

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA polymerase III PolC-type (Bacillus subtilis) | BDBM50182669 ((S)-3-{4-[1-(1-cyclopropyl-3-carboxy-4-oxo-6,8-dif...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GLSynthesis Inc. Curated by ChEMBL | Assay Description Binding affinity to Bacillus subtilis DNA polymerase3C | J Med Chem 49: 1455-65 (2006) Article DOI: 10.1021/jm0510023 BindingDB Entry DOI: 10.7270/Q23J3DR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Bacillus subtilis) | BDBM50182665 ((R)-3-{4-[1-(1-cyclopropyl-3-carboxy-4-oxo-6,8-dif...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GLSynthesis Inc. Curated by ChEMBL | Assay Description Binding affinity to Bacillus subtilis DNA polymerase3C | J Med Chem 49: 1455-65 (2006) Article DOI: 10.1021/jm0510023 BindingDB Entry DOI: 10.7270/Q23J3DR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Bacillus subtilis) | BDBM50182666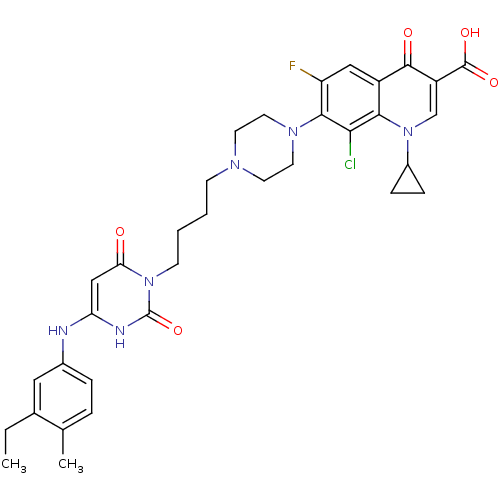 (3-{4-[1-(1-cyclopropyl-3-carboxy-4-oxo-6-fluoro-8-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GLSynthesis Inc. Curated by ChEMBL | Assay Description Binding affinity to Bacillus subtilis DNA polymerase3C | J Med Chem 49: 1455-65 (2006) Article DOI: 10.1021/jm0510023 BindingDB Entry DOI: 10.7270/Q23J3DR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Bacillus subtilis) | BDBM50182667 (3-{4-[1-(1-cyclopropyl-3-carboxy-4-oxo-6,8-difluor...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GLSynthesis Inc. Curated by ChEMBL | Assay Description Binding affinity to Bacillus subtilis DNA polymerase3C | J Med Chem 49: 1455-65 (2006) Article DOI: 10.1021/jm0510023 BindingDB Entry DOI: 10.7270/Q23J3DR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Bacillus subtilis) | BDBM50182662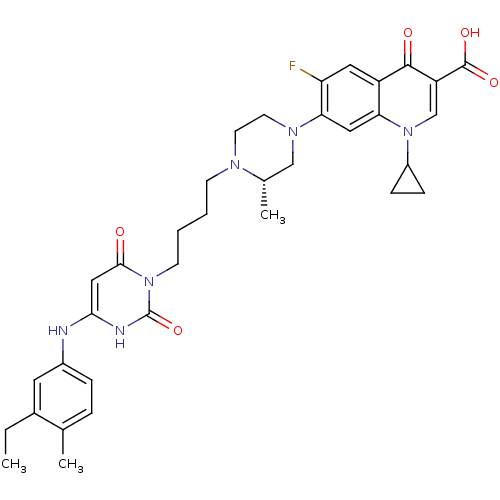 ((S)-3-{4-[1-(1-cyclopropyl-3-carboxy-4-oxo-6-fluor...