 Found 112 hits with Last Name = 'shiraki' and Initial = 'r'
Found 112 hits with Last Name = 'shiraki' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358251
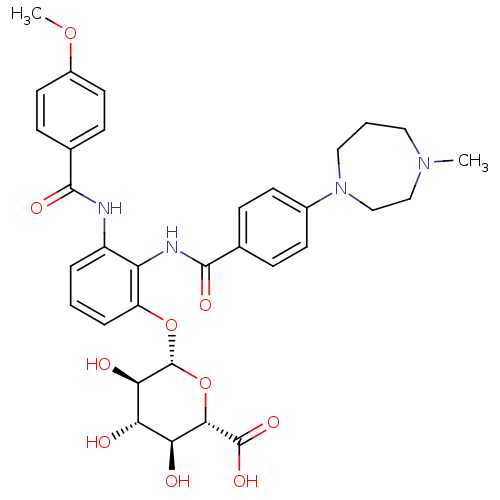
(CHEMBL1922344)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 |r| Show InChI InChI=1S/C33H38N4O10/c1-36-15-4-16-37(18-17-36)21-11-7-19(8-12-21)31(42)35-25-23(34-30(41)20-9-13-22(45-2)14-10-20)5-3-6-24(25)46-33-28(40)26(38)27(39)29(47-33)32(43)44/h3,5-14,26-29,33,38-40H,4,15-18H2,1-2H3,(H,34,41)(H,35,42)(H,43,44)/t26-,27-,28+,29-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by dixon plot analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358252
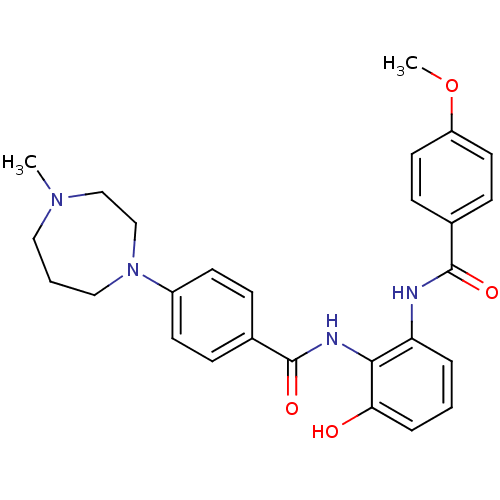
(CHEMBL1922235)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 Show InChI InChI=1S/C27H30N4O4/c1-30-15-4-16-31(18-17-30)21-11-7-19(8-12-21)27(34)29-25-23(5-3-6-24(25)32)28-26(33)20-9-13-22(35-2)14-10-20/h3,5-14,32H,4,15-18H2,1-2H3,(H,28,33)(H,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by dixon plot analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50358252

(CHEMBL1922235)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 Show InChI InChI=1S/C27H30N4O4/c1-30-15-4-16-31(18-17-30)21-11-7-19(8-12-21)27(34)29-25-23(5-3-6-24(25)32)28-26(33)20-9-13-22(35-2)14-10-20/h3,5-14,32H,4,15-18H2,1-2H3,(H,28,33)(H,29,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using chromogenic substrate S2302 by dixon plot analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50358251

(CHEMBL1922344)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 |r| Show InChI InChI=1S/C33H38N4O10/c1-36-15-4-16-37(18-17-36)21-11-7-19(8-12-21)31(42)35-25-23(34-30(41)20-9-13-22(45-2)14-10-20)5-3-6-24(25)46-33-28(40)26(38)27(39)29(47-33)32(43)44/h3,5-14,26-29,33,38-40H,4,15-18H2,1-2H3,(H,34,41)(H,35,42)(H,43,44)/t26-,27-,28+,29-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using chromogenic substrate S2302 by dixon plot analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50358252

(CHEMBL1922235)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 Show InChI InChI=1S/C27H30N4O4/c1-30-15-4-16-31(18-17-30)21-11-7-19(8-12-21)27(34)29-25-23(5-3-6-24(25)32)28-26(33)20-9-13-22(35-2)14-10-20/h3,5-14,32H,4,15-18H2,1-2H3,(H,28,33)(H,29,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin using chromogenic substrate S2238 by dixon plot analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50358251

(CHEMBL1922344)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 |r| Show InChI InChI=1S/C33H38N4O10/c1-36-15-4-16-37(18-17-36)21-11-7-19(8-12-21)31(42)35-25-23(34-30(41)20-9-13-22(45-2)14-10-20)5-3-6-24(25)46-33-28(40)26(38)27(39)29(47-33)32(43)44/h3,5-14,26-29,33,38-40H,4,15-18H2,1-2H3,(H,34,41)(H,35,42)(H,43,44)/t26-,27-,28+,29-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin using chromogenic substrate S2238 by dixon plot analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50381555

(CHEMBL2018097)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1ccc(Cl)c(Cc2cc3ccccc3s2)c1 |r| Show InChI InChI=1S/C21H21ClO5S/c22-15-6-5-12(21-20(26)19(25)18(24)16(10-23)27-21)7-13(15)9-14-8-11-3-1-2-4-17(11)28-14/h1-8,16,18-21,23-26H,9-10H2/t16-,18-,19+,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as [14C]AMG accumulation after 2 hrs by scintillation counting |
Bioorg Med Chem 20: 3263-79 (2012)
Article DOI: 10.1016/j.bmc.2012.03.051
BindingDB Entry DOI: 10.7270/Q2W66MSS |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50381554
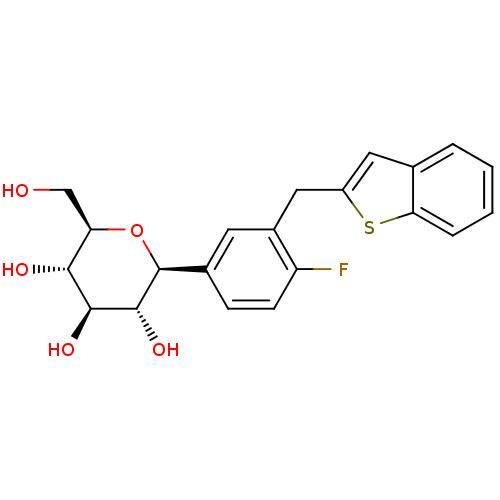
(CHEMBL2018096)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1ccc(F)c(Cc2cc3ccccc3s2)c1 |r| Show InChI InChI=1S/C21H21FO5S/c22-15-6-5-12(21-20(26)19(25)18(24)16(10-23)27-21)7-13(15)9-14-8-11-3-1-2-4-17(11)28-14/h1-8,16,18-21,23-26H,9-10H2/t16-,18-,19+,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as [14C]AMG accumulation after 2 hrs by scintillation counting |
Bioorg Med Chem 20: 3263-79 (2012)
Article DOI: 10.1016/j.bmc.2012.03.051
BindingDB Entry DOI: 10.7270/Q2W66MSS |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50381548
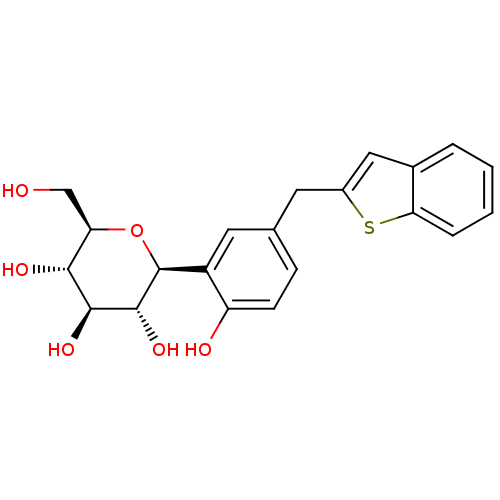
(CHEMBL2018090)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1cc(Cc2cc3ccccc3s2)ccc1O |r| Show InChI InChI=1S/C21H22O6S/c22-10-16-18(24)19(25)20(26)21(27-16)14-8-11(5-6-15(14)23)7-13-9-12-3-1-2-4-17(12)28-13/h1-6,8-9,16,18-26H,7,10H2/t16-,18-,19+,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as [14C]AMG accumulation after 2 hrs by scintillation counting |
Bioorg Med Chem 20: 3263-79 (2012)
Article DOI: 10.1016/j.bmc.2012.03.051
BindingDB Entry DOI: 10.7270/Q2W66MSS |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50381537

