

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor XI (Homo sapiens (Human)) | BDBM320260 (US10174020, Compound 8-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SICHUAN HAISCO PHARMACEUTICAL CO., LTD. US Patent | Assay Description The following method was used to test the in vitro inhibitory effect of the compounds of the present invention on the activity of human blood-coagula... | US Patent US10174020 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50277511 (3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human NK3 receptor | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50277511 (3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human NK2 receptor | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50386351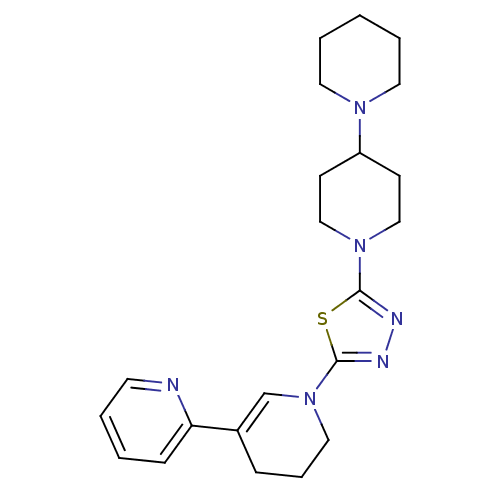 (CHEMBL2048589) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50386346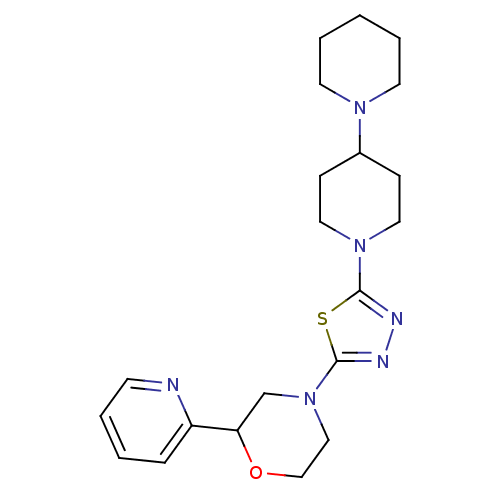 (CHEMBL2048594) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50386346 (CHEMBL2048594) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50386364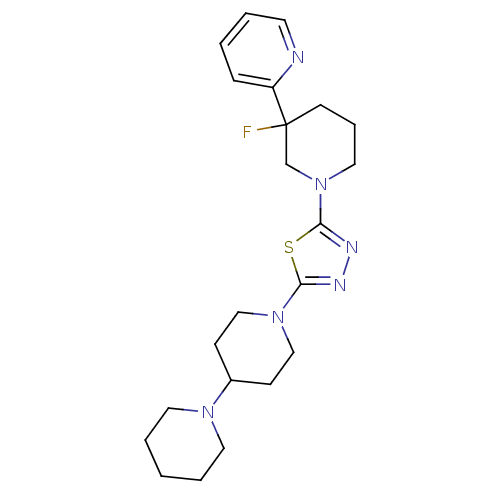 (CHEMBL2048592) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50386346 (CHEMBL2048594) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50386346 (CHEMBL2048594) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM320262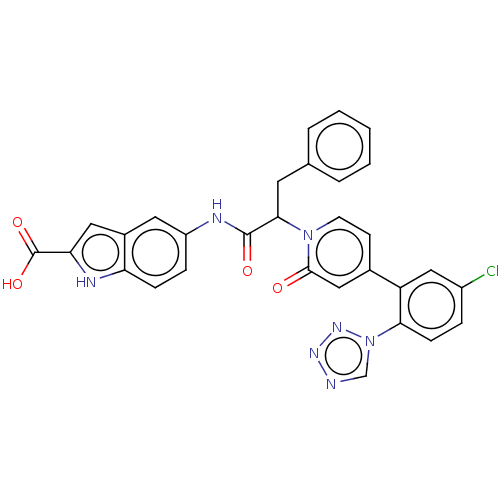 (5-[[2-[4-[5-chloro-2-(tetrazol-1-yl)phenyl]-2-oxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SICHUAN HAISCO PHARMACEUTICAL CO., LTD. US Patent | Assay Description The following method was used to test the in vitro inhibitory effect of the compounds of the present invention on the activity of human blood-coagula... | US Patent US10174020 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50386351 (CHEMBL2048589) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50432672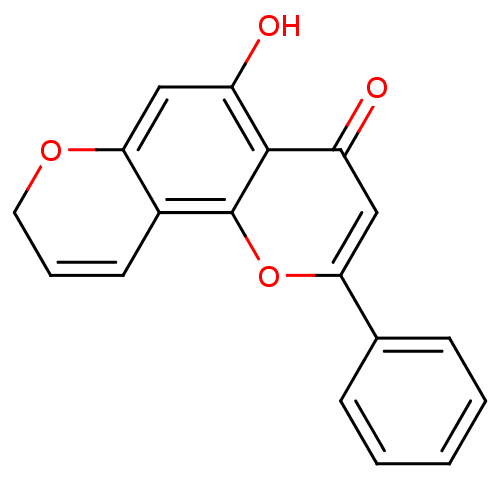 (CHEMBL2347912) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP1B1-dependent ethoxyresorufin-O-deethylase activity by spectrofluorimetric analysis in presence of NADPH regenerati... | J Med Chem 58: 6481-93 (2015) Article DOI: 10.1021/acs.jmedchem.5b00494 BindingDB Entry DOI: 10.7270/Q2183895 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50386364 (CHEMBL2048592) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50386347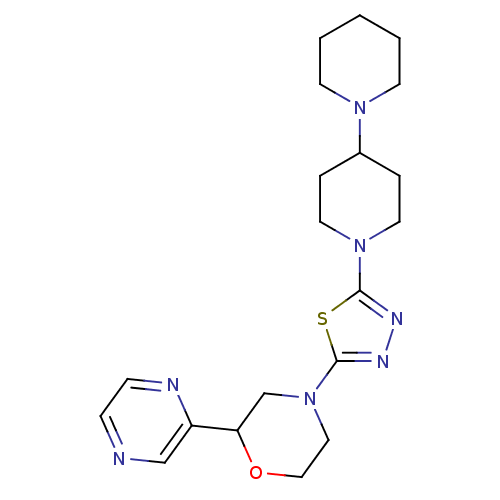 (CHEMBL2048595) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM320255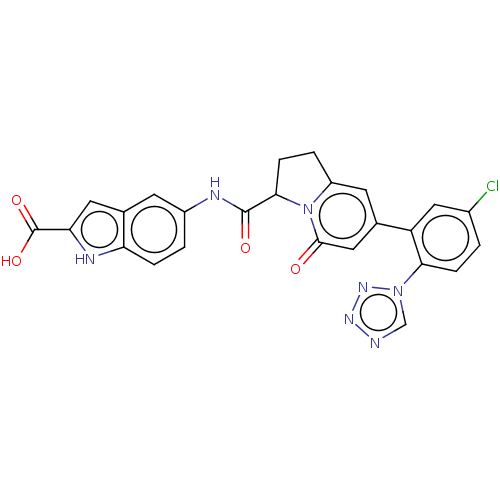 (N-(2-carboxyindol-5-yl)-7-(5-chloro-2-(1H-tetrazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SICHUAN HAISCO PHARMACEUTICAL CO., LTD. US Patent | Assay Description The following method was used to test the in vitro inhibitory effect