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GLSynthesis Inc. Curated by ChEMBL | Assay Description Binding affinity to Bacillus subtilis DNA polymerase3C | J Med Chem 49: 1455-65 (2006) Article DOI: 10.1021/jm0510023 BindingDB Entry DOI: 10.7270/Q23J3DR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Bacillus subtilis) | BDBM50182674 ((R)-3-{4-[1-(1-cyclopropyl-3-carboxy-4-oxo-6-fluor...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GLSynthesis Inc. Curated by ChEMBL | Assay Description Binding affinity to Bacillus subtilis DNA polymerase3C | J Med Chem 49: 1455-65 (2006) Article DOI: 10.1021/jm0510023 BindingDB Entry DOI: 10.7270/Q23J3DR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Bacillus subtilis) | BDBM50182659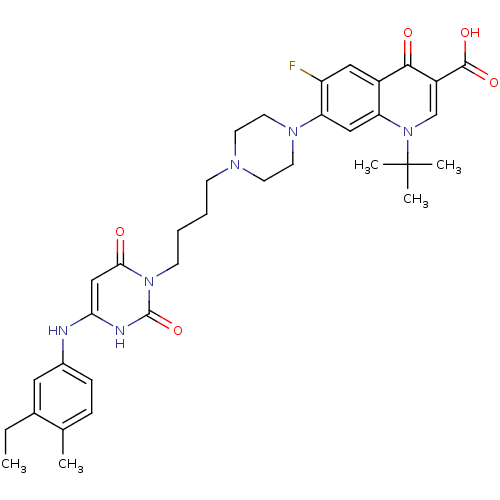 (3-{4-[1-(1-tert-butyl-3-carboxy-4-oxo-6-fluoro-7-q...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GLSynthesis Inc. Curated by ChEMBL | Assay Description Binding affinity to Bacillus subtilis DNA polymerase3C | J Med Chem 49: 1455-65 (2006) Article DOI: 10.1021/jm0510023 BindingDB Entry DOI: 10.7270/Q23J3DR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Bacillus subtilis) | BDBM50182663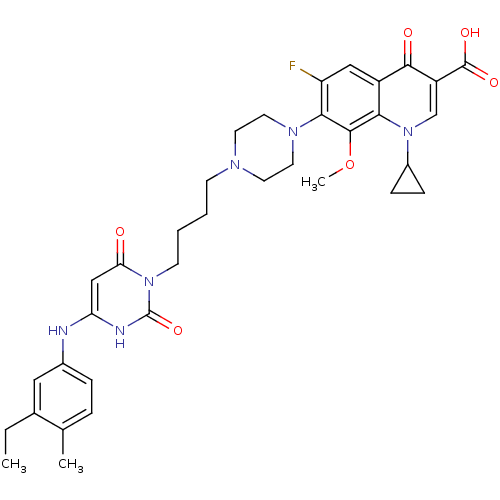 (3-{4-[1-(1-cyclopropyl-3-carboxy-4-oxo-6-fluoro-8-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GLSynthesis Inc. Curated by ChEMBL | Assay Description Binding affinity to Bacillus subtilis DNA polymerase3C | J Med Chem 49: 1455-65 (2006) Article DOI: 10.1021/jm0510023 BindingDB Entry DOI: 10.7270/Q23J3DR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Bacillus subtilis) | BDBM50182673 (3-{4-[1-(1-(4-fluorophenyl)-3-carboxy-4-oxo-6-fluo...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GLSynthesis Inc. Curated by ChEMBL | Assay Description Binding affinity to Bacillus subtilis DNA polymerase3C | J Med Chem 49: 1455-65 (2006) Article DOI: 10.1021/jm0510023 BindingDB Entry DOI: 10.7270/Q23J3DR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Bacillus