 Found 1178 hits with Last Name = 'doran' and Initial = 'sd'
Found 1178 hits with Last Name = 'doran' and Initial = 'sd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dipeptidyl peptidase 4
(Rattus norvegicus (rat)) | BDBM50338518

(1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...)Show SMILES N[C@H]1CN(C[C@@H]1N1CC(F)(F)CCC1=O)c1ncnc(n1)N1CCC(F)(F)C1 |r| Show InChI InChI=1S/C16H21F4N7O/c17-15(18)2-1-12(28)27(8-15)11-6-26(5-10(11)21)14-23-9-22-13(24-14)25-4-3-16(19,20)7-25/h9-11H,1-8,21H2/t10-,11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in rat plasma |
Bioorg Med Chem Lett 21: 1810-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.055
BindingDB Entry DOI: 10.7270/Q28W3DM7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50338518

(1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...)Show SMILES N[C@H]1CN(C[C@@H]1N1CC(F)(F)CCC1=O)c1ncnc(n1)N1CCC(F)(F)C1 |r| Show InChI InChI=1S/C16H21F4N7O/c17-15(18)2-1-12(28)27(8-15)11-6-26(5-10(11)21)14-23-9-22-13(24-14)25-4-3-16(19,20)7-25/h9-11H,1-8,21H2/t10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 21: 1810-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.055
BindingDB Entry DOI: 10.7270/Q28W3DM7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 3
(Homo sapiens (Human)) | BDBM50338518

(1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...)Show SMILES N[C@H]1CN(C[C@@H]1N1CC(F)(F)CCC1=O)c1ncnc(n1)N1CCC(F)(F)C1 |r| Show InChI InChI=1S/C16H21F4N7O/c17-15(18)2-1-12(28)27(8-15)11-6-26(5-10(11)21)14-23-9-22-13(24-14)25-4-3-16(19,20)7-25/h9-11H,1-8,21H2/t10-,11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of DPP3 |
Bioorg Med Chem Lett 21: 1810-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.055
BindingDB Entry DOI: 10.7270/Q28W3DM7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50338518

(1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...)Show SMILES N[C@H]1CN(C[C@@H]1N1CC(F)(F)CCC1=O)c1ncnc(n1)N1CCC(F)(F)C1 |r| Show InChI InChI=1S/C16H21F4N7O/c17-15(18)2-1-12(28)27(8-15)11-6-26(5-10(11)21)14-23-9-22-13(24-14)25-4-3-16(19,20)7-25/h9-11H,1-8,21H2/t10-,11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 |
Bioorg Med Chem Lett 21: 1810-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.055
BindingDB Entry DOI: 10.7270/Q28W3DM7 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50338518

(1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...)Show SMILES N[C@H]1CN(C[C@@H]1N1CC(F)(F)CCC1=O)c1ncnc(n1)N1CCC(F)(F)C1 |r| Show InChI InChI=1S/C16H21F4N7O/c17-15(18)2-1-12(28)27(8-15)11-6-26(5-10(11)21)14-23-9-22-13(24-14)25-4-3-16(19,20)7-25/h9-11H,1-8,21H2/t10-,11-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of POP |
Bioorg Med Chem Lett 21: 1810-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.055
BindingDB Entry DOI: 10.7270/Q28W3DM7 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase FAP
(Homo sapiens (Human)) | BDBM50338518

(1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...)Show SMILES N[C@H]1CN(C[C@@H]1N1CC(F)(F)CCC1=O)c1ncnc(n1)N1CCC(F)(F)C1 |r| Show InChI InChI=1S/C16H21F4N7O/c17-15(18)2-1-12(28)27(8-15)11-6-26(5-10(11)21)14-23-9-22-13(24-14)25-4-3-16(19,20)7-25/h9-11H,1-8,21H2/t10-,11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FAP |
Bioorg Med Chem Lett 21: 1810-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.055
BindingDB Entry DOI: 10.7270/Q28W3DM7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50338518