(CHEMBL2017954)Show SMILES CCc1ccc(Cc2cccc(c2)[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C21H26O5/c1-2-13-6-8-14(9-7-13)10-15-4-3-5-16(11-15)21-20(25)19(24)18(23)17(12-22)26-21/h3-9,11,17-25H,2,10,12H2,1H3/t17-,18-,19+,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as [14C]AMG accumulation after 2 hrs by scintillation counting |
Bioorg Med Chem 20: 3263-79 (2012)
Article DOI: 10.1016/j.bmc.2012.03.051
BindingDB Entry DOI: 10.7270/Q2W66MSS |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50246785
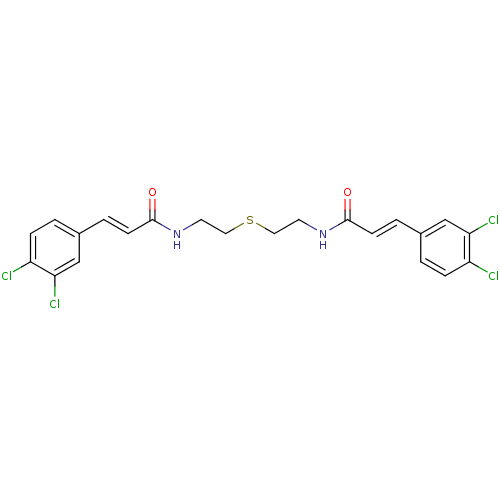
((2E,2'E)-N,N'-(Thiodiethane-2,1-diyl)bis[3-(3,4-di...)Show SMILES Clc1ccc(\C=C\C(=O)NCCSCCNC(=O)\C=C\c2ccc(Cl)c(Cl)c2)cc1Cl Show InChI InChI=1S/C22H20Cl4N2O2S/c23-17-5-1-15(13-19(17)25)3-7-21(29)27-9-11-31-12-10-28-22(30)8-4-16-2-6-18(24)20(26)14-16/h1-8,13-14H,9-12H2,(H,27,29)(H,28,30)/b7-3+,8-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase a in presence of glucose |
Bioorg Med Chem 16: 8627-34 (2008)
Article DOI: 10.1016/j.bmc.2008.08.010
BindingDB Entry DOI: 10.7270/Q2T72H81 |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50381549
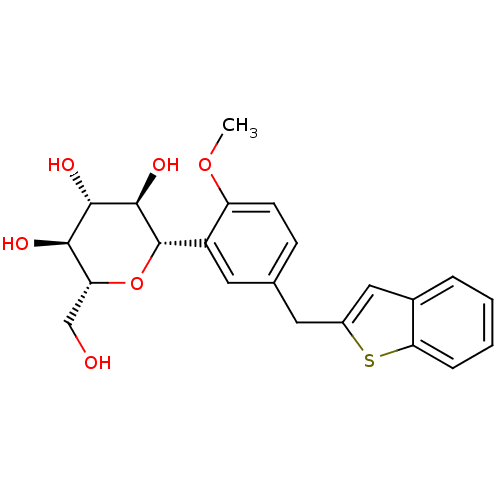
(CHEMBL2018091)Show SMILES COc1ccc(Cc2cc3ccccc3s2)cc1[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C22H24O6S/c1-27-16-7-6-12(8-14-10-13-4-2-3-5-18(13)29-14)9-15(16)22-21(26)20(25)19(24)17(11-23)28-22/h2-7,9-10,17,19-26H,8,11H2,1H3/t17-,19-,20+,21-,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as [14C]AMG accumulation after 2 hrs by scintillation counting |
Bioorg Med Chem 20: 3263-79 (2012)
Article DOI: 10.1016/j.bmc.2012.03.051
BindingDB Entry DOI: 10.7270/Q2W66MSS |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50381553
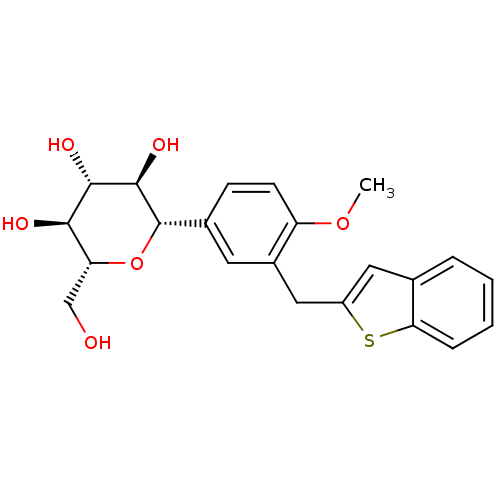
(CHEMBL2018095)Show SMILES COc1ccc(cc1Cc1cc2ccccc2s1)[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C22H24O6S/c1-27-16-7-6-13(22-21(26)20(25)19(24)17(11-23)28-22)8-14(16)10-15-9-12-4-2-3-5-18(12)29-15/h2-9,17,19-26H,10-11H2,1H3/t17-,19-,20+,21-,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as [14C]AMG accumulation after 2 hrs by scintillation counting |
Bioorg Med Chem 20: 3263-79 (2012)
Article DOI: 10.1016/j.bmc.2012.03.051
BindingDB Entry DOI: 10.7270/Q2W66MSS |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM35353