subtilis) | BDBM50182676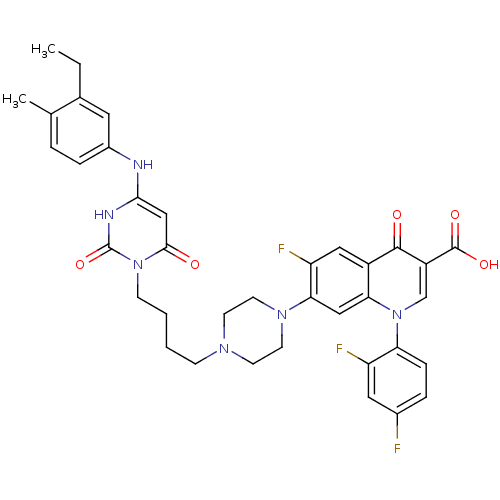 (3-{4-[1-(1-{2,4-difluorophenyl}-3-carboxy-4-oxo-6-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GLSynthesis Inc. Curated by ChEMBL | Assay Description Binding affinity to Bacillus subtilis DNA polymerase3C | J Med Chem 49: 1455-65 (2006) Article DOI: 10.1021/jm0510023 BindingDB Entry DOI: 10.7270/Q23J3DR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Bacillus subtilis) | BDBM50182672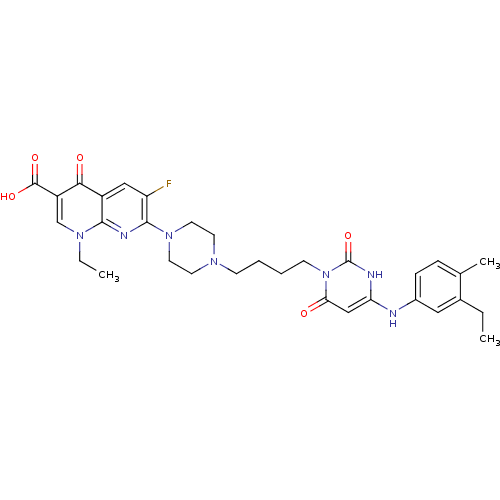 (1-ethyl-7-(4-(4-(4-(3-ethyl-4-methylphenylamino)-2...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GLSynthesis Inc. Curated by ChEMBL | Assay Description Binding affinity to Bacillus subtilis DNA polymerase3C | J Med Chem 49: 1455-65 (2006) Article DOI: 10.1021/jm0510023 BindingDB Entry DOI: 10.7270/Q23J3DR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Bacillus subtilis) | BDBM50182671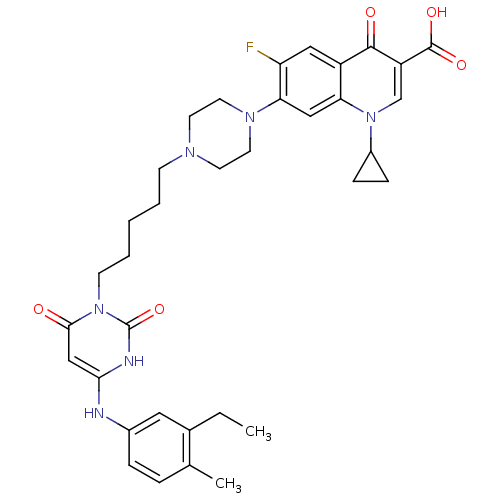 (3-{5-[1-(1-cyclopropyl-3-carboxy-4-oxo-6-fluoro-7-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GLSynthesis Inc. Curated by ChEMBL | Assay Description Binding affinity to Bacillus subtilis DNA polymerase3C | J Med Chem 49: 1455-65 (2006) Article DOI: 10.1021/jm0510023 BindingDB Entry DOI: 10.7270/Q23J3DR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Bacillus subtilis) | BDBM21688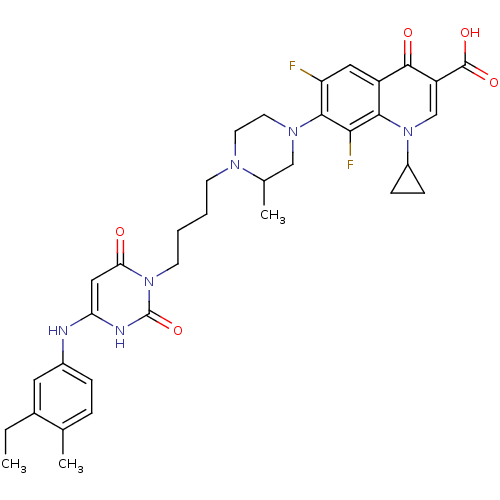 (1-cyclopropyl-7-[4-(4-{4-[(3-ethyl-4-methylphenyl)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GLSynthesis Inc. Curated by ChEMBL | Assay Description Binding affinity to Bacillus subtilis DNA polymerase3C | J Med Chem 49: 1455-65 (2006) Article DOI: 10.1021/jm0510023 BindingDB Entry DOI: 10.7270/Q23J3DR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Bacillus subtilis) | BDBM50182670 (3-{4-[1-(1-ethyl-3-carboxy-4-oxo-6,8-difluoro-7-qu...