 Found 169 hits with Last Name = 'britt' and Initial = 'sd'
Found 169 hits with Last Name = 'britt' and Initial = 'sd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50275934
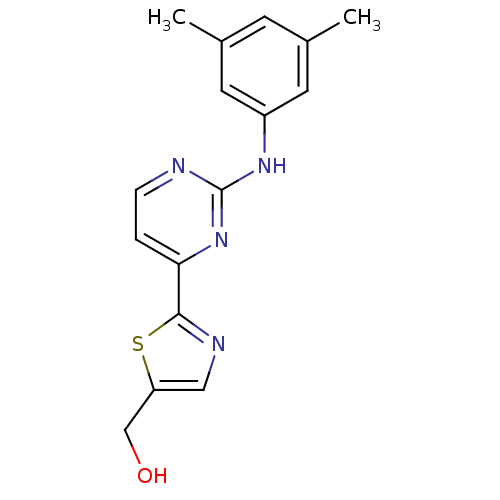
((2-(2-(3,5-dimethylphenylamino)pyrimidin-4-yl)thia...)Show InChI InChI=1S/C16H16N4OS/c1-10-5-11(2)7-12(6-10)19-16-17-4-3-14(20-16)15-18-8-13(9-21)22-15/h3-8,21H,9H2,1-2H3,(H,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50276015
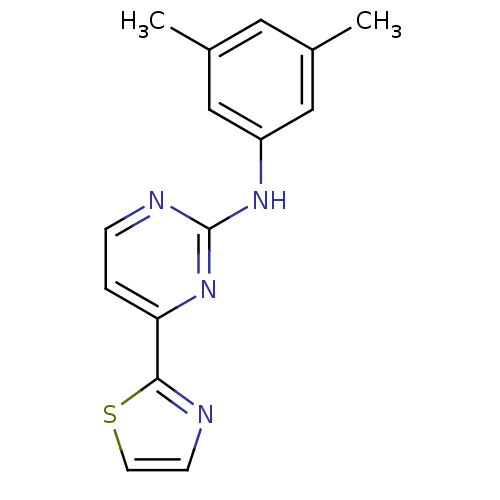
(CHEMBL509161 | N-(3,5-dimethylphenyl)-4-(thiazol-2...)Show InChI InChI=1S/C15H14N4S/c1-10-7-11(2)9-12(8-10)18-15-17-4-3-13(19-15)14-16-5-6-20-14/h3-9H,1-2H3,(H,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50275935
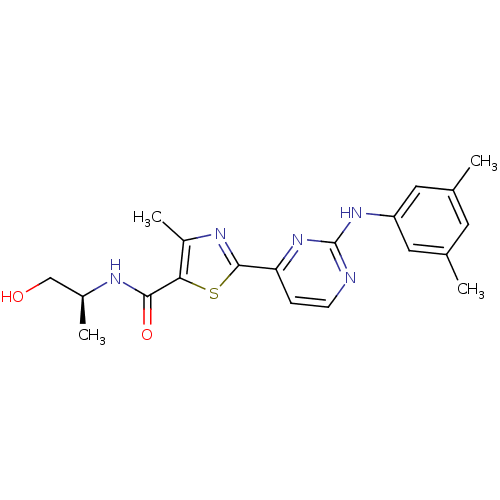
((S)-2-(2-(3,5-dimethylphenylamino)pyrimidin-4-yl)-...)Show SMILES C[C@@H](CO)NC(=O)c1sc(nc1C)-c1ccnc(Nc2cc(C)cc(C)c2)n1 |r| Show InChI InChI=1S/C20H23N5O2S/c1-11-7-12(2)9-15(8-11)24-20-21-6-5-16(25-20)19-23-14(4)17(28-19)18(27)22-13(3)10-26/h5-9,13,26H,10H2,1-4H3,(H,22,27)(H,21,24,25)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50275358
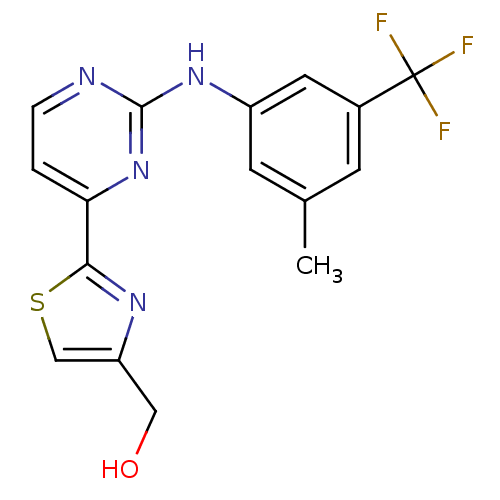
((2-(2-(3-methyl-5-(trifluoromethyl)phenylamino)pyr...)Show SMILES Cc1cc(Nc2nccc(n2)-c2nc(CO)cs2)cc(c1)C(F)(F)F Show InChI InChI=1S/C16H13F3N4OS/c1-9-4-10(16(17,18)19)6-11(5-9)22-15-20-3-2-13(23-15)14-21-12(7-24)8-25-14/h2-6,8,24H,7H2,1H3,(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50276065
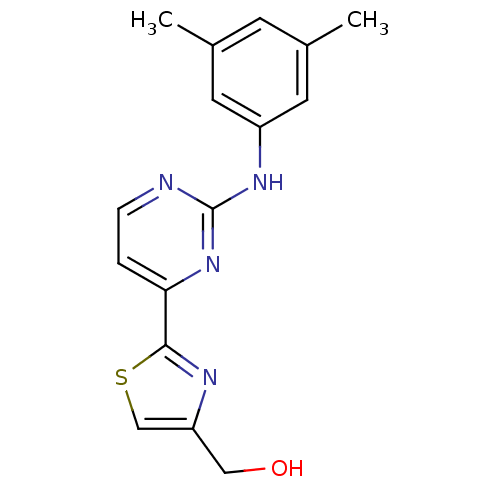
((2-(2-(3,5-dimethylphenylamino)pyrimidin-4-yl)thia...)Show InChI InChI=1S/C16H16N4OS/c1-10-5-11(2)7-12(6-10)19-16-17-4-3-14(20-16)15-18-13(8-21)9-22-15/h3-7,9,21H,8H2,1-2H3,(H,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50276018
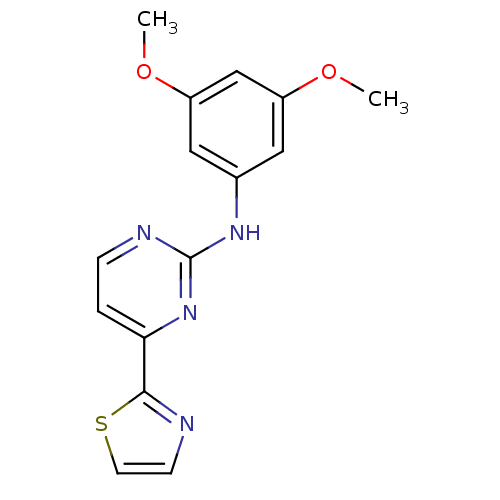
