

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123058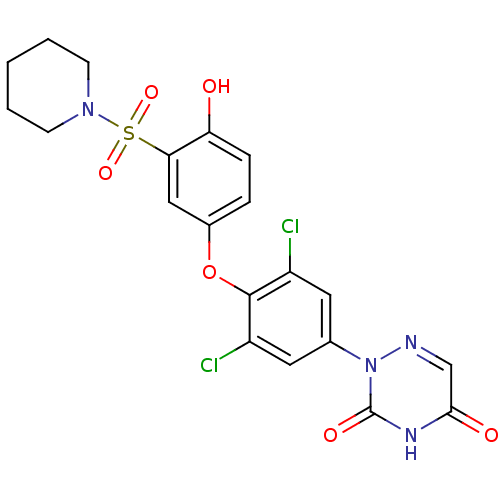 (2-(3,5-dichloro-4-(4-hydroxy-3-(piperidin-1-ylsulf...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123046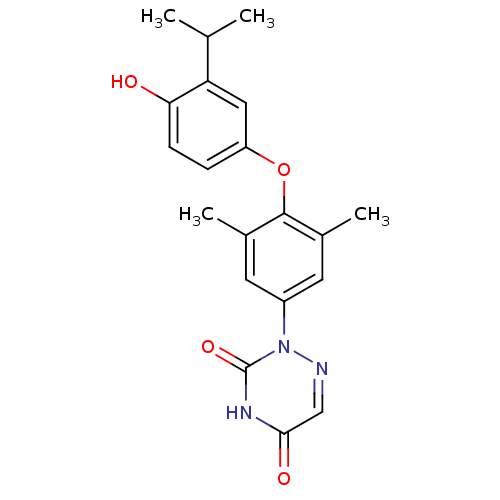 (2-[4-(4-Hydroxy-3-isopropyl-phenoxy)-3,5-dimethyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123044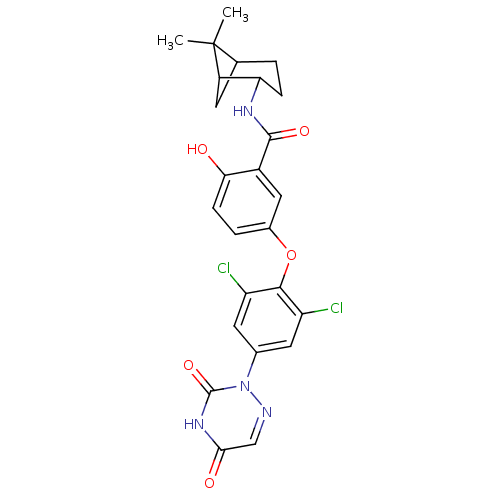 (5-(2,6-dichloro-4-(3,5-dioxo-4,5-dihydro-1,2,4-tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123044 (5-(2,6-dichloro-4-(3,5-dioxo-4,5-dihydro-1,2,4-tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123045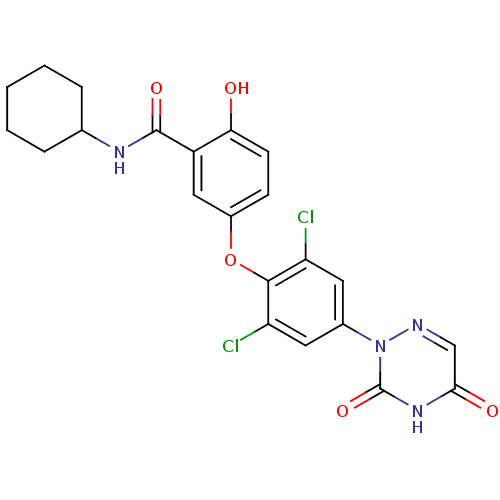 (CHEMBL413699 | N-Cyclohexyl-5-[2,6-dichloro-4-(3,5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18860 ((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123054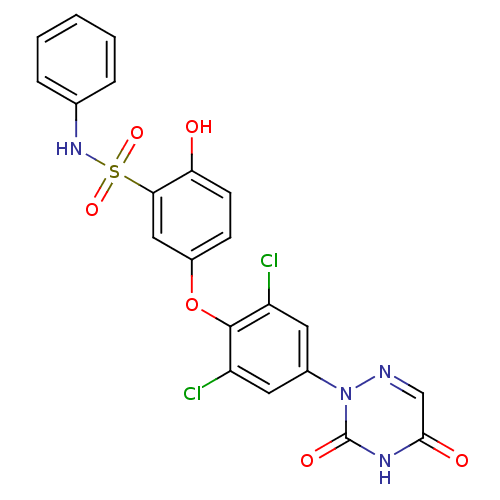 (5-(2,6-dichloro-4-(3,5-dioxo-4,5-dihydro-1,2,4-tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123064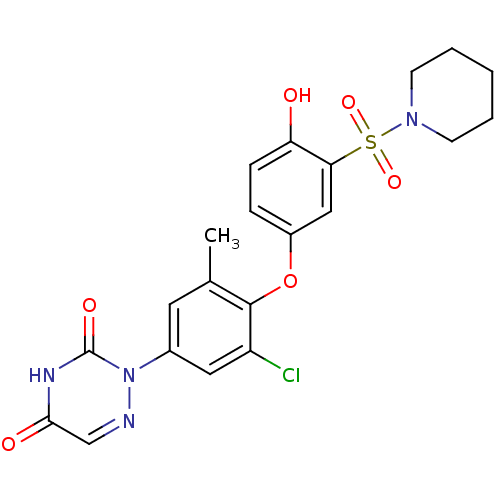 (2-(3-chloro-4-(4-hydroxy-3-(piperidin-1-ylsulfonyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123063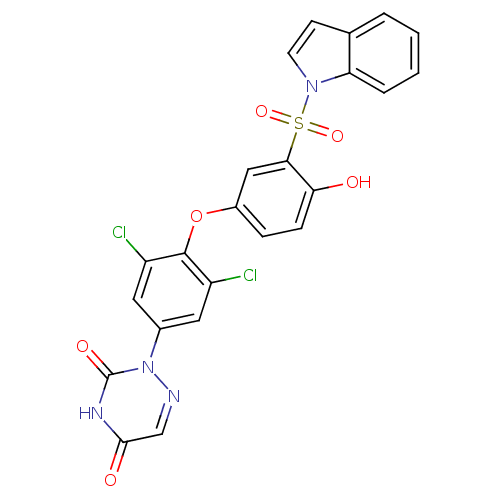 (2-{3,5-Dichloro-4-[4-hydroxy-3-(indole-1-sulfonyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123052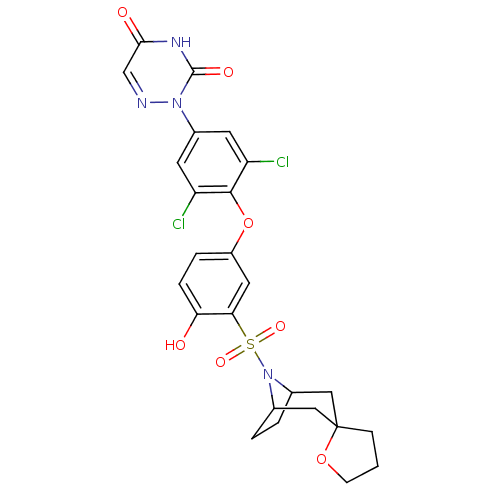 (2-[4-(3-{8-azaspiro[bicyclo[3.2.1]octane-3,2'-oxol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50128931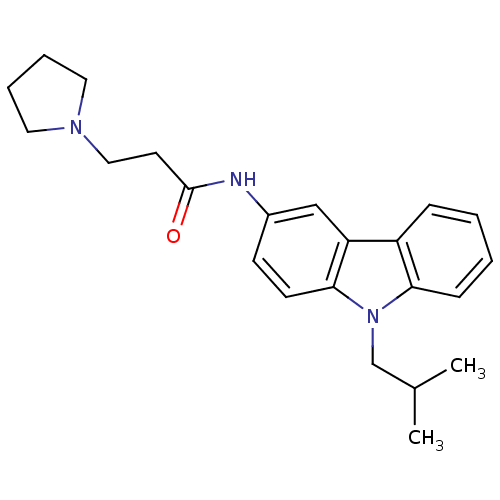 (CHEMBL61880 | N-(9-Isobutyl-9H-carbazol-3-yl)-3-py...