(1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...)Show SMILES N[C@H]1CN(C[C@@H]1N1CC(F)(F)CCC1=O)c1ncnc(n1)N1CCC(F)(F)C1 |r| Show InChI InChI=1S/C16H21F4N7O/c17-15(18)2-1-12(28)27(8-15)11-6-26(5-10(11)21)14-23-9-22-13(24-14)25-4-3-16(19,20)7-25/h9-11H,1-8,21H2/t10-,11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of DPP2 |
Bioorg Med Chem Lett 21: 1810-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.055
BindingDB Entry DOI: 10.7270/Q28W3DM7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM223332
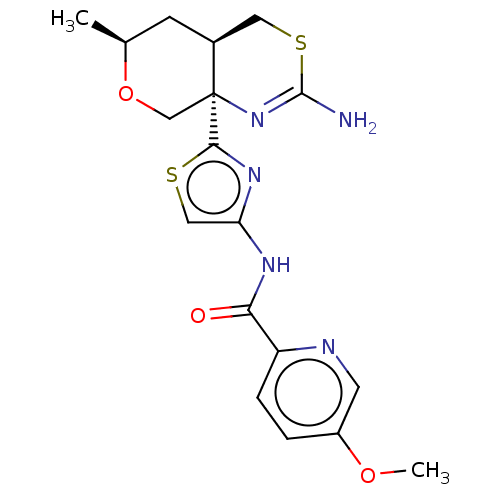
(US9315520, 19 | US9605007, Example 19 | US9744173,...)Show SMILES COc1ccc(nc1)C(=O)Nc1csc(n1)[C@]12CO[C@@H](C)C[C@H]1CSC(N)=N2 |r,c:29| Show InChI InChI=1S/C18H21N5O3S2/c1-10-5-11-7-28-17(19)23-18(11,9-26-10)16-22-14(8-27-16)21-15(24)13-4-3-12(25-2)6-20-13/h3-4,6,8,10-11H,5,7,9H2,1-2H3,(H2,19,23)(H,21,24)/t10-,11-,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... |
J Med Chem 61: 4476-4504 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00246
BindingDB Entry DOI: 10.7270/Q23J3GJ5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50452875
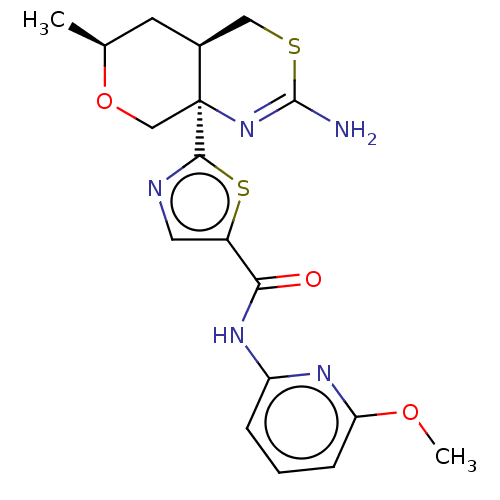
(CHEMBL4212046)Show SMILES [H][C@]12CSC(N)=N[C@]1(CO[C@@H](C)C2)c1ncc(s1)C(=O)Nc1cccc(OC)n1 |r,c:5| Show InChI InChI=1S/C18H21N5O3S2/c1-10-6-11-8-27-17(19)23-18(11,9-26-10)16-20-7-12(28-16)15(24)22-13-4-3-5-14(21-13)25-2/h3-5,7,10-11H,6,8-9H2,1-2H3,(H2,19,23)(H,21,22,24)/t10-,11-,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... |
J Med Chem 61: 4476-4504 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00246
BindingDB Entry DOI: 10.7270/Q23J3GJ5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50452884
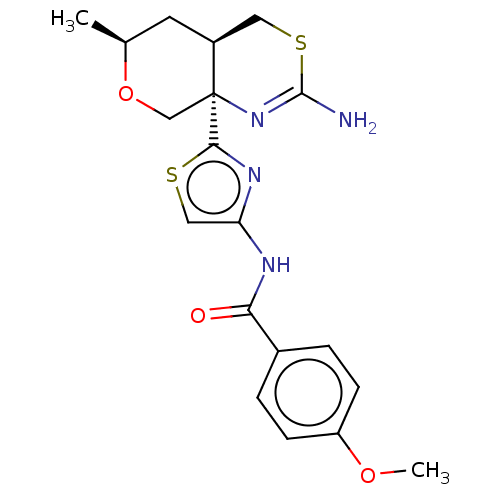
(CHEMBL4217023)Show SMILES [H][C@]12CSC(N)=N[C@]1(CO[C@@H](C)C2)c1nc(NC(=O)c2ccc(OC)cc2)cs1 |r,c:5| Show InChI InChI=1S/C19H22N4O3S2/c1-11-7-13-8-28-18(20)23-19(13,10-26-11)17-22-15(9-27-17)21-16(24)12-3-5-14(25-2)6-4-12/h3-6,9,11,13H,7-8,10H2,1-2H3,(H2,20,23)(H,21,24)/t11-,13-,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... |
J Med Chem 61: 4476-4504 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00246
BindingDB Entry DOI: 10.7270/Q23J3GJ5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50452883