(indole-2-carboxamide derivative, 25e (R-isomer))Show SMILES O[C@@H]1c2cc(F)c(NC(=O)c3cc4cc(Cl)ccc4[nH]3)c(F)c2CCC1(F)F |r| Show InChI InChI=1S/C19H13ClF4N2O2/c20-9-1-2-13-8(5-9)6-14(25-13)18(28)26-16-12(21)7-11-10(15(16)22)3-4-19(23,24)17(11)27/h1-2,5-7,17,25,27H,3-4H2,(H,26,28)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc.
| Assay Description
The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... |
Bioorg Med Chem 16: 10001-12 (2008)
Article DOI: 10.1016/j.bmc.2008.10.021
BindingDB Entry DOI: 10.7270/Q2K072MF |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50246632
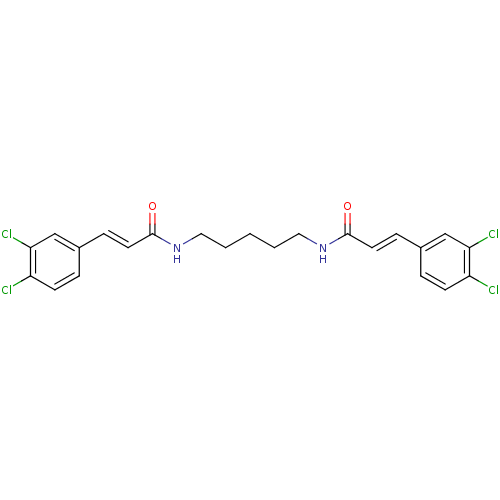
((2E,2'E)-N,N'-Pentane-1,5-diylbis[3-(3,4-dichlorop...)Show SMILES Clc1ccc(\C=C\C(=O)NCCCCCNC(=O)\C=C\c2ccc(Cl)c(Cl)c2)cc1Cl Show InChI InChI=1S/C23H22Cl4N2O2/c24-18-8-4-16(14-20(18)26)6-10-22(30)28-12-2-1-3-13-29-23(31)11-7-17-5-9-19(25)21(27)15-17/h4-11,14-15H,1-3,12-13H2,(H,28,30)(H,29,31)/b10-6+,11-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase a in presence of glucose |
Bioorg Med Chem 16: 8627-34 (2008)
Article DOI: 10.1016/j.bmc.2008.08.010
BindingDB Entry DOI: 10.7270/Q2T72H81 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50246752

((2E,2'E)-N,N'-(Oxydiethane-2,1-diyl)bis[3-(3,4-dic...)Show SMILES Clc1ccc(\C=C\C(=O)NCCOCCNC(=O)\C=C\c2ccc(Cl)c(Cl)c2)cc1Cl Show InChI InChI=1S/C22H20Cl4N2O3/c23-17-5-1-15(13-19(17)25)3-7-21(29)27-9-11-31-12-10-28-22(30)8-4-16-2-6-18(24)20(26)14-16/h1-8,13-14H,9-12H2,(H,27,29)(H,28,30)/b7-3+,8-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase a in presence of glucose |
Bioorg Med Chem 16: 8627-34 (2008)
Article DOI: 10.1016/j.bmc.2008.08.010
BindingDB Entry DOI: 10.7270/Q2T72H81 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50246786

((2E,2'E)-N,N'-(Sulfinyldiethane-2,1-diyl)bis[3-(3,...)Show SMILES Clc1ccc(\C=C\C(=O)NCCS(=O)CCNC(=O)\C=C\c2ccc(Cl)c(Cl)c2)cc1Cl Show InChI InChI=1S/C22H20Cl4N2O3S/c23-17-5-1-15(13-19(17)25)3-7-21(29)27-9-11-32(31)12-10-28-22(30)8-4-16-2-6-18(24)20(26)14-16/h1-8,13-14H,9-12H2,(H,27,29)(H,28,30)/b7-3+,8-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase a in presence of glucose |
Bioorg Med Chem 16: 8627-34 (2008)
Article DOI: 10.1016/j.bmc.2008.08.010
BindingDB Entry DOI: 10.7270/Q2T72H81 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358251