(1-(3-(cyclopenta-1,3-dienyl)benzyl)-3,5-diethylben...)Show InChI InChI=1S/C15H14N4O2S/c1-20-11-7-10(8-12(9-11)21-2)18-15-17-4-3-13(19-15)14-16-5-6-22-14/h3-9H,1-2H3,(H,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50249284
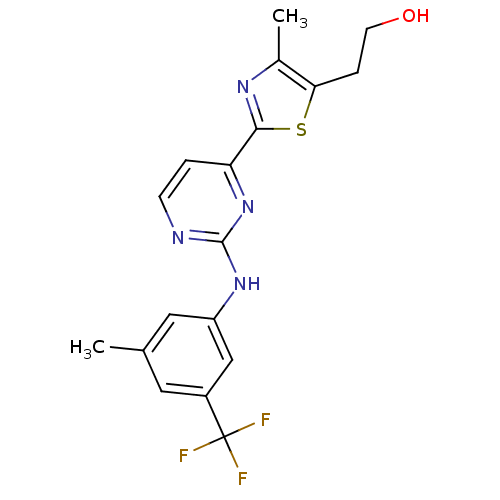
(2-(4-methyl-2-(2-(3-methyl-5-(trifluoromethyl)phen...)Show SMILES Cc1nc(sc1CCO)-c1ccnc(Nc2cc(C)cc(c2)C(F)(F)F)n1 Show InChI InChI=1S/C18H17F3N4OS/c1-10-7-12(18(19,20)21)9-13(8-10)24-17-22-5-3-14(25-17)16-23-11(2)15(27-16)4-6-26/h3,5,7-9,26H,4,6H2,1-2H3,(H,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50276064
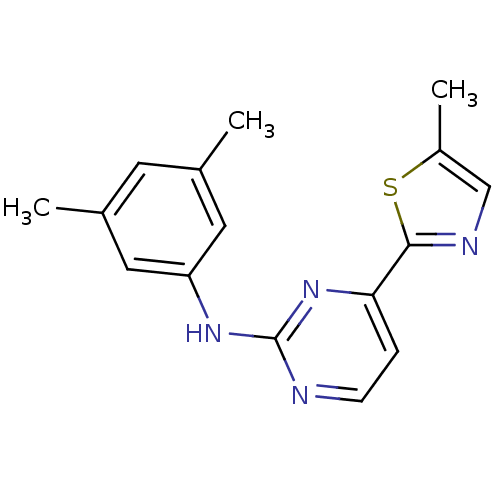
(CHEMBL470780 | N-(3,5-dimethylphenyl)-4-(5-methylt...)Show InChI InChI=1S/C16H16N4S/c1-10-6-11(2)8-13(7-10)19-16-17-5-4-14(20-16)15-18-9-12(3)21-15/h4-9H,1-3H3,(H,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50275357
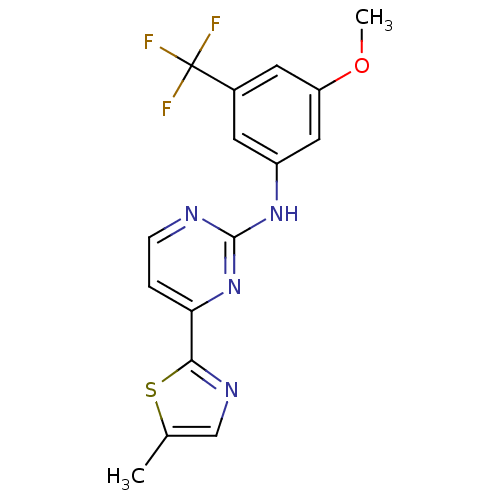
(CHEMBL451450 | N-(3-methoxy-5-(trifluoromethyl)phe...)Show SMILES COc1cc(Nc2nccc(n2)-c2ncc(C)s2)cc(c1)C(F)(F)F Show InChI InChI=1S/C16H13F3N4OS/c1-9-8-21-14(25-9)13-3-4-20-15(23-13)22-11-5-10(16(17,18)19)6-12(7-11)24-2/h3-8H,1-2H3,(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50249286
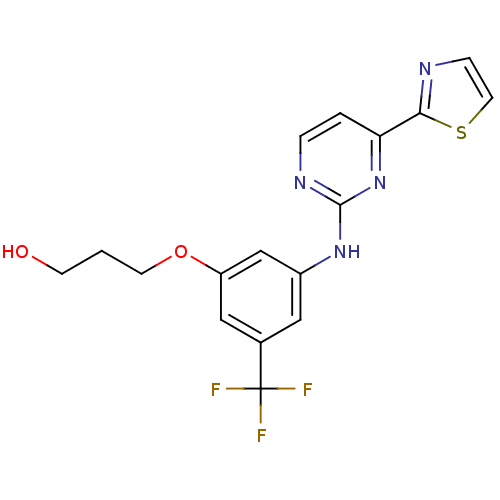
(3-(3-(4-(thiazol-2-yl)pyrimidin-2-ylamino)-5-(trif...)Show SMILES OCCCOc1cc(Nc2nccc(n2)-c2nccs2)cc(c1)C(F)(F)F Show InChI InChI=1S/C17H15F3N4O2S/c18-17(19,20)11-8-12(10-13(9-11)26-6-1-5-25)23-16-22-3-2-14(24-16)15-21-4-7-27-15/h2-4,7-10,25H,1,5-6H2,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50276016
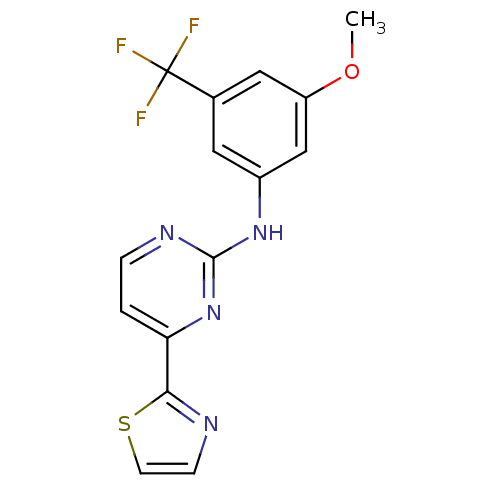
(CHEMBL512319 | N-(3-methoxy-5-(trifluoromethyl)phe...)Show InChI InChI=1S/C15H11F3N4OS/c1-23-11-7-9(15(16,17)18)6-10(8-11)21-14-20-3-2-12(22-14)13-19-4-5-24-13/h2-8H,1H3,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50249318