(CHEMBL4203860)Show SMILES [H][C@]12CSC(N)=N[C@]1(CO[C@@H](C)C2)c1ncc(s1)C(=O)Nc1cccc(OC)c1 |r,c:5| Show InChI InChI=1S/C19H22N4O3S2/c1-11-6-12-9-27-18(20)23-19(12,10-26-11)17-21-8-15(28-17)16(24)22-13-4-3-5-14(7-13)25-2/h3-5,7-8,11-12H,6,9-10H2,1-2H3,(H2,20,23)(H,22,24)/t11-,12-,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... |
J Med Chem 61: 4476-4504 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00246
BindingDB Entry DOI: 10.7270/Q23J3GJ5 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B
(Homo sapiens (Human)) | BDBM50344013
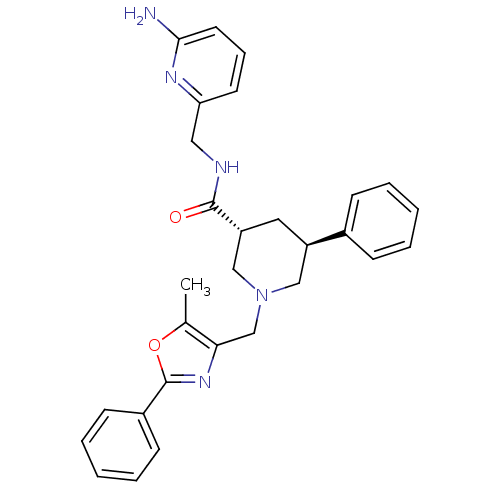
(CHEMBL1780094 | trans-(3R,5S)-N-((6-aminopyridin-2...)Show SMILES Cc1oc(nc1CN1C[C@@H](C[C@H](C1)c1ccccc1)C(=O)NCc1cccc(N)n1)-c1ccccc1 |r| Show InChI InChI=1S/C29H31N5O2/c1-20-26(33-29(36-20)22-11-6-3-7-12-22)19-34-17-23(21-9-4-2-5-10-21)15-24(18-34)28(35)31-16-25-13-8-14-27(30)32-25/h2-14,23-24H,15-19H2,1H3,(H2,30,32)(H,31,35)/t23-,24-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8B |
Bioorg Med Chem Lett 21: 3095-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.022
BindingDB Entry DOI: 10.7270/Q20002F8 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50314930
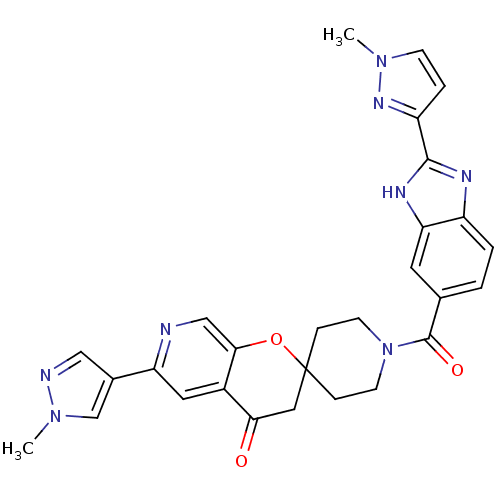
(1-(2-(1-methyl-1H-pyrazol-3-yl)-1H-benzo[d]imidazo...)Show SMILES Cn1ccc(n1)-c1nc2ccc(cc2[nH]1)C(=O)N1CCC2(CC1)CC(=O)c1cc(ncc1O2)-c1cnn(C)c1 Show InChI InChI=1S/C28H26N8O3/c1-34-8-5-21(33-34)26-31-20-4-3-17(11-23(20)32-26)27(38)36-9-6-28(7-10-36)13-24(37)19-12-22(29-15-25(19)39-28)18-14-30-35(2)16-18/h3-5,8,11-12,14-16H,6-7,9-10,13H2,1-2H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader |
Bioorg Med Chem Lett 20: 2383-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.04.091