(CHEMBL1922344)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 |r| Show InChI InChI=1S/C33H38N4O10/c1-36-15-4-16-37(18-17-36)21-11-7-19(8-12-21)31(42)35-25-23(34-30(41)20-9-13-22(45-2)14-10-20)5-3-6-24(25)46-33-28(40)26(38)27(39)29(47-33)32(43)44/h3,5-14,26-29,33,38-40H,4,15-18H2,1-2H3,(H,34,41)(H,35,42)(H,43,44)/t26-,27-,28+,29-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by fluorometric analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50381544
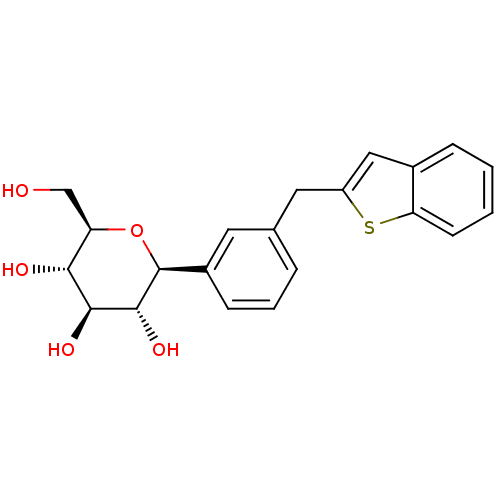
(CHEMBL2018084)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1cccc(Cc2cc3ccccc3s2)c1 |r| Show InChI InChI=1S/C21H22O5S/c22-11-16-18(23)19(24)20(25)21(26-16)14-6-3-4-12(8-14)9-15-10-13-5-1-2-7-17(13)27-15/h1-8,10,16,18-25H,9,11H2/t16-,18-,19+,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as [14C]AMG accumulation after 2 hrs by scintillation counting |
Bioorg Med Chem 20: 3263-79 (2012)
Article DOI: 10.1016/j.bmc.2012.03.051
BindingDB Entry DOI: 10.7270/Q2W66MSS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358264
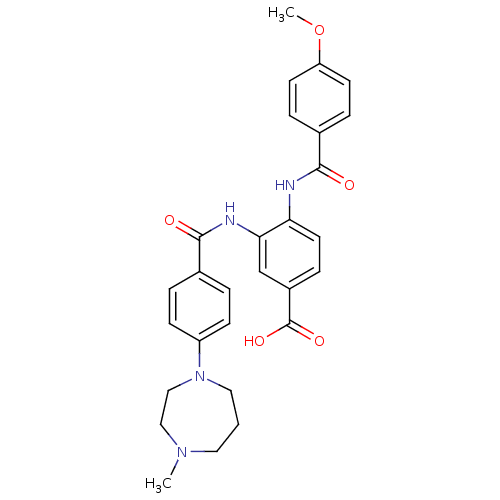
(CHEMBL1922234)Show SMILES COc1ccc(cc1)C(=O)Nc1ccc(cc1NC(=O)c1ccc(cc1)N1CCCN(C)CC1)C(O)=O Show InChI InChI=1S/C28H30N4O5/c1-31-14-3-15-32(17-16-31)22-9-4-19(5-10-22)27(34)30-25-18-21(28(35)36)8-13-24(25)29-26(33)20-6-11-23(37-2)12-7-20/h4-13,18H,3,14-17H2,1-2H3,(H,29,33)(H,30,34)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by fluorometric analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM35351
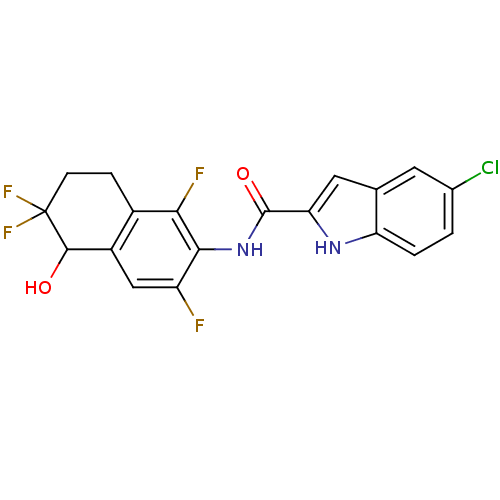
(indole-2-carboxamide derivative, 25e)Show SMILES OC1c2cc(F)c(NC(=O)c3cc4cc(Cl)ccc4[nH]3)c(F)c2CCC1(F)F Show InChI InChI=1S/C19H13ClF4N2O2/c20-9-1-2-13-8(5-9)6-14(25-13)18(28)26-16-12(21)7-11-10(15(16)22)3-4-19(23,24)17(11)27/h1-2,5-7,17,25,27H,3-4H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc.
| Assay Description
The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... |
Bioorg Med Chem 16: 10001-12 (2008)
Article DOI: 10.1016/j.bmc.2008.10.021
BindingDB Entry DOI: 10.7270/Q2K072MF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358276
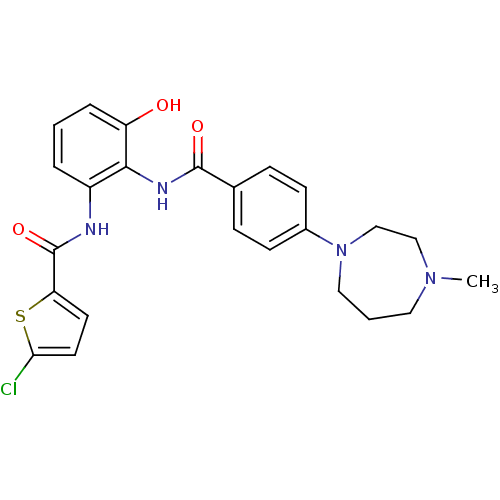
(CHEMBL1922342)Show SMILES CN1CCCN(CC1)c1ccc(cc1)C(=O)Nc1c(O)cccc1NC(=O)c1ccc(Cl)s1 Show InChI InChI=1S/C24H25ClN4O3S/c1-28-12-3-13-29(15-14-28)17-8-6-16(7-9-17)23(31)27-22-18(4-2-5-19(22)30)26-24(32)20-10-11-21(25)33-20/h2,4-11,30H,3,12-15H2,1H3,(H,26,32)(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 41.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by fluorometric analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358275
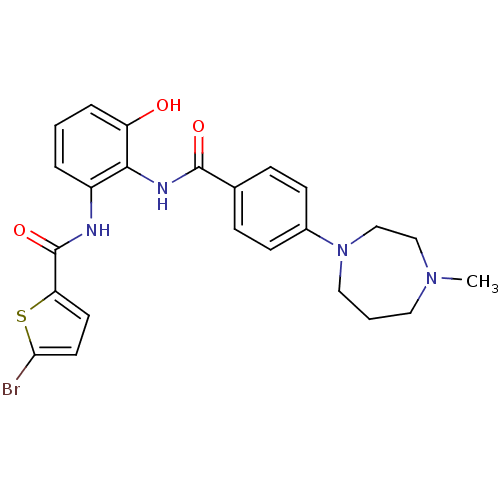
(CHEMBL1922343)Show SMILES CN1CCCN(CC1)c1ccc(cc1)C(=O)Nc1c(O)cccc1NC(=O)c1ccc(Br)s1 Show InChI InChI=1S/C24H25BrN4O3S/c1-28-12-3-13-29(15-14-28)17-8-6-16(7-9-17)23(31)27-22-18(4-2-5-19(22)30)26-24(32)20-10-11-21(25)33-20/h2,4-11,30H,3,12-15H2,1H3,(H,26,32)(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 41.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by fluorometric analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50246788

((2E,2'E)-N,N'-Butane-1,4-diylbis[3-(3,4-dichloroph...)Show SMILES Clc1ccc(\C=C\C(=O)NCCCCNC(=O)\C=C\c2ccc(Cl)c(Cl)c2)cc1Cl Show InChI InChI=1S/C22H20Cl4N2O2/c23-17-7-3-15(13-19(17)25)5-9-21(29)27-11-1-2-12-28-22(30)10-6-16-4-8-18(24)20(26)14-16/h3-10,13-14H,1-2,11-12H2,(H,27,29)(H,28,30)/b9-5+,10-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase a in presence of glucose |
Bioorg Med Chem 16: 8627-34 (2008)
Article DOI: 10.1016/j.bmc.2008.08.010
BindingDB Entry DOI: 10.7270/Q2T72H81 |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50381550
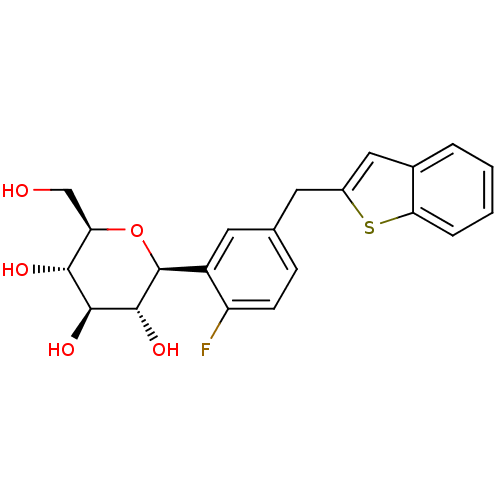
(CHEMBL2018092)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1cc(Cc2cc3ccccc3s2)ccc1F |r| Show InChI InChI=1S/C21H21FO5S/c22-15-6-5-11(7-13-9-12-3-1-2-4-17(12)28-13)8-14(15)21-20(26)19(25)18(24)16(10-23)27-21/h1-6,8-9,16,18-21,23-26H,7,10H2/t16-,18-,19+,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as [14C]AMG accumulation after 2 hrs by scintillation counting |
Bioorg Med Chem 20: 3263-79 (2012)
Article DOI: 10.1016/j.bmc.2012.03.051
BindingDB Entry DOI: 10.7270/Q2W66MSS |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM35350
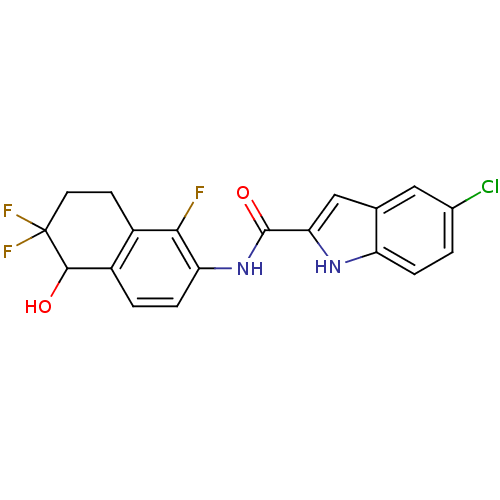
(indole-2-carboxamide derivative, 25d)Show SMILES OC1c2ccc(NC(=O)c3cc4cc(Cl)ccc4[nH]3)c(F)c2CCC1(F)F Show InChI InChI=1S/C19H14ClF3N2O2/c20-10-1-3-13-9(7-10)8-15(24-13)18(27)25-14-4-2-12-11(16(14)21)5-6-19(22,23)17(12)26/h1-4,7-8,17,24,26H,5-6H2,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc.
| Assay Description
The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... |
Bioorg Med Chem 16: 10001-12 (2008)
Article DOI: 10.1016/j.bmc.2008.10.021
BindingDB Entry DOI: 10.7270/Q2K072MF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358265

