

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Mus musculus) | BDBM50049168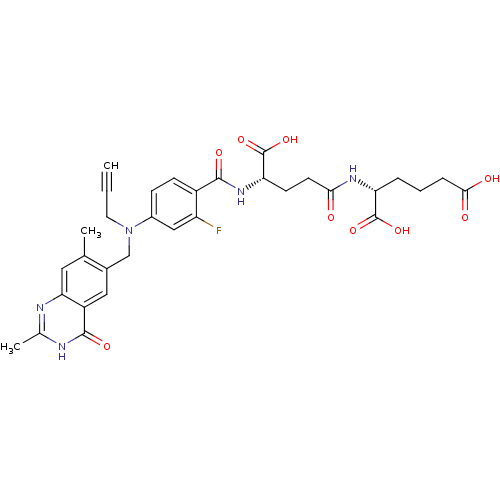 ((R)-2-((S)-4-Carboxy-4-{4-[(2,7-dimethyl-4-oxo-3,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity of the compound against L1210 Thymidylate synthase. | J Med Chem 39: 73-85 (1996) Article DOI: 10.1021/jm950471+ BindingDB Entry DOI: 10.7270/Q2WH2P2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50088159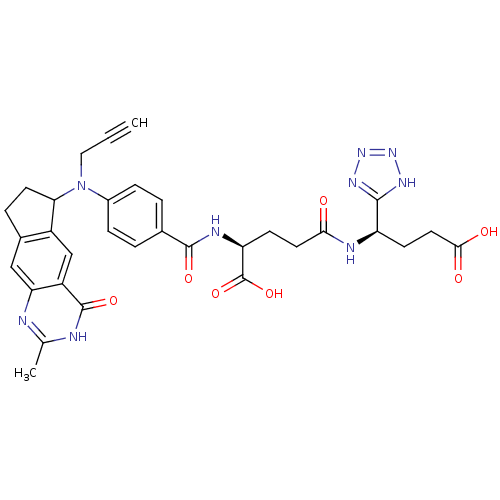 (4-[3-Carboxy-1-(1H-tetrazol-5-yl)-propylcarbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408128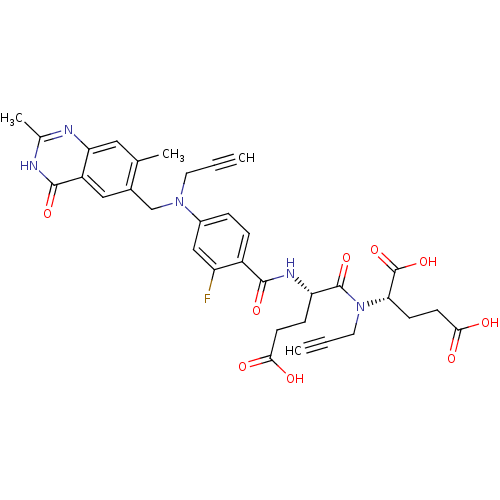 (CHEMBL30179) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of Thymidylate Synthase(TS) from mouse L1210 cells | J Med Chem 40: 1495-510 (1997) Article DOI: 10.1021/jm960878u BindingDB Entry DOI: 10.7270/Q2862HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50088168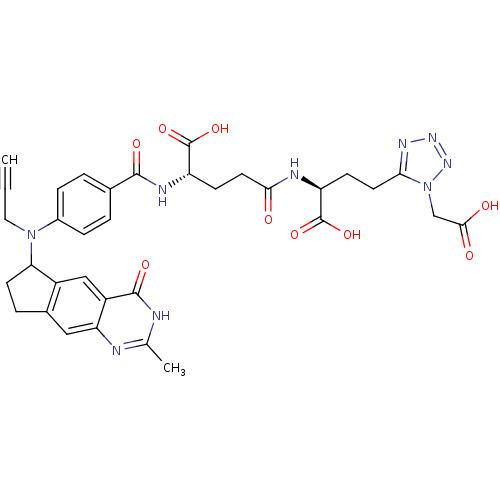 (2-(4-Carboxy-4-{4-[(2-methyl-4-oxo-4,6,7,8-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408130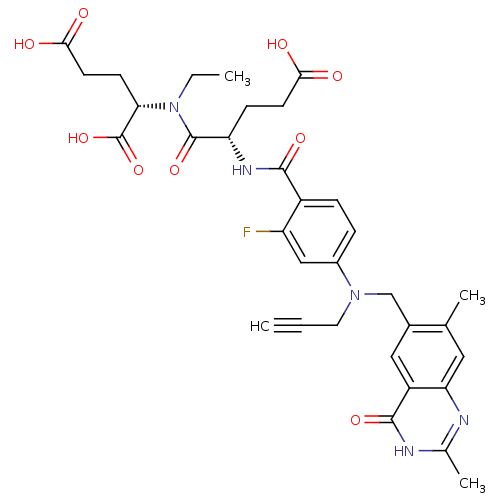 (CHEMBL35926) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of Thymidylate Synthase(TS) from mouse L1210 cells | J Med Chem 40: 1495-510 (1997) Article DOI: 10.1021/jm960878u BindingDB Entry DOI: 10.7270/Q2862HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50088161 (2-(4-Carboxy-4-{4-[(2-methyl-4-oxo-4,6,7,8-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50290520 (((R)-1-{(S)-1-[(2R,3S)-3-(2-tert-Butylsulfamoyl-et...