BindingDB Entry DOI: 10.7270/Q22N52DW |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50314931
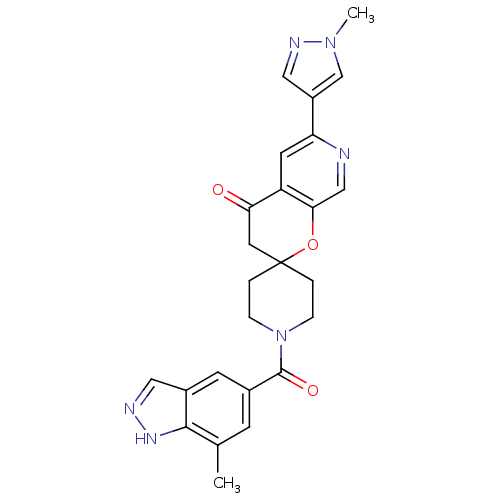
(1-(7-methyl-1H-indazole-5-carbonyl)-6'-(1-methyl-1...)Show SMILES Cc1cc(cc2cn[nH]c12)C(=O)N1CCC2(CC1)CC(=O)c1cc(ncc1O2)-c1cnn(C)c1 Show InChI InChI=1S/C25H24N6O3/c1-15-7-16(8-17-11-27-29-23(15)17)24(33)31-5-3-25(4-6-31)10-21(32)19-9-20(26-13-22(19)34-25)18-12-28-30(2)14-18/h7-9,11-14H,3-6,10H2,1-2H3,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader |
Bioorg Med Chem Lett 20: 2383-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.04.091
BindingDB Entry DOI: 10.7270/Q22N52DW |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50314932
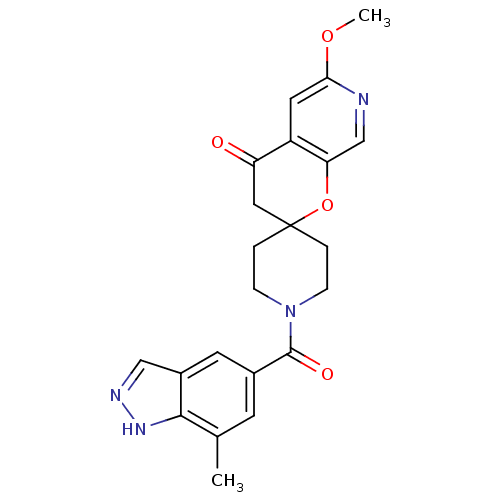
(6'-methoxy-1-(7-methyl-1H-indazole-5-carbonyl)spir...)Show SMILES COc1cc2C(=O)CC3(CCN(CC3)C(=O)c3cc(C)c4[nH]ncc4c3)Oc2cn1 Show InChI InChI=1S/C22H22N4O4/c1-13-7-14(8-15-11-24-25-20(13)15)21(28)26-5-3-22(4-6-26)10-17(27)16-9-19(29-2)23-12-18(16)30-22/h7-9,11-12H,3-6,10H2,1-2H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader |
Bioorg Med Chem Lett 20: 2383-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.04.091
BindingDB Entry DOI: 10.7270/Q22N52DW |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM47353
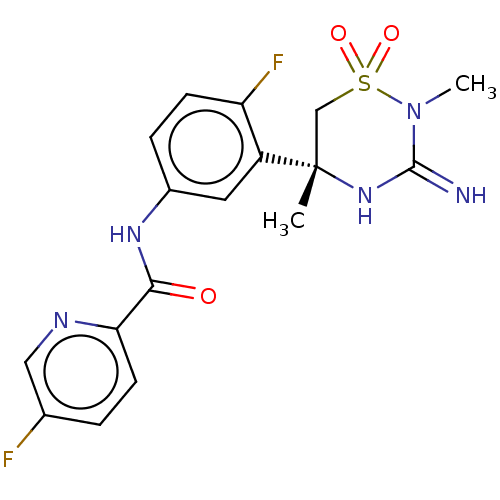
(BDBM143220 | US9029362, 173 | US9687494, 25)Show SMILES CN1C(=N)N[C@@](C)(CS1(=O)=O)c1cc(NC(=O)c2ccc(F)cn2)ccc1F |r| Show InChI InChI=1S/C17H17F2N5O3S/c1-17(9-28(26,27)24(2)16(20)23-17)12-7-11(4-5-13(12)19)22-15(25)14-6-3-10(18)8-21-14/h3-8H,9H2,1-2H3,(H2,20,23)(H,22,25)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human BACE2 |
J Med Chem 61: 4476-4504 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00246