(CHEMBL1922236)Show SMILES COc1ccc(cc1)C(=O)Nc1ccc(O)cc1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 Show InChI InChI=1S/C27H30N4O4/c1-30-14-3-15-31(17-16-30)21-8-4-19(5-9-21)27(34)29-25-18-22(32)10-13-24(25)28-26(33)20-6-11-23(35-2)12-7-20/h4-13,18,32H,3,14-17H2,1-2H3,(H,28,33)(H,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by fluorometric analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358252

(CHEMBL1922235)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 Show InChI InChI=1S/C27H30N4O4/c1-30-15-4-16-31(18-17-30)21-11-7-19(8-12-21)27(34)29-25-23(5-3-6-24(25)32)28-26(33)20-9-13-22(35-2)14-10-20/h3,5-14,32H,4,15-18H2,1-2H3,(H,28,33)(H,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 54.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by fluorometric analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358272
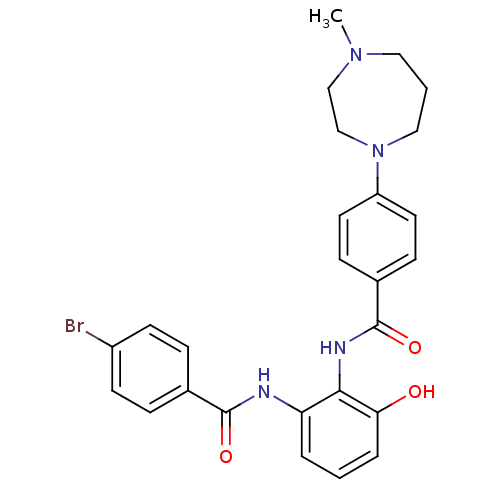
(CHEMBL1922340)Show SMILES CN1CCCN(CC1)c1ccc(cc1)C(=O)Nc1c(O)cccc1NC(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C26H27BrN4O3/c1-30-14-3-15-31(17-16-30)21-12-8-19(9-13-21)26(34)29-24-22(4-2-5-23(24)32)28-25(33)18-6-10-20(27)11-7-18/h2,4-13,32H,3,14-17H2,1H3,(H,28,33)(H,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 59.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by fluorometric analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358259
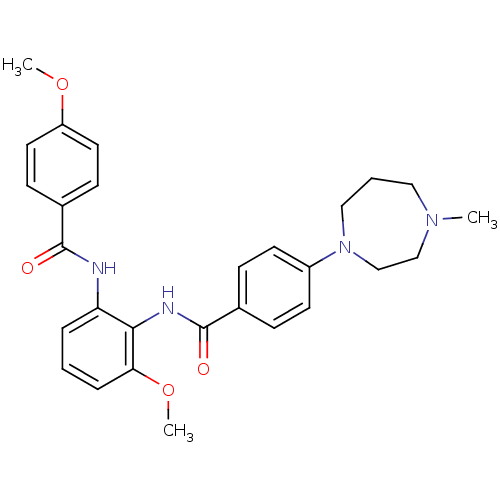
(CHEMBL1922229)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(OC)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 Show InChI InChI=1S/C28H32N4O4/c1-31-16-5-17-32(19-18-31)22-12-8-20(9-13-22)28(34)30-26-24(6-4-7-25(26)36-3)29-27(33)21-10-14-23(35-2)15-11-21/h4,6-15H,5,16-19H2,1-3H3,(H,29,33)(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 62.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by fluorometric analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM35349
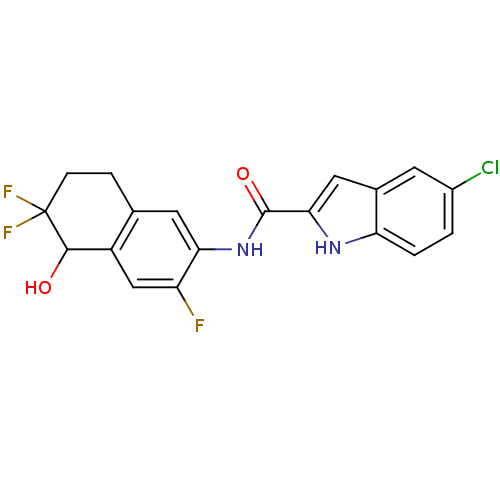
(indole-2-carboxamide derivative, 25c)Show SMILES OC1c2cc(F)c(NC(=O)c3cc4cc(Cl)ccc4[nH]3)cc2CCC1(F)F Show InChI InChI=1S/C19H14ClF3N2O2/c20-11-1-2-14-10(5-11)7-16(24-14)18(27)25-15-6-9-3-4-19(22,23)17(26)12(9)8-13(15)21/h1-2,5-8,17,24,26H,3-4H2,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc.
| Assay Description
The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... |
Bioorg Med Chem 16: 10001-12 (2008)
Article DOI: 10.1016/j.bmc.2008.10.021
BindingDB Entry DOI: 10.7270/Q2K072MF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358273
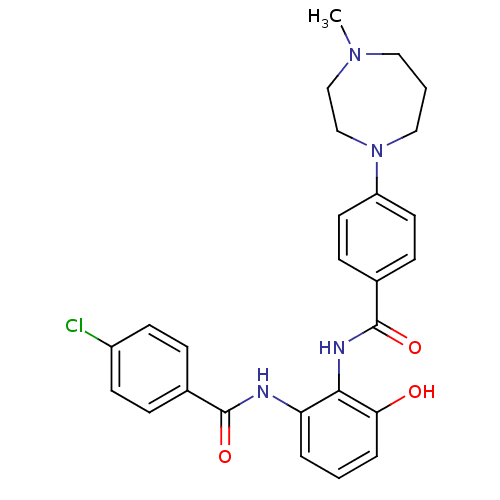
(CHEMBL1922339)Show SMILES CN1CCCN(CC1)c1ccc(cc1)C(=O)Nc1c(O)cccc1NC(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C26H27ClN4O3/c1-30-14-3-15-31(17-16-30)21-12-8-19(9-13-21)26(34)29-24-22(4-2-5-23(24)32)28-25(33)18-6-10-20(27)11-7-18/h2,4-13,32H,3,14-17H2,1H3,(H,28,33)(H,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 64.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by fluorometric analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM35345
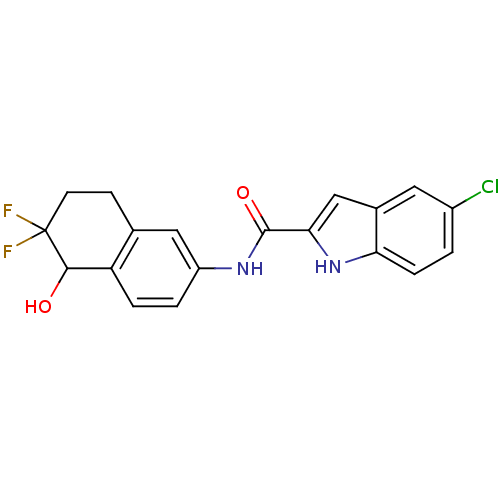
(indole-2-carboxamide derivative, 15)Show SMILES OC1c2ccc(NC(=O)c3cc4cc(Cl)ccc4[nH]3)cc2CCC1(F)F Show InChI InChI=1S/C19H15ClF2N2O2/c20-12-1-4-15-11(7-12)9-16(24-15)18(26)23-13-2-3-14-10(8-13)5-6-19(21,22)17(14)25/h1-4,7-9,17,24-25H,5-6H2,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc.
| Assay Description
The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... |
Bioorg Med Chem 16: 10001-12 (2008)
Article DOI: 10.1016/j.bmc.2008.10.021
BindingDB Entry DOI: 10.7270/Q2K072MF |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50381539
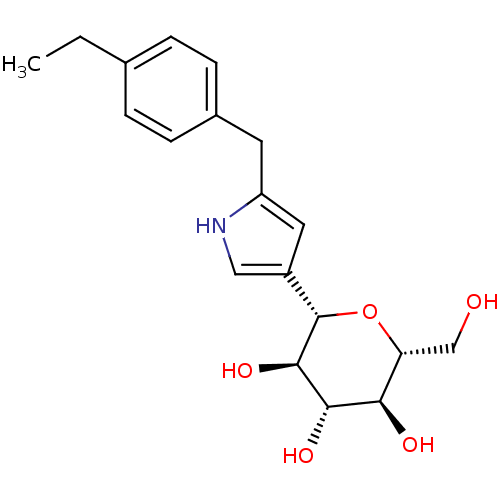
(CHEMBL2017956)Show SMILES CCc1ccc(Cc2cc(c[nH]2)[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C19H25NO5/c1-2-11-3-5-12(6-4-11)7-14-8-13(9-20-14)19-18(24)17(23)16(22)15(10-21)25-19/h3-6,8-9,15-24H,2,7,10H2,1H3/t15-,16-,17+,18-,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as [14C]AMG accumulation after 2 hrs by scintillation counting |
Bioorg Med Chem 20: 3263-79 (2012)
Article DOI: 10.1016/j.bmc.2012.03.051
BindingDB Entry DOI: 10.7270/Q2W66MSS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358261