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 7: 2635-2638 (1997) Article DOI: 10.1016/S0960-894X(97)10054-3 BindingDB Entry DOI: 10.7270/Q2G44Q84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50088164 ((S)-2-{4-[(2-Methyl-4-oxo-4,6,7,8-tetrahydro-3H-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408876 (CHEMBL434602) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50290527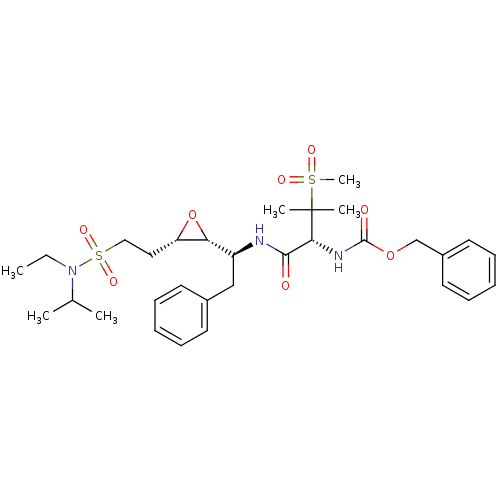 (CHEMBL86971 | [(R)-1-((S)-1-{(2R,3S)-3-[2-(Ethyl-i...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 7: 2635-2638 (1997) Article DOI: 10.1016/S0960-894X(97)10054-3 BindingDB Entry DOI: 10.7270/Q2G44Q84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22904 ((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of Histamine H3 receptor | J Med Chem 46: 1980-8 (2003) Article DOI: 10.1021/jm020415q BindingDB Entry DOI: 10.7270/Q2XG9QG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50049159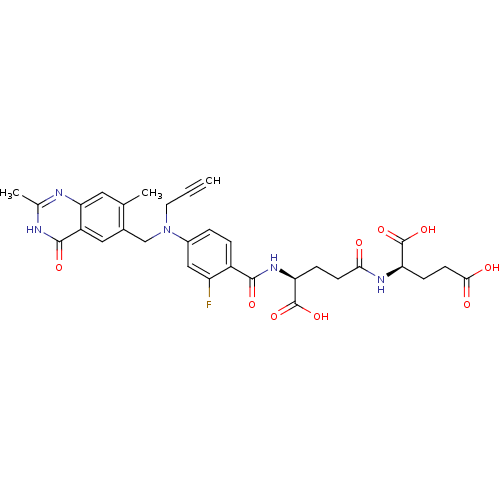 ((R)-2-((S)-4-Carboxy-4-{4-[(2,7-dimethyl-4-oxo-3,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity of the compound against L1210 Thymidylate synthase. | J Med Chem 39: 73-85 (1996) Article DOI: 10.1021/jm950471+ BindingDB Entry DOI: 10.7270/Q2WH2P2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM331621 (N-((S)-2-((3aS,6S,6aS)-6-chloro-3- oxotetrahydro-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GRUNENTHAL GMBH US Patent | Assay Description Recombinant human cathepsins (CatS, CatK, CatL) were purchased from a Enzo Life Sciences. All assays were carried out in 96-well format using a buffe... | US Patent US9725459 (2017) BindingDB Entry DOI: 10.7270/Q2JH3P8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408122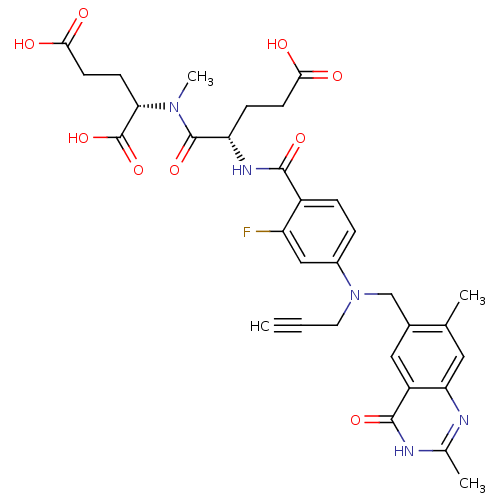 (CHEMBL37106) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of Thymidylate Synthase(TS) from mouse L1210 cells | J Med Chem 40: 1495-510 (1997) Article DOI: 10.1021/jm960878u