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using 125[I]-PYY as radioligand | Bioorg Med Chem Lett 13: 1989-92 (2003) BindingDB Entry DOI: 10.7270/Q2057F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123061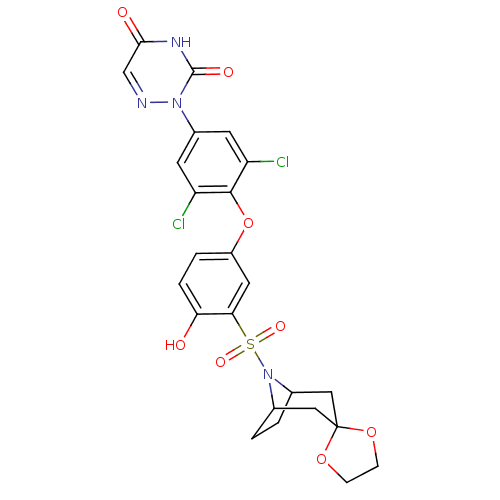 (2-[4-(3-{8-azaspiro[bicyclo[3.2.1]octane-3,2'-[1,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123059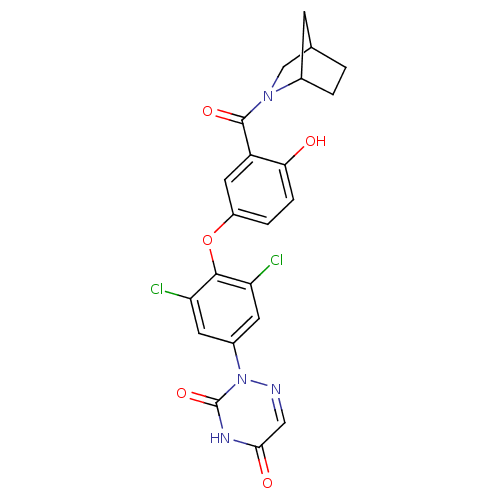 (2-{4-[3-(2-Aza-bicyclo[2.2.1]heptane-2-carbonyl)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123059 (2-{4-[3-(2-Aza-bicyclo[2.2.1]heptane-2-carbonyl)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123057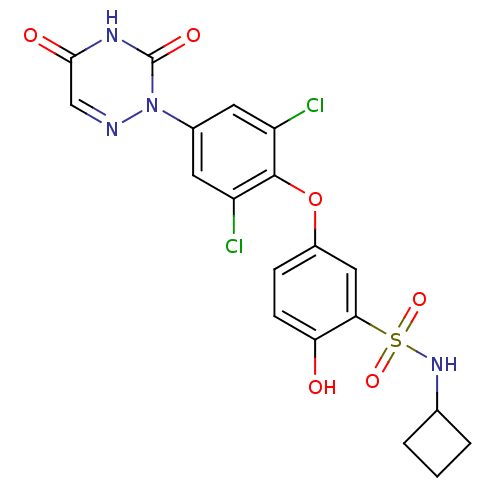 (CHEMBL124039 | N-Cyclobutyl-5-[2,6-dichloro-4-(3,5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123062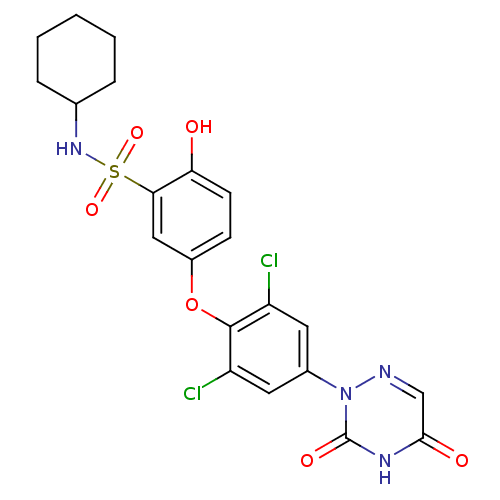 (CHEMBL340158 | N-Cyclohexyl-5-[2,6-dichloro-4-(3,5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123056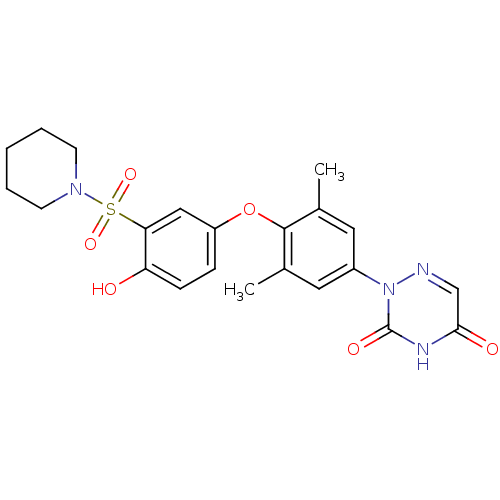 (2-(4-(4-hydroxy-3-(piperidin-1-ylsulfonyl)phenoxy)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123059 (2-{4-[3-(2-Aza-bicyclo[2.2.1]heptane-2-carbonyl)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123049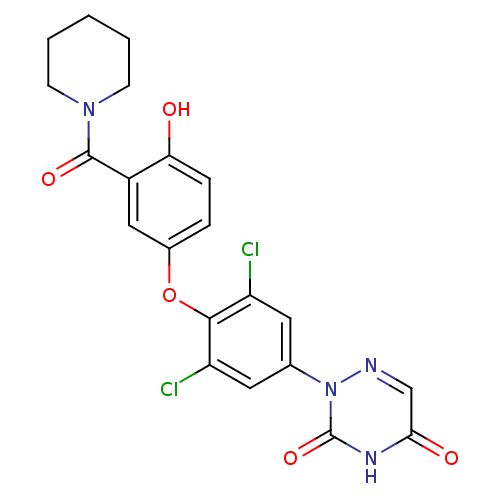 (2-(3,5-dichloro-4-(4-hydroxy-3-(piperidine-1-carbo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123050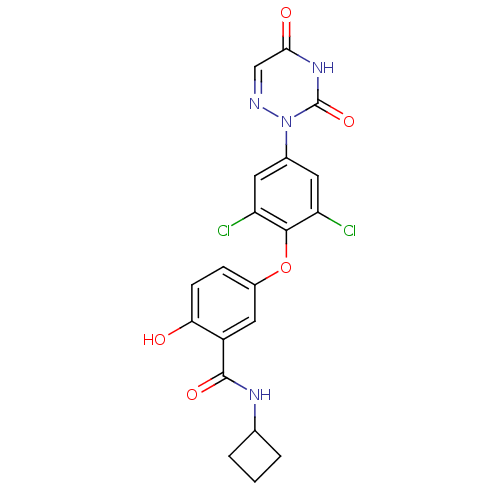 (CHEMBL124318 | N-Cyclobutyl-5-[2,6-dichloro-4-(3,5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123055 (2-(3,5-dichloro-4-(4-hydroxy-3-(morpholine-4-carbo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50128927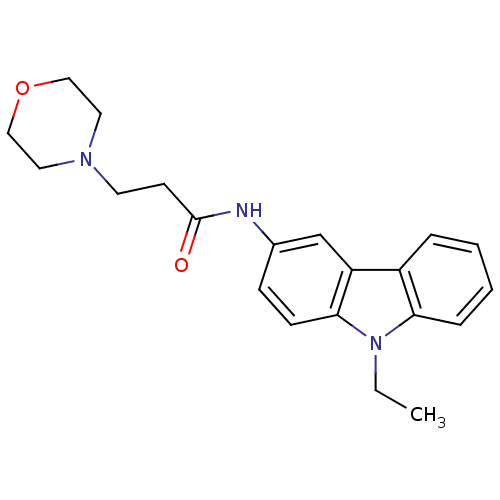 (CHEMBL59680 | N-(9-Ethyl-9H-carbazol-3-yl)-3-morph...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using 125[I]-PYY as radioligand | Bioorg Med Chem Lett 13: 1989-92 (2003) BindingDB Entry DOI: 10.7270/Q2057F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50128943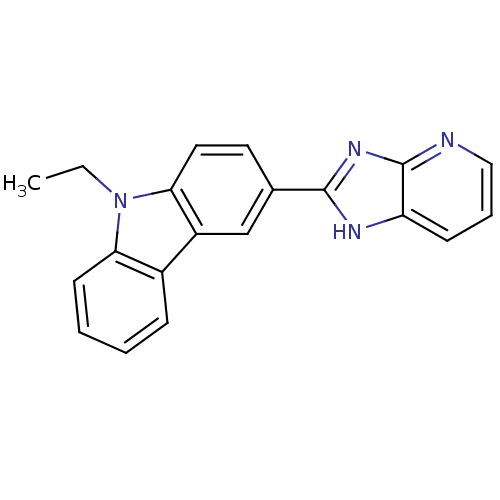 (9-Ethyl-3-(3H-imidazo[4,5-b]pyridin-2-yl)-9H-carba...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using 125[I]-PYY as radioligand | Bioorg Med Chem Lett 13: 1989-92 (2003) BindingDB Entry DOI: 10.7270/Q2057F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50133761 (3-[5-(3-Trifluoromethyl-phenyl)-1H-imidazol-2-yl]-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using [125I]-PYY as radiogigand in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 13: 3593-6 (2003) BindingDB Entry DOI: 10.7270/Q2DF6QKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123060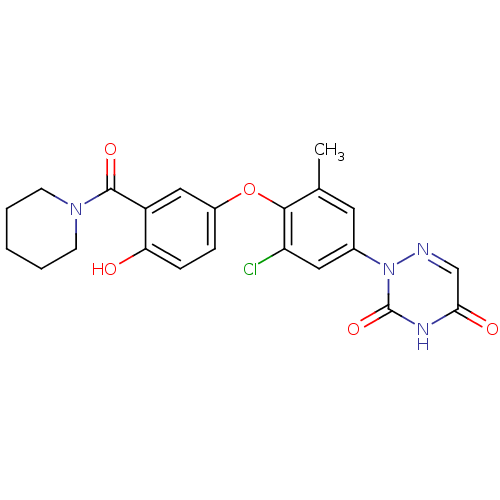 (2-{3-Chloro-4-[4-hydroxy-3-(piperidine-1-carbonyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123053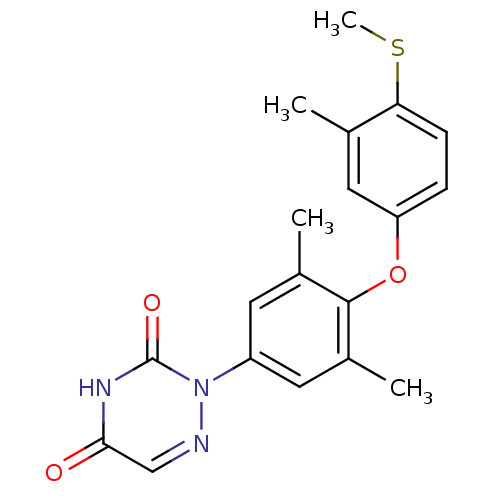 (2-[3,5-Dimethyl-4-(3-methyl-4-methylsulfanyl-pheno...