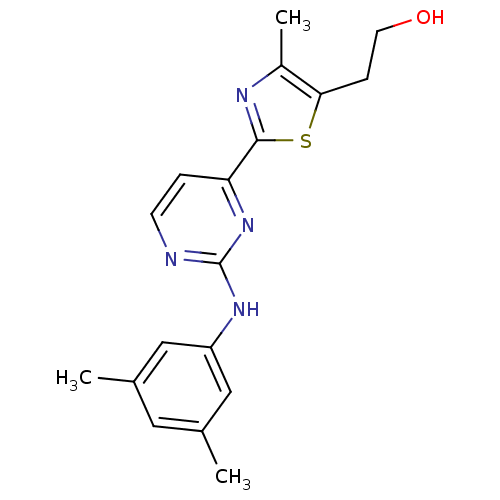
(2-(2-(2-(3,5-dimethylphenylamino)pyrimidin-4-yl)-4...)Show InChI InChI=1S/C18H20N4OS/c1-11-8-12(2)10-14(9-11)21-18-19-6-4-15(22-18)17-20-13(3)16(24-17)5-7-23/h4,6,8-10,23H,5,7H2,1-3H3,(H,19,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50275355
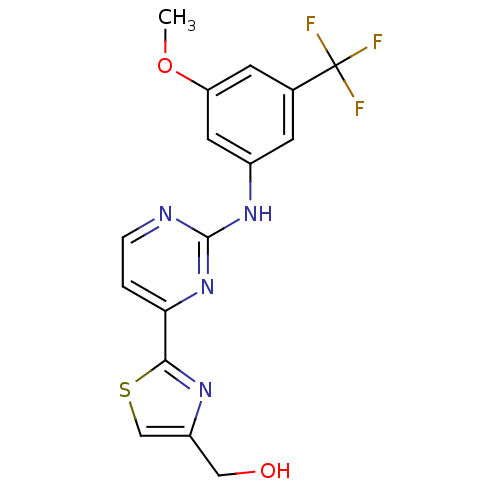
((2-(2-(3-methoxy-5-(trifluoromethyl)phenylamino)py...)Show SMILES COc1cc(Nc2nccc(n2)-c2nc(CO)cs2)cc(c1)C(F)(F)F Show InChI InChI=1S/C16H13F3N4O2S/c1-25-12-5-9(16(17,18)19)4-10(6-12)22-15-20-3-2-13(23-15)14-21-11(7-24)8-26-14/h2-6,8,24H,7H2,1H3,(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50276017
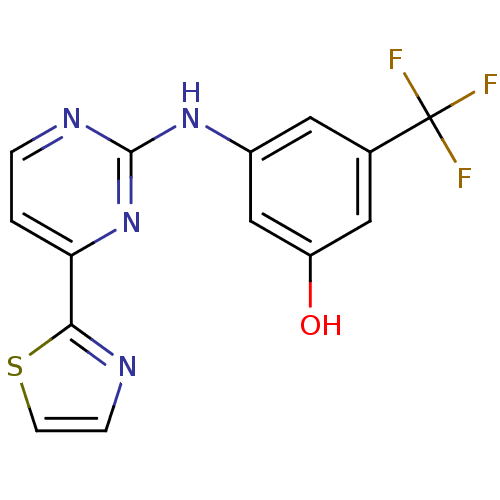
(3-(4-(thiazol-2-yl)pyrimidin-2-ylamino)-5-(trifluo...)Show InChI InChI=1S/C14H9F3N4OS/c15-14(16,17)8-5-9(7-10(22)6-8)20-13-19-2-1-11(21-13)12-18-3-4-23-12/h1-7,22H,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50275356
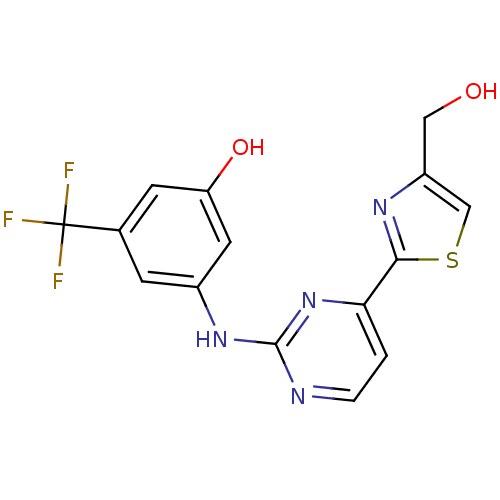
(3-(4-(4-(hydroxymethyl)thiazol-2-yl)pyrimidin-2-yl...)Show SMILES OCc1csc(n1)-c1ccnc(Nc2cc(O)cc(c2)C(F)(F)F)n1 Show InChI InChI=1S/C15H11F3N4O2S/c16-15(17,18)8-3-9(5-11(24)4-8)21-14-19-2-1-12(22-14)13-20-10(6-23)7-25-13/h1-5,7,23-24H,6H2,(H,19,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50249356
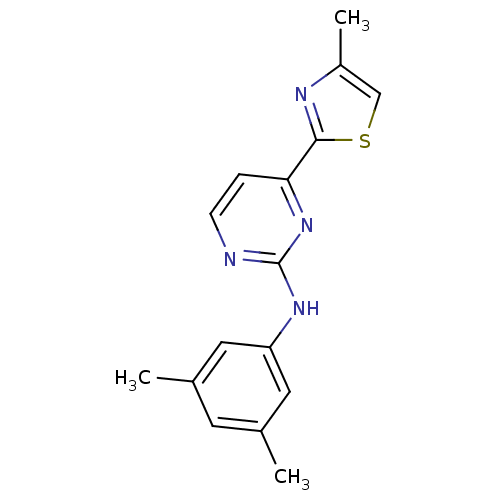
(CHEMBL453217 | N-(3,5-dimethylphenyl)-4-(4-methylt...)Show InChI InChI=1S/C16H16N4S/c1-10-6-11(2)8-13(7-10)19-16-17-5-4-14(20-16)15-18-12(3)9-21-15/h4-9H,1-3H3,(H,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50276063
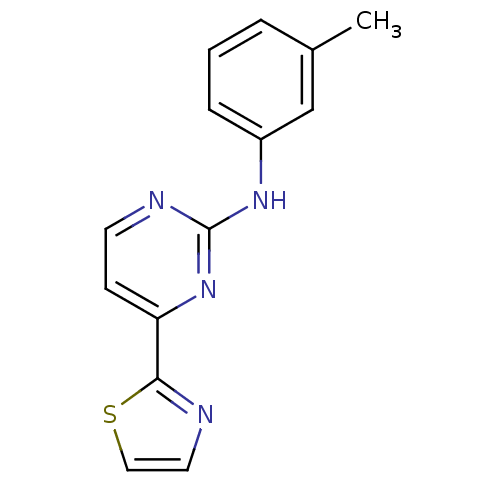
(4-(thiazol-2-yl)-N-m-tolylpyrimidin-2-amine | CHEM...)Show InChI InChI=1S/C14H12N4S/c1-10-3-2-4-11(9-10)17-14-16-6-5-12(18-14)13-15-7-8-19-13/h2-9H,1H3,(H,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50249356

