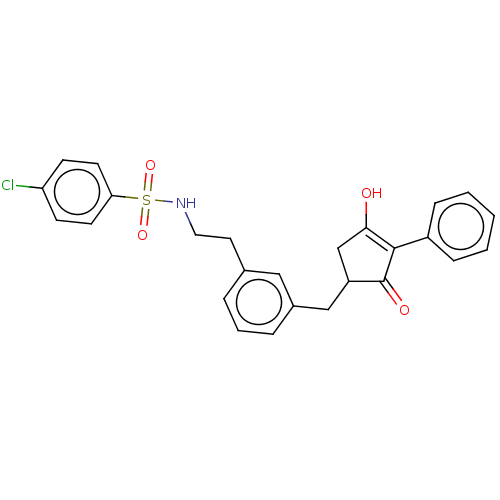 (CHEMBL3335482) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Antagonist activity against human thromboxane A2 receptor alpha expressed in QBI-HEK293A cells assessed as reduction in I-BOP-induced inositol monoph... | ACS Med Chem Lett 5: 1015-20 (2014) Article DOI: 10.1021/ml5002085 BindingDB Entry DOI: 10.7270/Q27M09HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50059171 (CHEMBL3393296) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant 5'-c-myc, His6-tagged p38-alpha using MK2 (46 to 400) as substrate assessed as inhibition of phosphorylation after 60... | J Med Chem 58: 278-93 (2015) Article DOI: 10.1021/jm501038s BindingDB Entry DOI: 10.7270/Q269758W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Procathepsin L [114-333,T223A] (Homo sapiens (Human)) | BDBM31990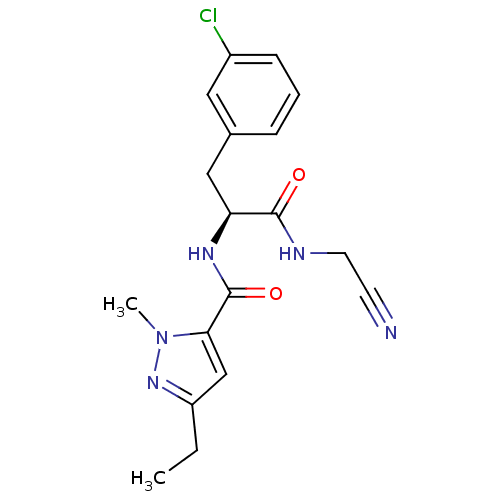 (Dipeptidyl nitrile inhibitor, 23) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca | Assay Description IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. | Bioorg Med Chem Lett 19: 4280-3 (2009) Article DOI: 10.1016/j.bmcl.2009.05.071 BindingDB Entry DOI: 10.7270/Q2TT4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50059188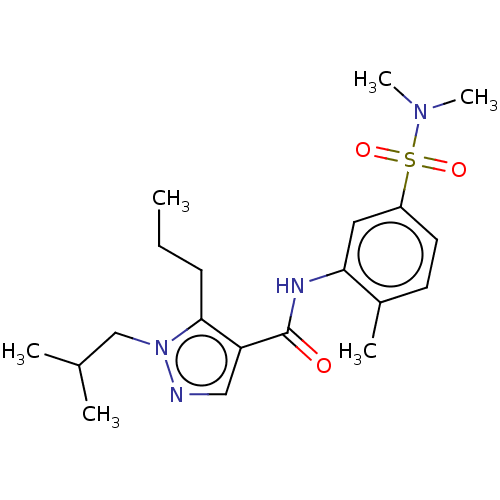 (CHEMBL3393307) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant 5'-c-myc, His6-tagged p38-alpha using MK2 (46 to 400) as substrate assessed as inhibition of phosphorylation after 60... | J Med Chem 58: 278-93 (2015) Article DOI: 10.1021/jm501038s BindingDB Entry DOI: 10.7270/Q269758W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L [114-333,T223A] (Homo sapiens (Human)) | BDBM31991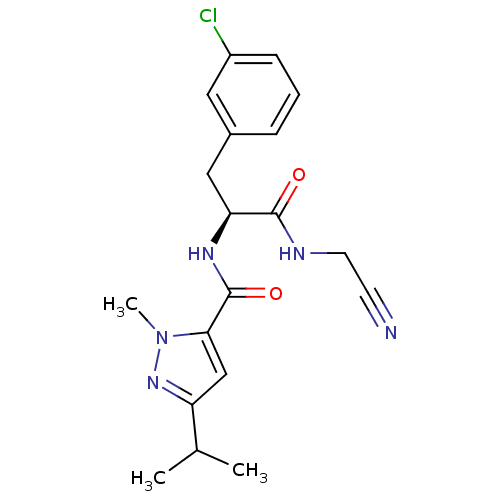 (Dipeptidyl nitrile inhibitor, 24) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca | Assay Description IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. | Bioorg Med Chem Lett 19: 4280-3 (2009) Article DOI: 10.1016/j.bmcl.2009.05.071 BindingDB Entry DOI: 10.7270/Q2TT4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L [114-333,T223A] (Homo sapiens (Human)) | BDBM31978 (Dipeptidyl nitrile inhibitor, 11) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca | Assay Description IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. | Bioorg Med Chem Lett 19: 4280-3 (2009) Article DOI: 10.1016/j.bmcl.2009.05.071 BindingDB Entry DOI: 10.7270/Q2TT4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L [114-333,T223A] (Homo sapiens (Human)) | BDBM31977 (Dipeptidyl nitrile inhibitor, 10) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca | Assay Description IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. | Bioorg Med Chem Lett 19: 4280-3 (2009) Article DOI: 10.1016/j.bmcl.2009.05.071 BindingDB Entry DOI: 10.7270/Q2TT4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L [114-333,T223A] (Homo sapiens (Human)) | BDBM31989 (Dipeptidyl nitrile inhibitor, 22) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca | Assay Description IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. | Bioorg Med Chem Lett 19: 4280-3 (2009) Article DOI: 10.1016/j.bmcl.2009.05.071 BindingDB Entry DOI: 10.7270/Q2TT4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50059172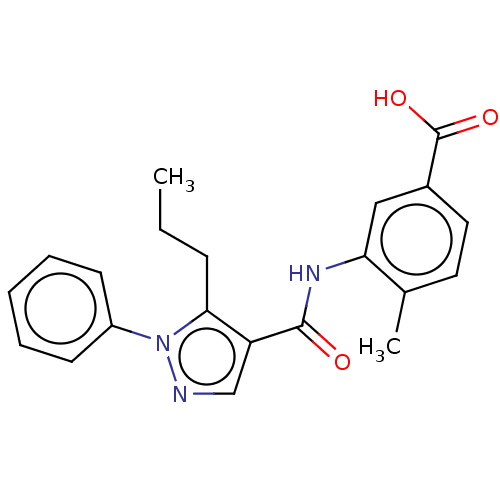 (CHEMBL3393303) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant 5'-c-myc, His6-tagged p38-alpha using MK2 (46 to 400) as substrate assessed as inhibition of phosphorylation after 60... | J Med Chem 58: 278-93 (2015) Article DOI: 10.1021/jm501038s BindingDB Entry DOI: 10.7270/Q269758W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L [114-333,T223A] (Homo sapiens (Human)) | BDBM31983 (Dipeptidyl nitrile inhibitor, 16) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca | Assay Description IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. | Bioorg Med Chem Lett 19: 4280-3 (2009) Article DOI: 10.1016/j.bmcl.2009.05.071 BindingDB Entry DOI: 10.7270/Q2TT4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50059181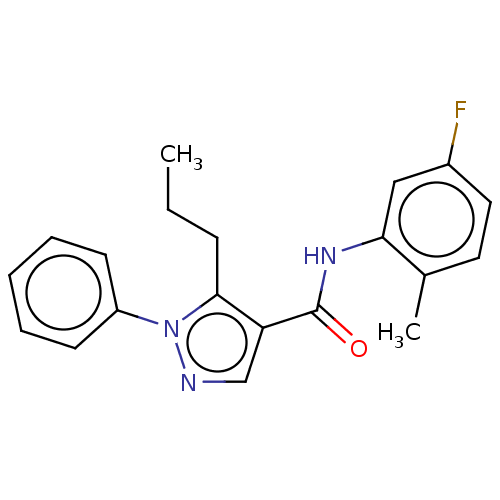 (CHEMBL3393297) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant 5'-c-myc, His6-tagged p38-alpha using MK2 (46 to 400) as substrate assessed as inhibition of phosphorylation after 60... | J Med Chem 58: 278-93 (2015) Article DOI: 10.1021/jm501038s BindingDB Entry DOI: 10.7270/Q269758W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM31970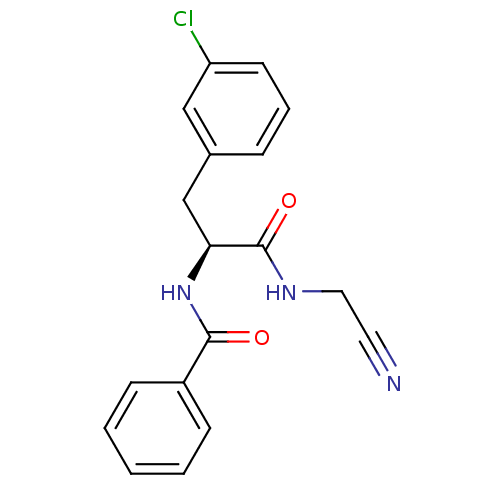 (Dipeptidyl nitrile inhibitor, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca | Assay Description IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. | Bioorg Med Chem Lett 19: 4280-3 (2009) Article DOI: 10.1016/j.bmcl.2009.05.071 BindingDB Entry DOI: 10.7270/Q2TT4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50059177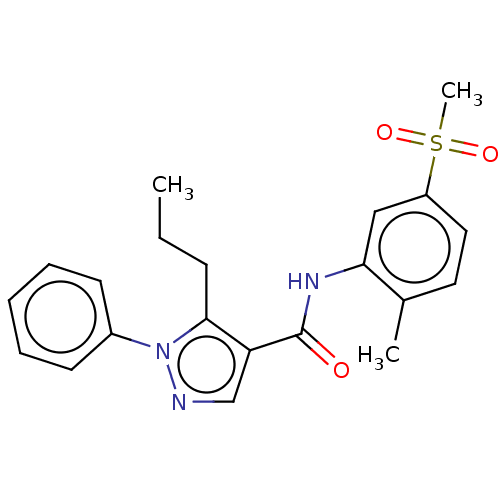 (CHEMBL3393302) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant 5'-c-myc, His6-tagged p38-alpha using MK2 (46 to 400) as substrate assessed as inhibition of phosphorylation after 60... | J Med Chem 58: 278-93 (2015) Article DOI: 10.1021/jm501038s BindingDB Entry DOI: 10.7270/Q269758W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50353640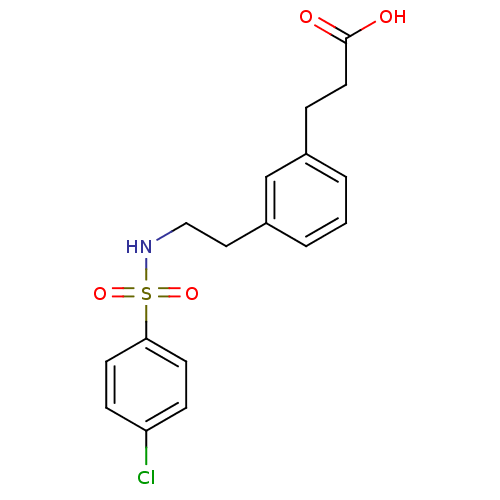 (CHEMBL65121) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human TP receptor expressed in QBI-HEK 293A cells assessed as IP3 metabolite level after 1 hr by HTRF assay | Bioorg Med Chem Lett 24: 4171-5 (2014) Article DOI: 10.1016/j.bmcl.2014.07.047 BindingDB Entry DOI: 10.7270/Q2QF8VJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM31977 (Dipeptidyl nitrile inhibitor, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca | Assay Description IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. | Bioorg Med Chem Lett 19: 4280-3 (2009) Article DOI: 10.1016/j.bmcl.2009.05.071 BindingDB Entry DOI: 10.7270/Q2TT4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM31988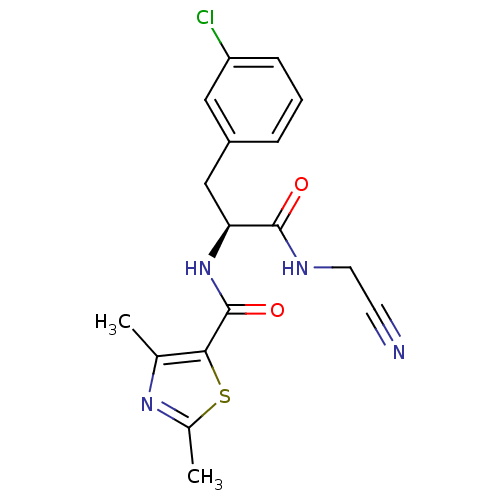 (Dipeptidyl nitrile inhibitor, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca | Assay