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50128938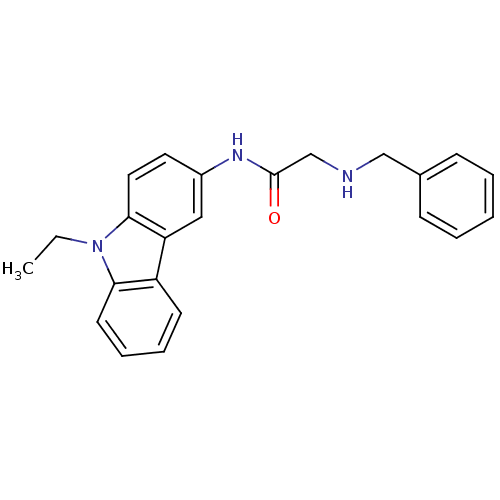 (2-Benzylamino-N-(9-ethyl-9H-carbazol-3-yl)-acetami...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using 125[I]-PYY as radioligand | Bioorg Med Chem Lett 13: 1989-92 (2003) BindingDB Entry DOI: 10.7270/Q2057F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50128928 (CHEMBL301638 | N-(9-Isopropyl-9H-carbazol-3-yl)-3-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using 125[I]-PYY as radioligand | Bioorg Med Chem Lett 13: 1989-92 (2003) BindingDB Entry DOI: 10.7270/Q2057F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50133775 (4-[5-(3,4-Dichloro-phenyl)-1H-imidazol-2-yl]-pyrid...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using [125I]-PYY as radiogigand in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 13: 3593-6 (2003) BindingDB Entry DOI: 10.7270/Q2DF6QKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50133780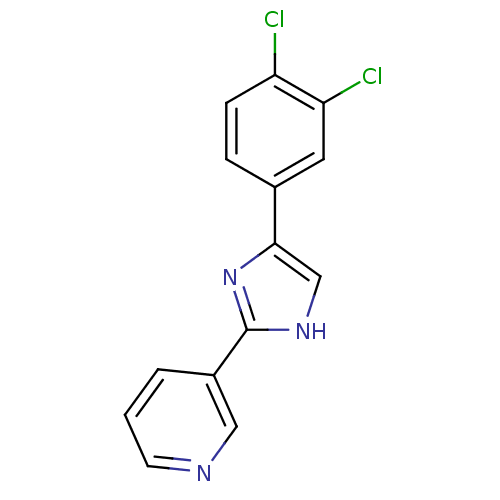 (3-[5-(3,4-Dichloro-phenyl)-1H-imidazol-2-yl]-pyrid...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using [125I]-PYY as radiogigand in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 13: 3593-6 (2003) BindingDB Entry DOI: 10.7270/Q2DF6QKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50128935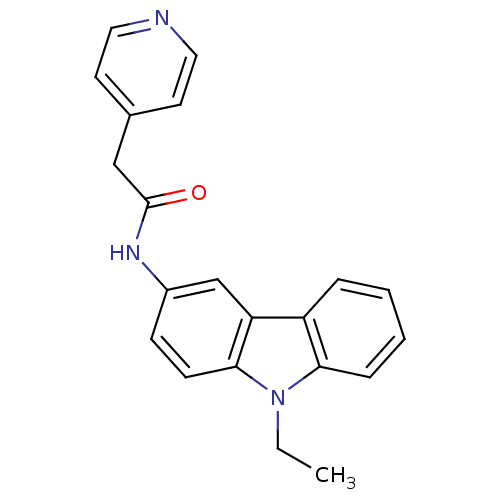 (CHEMBL294305 | N-(9-Ethyl-9H-carbazol-3-yl)-2-pyri...