of the compounds of the present invention on the activity of human blood-coagula... | US Patent US10174020 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50386350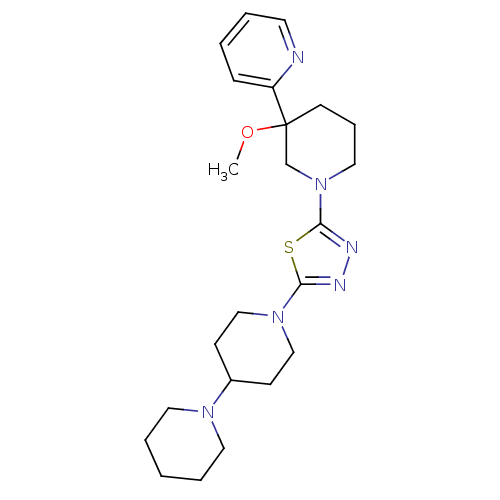 (CHEMBL2048590) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50386353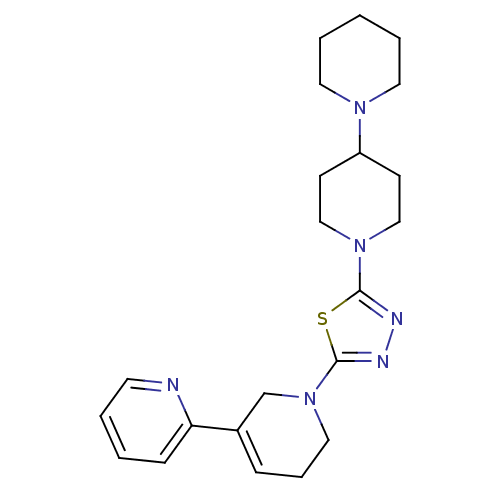 (CHEMBL2048587) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM320258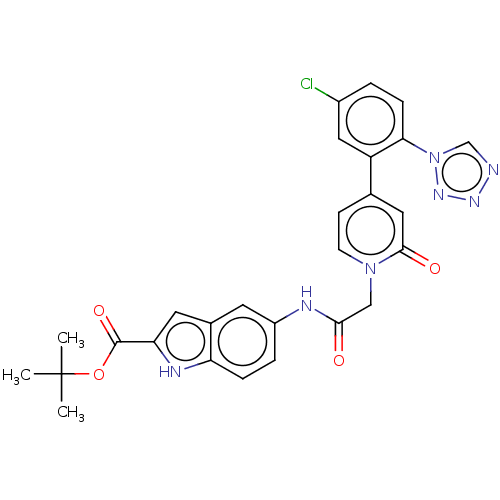 (N-(2-carboxyindol-5-yl)-2-(4-(5-chloro-2-(1H-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SICHUAN HAISCO PHARMACEUTICAL CO., LTD. US Patent | Assay Description The following method was used to test the in vitro inhibitory effect of the compounds of the present invention on the activity of human blood-coagula... | US Patent US10174020 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50432672 (CHEMBL2347912) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP1A2-dependent methoxyresorufin-O-demethylase activity by spectrofluorimetric analysis in presence of NADPH regenera... | J Med Chem 58: 6481-93 (2015) Article DOI: 10.1021/acs.jmedchem.5b00494 BindingDB Entry DOI: 10.7270/Q2183895 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50386352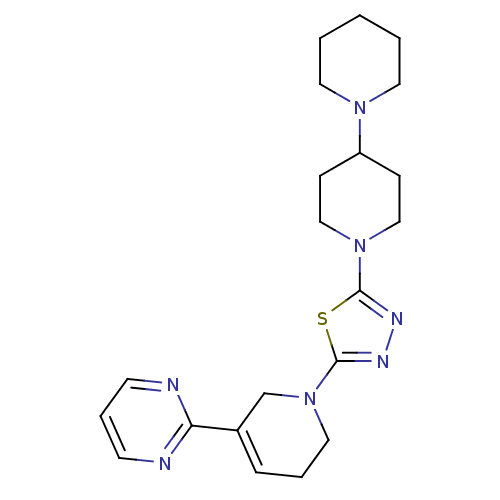 (CHEMBL2048588) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50386359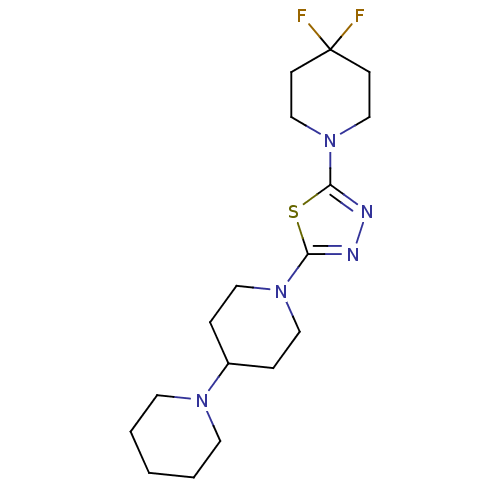 (CHEMBL2048581) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50014323 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP1B1-dependent ethoxyresorufin-O-deethylase activity by spectrofluorimetric analysis in presence of NADPH regenerati... | J Med Chem 58: 6481-93 (2015) Article DOI: 10.1021/acs.jmedchem.5b00494 BindingDB Entry DOI: 10.7270/Q2183895 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50386347 (CHEMBL2048595) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM320256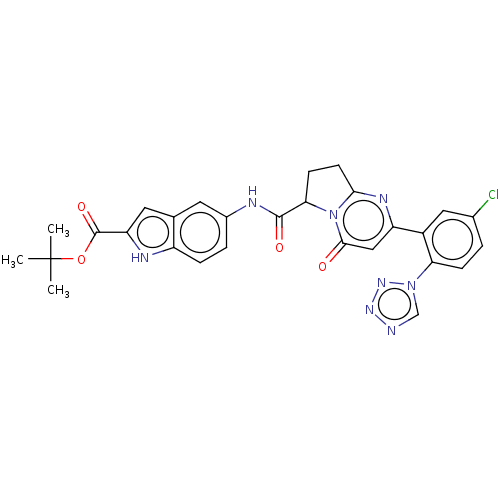 (N-(2-carboxyindol-5-yl)-2-(5-chloro-2-(1H-tetrazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SICHUAN HAISCO PHARMACEUTICAL CO., LTD. US Patent | Assay Description The following method was used to test the in vitro inhibitory effect of the compounds of the present invention on the activity of human blood-coagula... | US Patent US10174020 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50386359 (CHEMBL2048581) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50014323 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP1A2-dependent methoxyresorufin-O-demethylase activity by spectrofluorimetric analysis in presence of NADPH regenera... | J Med Chem 58: 6481-93 (2015) Article DOI: 10.1021/acs.jmedchem.5b00494 BindingDB Entry DOI: 10.7270/Q2183895 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50386362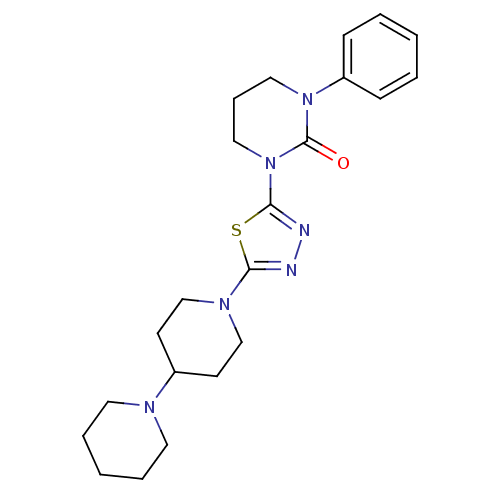 (CHEMBL2048412) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50113259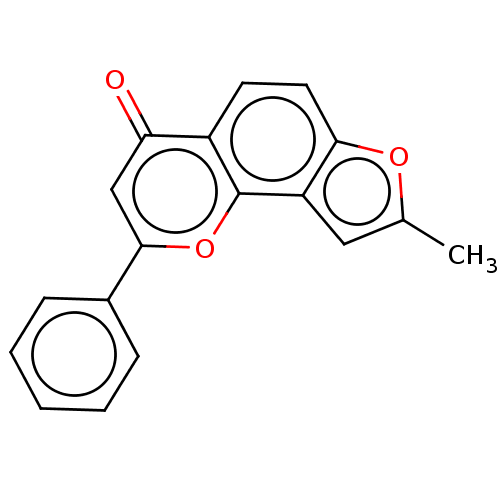 (CHEMBL3601433) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP1A2-dependent methoxyresorufin-O-demethylase activity by spectrofluorimetric analysis in presence of NADPH regenera... | J Med Chem 58: 6481-93 (2015) Article DOI: 10.1021/acs.jmedchem.5b00494 BindingDB Entry DOI: 10.7270/Q2183895 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50386362 (CHEMBL2048412) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50386363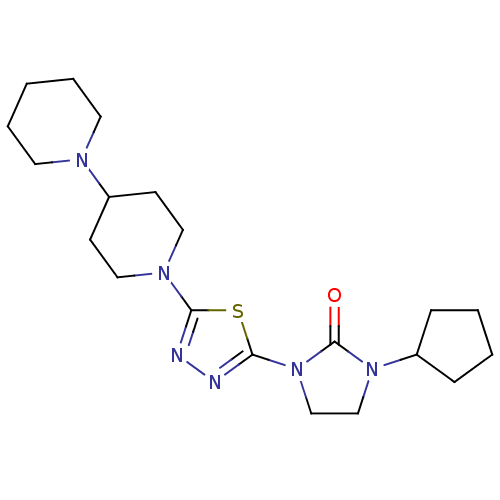 (CHEMBL2048411) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50386346 (CHEMBL2048594) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50386354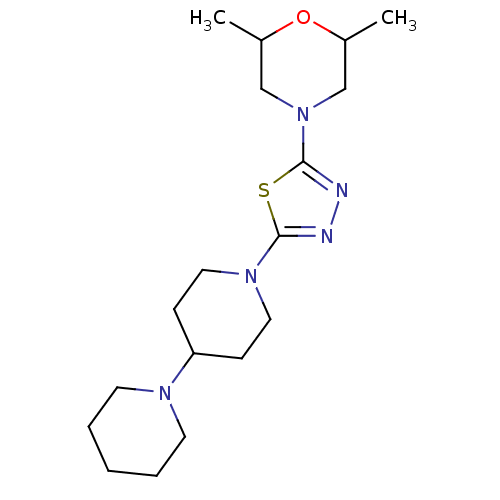 (CHEMBL2048586) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50386357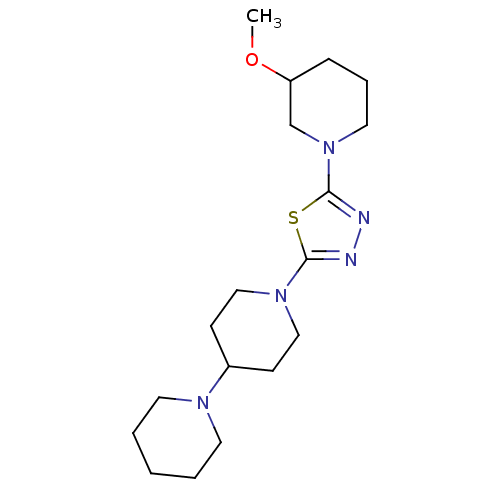 (CHEMBL2048583) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50113261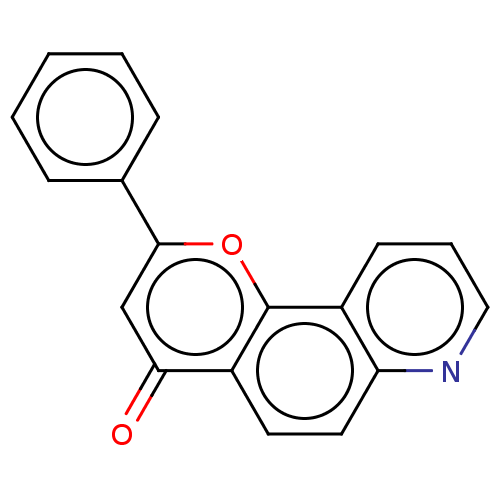 (CHEMBL3601435) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP1A1-dependent ethoxyresorufin-O-deethylase activity by spectrofluorimetric analysis in presence of NADPH regenerati... | J Med Chem 58: 6481-93 (2015) Article DOI: 10.1021/acs.jmedchem.5b00494 BindingDB Entry DOI: 10.7270/Q2183895 