BindingDB Entry DOI: 10.7270/Q2862HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408872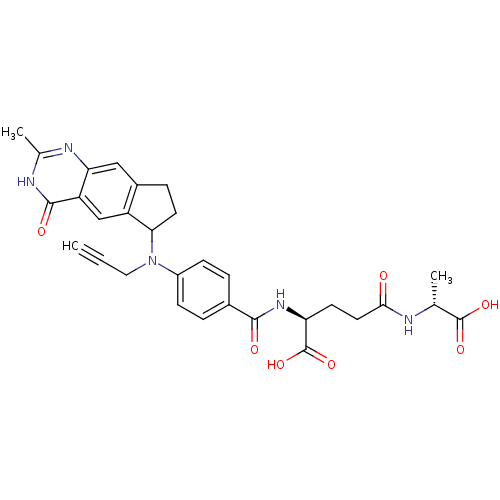 (CHEMBL58548) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50127610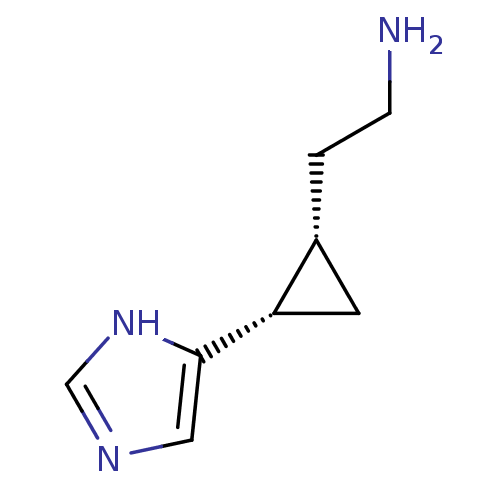 ((1S,2S)-2-(2-aminoethyl)-1-(1H-imidazol-4-yl)cyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Affinity against rat Histamine H3 receptor using [3H]-N-alpha-methyl histamine | J Med Chem 46: 1980-8 (2003) Article DOI: 10.1021/jm020415q BindingDB Entry DOI: 10.7270/Q2XG9QG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50046213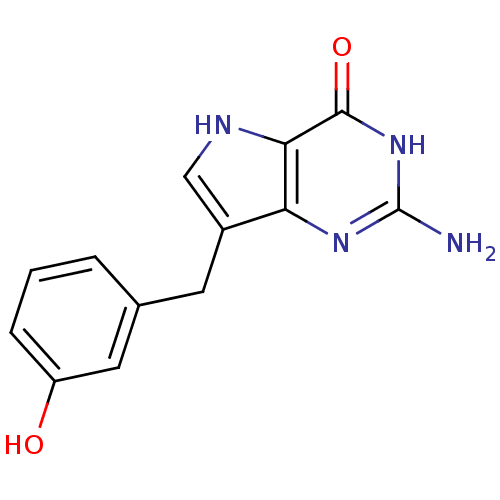 (2-Amino-7-(3-hydroxy-benzyl)-3,5-dihydro-pyrrolo[3...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using 2-amino-6-mercapto-7-methyl purine ribonucleoside (MESG) as subst... | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408871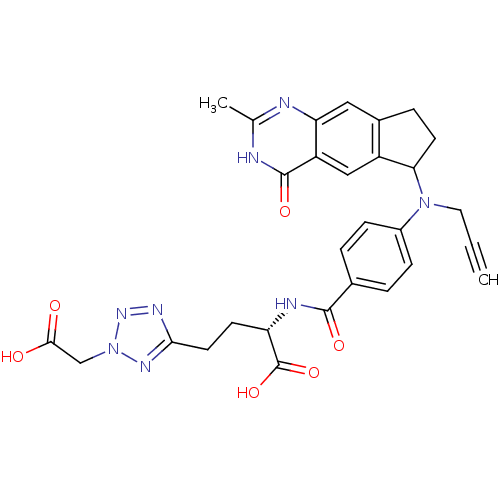 (CHEMBL60700) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408877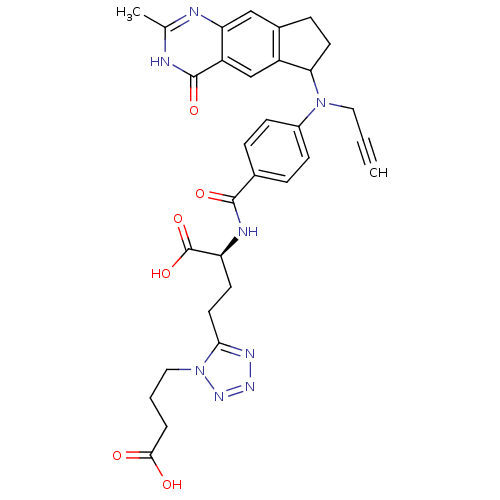 (CHEMBL412127) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Compound was tested for the binding affinity against thymidylate synthase from L1210 mouse leukemia cells; value given as 0.9, 2.0 | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50049170 ((R)-2-((S)-4-Carboxy-4-{4-[(2,7-dimethyl-4-oxo-3,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408135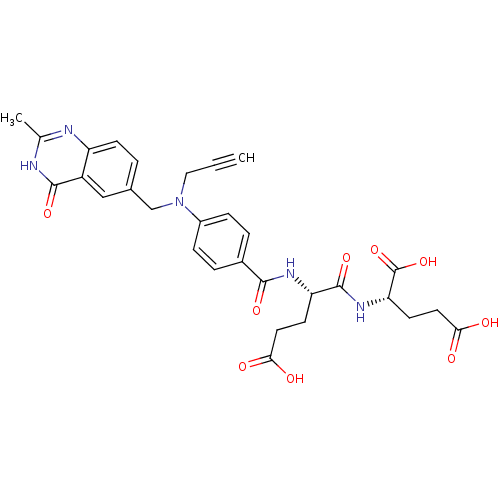 (CHEMBL288666) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of Thymidylate Synthase(TS) from mouse L1210 cells | J Med Chem 40: 1495-510 (1997) Article DOI: 10.1021/jm960878u BindingDB Entry DOI: 10.7270/Q2862HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50088157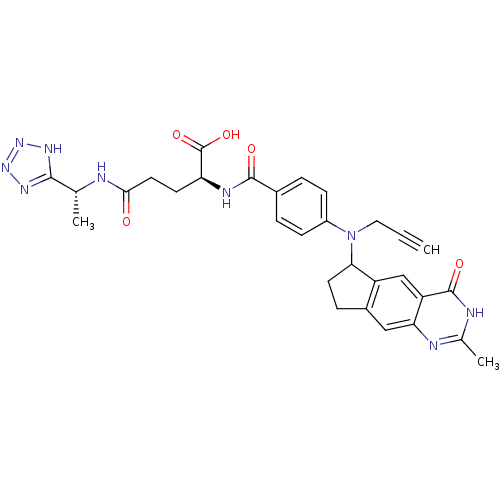 (2-{4-[(2-Methyl-4-oxo-4,6,7,8-tetrahydro-3H-cyclop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408874 (CHEMBL299062) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50088169 (4-(2-Benzenesulfonylamino-1-methyl-2-oxo-ethylcarb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408873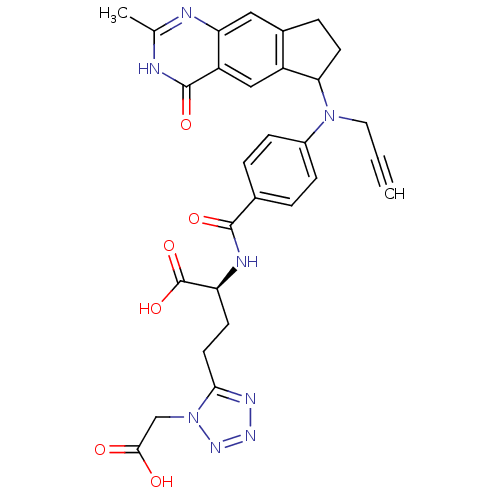 (CHEMBL292920) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Compound was tested for the binding affinity against thymidylate synthase from L1210 mouse leukemia cells; value given as 2.4,1.8 | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50127604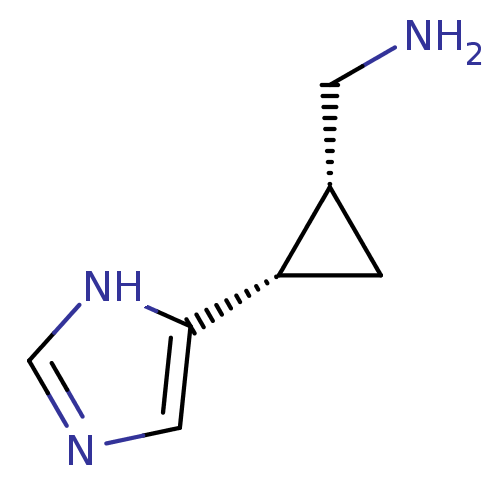 (C-[2-(1H-Imidazol-4-yl)-cyclopropyl]-methylamine |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Affinity against rat Histamine H3 receptor using [3H]-N-alpha-methyl histamine | J Med Chem 46: 1980-8 (2003) Article DOI: 10.1021/jm020415q BindingDB Entry DOI: 10.7270/Q2XG9QG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408136 (CHEMBL291084) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of Thymidylate Synthase(TS) from mouse L1210 cells | J Med Chem 40: 1495-510 (1997) Article DOI: 10.1021/jm960878u BindingDB Entry DOI: 10.7270/Q2862HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408875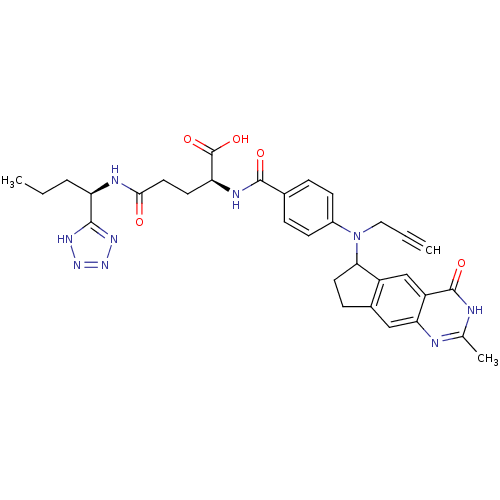 (CHEMBL57549) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 43: 1910-26 (2000) BindingDB Entry DOI: 10.7270/Q2KP81DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of rat Histamine H3 receptor using [3H]-N-alpha-methyl histamine | J Med Chem 46: 1980-8 (2003) Article DOI: 10.1021/jm020415q BindingDB Entry DOI: 10.7270/Q2XG9QG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50290525 (CHEMBL92097 | [(R)-1-((S)-1-{(2R,3S)-3-[2-(Ethyl-i...