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using 125[I]-PYY as radioligand | Bioorg Med Chem Lett 13: 1989-92 (2003) BindingDB Entry DOI: 10.7270/Q2057F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50133762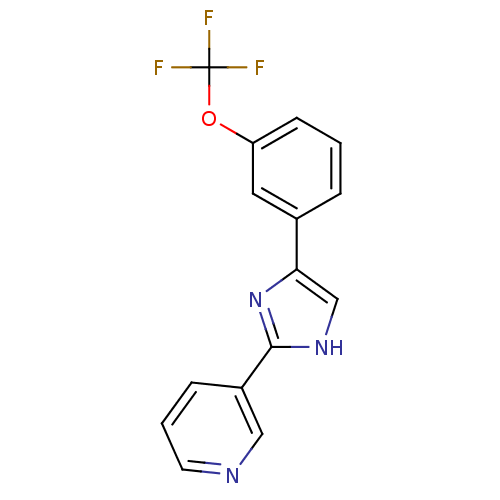 (3-[5-(3-Trifluoromethoxy-phenyl)-1H-imidazol-2-yl]...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using [125I]-PYY as radiogigand in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 13: 3593-6 (2003) BindingDB Entry DOI: 10.7270/Q2DF6QKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50386955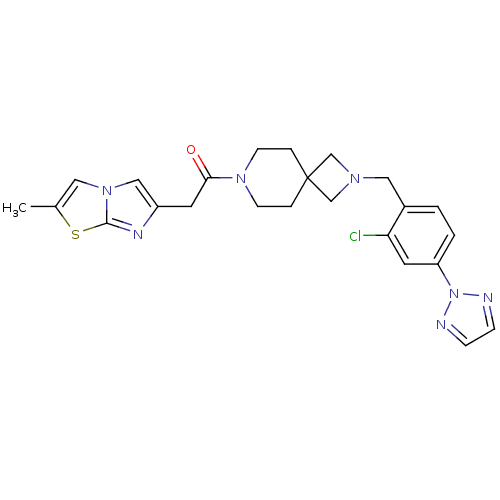 (CHEMBL2048820) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA | Bioorg Med Chem Lett 22: 4281-7 (2012) Article DOI: 10.1016/j.bmcl.2012.05.024 BindingDB Entry DOI: 10.7270/Q2P2705D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50128944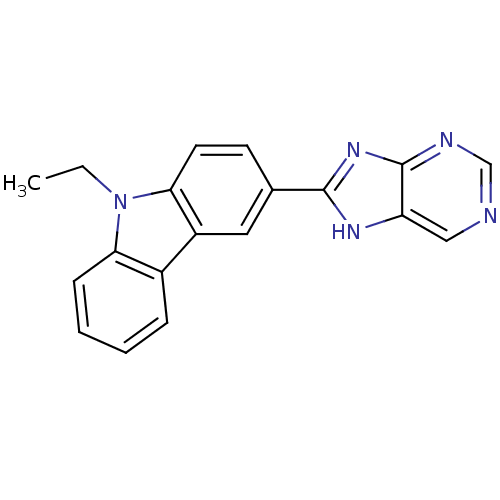 (9-Ethyl-3-(9H-purin-8-yl)-9H-carbazole | CHEMBL611...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using 125[I]-PYY as radioligand | Bioorg Med Chem Lett 13: 1989-92 (2003) BindingDB Entry DOI: 10.7270/Q2057F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50133779 (3-[5-(3-Chloro-phenyl)-1H-imidazol-2-yl]-pyridine ...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using [125I]-PYY as radiogigand in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 13: 3593-6 (2003) BindingDB Entry DOI: 10.7270/Q2DF6QKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50128934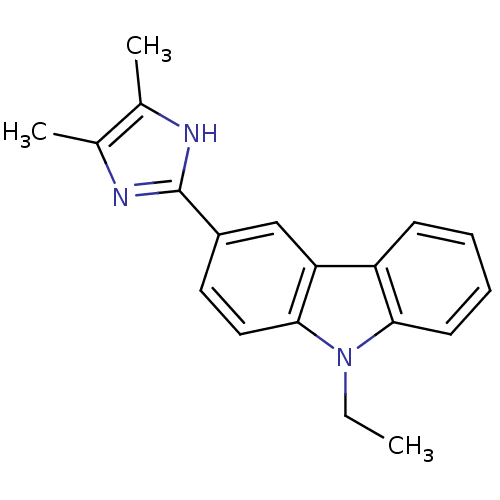 (3-(4,5-Dimethyl-1H-imidazol-2-yl)-9-ethyl-9H-carba...