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GLSynthesis Inc. Curated by ChEMBL | Assay Description Binding affinity to Bacillus subtilis DNA polymerase3C | J Med Chem 49: 1455-65 (2006) Article DOI: 10.1021/jm0510023 BindingDB Entry DOI: 10.7270/Q23J3DR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Bacillus subtilis) | BDBM50182668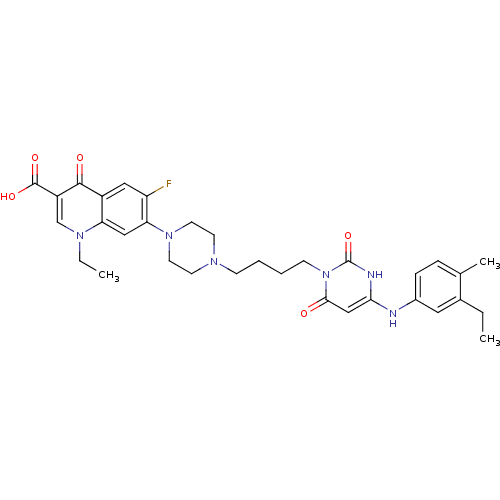 (3-{4-[1-(1-ethyl-3-carboxy-4-oxo-6-fluoro-7-quinol...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GLSynthesis Inc. Curated by ChEMBL | Assay Description Binding affinity to Bacillus subtilis DNA polymerase3C | J Med Chem 49: 1455-65 (2006) Article DOI: 10.1021/jm0510023 BindingDB Entry DOI: 10.7270/Q23J3DR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Bacillus subtilis) | BDBM50182675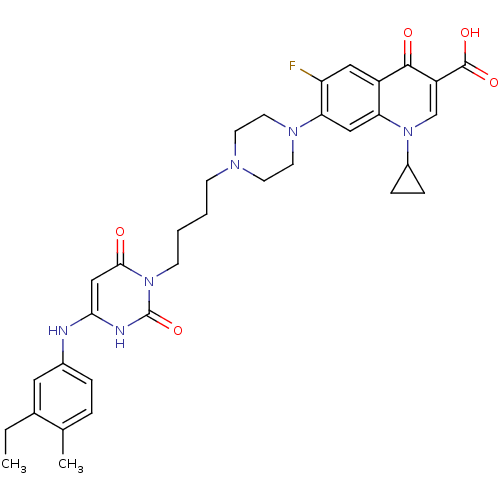 (3-{4-[1-(1-cyclopropyl-3-carboxy-4-oxo-6-fluoro-7-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GLSynthesis Inc. Curated by ChEMBL | Assay Description Binding affinity to Bacillus subtilis DNA polymerase3C | J Med Chem 49: 1455-65 (2006) Article DOI: 10.1021/jm0510023 BindingDB Entry DOI: 10.7270/Q23J3DR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Bacillus subtilis) | BDBM50182656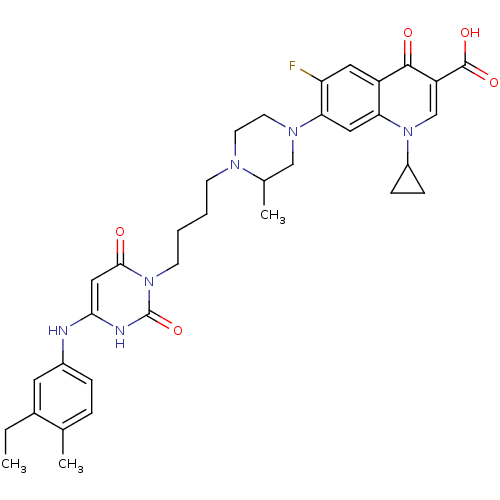 (3-{4-[1-(1-cyclopropyl-3-carboxy-4-oxo-6-fluoro-7-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GLSynthesis Inc. Curated by ChEMBL | Assay Description Binding affinity to Bacillus subtilis DNA polymerase3C | J Med Chem 49: 1455-65 (2006) Article DOI: 10.1021/jm0510023 BindingDB Entry DOI: 10.7270/Q23J3DR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Bacillus subtilis) | BDBM50182657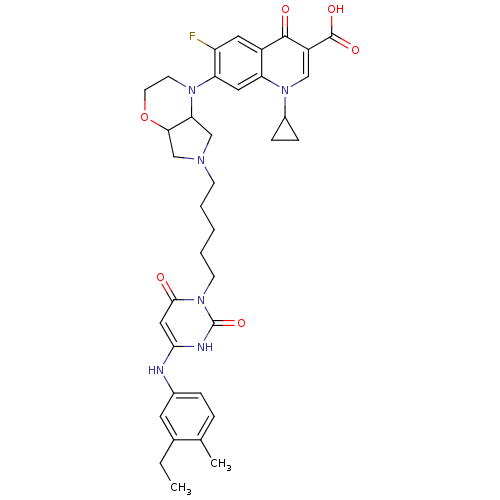 (3-{5-[1-(1-cyclopropyl-3-carboxy-4-oxo-6-fluoro-7-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GLSynthesis Inc. Curated by ChEMBL | Assay Description Binding affinity to Bacillus subtilis DNA polymerase3C | J Med Chem 49: 1455-65 (2006) Article DOI: 10.1021/jm0510023 BindingDB Entry DOI: 10.7270/Q23J3DR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Bacillus subtilis) | BDBM50182664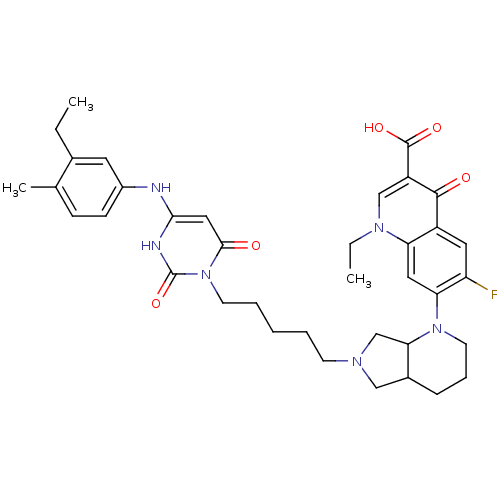 (3-{5-[1-(1-ethyl-3-carboxy-4-oxo-6-fluoro-7-quinol...