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 7: 2635-2638 (1997) Article DOI: 10.1016/S0960-894X(97)10054-3 BindingDB Entry DOI: 10.7270/Q2G44Q84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM331540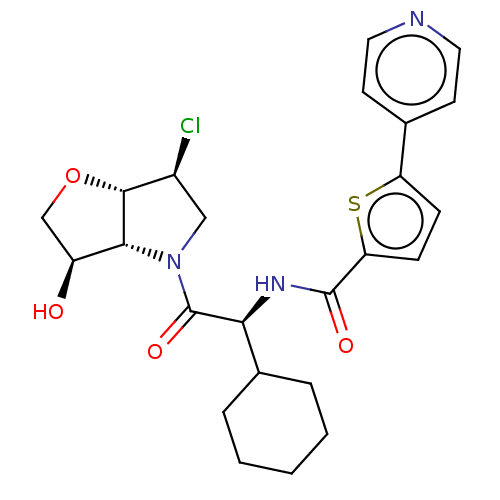 (N-((S)-2-((3aS,6S,6aS)-6-chloro-3-oxotetrahydro-2H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GRUNENTHAL GMBH US Patent | Assay Description Recombinant human cathepsins (CatS, CatK, CatL) were purchased from a Enzo Life Sciences. All assays were carried out in 96-well format using a buffe... | US Patent US9725459 (2017) BindingDB Entry DOI: 10.7270/Q2JH3P8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408129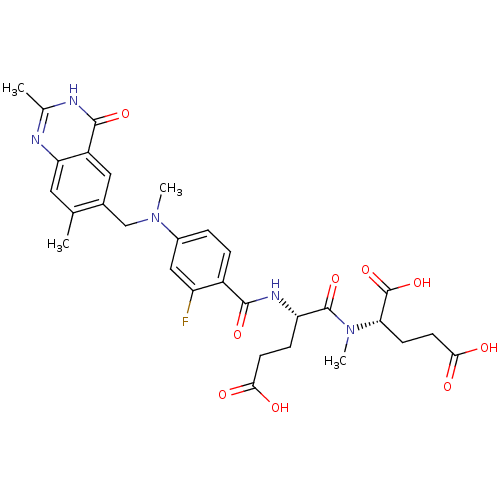 (CHEMBL289749) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of Thymidylate Synthase(TS) from mouse L1210 cells | J Med Chem 40: 1495-510 (1997) Article DOI: 10.1021/jm960878u BindingDB Entry DOI: 10.7270/Q2862HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50290523 (CHEMBL327222 | Thiophene-2-carboxylic acid [(R)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 7: 2635-2638 (1997) Article DOI: 10.1016/S0960-894X(97)10054-3 BindingDB Entry DOI: 10.7270/Q2G44Q84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50039542 (2-Amino-7-pyridin-3-ylmethyl-3,5-dihydro-pyrrolo[3...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using 2-amino-6-mercapto-7-methyl purine ribonucleoside (MESG) as subst... | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50048046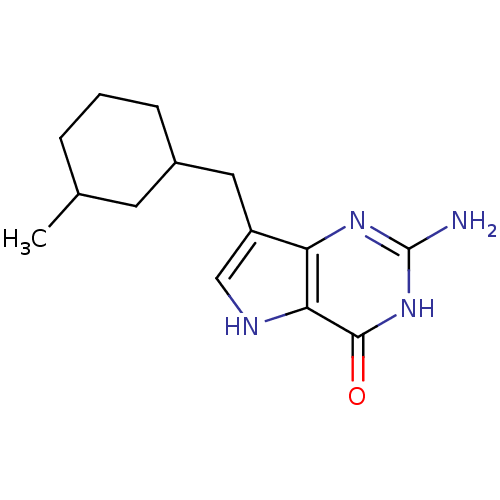 (2-Amino-7-(3-methyl-cyclohexylmethyl)-3,5-dihydro-...