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50113260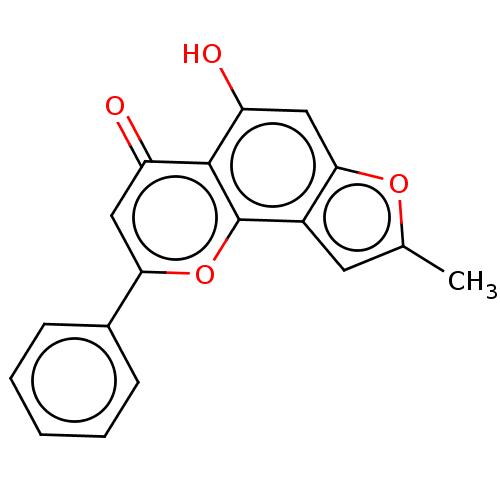 (CHEMBL3601434) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP1A2-dependent methoxyresorufin-O-demethylase activity by spectrofluorimetric analysis in presence of NADPH regenera... | J Med Chem 58: 6481-93 (2015) Article DOI: 10.1021/acs.jmedchem.5b00494 BindingDB Entry DOI: 10.7270/Q2183895 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50386352 (CHEMBL2048588) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50014323 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP1A1-dependent ethoxyresorufin-O-deethylase activity by spectrofluorimetric analysis in presence of NADPH regenerati... | J Med Chem 58: 6481-93 (2015) Article DOI: 10.1021/acs.jmedchem.5b00494 BindingDB Entry DOI: 10.7270/Q2183895 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50386357 (CHEMBL2048583) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50386356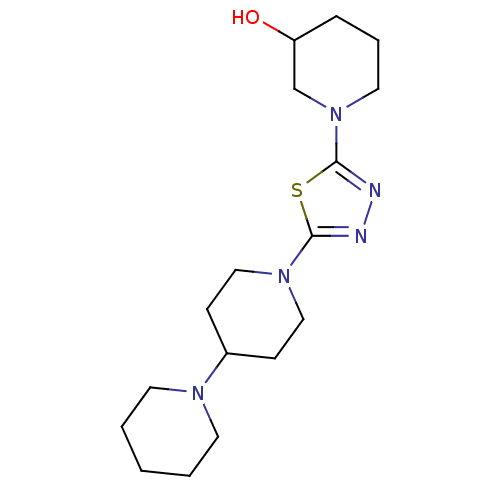 (CHEMBL2048584) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50333504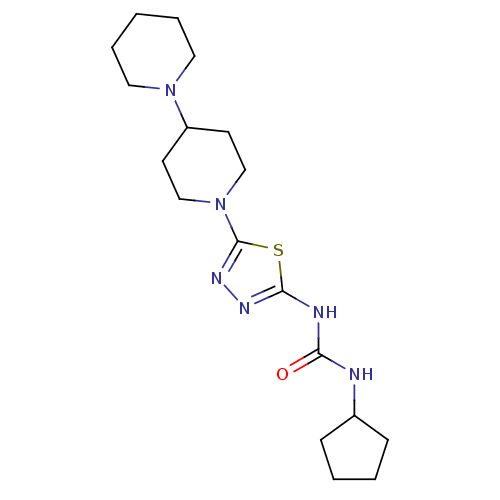 (1-(5-(1,4'-bipiperidin-1'-yl)-1,3,4-thiadiazol-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50386353 (CHEMBL2048587) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50333504 (1-(5-(1,4'-bipiperidin-1'-yl)-1,3,4-thiadiazol-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50432677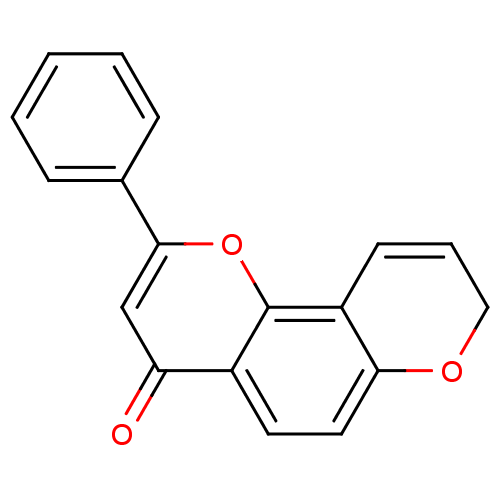 (CHEMBL2347756) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP1B1-dependent ethoxyresorufin-O-deethylase activity by spectrofluorimetric analysis in presence of NADPH regenerati... | J Med Chem 58: 6481-93 (2015) Article DOI: 10.1021/acs.jmedchem.5b00494 BindingDB Entry DOI: 10.7270/Q2183895 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50386363 (CHEMBL2048411) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50386354 (CHEMBL2048586) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50386349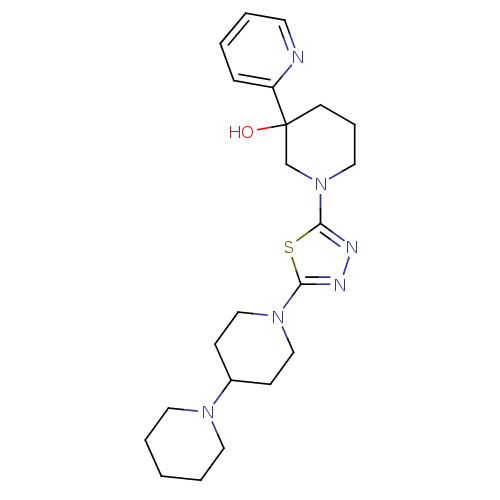 (CHEMBL2048591) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50386350 (CHEMBL2048590) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50386349 (CHEMBL2048591) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50386355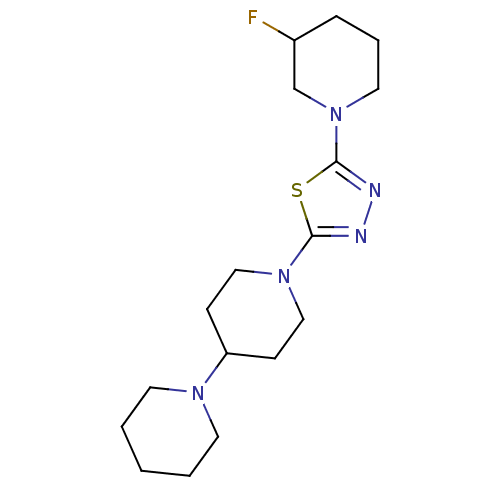 (CHEMBL2048585) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50432677 (CHEMBL2347756) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP1A2-dependent methoxyresorufin-O-demethylase activity by spectrofluorimetric analysis in presence of NADPH regenera... | J Med Chem 58: 6481-93 (2015) Article DOI: 10.1021/acs.jmedchem.5b00494 BindingDB Entry DOI: 10.7270/Q2183895 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1976 total ) | Next | Last >> |