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GLSynthesis Inc. Curated by ChEMBL | Assay Description Binding affinity to Bacillus subtilis DNA polymerase3C | J Med Chem 49: 1455-65 (2006) Article DOI: 10.1021/jm0510023 BindingDB Entry DOI: 10.7270/Q23J3DR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Bacillus subtilis) | BDBM50182658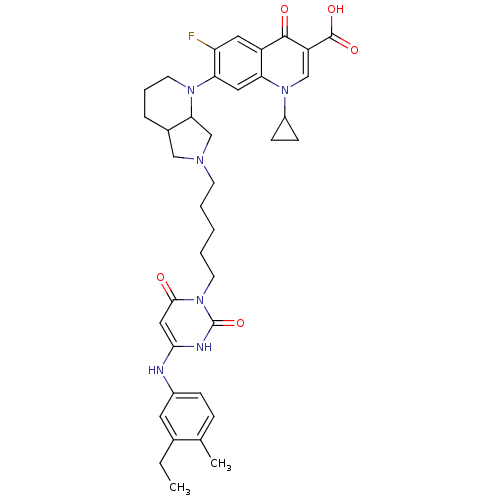 (3-{5-[1-(1-cyclopropyl-3-carboxy-6-fluoro-4-oxo-7-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GLSynthesis Inc. Curated by ChEMBL | Assay Description Binding affinity to Bacillus subtilis DNA polymerase3C | J Med Chem 49: 1455-65 (2006) Article DOI: 10.1021/jm0510023 BindingDB Entry DOI: 10.7270/Q23J3DR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Bacillus subtilis) | BDBM21686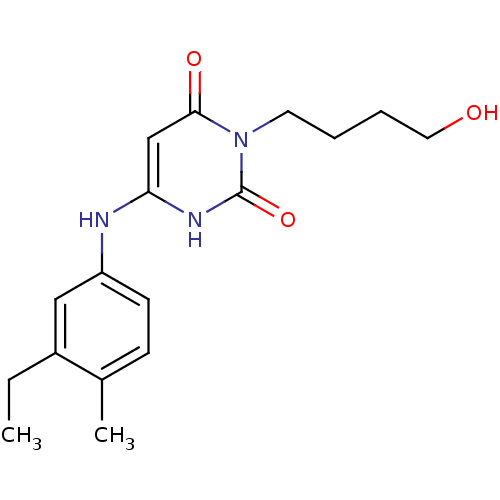 (3-(4-hydroxybutyl)-6-(3-ethyl-4-methylanilino)urac...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GLSynthesis Inc. Curated by ChEMBL | Assay Description Binding affinity to Bacillus subtilis DNA polymerase3C | J Med Chem 49: 1455-65 (2006) Article DOI: 10.1021/jm0510023 BindingDB Entry DOI: 10.7270/Q23J3DR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Bacillus subtilis) | BDBM50182660 (3-{4-[1-(1-cyclopropyl-3-carboxy-4-oxo-6-fluoro-7-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GLSynthesis Inc. Curated by ChEMBL | Assay Description Binding affinity to Bacillus subtilis DNA polymerase3C | J Med Chem 49: 1455-65 (2006) Article DOI: 10.1021/jm0510023 BindingDB Entry DOI: 10.7270/Q23J3DR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase III PolC-type (Bacillus subtilis) | BDBM50182661 (3-{4-[1-(1-cyclopropyl-3-carboxy-4-oxo-6-fluoro-7-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GLSynthesis