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using 2-amino-6-mercapto-7-methyl purine ribonucleoside (MESG) as subst... | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50039544 (2-Amino-7-thiophen-3-ylmethyl-3,5-dihydro-pyrrolo[...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using 2-amino-6-mercapto-7-methyl purine ribonucleoside (MESG) as subst... | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50039547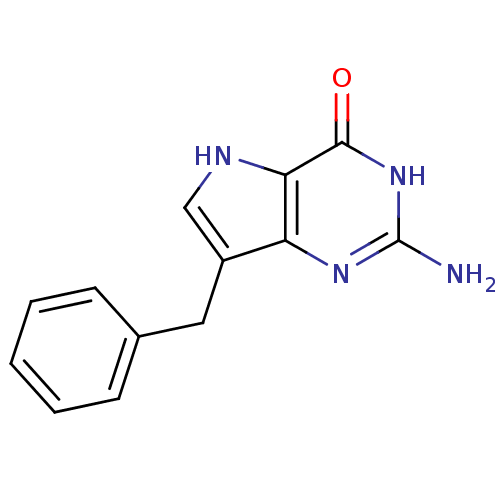 (2-Amino-7-benzyl-3,5-dihydro-pyrrolo[3,2-d]pyrimid...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using 2-amino-6-mercapto-7-methyl purine ribonucleoside (MESG) as subst... | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50290524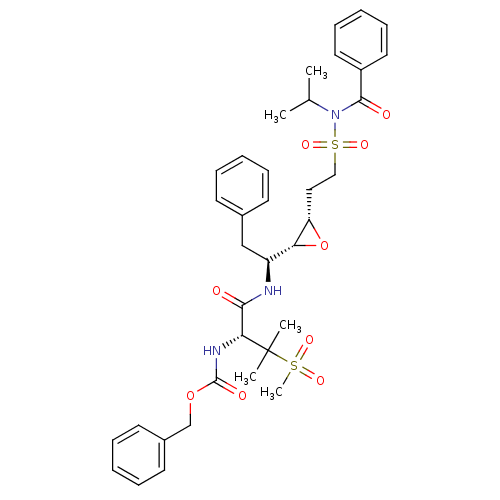 (CHEMBL404933 | [(R)-1-((S)-1-{(2R,3S)-3-[2-(Benzoy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 7: 2635-2638 (1997) Article DOI: 10.1016/S0960-894X(97)10054-3 BindingDB Entry DOI: 10.7270/Q2G44Q84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50048053 (2-Amino-7-(3-trifluoromethyl-cyclohexylmethyl)-3,5...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using 2-amino-6-mercapto-7-methyl purine ribonucleoside (MESG) as subst... | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50046219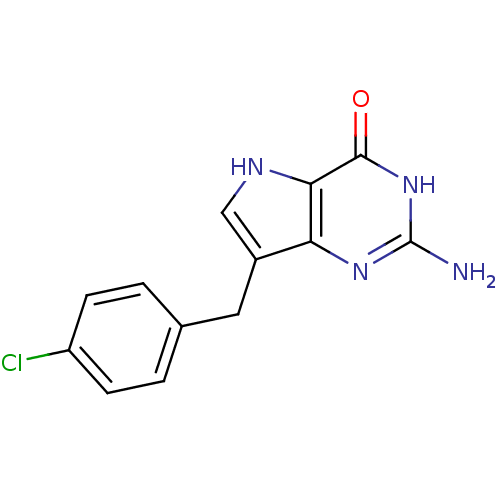 (2-Amino-7-(4-chloro-benzyl)-3,5-dihydro-pyrrolo[3,...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity of the compound against calf spleen Purine nucleoside phosphorylase (PNP) using 2-amino-6-mercapto-7-methyl purine ribonucleoside... | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50078452 ((S)-3-(2-Amino-4-oxo-4,5-dihydro-3H-pyrrolo[3,2-d]...