(CHEMBL453217 | N-(3,5-dimethylphenyl)-4-(4-methylt...)Show InChI InChI=1S/C16H16N4S/c1-10-6-11(2)8-13(7-10)19-16-17-5-4-14(20-16)15-18-12(3)9-21-15/h4-9H,1-3H3,(H,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50135430

(CHEMBL434033 | Naphthalene-2-carboxylic acid (3R,5...)Show SMILES CC[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@@H](NC(=O)[C@H](NC(=O)CCCCC(O)=O)C(C)C)C(C)C)OC(=O)c1ccc2ccccc2c1)C=O Show InChI InChI=1S/C36H48N4O9/c1-6-26(20-41)37-33(45)28-18-27(49-36(48)25-16-15-23-11-7-8-12-24(23)17-25)19-40(28)35(47)32(22(4)5)39-34(46)31(21(2)3)38-29(42)13-9-10-14-30(43)44/h7-8,11-12,15-17,20-22,26-28,31-32H,6,9-10,13-14,18-19H2,1-5H3,(H,37,45)(H,38,42)(H,39,46)(H,43,44)/t26-,27+,28-,31+,32-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HCV (Hepatitis C Virus) NS3-4A protease. |
Bioorg Med Chem Lett 13: 4059-63 (2003)
BindingDB Entry DOI: 10.7270/Q2028QX4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50249416
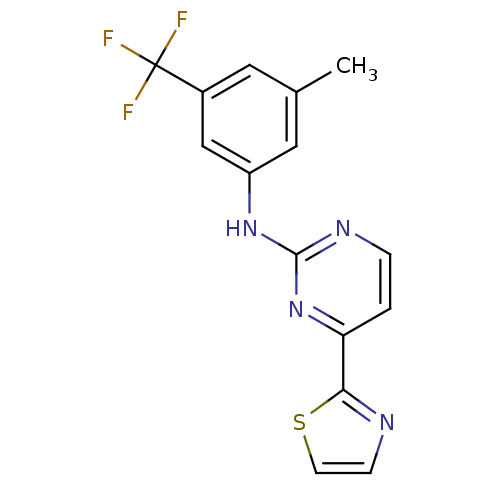
(CHEMBL471776 | N-(3-methyl-5-(trifluoromethyl)phen...)Show InChI InChI=1S/C15H11F3N4S/c1-9-6-10(15(16,17)18)8-11(7-9)21-14-20-3-2-12(22-14)13-19-4-5-23-13/h2-8H,1H3,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50249258
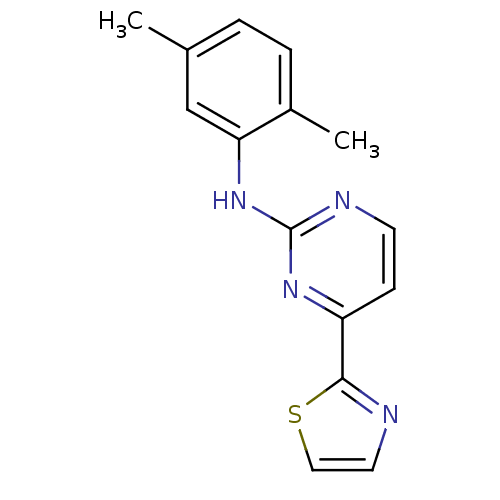
(CHEMBL475570 | N-(2,5-dimethylphenyl)-4-(thiazol-2...)Show InChI InChI=1S/C15H14N4S/c1-10-3-4-11(2)13(9-10)19-15-17-6-5-12(18-15)14-16-7-8-20-14/h3-9H,1-2H3,(H,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 153 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SRC (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50141199
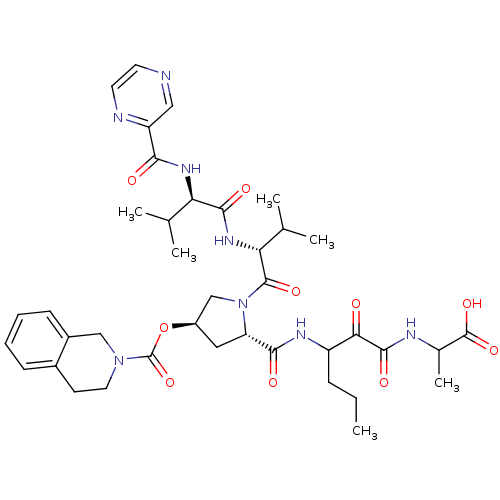
(3,4-Dihydro-1H-isoquinoline-2-carboxylic acid 5-[(...)Show SMILES CCCC(NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OC(=O)N1CCc2ccccc2C1)C(=O)C(=O)NC(C)C(O)=O Show InChI InChI=1S/C39H52N8O10/c1-7-10-27(32(48)36(52)42-23(6)38(54)55)43-34(50)29-17-26(57-39(56)46-16-13-24-11-8-9-12-25(24)19-46)20-47(29)37(53)31(22(4)5)45-35(51)30(21(2)3)44-33(49)28-18-40-14-15-41-28/h8-9,11-12,14-15,18,21-23,26-27,29-31H,7,10,13,16-17,19-20H2,1-6H3,(H,42,52)(H,43,50)(H,44,49)(H,45,51)(H,54,55)/t23?,26-,27?,29+,30-,31-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding inhibition of hepatitis C virus NS3.4A protease 2 using p-nitroaniline assay (pNA); <0.2 (0.18) |
Bioorg Med Chem Lett 14: 1441-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.022
BindingDB Entry DOI: 10.7270/Q24Q7TD2 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50141198
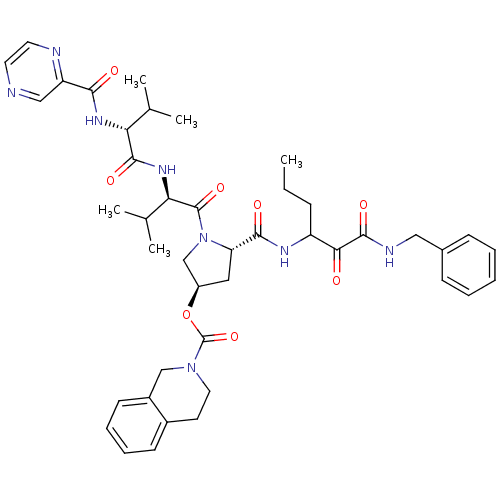
(3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...)Show SMILES CCCC(NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OC(=O)N1CCc2ccccc2C1)C(=O)C(=O)NCc1ccccc1 Show InChI InChI=1S/C43H54N8O8/c1-6-12-32(37(52)41(56)46-22-28-13-8-7-9-14-28)47-39(54)34-21-31(59-43(58)50-20-17-29-15-10-11-16-30(29)24-50)25-51(34)42(57)36(27(4)5)49-40(55)35(26(2)3)48-38(53)33-23-44-18-19-45-33/h7-11,13-16,18-19,23,26-27,31-32,34-36H,6,12,17,20-22,24-25H2,1-5H3,(H,46,56)(H,47,54)(H,48,53)(H,49,55)/t31-,32?,34+,35-,36-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding inhibition of hepatitis C virus NS3.4A protease 2 using p-nitroaniline assay (pNA); <0.2 (0.15) |
Bioorg Med Chem Lett 14: 1441-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.022
BindingDB Entry DOI: 10.7270/Q24Q7TD2 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50141196
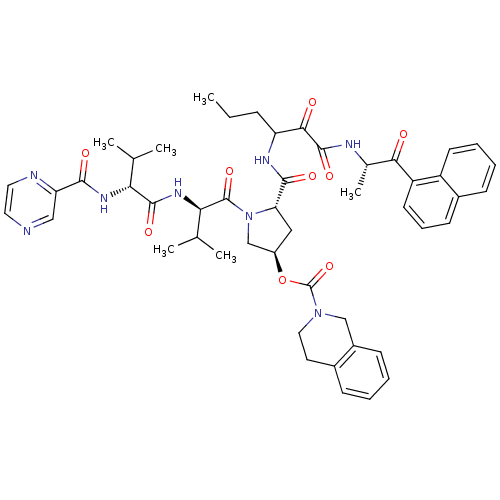
(1-[3-methyl-2-[2-methyl-1-(2-pyrazinylcarboxamido)...)Show SMILES CCCC(NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OC(=O)N1CCc2ccccc2C1)C(=O)C(=O)N[C@@H](C)C(=O)c1cccc2ccccc12 Show InChI InChI=1S/C49H58N8O9/c1-7-13-37(43(59)47(63)52-30(6)42(58)36-19-12-17-32-15-10-11-18-35(32)36)53-45(61)39-24-34(66-49(65)56-23-20-31-14-8-9-16-33(31)26-56)27-57(39)48(64)41(29(4)5)55-46(62)40(28(2)3)54-44(60)38-25-50-21-22-51-38/h8-12,14-19,21-22,25,28-30,34,37,39-41H,7,13,20,23-24,26-27H2,1-6H3,(H,52,63)(H,53,61)(H,54,60)(H,55,62)/t30-,34+,37?,39-,40+,41+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding inhibition of hepatitis C virus NS3.4A protease 2 using p-nitroaniline assay (pNA); <0.2 (0.18) |
Bioorg Med Chem Lett 14: 1441-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.022
BindingDB Entry DOI: 10.7270/Q24Q7TD2 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50141210
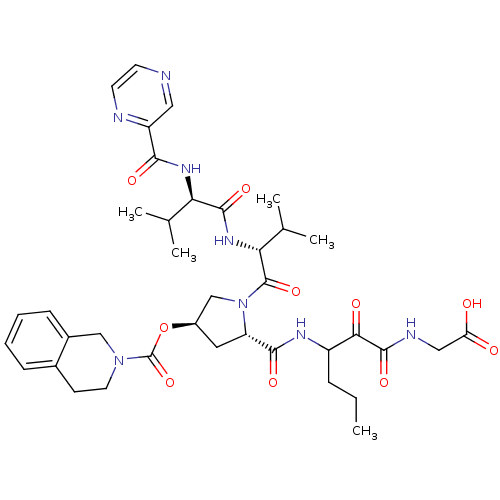
(3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...)Show SMILES CCCC(NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OC(=O)N1CCc2ccccc2C1)C(=O)C(=O)NCC(O)=O Show InChI InChI=1S/C38H50N8O10/c1-6-9-26(32(49)36(53)41-18-29(47)48)42-34(51)28-16-25(56-38(55)45-15-12-23-10-7-8-11-24(23)19-45)20-46(28)37(54)31(22(4)5)44-35(52)30(21(2)3)43-33(50)27-17-39-13-14-40-27/h7-8,10-11,13-14,17,21-22,25-26,28,30-31H,6,9,12,15-16,18-20H2,1-5H3,(H,41,53)(H,42,51)(H,43,50)(H,44,52)(H,47,48)/t25-,26?,28+,30-,31-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding inhibition of hepatitis C virus NS3.4A protease 2 using p-nitroaniline assay (pNA); <0.2 (0.15) |
Bioorg Med Chem Lett 14: 1441-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.022
BindingDB Entry DOI: 10.7270/Q24Q7TD2 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50141206