Inc. Curated by ChEMBL | Assay Description Binding affinity to Bacillus subtilis DNA polymerase3C | J Med Chem 49: 1455-65 (2006) Article DOI: 10.1021/jm0510023 BindingDB Entry DOI: 10.7270/Q23J3DR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM630 ((2R,4S)-2-[(R)-({[4-(dimethylamino)phenyl]methyl}c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3120-8 (1993) Article DOI: 10.1021/jm00073a011 BindingDB Entry DOI: 10.7270/Q24F1NXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM626 ((2R,4S)-2-[(R)-(benzylcarbamoyl)[1-(pyridin-2-yl)a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3120-8 (1993) Article DOI: 10.1021/jm00073a011 BindingDB Entry DOI: 10.7270/Q24F1NXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM3386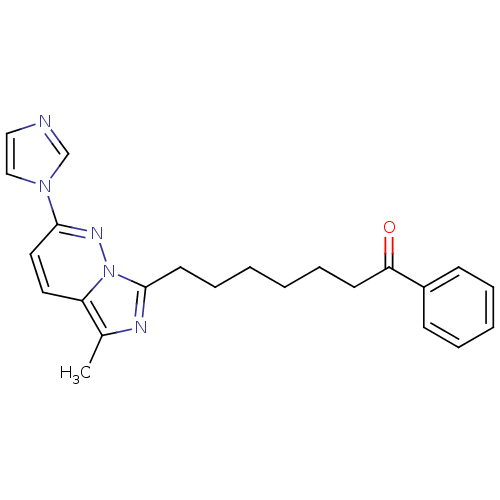 (7-[2-(1H-Imidazol-l-yl)-5-methylimidazo[1,5-b]pyri...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 3784-94 (1993) Article DOI: 10.1021/jm00076a005 BindingDB Entry DOI: 10.7270/Q2PR7T57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM591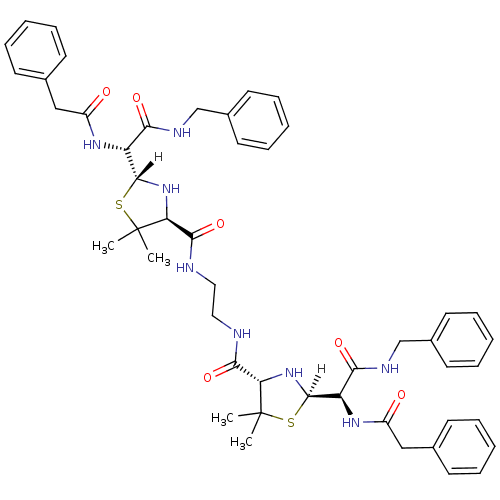 ((2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phenylacetamido...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM591 ((2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phenylacetamido...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3129-36 (1993) Article DOI: 10.1021/jm00073a012 BindingDB Entry DOI: 10.7270/Q20P0X6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM591 ((2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phenylacetamido...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3120-8 (1993) Article DOI: 10.1021/jm00073a011 BindingDB Entry DOI: 10.7270/Q24F1NXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4942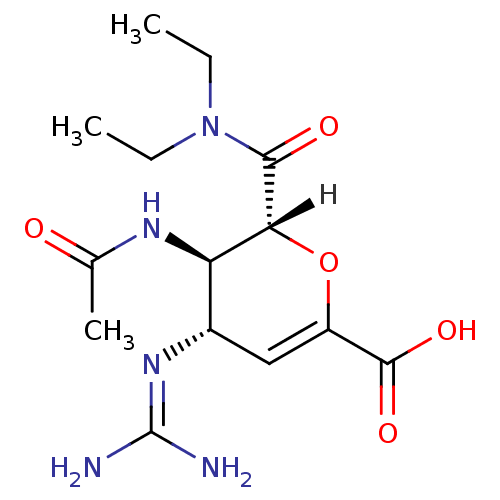 ((2R,3R,4S)-4-carbamimidamido-2-(diethylcarbamoyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4942 ((2R,3R,4S)-4-carbamimidamido-2-(diethylcarbamoyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4940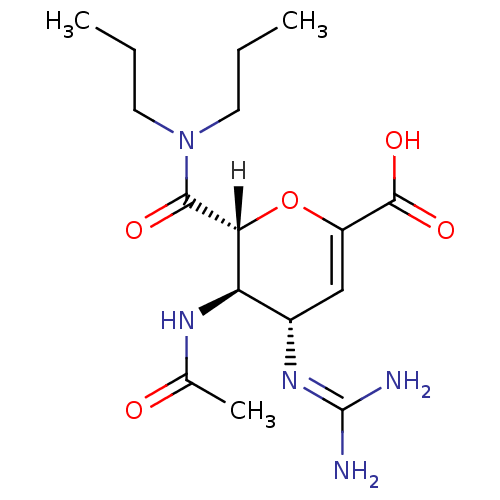 ((2R,3R,4S)-4-carbamimidamido-2-(dipropylcarbamoyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4972 ((2R,3R,4S)-4-amino-3-acetamido-2-{[2-(3-phenylphen...