(CHEMBL1922230)Show SMILES COc1ccc(cc1)C(=O)Nc1ccc(OC)cc1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 Show InChI InChI=1S/C28H32N4O4/c1-31-15-4-16-32(18-17-31)22-9-5-20(6-10-22)28(34)30-26-19-24(36-3)13-14-25(26)29-27(33)21-7-11-23(35-2)12-8-21/h5-14,19H,4,15-18H2,1-3H3,(H,29,33)(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 73.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by fluorometric analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50381552
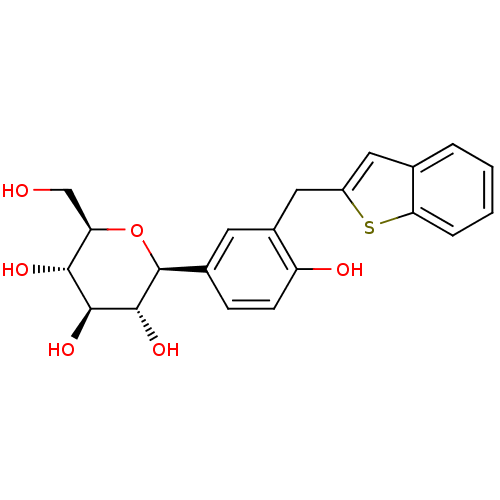
(CHEMBL2018094)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1ccc(O)c(Cc2cc3ccccc3s2)c1 |r| Show InChI InChI=1S/C21H22O6S/c22-10-16-18(24)19(25)20(26)21(27-16)12-5-6-15(23)13(7-12)9-14-8-11-3-1-2-4-17(11)28-14/h1-8,16,18-26H,9-10H2/t16-,18-,19+,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as [14C]AMG accumulation after 2 hrs by scintillation counting |
Bioorg Med Chem 20: 3263-79 (2012)
Article DOI: 10.1016/j.bmc.2012.03.051
BindingDB Entry DOI: 10.7270/Q2W66MSS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358274
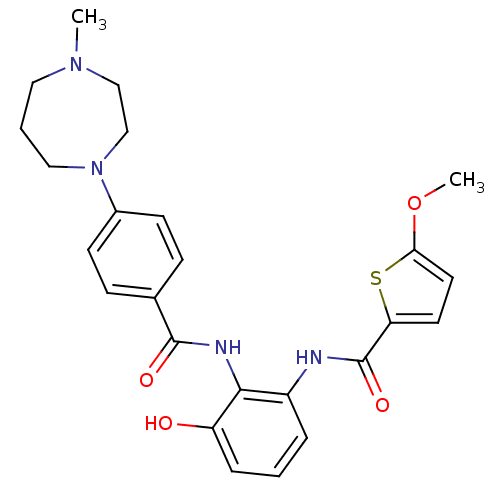
(CHEMBL1922341)Show SMILES COc1ccc(s1)C(=O)Nc1cccc(O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 Show InChI InChI=1S/C25H28N4O4S/c1-28-13-4-14-29(16-15-28)18-9-7-17(8-10-18)24(31)27-23-19(5-3-6-20(23)30)26-25(32)21-11-12-22(33-2)34-21/h3,5-12,30H,4,13-16H2,1-2H3,(H,26,32)(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 86.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by fluorometric analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50381551
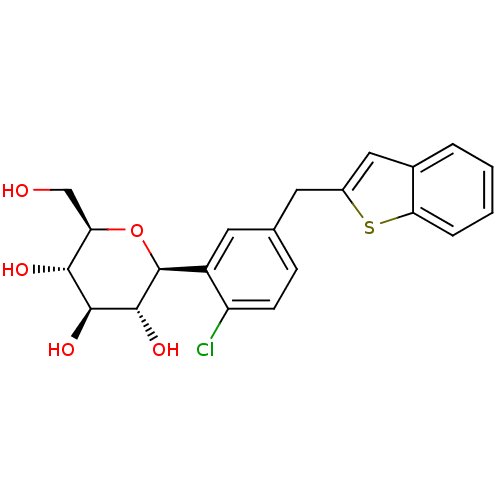
(CHEMBL2018093)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1cc(Cc2cc3ccccc3s2)ccc1Cl |r| Show InChI InChI=1S/C21H21ClO5S/c22-15-6-5-11(7-13-9-12-3-1-2-4-17(12)28-13)8-14(15)21-20(26)19(25)18(24)16(10-23)27-21/h1-6,8-9,16,18-21,23-26H,7,10H2/t16-,18-,19+,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as [14C]AMG accumulation after 2 hrs by scintillation counting |
Bioorg Med Chem 20: 3263-79 (2012)
Article DOI: 10.1016/j.bmc.2012.03.051
BindingDB Entry DOI: 10.7270/Q2W66MSS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358254
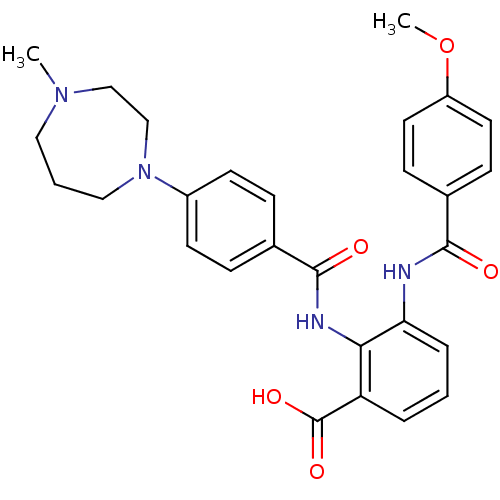
(CHEMBL1922233)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(C(O)=O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 Show InChI InChI=1S/C28H30N4O5/c1-31-15-4-16-32(18-17-31)21-11-7-19(8-12-21)27(34)30-25-23(28(35)36)5-3-6-24(25)29-26(33)20-9-13-22(37-2)14-10-20/h3,5-14H,4,15-18H2,1-2H3,(H,29,33)(H,30,34)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 99.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by fluorometric analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50381538
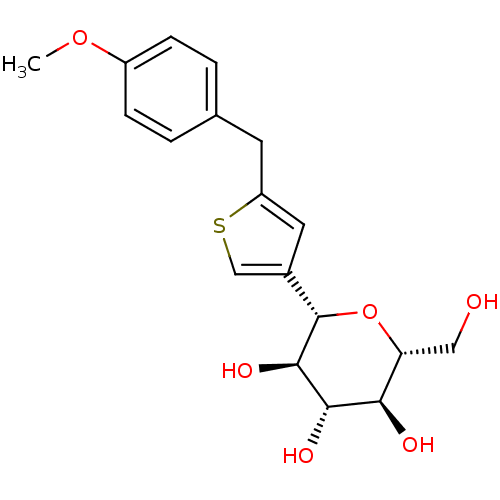
(CHEMBL2017955)Show SMILES COc1ccc(Cc2cc(cs2)[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C18H22O6S/c1-23-12-4-2-10(3-5-12)6-13-7-11(9-25-13)18-17(22)16(21)15(20)14(8-19)24-18/h2-5,7,9,14-22H,6,8H2,1H3/t14-,15-,16+,17-,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as [14C]AMG accumulation after 2 hrs by scintillation counting |
Bioorg Med Chem 20: 3263-79 (2012)
Article DOI: 10.1016/j.bmc.2012.03.051
BindingDB Entry DOI: 10.7270/Q2W66MSS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358257
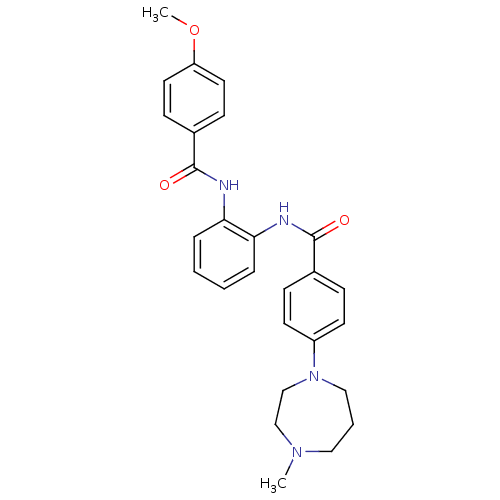
(CHEMBL1922226)Show SMILES COc1ccc(cc1)C(=O)Nc1ccccc1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 Show InChI InChI=1S/C27H30N4O3/c1-30-16-5-17-31(19-18-30)22-12-8-20(9-13-22)26(32)28-24-6-3-4-7-25(24)29-27(33)21-10-14-23(34-2)15-11-21/h3-4,6-15H,5,16-19H2,1-2H3,(H,28,32)(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by fluorometric analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358253
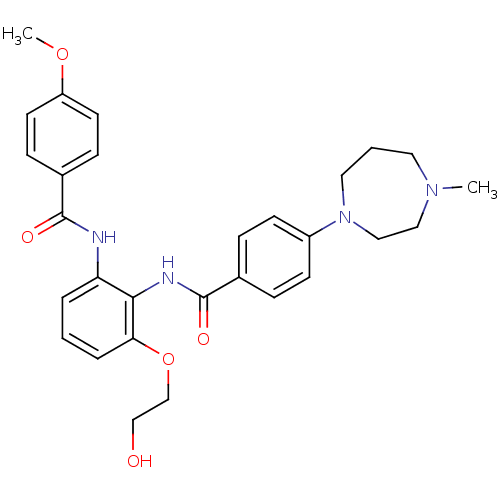
(CHEMBL1922240)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(OCCO)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 Show InChI InChI=1S/C29H34N4O5/c1-32-15-4-16-33(18-17-32)23-11-7-21(8-12-23)29(36)31-27-25(5-3-6-26(27)38-20-19-34)30-28(35)22-9-13-24(37-2)14-10-22/h3,5-14,34H,4,15-20H2,1-2H3,(H,30,35)(H,31,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by fluorometric analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358262