(3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...)Show SMILES CCCC(NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OC(=O)N1CCc2ccccc2C1)C(=O)C(=O)N[C@@H](C)c1ccccc1 Show InChI InChI=1S/C44H56N8O8/c1-7-13-33(38(53)42(57)47-28(6)29-14-9-8-10-15-29)48-40(55)35-22-32(60-44(59)51-21-18-30-16-11-12-17-31(30)24-51)25-52(35)43(58)37(27(4)5)50-41(56)36(26(2)3)49-39(54)34-23-45-19-20-46-34/h8-12,14-17,19-20,23,26-28,32-33,35-37H,7,13,18,21-22,24-25H2,1-6H3,(H,47,57)(H,48,55)(H,49,54)(H,50,56)/t28-,32+,33?,35-,36+,37+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding inhibition of hepatitis C virus NS3.4A protease 2 using p-nitroaniline assay (pNA); <0.2 (0.10) |
Bioorg Med Chem Lett 14: 1441-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.022
BindingDB Entry DOI: 10.7270/Q24Q7TD2 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50141194

(3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...)Show SMILES CCCC(NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OC(=O)N1CCc2ccccc2C1)C(=O)C(=O)c1ccccc1 Show InChI InChI=1S/C42H51N7O8/c1-6-12-31(37(51)36(50)28-14-8-7-9-15-28)45-39(53)33-21-30(57-42(56)48-20-17-27-13-10-11-16-29(27)23-48)24-49(33)41(55)35(26(4)5)47-40(54)34(25(2)3)46-38(52)32-22-43-18-19-44-32/h7-11,13-16,18-19,22,25-26,30-31,33-35H,6,12,17,20-21,23-24H2,1-5H3,(H,45,53)(H,46,52)(H,47,54)/t30-,31?,33+,34-,35-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding inhibition of hepatitis C virus NS3.4A protease 2 using p-nitroaniline assay (pNA); <0.2 (0.03) |
Bioorg Med Chem Lett 14: 1441-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.022
BindingDB Entry DOI: 10.7270/Q24Q7TD2 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50141203