Description IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. | Bioorg Med Chem Lett 19: 4280-3 (2009) Article DOI: 10.1016/j.bmcl.2009.05.071 BindingDB Entry DOI: 10.7270/Q2TT4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin L2 (Homo sapiens (Human)) | BDBM31980 (Dipeptidyl nitrile inhibitor, 13) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca | Assay Description IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. | Bioorg Med Chem Lett 19: 4280-3 (2009) Article DOI: 10.1016/j.bmcl.2009.05.071 BindingDB Entry DOI: 10.7270/Q2TT4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin L2 (Homo sapiens (Human)) | BDBM31992 (Dipeptidyl nitrile inhibitor, 25) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca | Assay Description IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. | Bioorg Med Chem Lett 19: 4280-3 (2009) Article DOI: 10.1016/j.bmcl.2009.05.071 BindingDB Entry DOI: 10.7270/Q2TT4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin L2 (Homo sapiens (Human)) | BDBM31977 (Dipeptidyl nitrile inhibitor, 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca | Assay Description IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. | Bioorg Med Chem Lett 19: 4280-3 (2009) Article DOI: 10.1016/j.bmcl.2009.05.071 BindingDB Entry DOI: 10.7270/Q2TT4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50059175 (CHEMBL3393298) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of p38-alpha in human TC28 cells assessed as inhibition of IL-1-induced PGE2 production incubated for 20 mins prior to IL-1 challenge meas... | J Med Chem 58: 278-93 (2015) Article DOI: 10.1021/jm501038s BindingDB Entry DOI: 10.7270/Q269758W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L [114-333,T223A] (Homo sapiens (Human)) | BDBM31981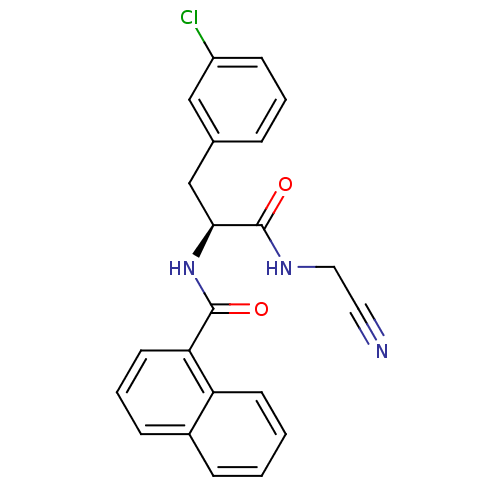 (Dipeptidyl nitrile inhibitor, 14) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 251 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca | Assay Description IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. | Bioorg Med Chem Lett 19: 4280-3 (2009) Article DOI: 10.1016/j.bmcl.2009.05.071 BindingDB Entry DOI: 10.7270/Q2TT4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L [114-333,T223A] (Homo sapiens (Human)) | BDBM31970 (Dipeptidyl nitrile inhibitor, 1) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 251 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca | Assay Description IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. | Bioorg Med Chem Lett 19: 4280-3 (2009) Article DOI: 10.1016/j.bmcl.2009.05.071 BindingDB Entry DOI: 10.7270/Q2TT4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L [114-333,T223A] (Homo sapiens (Human)) | BDBM31974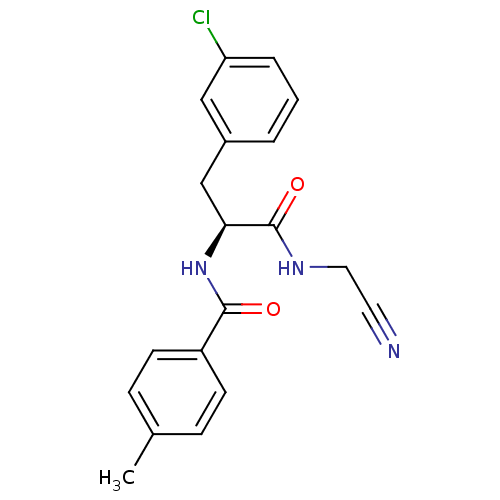 (Dipeptidyl nitrile inhibitor, 7) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 251 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca | Assay Description IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. | Bioorg Med Chem Lett 19: 4280-3 (2009) Article DOI: 10.1016/j.bmcl.2009.05.071 BindingDB Entry DOI: 10.7270/Q2TT4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin L2 (Homo sapiens (Human)) | BDBM31988 (Dipeptidyl nitrile inhibitor, 21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca | Assay Description IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. | Bioorg Med Chem Lett 19: 4280-3 (2009) Article DOI: 10.1016/j.bmcl.2009.05.071 BindingDB Entry DOI: 10.7270/Q2TT4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L [114-333,T223A] (Homo sapiens (Human)) | BDBM31975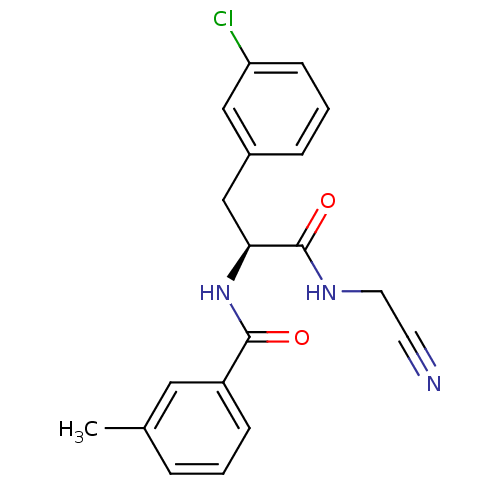 (Dipeptidyl nitrile inhibitor, 8) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 251 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca | Assay Description IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. | Bioorg Med Chem Lett 19: 4280-3 (2009) Article DOI: 10.1016/j.bmcl.2009.05.071 BindingDB Entry DOI: 10.7270/Q2TT4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50059186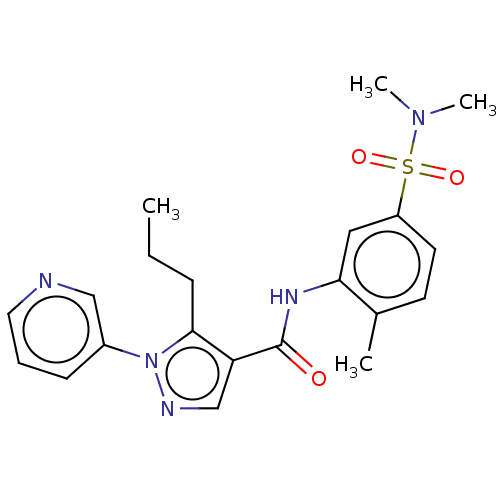 (CHEMBL3393305) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant 5'-c-myc, His6-tagged p38-alpha using MK2 (46 to 400) as substrate assessed as inhibition of phosphorylation after 60... | J Med Chem 58: 278-93 (2015) Article DOI: 10.1021/jm501038s BindingDB Entry DOI: 10.7270/Q269758W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM31980 (Dipeptidyl nitrile inhibitor, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca | Assay Description IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. | Bioorg Med Chem Lett 19: 4280-3 (2009) Article DOI: 10.1016/j.bmcl.2009.05.071 BindingDB Entry DOI: 10.7270/Q2TT4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L [114-333,T223A] (Homo sapiens (Human)) | BDBM31979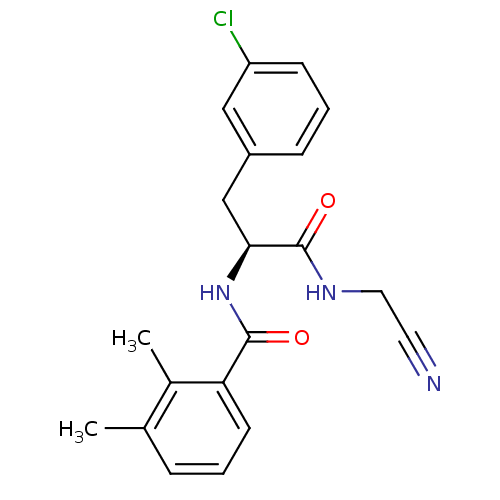 (Dipeptidyl nitrile inhibitor, 12) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca | Assay Description IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. | Bioorg Med Chem Lett 19: 4280-3 (2009) Article DOI: 10.1016/j.bmcl.2009.05.071 BindingDB Entry DOI: 10.7270/Q2TT4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L [114-333,T223A] (Homo sapiens (Human)) | BDBM31973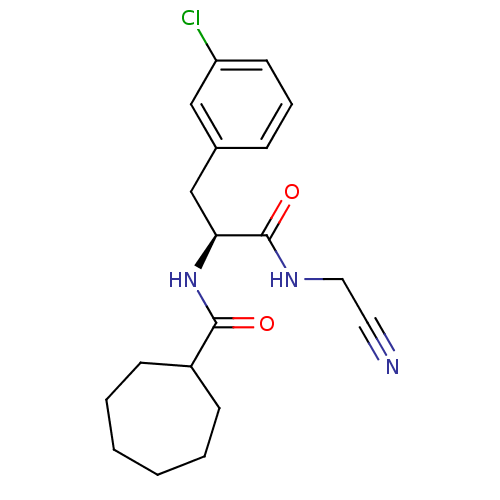 (Dipeptidyl nitrile inhibitor, 6) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca | Assay Description IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. | Bioorg Med Chem Lett 19: 4280-3 (2009) Article DOI: 10.1016/j.bmcl.2009.05.071 BindingDB Entry DOI: 10.7270/Q2TT4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50059176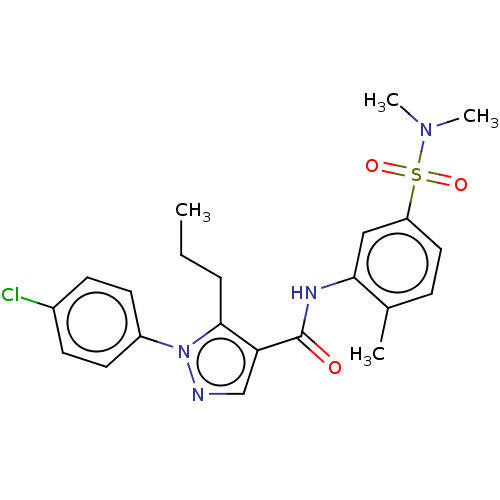 (CHEMBL3393304) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant 5'-c-myc, His6-tagged p38-alpha using MK2 (46 to 400) as substrate assessed as inhibition of phosphorylation after 60... | J Med Chem 58: 278-93 (2015) Article DOI: 10.1021/jm501038s BindingDB Entry DOI: 10.7270/Q269758W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 238 total ) | Next | Last >> |