(CHEMBL1922231)Show SMILES COc1ccc(cc1)C(=O)Nc1cc(OC)ccc1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 Show InChI InChI=1S/C28H32N4O4/c1-31-15-4-16-32(18-17-31)22-9-5-20(6-10-22)27(33)29-25-14-13-24(36-3)19-26(25)30-28(34)21-7-11-23(35-2)12-8-21/h5-14,19H,4,15-18H2,1-3H3,(H,29,33)(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by fluorometric analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358268
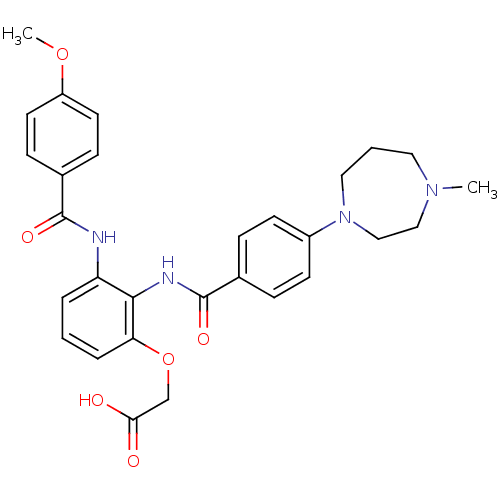
(CHEMBL1922239)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(OCC(O)=O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 Show InChI InChI=1S/C29H32N4O6/c1-32-15-4-16-33(18-17-32)22-11-7-20(8-12-22)29(37)31-27-24(5-3-6-25(27)39-19-26(34)35)30-28(36)21-9-13-23(38-2)14-10-21/h3,5-14H,4,15-19H2,1-2H3,(H,30,36)(H,31,37)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by fluorometric analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358260
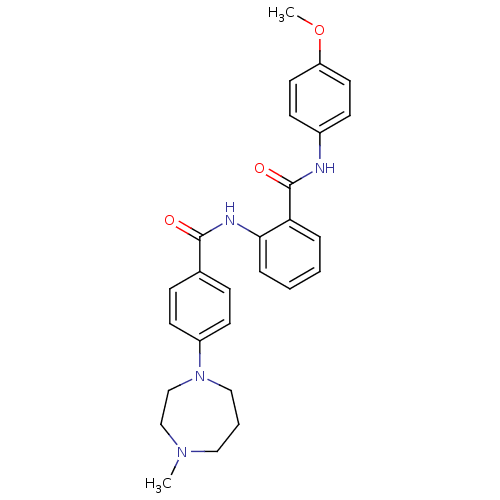
(CHEMBL1922228)Show SMILES COc1ccc(NC(=O)c2ccccc2NC(=O)c2ccc(cc2)N2CCCN(C)CC2)cc1 Show InChI InChI=1S/C27H30N4O3/c1-30-16-5-17-31(19-18-30)22-12-8-20(9-13-22)26(32)29-25-7-4-3-6-24(25)27(33)28-21-10-14-23(34-2)15-11-21/h3-4,6-15H,5,16-19H2,1-2H3,(H,28,33)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by fluorometric analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358267