(3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...)Show SMILES CCCC(NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OC(=O)N1CCc2ccccc2C1)C(=O)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C44H56N8O8/c1-6-12-33(38(53)42(57)47-19-17-29-13-8-7-9-14-29)48-40(55)35-23-32(60-44(59)51-22-18-30-15-10-11-16-31(30)25-51)26-52(35)43(58)37(28(4)5)50-41(56)36(27(2)3)49-39(54)34-24-45-20-21-46-34/h7-11,13-16,20-21,24,27-28,32-33,35-37H,6,12,17-19,22-23,25-26H2,1-5H3,(H,47,57)(H,48,55)(H,49,54)(H,50,56)/t32-,33?,35+,36-,37-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding inhibition of hepatitis C virus NS3.4A protease 2 using p-nitroaniline assay (pNA) |
Bioorg Med Chem Lett 14: 1441-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.022
BindingDB Entry DOI: 10.7270/Q24Q7TD2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50249540
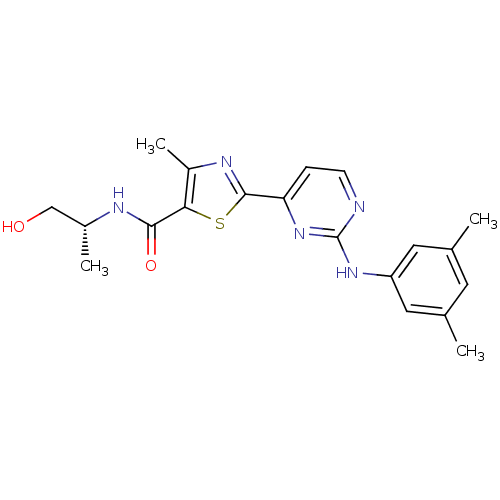
((R)-2-(2-(3,5-dimethylphenylamino)pyrimidin-4-yl)-...)Show SMILES C[C@H](CO)NC(=O)c1sc(nc1C)-c1ccnc(Nc2cc(C)cc(C)c2)n1 |r| Show InChI InChI=1S/C20H23N5O2S/c1-11-7-12(2)9-15(8-11)24-20-21-6-5-16(25-20)19-23-14(4)17(28-19)18(27)22-13(3)10-26/h5-9,13,26H,10H2,1-4H3,(H,22,27)(H,21,24,25)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50141202

(3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...)Show SMILES CCCC(NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OC(=O)N1CCc2ccccc2C1)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C39H52N8O8/c1-6-9-28(33(48)37(52)42-26-12-13-26)43-35(50)30-18-27(55-39(54)46-17-14-24-10-7-8-11-25(24)20-46)21-47(30)38(53)32(23(4)5)45-36(51)31(22(2)3)44-34(49)29-19-40-15-16-41-29/h7-8,10-11,15-16,19,22-23,26-28,30-32H,6,9,12-14,17-18,20-21H2,1-5H3,(H,42,52)(H,43,50)(H,44,49)(H,45,51)/t27-,28?,30+,31-,32-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding inhibition of hepatitis C virus NS3.4A protease 2 using p-nitroaniline assay (pNA); <0.2 (0.15) |
Bioorg Med Chem Lett 14: 1441-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.022
BindingDB Entry DOI: 10.7270/Q24Q7TD2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50275935

((S)-2-(2-(3,5-dimethylphenylamino)pyrimidin-4-yl)-...)Show SMILES C[C@@H](CO)NC(=O)c1sc(nc1C)-c1ccnc(Nc2cc(C)cc(C)c2)n1 |r| Show InChI InChI=1S/C20H23N5O2S/c1-11-7-12(2)9-15(8-11)24-20-21-6-5-16(25-20)19-23-14(4)17(28-19)18(27)22-13(3)10-26/h5-9,13,26H,10H2,1-4H3,(H,22,27)(H,21,24,25)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ZAP70 (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50275934

((2-(2-(3,5-dimethylphenylamino)pyrimidin-4-yl)thia...)Show InChI InChI=1S/C16H16N4OS/c1-10-5-11(2)7-12(6-10)19-16-17-4-3-14(20-16)15-18-8-13(9-21)22-15/h3-8,21H,9H2,1-2H3,(H,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ZAP70 (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50141186
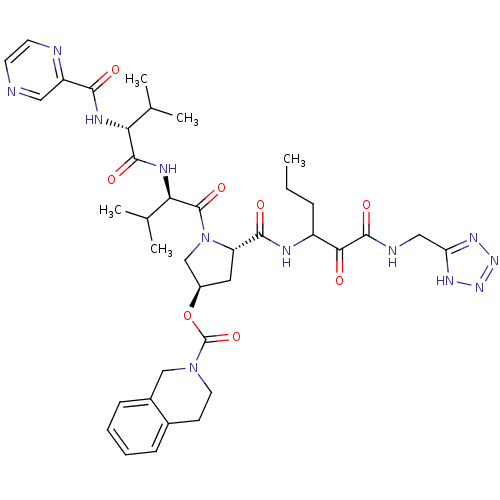
(3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...)Show SMILES CCCC(NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OC(=O)N1CCc2ccccc2C1)C(=O)C(=O)NCc1nnn[nH]1 Show InChI InChI=1S/C38H50N12O8/c1-6-9-26(32(51)36(55)41-18-29-45-47-48-46-29)42-34(53)28-16-25(58-38(57)49-15-12-23-10-7-8-11-24(23)19-49)20-50(28)37(56)31(22(4)5)44-35(54)30(21(2)3)43-33(52)27-17-39-13-14-40-27/h7-8,10-11,13-14,17,21-22,25-26,28,30-31H,6,9,12,15-16,18-20H2,1-5H3,(H,41,55)(H,42,53)(H,43,52)(H,44,54)(H,45,46,47,48)/t25-,26?,28+,30-,31-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding inhibition of hepatitis C virus NS3.4A protease 2 using p-nitroaniline assay (pNA) |
Bioorg Med Chem Lett 14: 1441-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.022
BindingDB Entry DOI: 10.7270/Q24Q7TD2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50276018