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using 125[I]-PYY as radioligand | Bioorg Med Chem Lett 13: 1989-92 (2003) BindingDB Entry DOI: 10.7270/Q2057F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50386949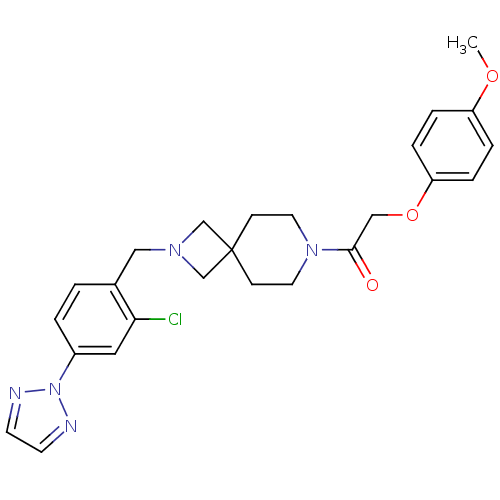 (CHEMBL2048814) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA | Bioorg Med Chem Lett 22: 4281-7 (2012) Article DOI: 10.1016/j.bmcl.2012.05.024 BindingDB Entry DOI: 10.7270/Q2P2705D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50128939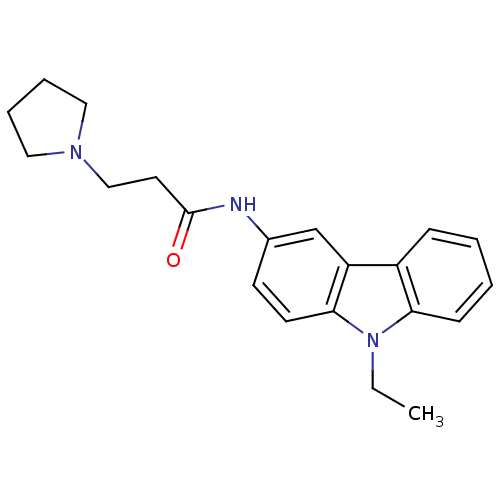 (CHEMBL292729 | N-(9-Ethyl-9H-carbazol-3-yl)-3-pyrr...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using 125[I]-PYY as radioligand | Bioorg Med Chem Lett 13: 1989-92 (2003) BindingDB Entry DOI: 10.7270/Q2057F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50128926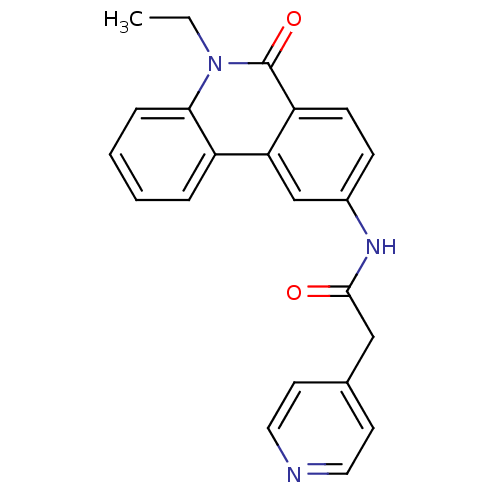 (CHEMBL304896 | N-(5-Ethyl-6-oxo-5,6-dihydro-phenan...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using 125[I]-PYY as radioligand | Bioorg Med Chem Lett 13: 1989-92 (2003) BindingDB Entry DOI: 10.7270/Q2057F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123047 (2-{3-Chloro-4-[4-hydroxy-3-(4-methyl-piperazine-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50133769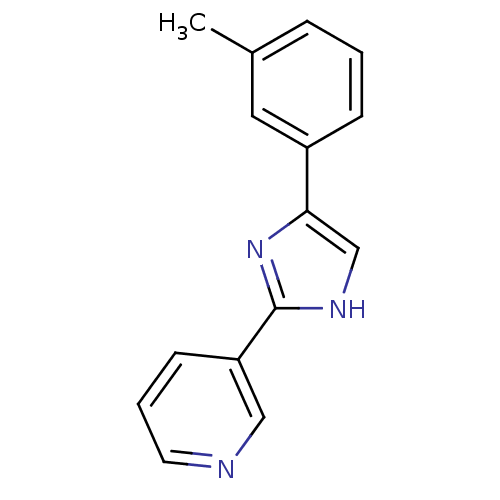 (3-(5-m-Tolyl-1H-imidazol-2-yl)-pyridine | CHEMBL33...