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using inosine as substrate at a concentration of 10 uM. | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM331568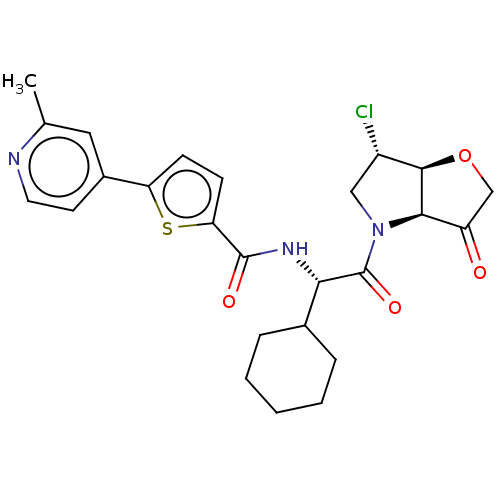 (N-((S)-2-((3aS,6S,6aS)-6-chloro-3- oxotetrahydro-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GRUNENTHAL GMBH US Patent | Assay Description Recombinant human cathepsins (CatS, CatK, CatL) were purchased from a Enzo Life Sciences. All assays were carried out in 96-well format using a buffe... | US Patent US9725459 (2017) BindingDB Entry DOI: 10.7270/Q2JH3P8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM331622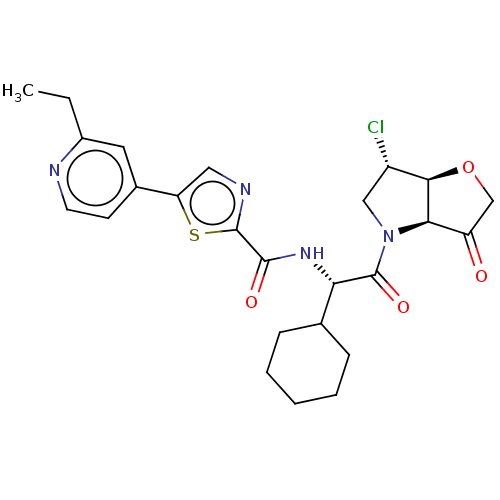 (N-((S)-2-((3aS,6S,6aS)-6-chloro-3- oxotetrahydro-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GRUNENTHAL GMBH US Patent | Assay Description Recombinant human cathepsins (CatS, CatK, CatL) were purchased from a Enzo Life Sciences. All assays were carried out in 96-well format using a buffe... | US Patent US9725459 (2017) BindingDB Entry DOI: 10.7270/Q2JH3P8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50408127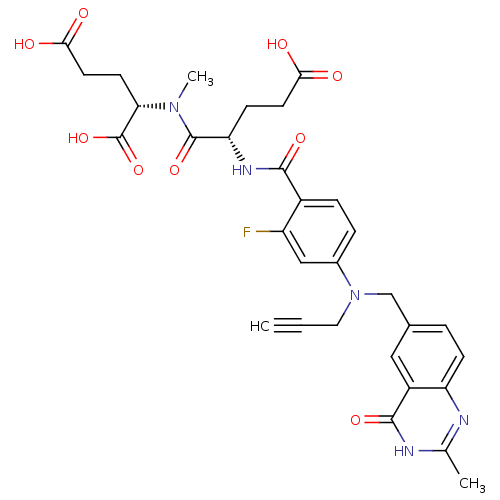 (CHEMBL264609) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of Thymidylate Synthase(TS) from mouse L1210 cells | J Med Chem 40: 1495-510 (1997) Article DOI: 10.1021/jm960878u BindingDB Entry DOI: 10.7270/Q2862HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50127605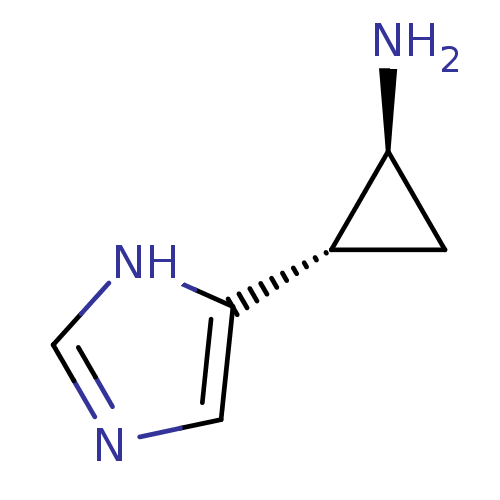 ((1S,2S)-2-(1H-Imidazol-4-yl)-cyclopropylamine | (1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Affinity against rat Histamine H3 receptor using [3H]-N-alpha-methyl histamine | J Med Chem 46: 1980-8 (2003) Article DOI: 10.1021/jm020415q BindingDB Entry DOI: 10.7270/Q2XG9QG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50042804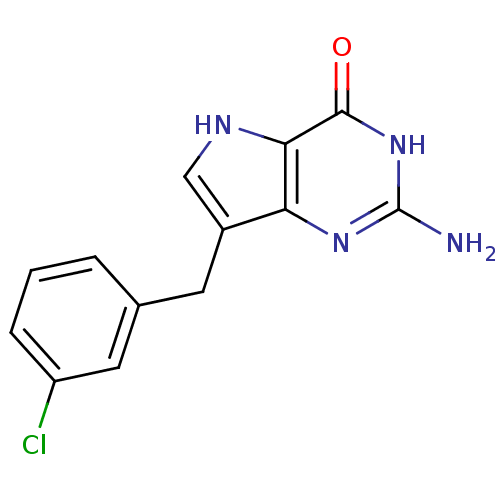 (2-Amino-7-(3-chloro-benzyl)-3,5-dihydro-pyrrolo[3,...