(1-(3-(cyclopenta-1,3-dienyl)benzyl)-3,5-diethylben...)Show InChI InChI=1S/C15H14N4O2S/c1-20-11-7-10(8-12(9-11)21-2)18-15-17-4-3-13(19-15)14-16-5-6-22-14/h3-9H,1-2H3,(H,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 318 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ZAP70 (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50276064

(CHEMBL470780 | N-(3,5-dimethylphenyl)-4-(5-methylt...)Show InChI InChI=1S/C16H16N4S/c1-10-6-11(2)8-13(7-10)19-16-17-5-4-14(20-16)15-18-9-12(3)21-15/h4-9H,1-3H3,(H,17,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 348 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SRC (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50135432

(CHEMBL340890 | Naphthalene-2-carboxylic acid (3R,5...)Show SMILES CC[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OC(=O)c1ccc2ccccc2c1)C=O Show InChI InChI=1S/C35H42N6O7/c1-6-25(19-42)38-32(44)28-16-26(48-35(47)24-12-11-22-9-7-8-10-23(22)15-24)18-41(28)34(46)30(21(4)5)40-33(45)29(20(2)3)39-31(43)27-17-36-13-14-37-27/h7-15,17,19-21,25-26,28-30H,6,16,18H2,1-5H3,(H,38,44)(H,39,43)(H,40,45)/t25-,26+,28-,29+,30+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HCV (Hepatitis C Virus) NS3-4A protease. |
Bioorg Med Chem Lett 13: 4059-63 (2003)
BindingDB Entry DOI: 10.7270/Q2028QX4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50276064

(CHEMBL470780 | N-(3,5-dimethylphenyl)-4-(5-methylt...)Show InChI InChI=1S/C16H16N4S/c1-10-6-11(2)8-13(7-10)19-16-17-5-4-14(20-16)15-18-9-12(3)21-15/h4-9H,1-3H3,(H,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ZAP70 (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50276015

(CHEMBL509161 | N-(3,5-dimethylphenyl)-4-(thiazol-2...)Show InChI InChI=1S/C15H14N4S/c1-10-7-11(2)9-12(8-10)18-15-17-4-3-13(19-15)14-16-5-6-20-14/h3-9H,1-2H3,(H,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 419 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ZAP70 (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50249286

(3-(3-(4-(thiazol-2-yl)pyrimidin-2-ylamino)-5-(trif...)Show SMILES OCCCOc1cc(Nc2nccc(n2)-c2nccs2)cc(c1)C(F)(F)F Show InChI InChI=1S/C17H15F3N4O2S/c18-17(19,20)11-8-12(10-13(9-11)26-6-1-5-25)23-16-22-3-2-14(24-16)15-21-4-7-27-15/h2-4,7-10,25H,1,5-6H2,(H,22,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 422 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SRC (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50141207
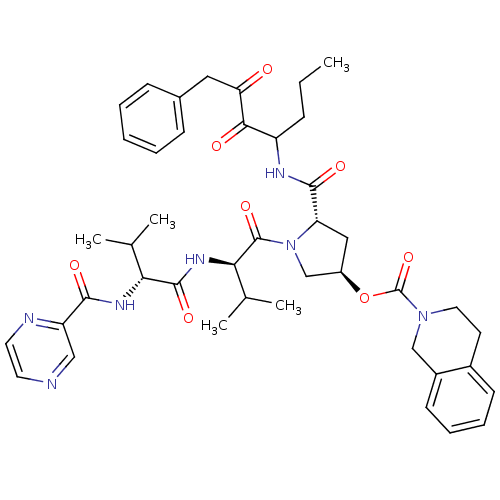
(3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...)Show SMILES CCCC(NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OC(=O)N1CCc2ccccc2C1)C(=O)C(=O)Cc1ccccc1 Show InChI InChI=1S/C43H53N7O8/c1-6-12-32(38(52)35(51)21-28-13-8-7-9-14-28)46-40(54)34-22-31(58-43(57)49-20-17-29-15-10-11-16-30(29)24-49)25-50(34)42(56)37(27(4)5)48-41(55)36(26(2)3)47-39(53)33-23-44-18-19-45-33/h7-11,13-16,18-19,23,26-27,31-32,34,36-37H,6,12,17,20-22,24-25H2,1-5H3,(H,46,54)(H,47,53)(H,48,55)/t31-,32?,34+,36-,37-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding inhibition of hepatitis C virus NS3.4A protease 2 using p-nitroaniline assay (pNA) |
Bioorg Med Chem Lett 14: 1441-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.022
BindingDB Entry DOI: 10.7270/Q24Q7TD2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50276065

((2-(2-(3,5-dimethylphenylamino)pyrimidin-4-yl)thia...)Show InChI InChI=1S/C16H16N4OS/c1-10-5-11(2)7-12(6-10)19-16-17-4-3-14(20-16)15-18-13(8-21)9-22-15/h3-7,9,21H,8H2,1-2H3,(H,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ZAP70 (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50141193