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using [125I]-PYY as radiogigand in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 13: 3593-6 (2003) BindingDB Entry DOI: 10.7270/Q2DF6QKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50128929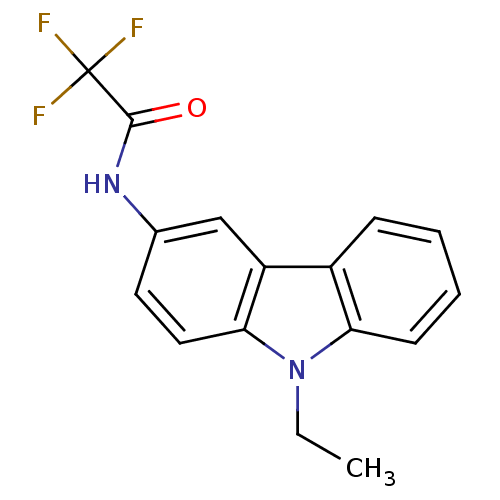 (CHEMBL61879 | N-(9-Ethyl-9H-carbazol-3-yl)-2,2,2-t...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using 125[I]-PYY as radioligand | Bioorg Med Chem Lett 13: 1989-92 (2003) BindingDB Entry DOI: 10.7270/Q2057F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50386957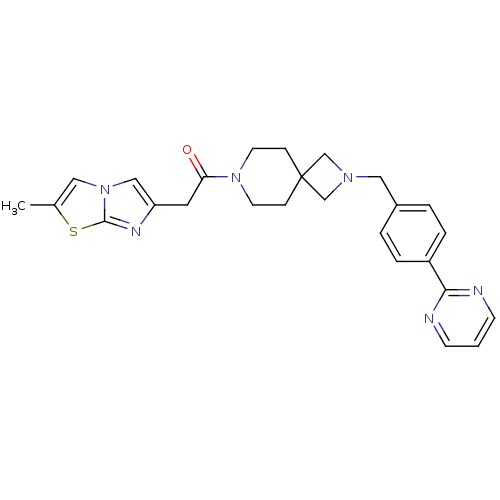 (CHEMBL2048822) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA | Bioorg Med Chem Lett 22: 4281-7 (2012) Article DOI: 10.1016/j.bmcl.2012.05.024 BindingDB Entry DOI: 10.7270/Q2P2705D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50386952 (CHEMBL2048817) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA | Bioorg Med Chem Lett 22: 4281-7 (2012) Article DOI: 10.1016/j.bmcl.2012.05.024 BindingDB Entry DOI: 10.7270/Q2P2705D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50386948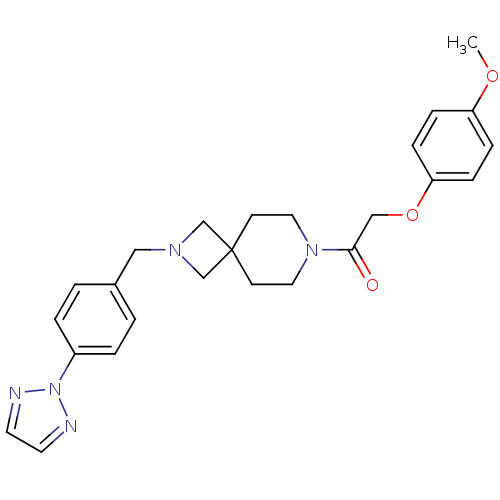 (CHEMBL2048813) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA | Bioorg Med Chem Lett 22: 4281-7 (2012) Article DOI: 10.1016/j.bmcl.2012.05.024 BindingDB Entry DOI: 10.7270/Q2P2705D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50128936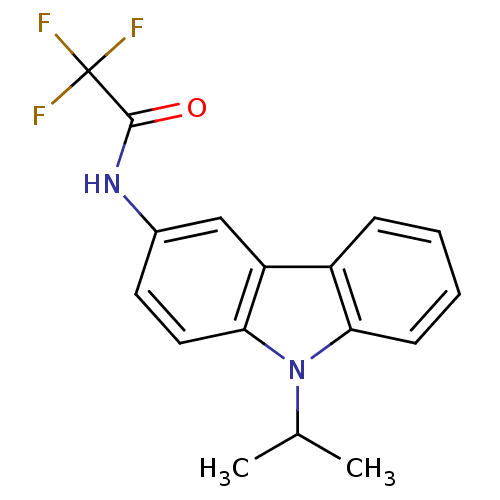 (2,2,2-Trifluoro-N-(9-isopropyl-9H-carbazol-3-yl)-a...