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using 2-amino-6-mercapto-7-methyl purine ribonucleoside (MESG) as subst... | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50290526 (CHEMBL328036 | [(R)-1-((S)-1-{(2R,3S)-3-[2-(Ethyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 7: 2635-2638 (1997) Article DOI: 10.1016/S0960-894X(97)10054-3 BindingDB Entry DOI: 10.7270/Q2G44Q84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50039549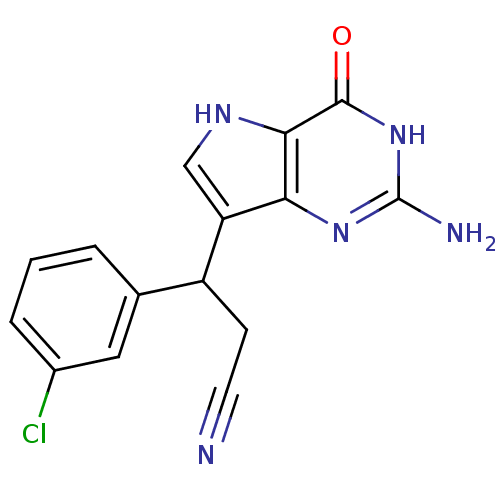 (3-(2-Amino-4-oxo-4,5-dihydro-3H-pyrrolo[3,2-d]pyri...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibitory activity against calf spleen purine nucleoside phosphorylase (PNP) using 2-amino-6-mercapto-7-methyl purine ribonucleoside (MESG) as subst... | J Med Chem 42: 2422-31 (1999) Article DOI: 10.1021/jm990037y BindingDB Entry DOI: 10.7270/Q2CR5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50127608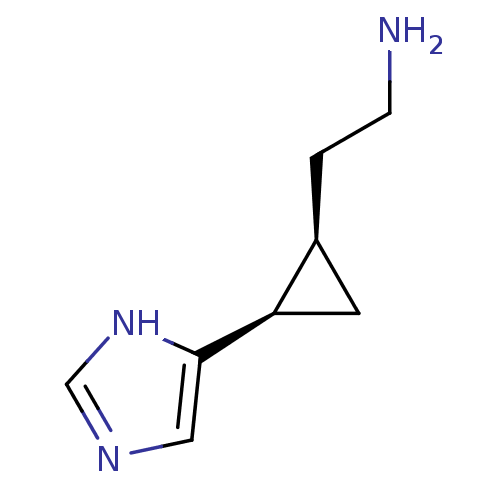 (2-[2-(1H-Imidazol-4-yl)-cyclopropyl]-ethylamine | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Affinity against rat Histamine H3 receptor using [3H]-N-alpha-methyl histamine | J Med Chem 46: 1980-8 (2003) Article DOI: 10.1021/jm020415q BindingDB Entry DOI: 10.7270/Q2XG9QG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM331566 (N-((S)-2-((3aS,6S,6aS)-6-chloro-3- oxotetrahydro-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GRUNENTHAL GMBH US Patent | Assay Description Recombinant human cathepsins (CatS, CatK, CatL) were purchased from a Enzo Life Sciences. All assays were carried out in 96-well format using a buffe... | US Patent US9725459 (2017) BindingDB Entry DOI: 10.7270/Q2JH3P8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4987 total ) | Next | Last >> |