(CHEMBL1922238)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(F)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 Show InChI InChI=1S/C27H29FN4O3/c1-31-15-4-16-32(18-17-31)21-11-7-19(8-12-21)27(34)30-25-23(28)5-3-6-24(25)29-26(33)20-9-13-22(35-2)14-10-20/h3,5-14H,4,15-18H2,1-2H3,(H,29,33)(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by fluorometric analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358266

(CHEMBL1922237)Show SMILES COc1ccc(cc1)C(=O)Nc1cc(O)ccc1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 Show InChI InChI=1S/C27H30N4O4/c1-30-14-3-15-31(17-16-30)21-8-4-19(5-9-21)26(33)28-24-13-10-22(32)18-25(24)29-27(34)20-6-11-23(35-2)12-7-20/h4-13,18,32H,3,14-17H2,1-2H3,(H,28,33)(H,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 157 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by fluorometric analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM35352
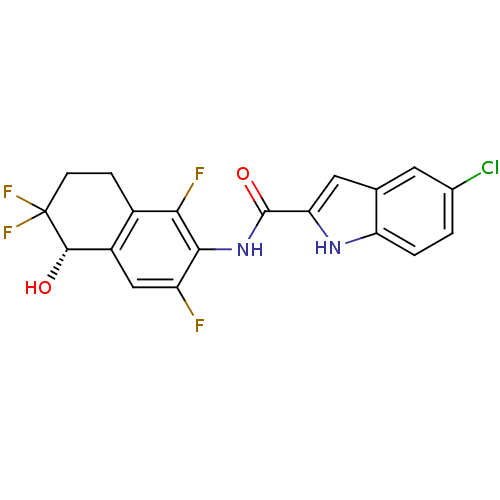
(indole-2-carboxamide derivative, 25e (S-isomer))Show SMILES O[C@H]1c2cc(F)c(NC(=O)c3cc4cc(Cl)ccc4[nH]3)c(F)c2CCC1(F)F |r| Show InChI InChI=1S/C19H13ClF4N2O2/c20-9-1-2-13-8(5-9)6-14(25-13)18(28)26-16-12(21)7-11-10(15(16)22)3-4-19(23,24)17(11)27/h1-2,5-7,17,25,27H,3-4H2,(H,26,28)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc.
| Assay Description
The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... |
Bioorg Med Chem 16: 10001-12 (2008)
Article DOI: 10.1016/j.bmc.2008.10.021
BindingDB Entry DOI: 10.7270/Q2K072MF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358263
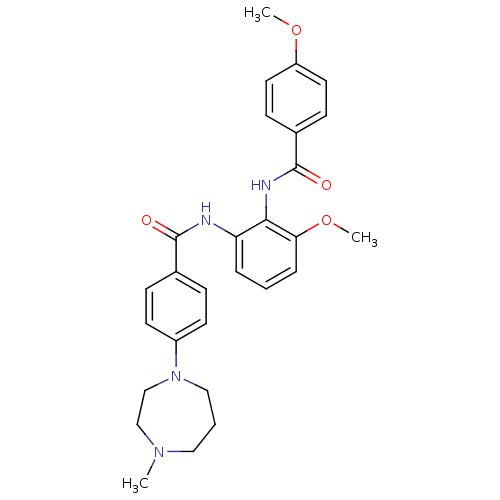
(CHEMBL1922232)Show SMILES COc1ccc(cc1)C(=O)Nc1c(NC(=O)c2ccc(cc2)N2CCCN(C)CC2)cccc1OC Show InChI InChI=1S/C28H32N4O4/c1-31-16-5-17-32(19-18-31)22-12-8-20(9-13-22)27(33)29-24-6-4-7-25(36-3)26(24)30-28(34)21-10-14-23(35-2)15-11-21/h4,6-15H,5,16-19H2,1-3H3,(H,29,33)(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 213 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by fluorometric analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM35636

(5-chloroindolecarboxamide, 13a)Show InChI InChI=1S/C16H14ClN3O3/c17-11-2-3-12-10(5-11)6-13(19-12)16(23)20-15-4-1-9(7-18-15)14(22)8-21/h1-7,14,19,21-22H,8H2,(H,18,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc.
| Assay Description
The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... |
Bioorg Med Chem 16: 5452-64 (2008)
Article DOI: 10.1016/j.bmc.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J101H2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data