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using 125[I]-PYY as radioligand | Bioorg Med Chem Lett 13: 1989-92 (2003) BindingDB Entry DOI: 10.7270/Q2057F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50128932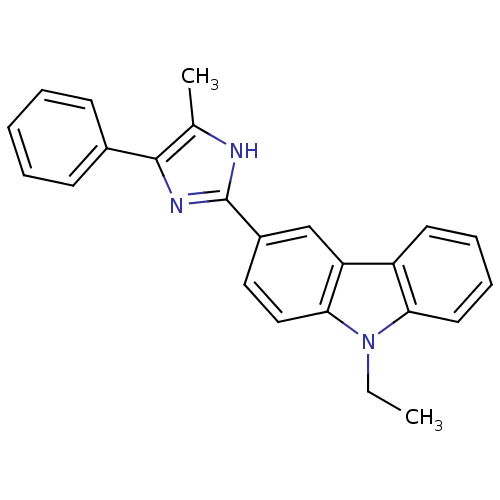 (9-Ethyl-3-(4-methyl-5-phenyl-1H-imidazol-2-yl)-9H-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using 125[I]-PYY as radioligand | Bioorg Med Chem Lett 13: 1989-92 (2003) BindingDB Entry DOI: 10.7270/Q2057F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50128925 (9-Ethyl-3-(1H-imidazo[4,5-c]pyridin-2-yl)-9H-carba...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using 125[I]-PYY as radioligand | Bioorg Med Chem Lett 13: 1989-92 (2003) BindingDB Entry DOI: 10.7270/Q2057F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50133782 (3-[5-(4-Chloro-phenyl)-1H-imidazol-2-yl]-pyridine ...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using [125I]-PYY as radiogigand in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 13: 3593-6 (2003) BindingDB Entry DOI: 10.7270/Q2DF6QKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50128942 (CHEMBL59866 | N-(5-Isopropyl-6-oxo-5,6-dihydro-phe...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using 125[I]-PYY as radioligand | Bioorg Med Chem Lett 13: 1989-92 (2003) BindingDB Entry DOI: 10.7270/Q2057F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 146 total ) | Next | Last >> |