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4940 ((2R,3R,4S)-4-carbamimidamido-2-(dipropylcarbamoyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4940 ((2R,3R,4S)-4-carbamimidamido-2-(dipropylcarbamoyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza sialidase was determined by measuring the ability to inhibit the hydrolysis of MUN by A/Aichi virus grown in hen eggs | Bioorg Med Chem Lett 6: 1805-1808 (1996) Article DOI: 10.1016/0960-894X(96)00318-6 BindingDB Entry DOI: 10.7270/Q2T1544R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4945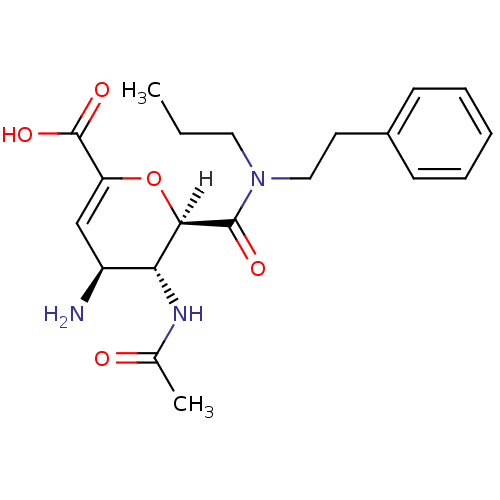 ((2R,3R,4S)-4-amino-3-acetamido-2-[(2-phenylethyl)(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited Curated by ChEMBL | Assay Description Compound was tested for inhibitory concentration against Influenza sialidase type A | J Med Chem 41: 798-807 (1998) Article DOI: 10.1021/jm9703754 BindingDB Entry DOI: 10.7270/Q2M32WFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4945 ((2R,3R,4S)-4-amino-3-acetamido-2-[(2-phenylethyl)(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4967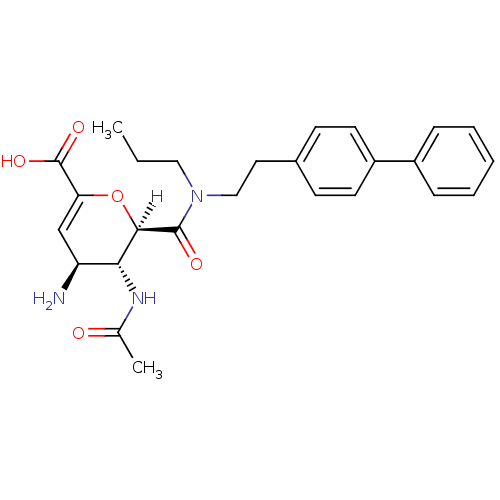 ((2R,3R,4S)-4-amino-3-acetamido-2-{[2-(4-phenylphen...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM613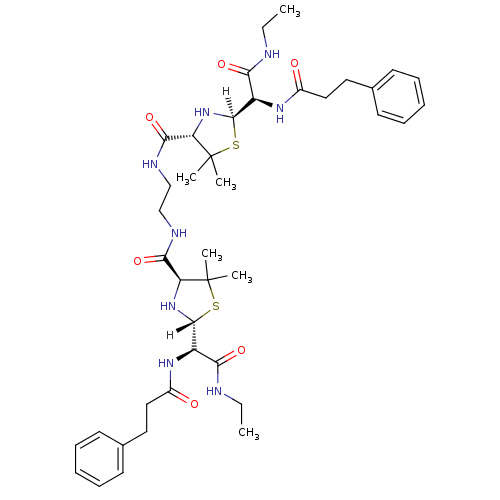 ((2R,4S)-2-[(R)-(ethylcarbamoyl)(3-phenylpropanamid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3120-8 (1993) Article DOI: 10.1021/jm00073a011 BindingDB Entry DOI: 10.7270/Q24F1NXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492164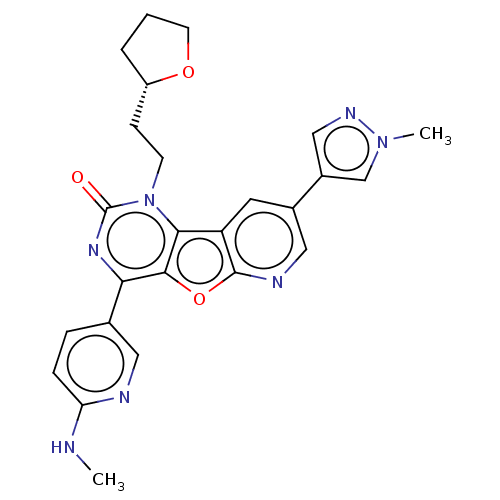 (CHEMBL2381561) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492156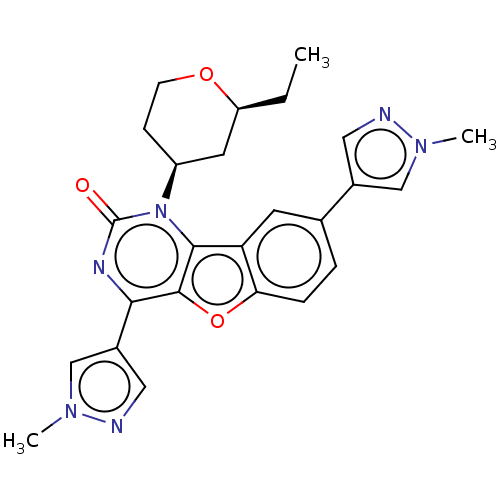 (CHEMBL2397560) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM625 ((2R,4S)-2-[(R)-(ethylcarbamoyl)[(2Z)-3-phenylprop-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3120-8 (1993) Article DOI: 10.1021/jm00073a011 BindingDB Entry DOI: 10.7270/Q24F1NXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492155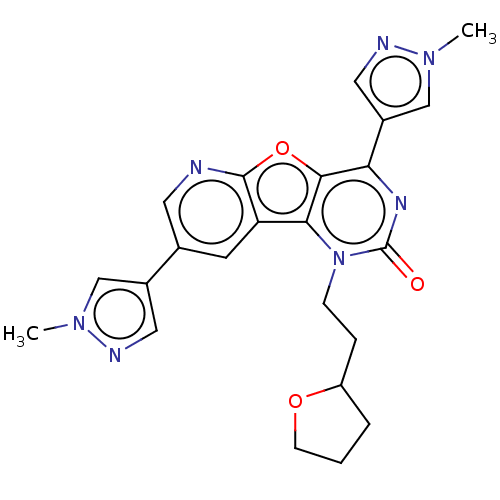 (CHEMBL2397554) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4929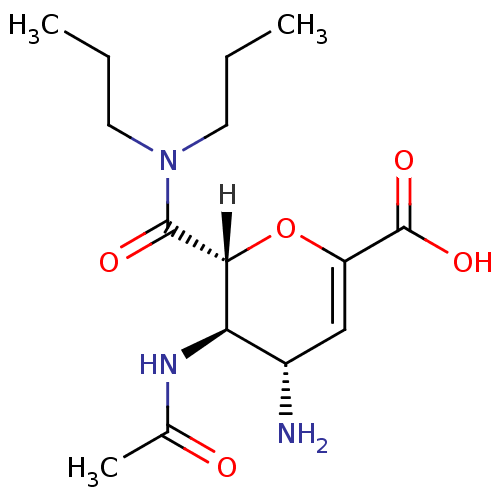 ((2R,3R,4S)-4-amino-2-(dipropylcarbamoyl)-3-acetami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4966 ((2R,3R,4S)-4-amino-3-acetamido-2-{[2-(3-hydroxyphe...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4967 ((2R,3R,4S)-4-amino-3-acetamido-2-{[2-(4-phenylphen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of influenza A sialidase (neuraminidase) | Bioorg Med Chem Lett 6: 2931-2936 (1996) Article DOI: 10.1016/S0960-894X(96)00542-2 BindingDB Entry DOI: 10.7270/Q24B31TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4937 ((2R,3R,4S)-4-amino-2-(diethylcarbamoyl)-3-acetamid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM589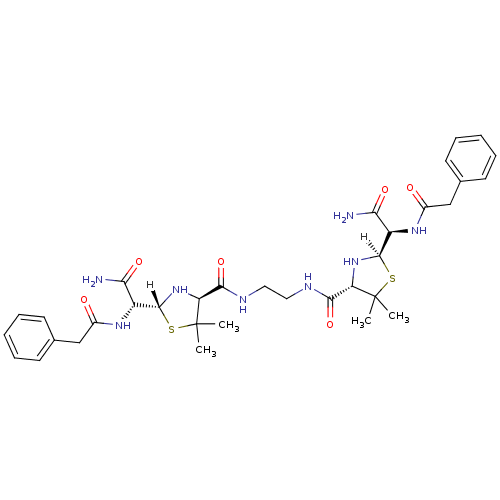 ((2R,4S)-2-[(R)-carbamoyl(1-phenylacetamido)methyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3120-8 (1993) Article DOI: 10.1021/jm00073a011 BindingDB Entry DOI: 10.7270/Q24F1NXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM4941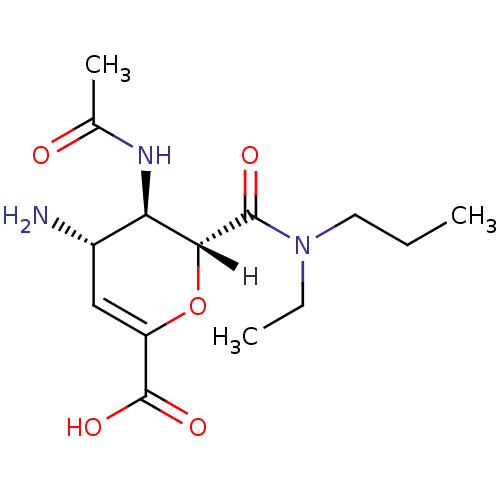 ((2R,3R,4S)-4-amino-3-acetamido-2-[ethyl(propyl)car...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Limited | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 41: 787-97 (1998) Article DOI: 10.1021/jm970374b BindingDB Entry DOI: 10.7270/Q2RF5S7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492176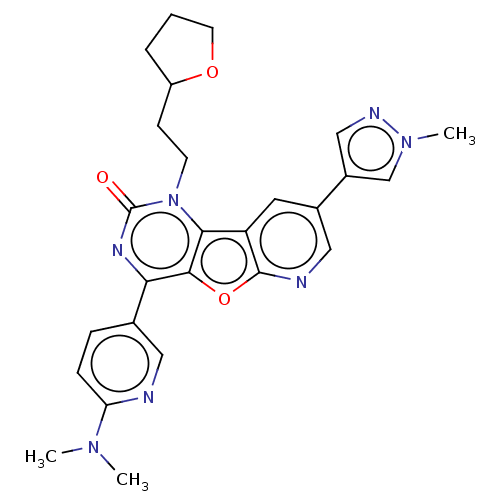 (CHEMBL2397567) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 585 total ) | Next | Last >> |