(3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...)Show SMILES CCCC(NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OC(=O)N1CCc2ccccc2C1)C(=O)C(=O)NC(C)C Show InChI InChI=1S/C39H54N8O8/c1-8-11-28(33(48)37(52)42-24(6)7)43-35(50)30-18-27(55-39(54)46-17-14-25-12-9-10-13-26(25)20-46)21-47(30)38(53)32(23(4)5)45-36(51)31(22(2)3)44-34(49)29-19-40-15-16-41-29/h9-10,12-13,15-16,19,22-24,27-28,30-32H,8,11,14,17-18,20-21H2,1-7H3,(H,42,52)(H,43,50)(H,44,49)(H,45,51)/t27-,28?,30+,31-,32-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding inhibition of hepatitis C virus NS3.4A protease 2 using p-nitroaniline assay (pNA) |
Bioorg Med Chem Lett 14: 1441-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.022
BindingDB Entry DOI: 10.7270/Q24Q7TD2 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50141212
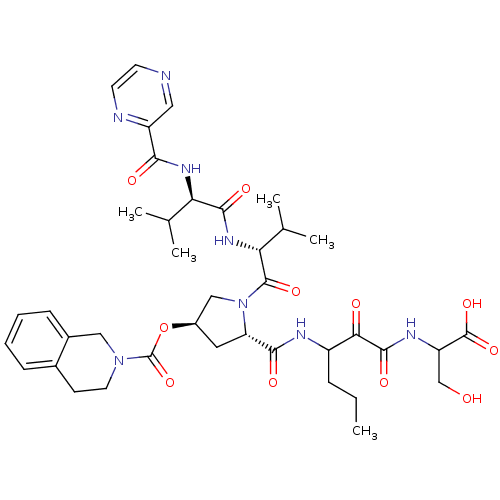
(3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...)Show SMILES CCCC(NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OC(=O)N1CCc2ccccc2C1)C(=O)C(=O)NC(CO)C(O)=O Show InChI InChI=1S/C39H52N8O11/c1-6-9-26(32(49)36(53)43-28(20-48)38(55)56)42-34(51)29-16-25(58-39(57)46-15-12-23-10-7-8-11-24(23)18-46)19-47(29)37(54)31(22(4)5)45-35(52)30(21(2)3)44-33(50)27-17-40-13-14-41-27/h7-8,10-11,13-14,17,21-22,25-26,28-31,48H,6,9,12,15-16,18-20H2,1-5H3,(H,42,51)(H,43,53)(H,44,50)(H,45,52)(H,55,56)/t25-,26?,28?,29+,30-,31-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding inhibition of hepatitis C virus NS3.4A protease 2 using p-nitroaniline assay (pNA) |
Bioorg Med Chem Lett 14: 1441-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.022
BindingDB Entry DOI: 10.7270/Q24Q7TD2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50249318

(2-(2-(2-(3,5-dimethylphenylamino)pyrimidin-4-yl)-4...)Show InChI InChI=1S/C18H20N4OS/c1-11-8-12(2)10-14(9-11)21-18-19-6-4-15(22-18)17-20-13(3)16(24-17)5-7-23/h4,6,8-10,23H,5,7H2,1-3H3,(H,19,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ZAP70 (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50249226
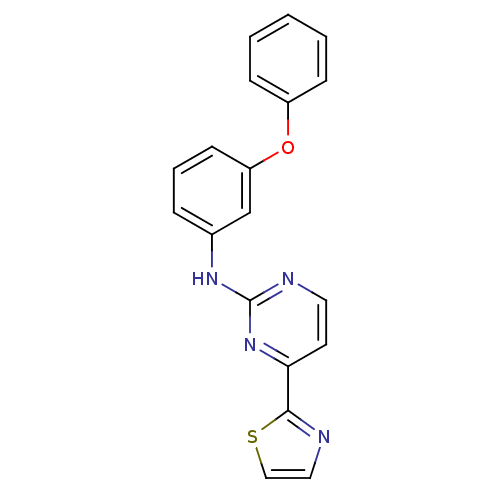
(CHEMBL513985 | N-(3-phenoxyphenyl)-4-(thiazol-2-yl...)Show InChI InChI=1S/C19H14N4OS/c1-2-6-15(7-3-1)24-16-8-4-5-14(13-16)22-19-21-10-9-17(23-19)18-20-11-12-25-18/h1-13H,(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50249286

(3-(3-(4-(thiazol-2-yl)pyrimidin-2-ylamino)-5-(trif...)Show SMILES OCCCOc1cc(Nc2nccc(n2)-c2nccs2)cc(c1)C(F)(F)F Show InChI InChI=1S/C17H15F3N4O2S/c18-17(19,20)11-8-12(10-13(9-11)26-6-1-5-25)23-16-22-3-2-14(24-16)15-21-4-7-27-15/h2-4,7-10,25H,1,5-6H2,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 677 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ZAP70 (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50275356

(3-(4-(4-(hydroxymethyl)thiazol-2-yl)pyrimidin-2-yl...)Show SMILES OCc1csc(n1)-c1ccnc(Nc2cc(O)cc(c2)C(F)(F)F)n1 Show InChI InChI=1S/C15H11F3N4O2S/c16-15(17,18)8-3-9(5-11(24)4-8)21-14-19-2-1-12(22-14)13-20-10(6-23)7-25-13/h1-5,7,23-24H,6H2,(H,19,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ZAP70 (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50275358

((2-(2-(3-methyl-5-(trifluoromethyl)phenylamino)pyr...)Show SMILES Cc1cc(Nc2nccc(n2)-c2nc(CO)cs2)cc(c1)C(F)(F)F Show InChI InChI=1S/C16H13F3N4OS/c1-9-4-10(16(17,18)19)6-11(5-9)22-15-20-3-2-13(23-15)14-21-12(7-24)8-25-14/h2-6,8,24H,7H2,1H3,(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ZAP70 (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50276017

(3-(4-(thiazol-2-yl)pyrimidin-2-ylamino)-5-(trifluo...)Show InChI InChI=1S/C14H9F3N4OS/c15-14(16,17)8-5-9(7-10(22)6-8)20-13-19-2-1-11(21-13)12-18-3-4-23-12/h1-7,22H,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 754 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ZAP70 (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50141188

(3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...)Show SMILES CCCC(NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OC(=O)N1CCc2ccccc2C1)C(=O)C(=O)NC1CCC1 Show InChI InChI=1S/C40H54N8O8/c1-6-10-29(34(49)38(53)43-27-13-9-14-27)44-36(51)31-19-28(56-40(55)47-18-15-25-11-7-8-12-26(25)21-47)22-48(31)39(54)33(24(4)5)46-37(52)32(23(2)3)45-35(50)30-20-41-16-17-42-30/h7-8,11-12,16-17,20,23-24,27-29,31-33H,6,9-10,13-15,18-19,21-22H2,1-5H3,(H,43,53)(H,44,51)(H,45,50)(H,46,52)/t28-,29?,31+,32-,33-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding inhibition of hepatitis C virus NS3.4A protease 2 using p-nitroaniline assay (pNA) |
Bioorg Med Chem Lett 14: 1441-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.022
BindingDB Entry DOI: 10.7270/Q24Q7TD2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data