BindingDB Entry DOI: 10.7270/Q23J3GJ5 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50263791
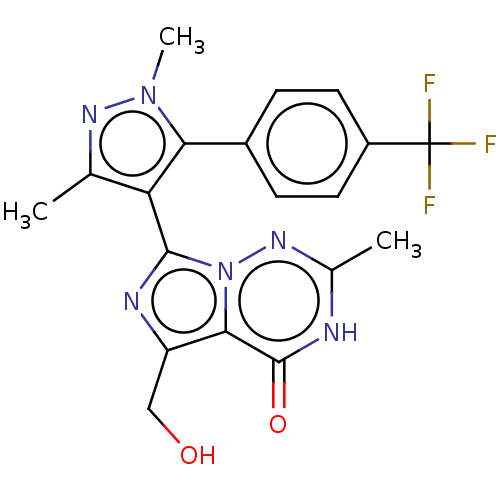
(CHEMBL4062397)Show SMILES Cc1nn(C)c(c1-c1nc(CO)c2n1nc(C)[nH]c2=O)-c1ccc(cc1)C(F)(F)F |(22.28,-12.54,;21.03,-13.44,;21.02,-14.98,;19.55,-15.45,;19.07,-16.91,;18.65,-14.2,;19.56,-12.96,;19.08,-11.49,;19.99,-10.25,;19.09,-9,;19.57,-7.53,;21.07,-7.21,;17.62,-9.47,;17.62,-11.01,;16.28,-11.78,;14.95,-11.01,;13.61,-11.77,;14.96,-9.46,;16.29,-8.7,;16.3,-7.15,;17.11,-14.2,;16.33,-15.53,;14.8,-15.53,;14.02,-14.19,;14.8,-12.86,;16.34,-12.87,;12.47,-14.18,;12.47,-15.72,;11.14,-14.94,;11.72,-12.85,)| Show InChI InChI=1S/C19H17F3N6O2/c1-9-14(17-24-13(8-29)16-18(30)23-10(2)26-28(16)17)15(27(3)25-9)11-4-6-12(7-5-11)19(20,21)22/h4-7,29H,8H2,1-3H3,(H,23,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PDE2A1 using 3',5'-[3H]cGMP as substrate measured after 30 mins by Yttrium silicate scintillation proximity assay |
ACS Med Chem Lett 9: 68-72 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00343
BindingDB Entry DOI: 10.7270/Q2MG7S23 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM143218

(US8940748, 34 | US9029362, 34 | US9687494, 34)Show SMILES CN1C(=N)N[C@@](C)(CS1(=O)=O)c1cc(NC(=O)c2ccc(Cl)cn2)ccc1F |r| Show InChI InChI=1S/C17H17ClFN5O3S/c1-17(9-28(26,27)24(2)16(20)23-17)12-7-11(4-5-13(12)19)22-15(25)14-6-3-10(18)8-21-14/h3-8H,9H2,1-2H3,(H2,20,23)(H,22,25)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... |
J Med Chem 61: 4476-4504 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00246
BindingDB Entry DOI: 10.7270/Q23J3GJ5 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50286695
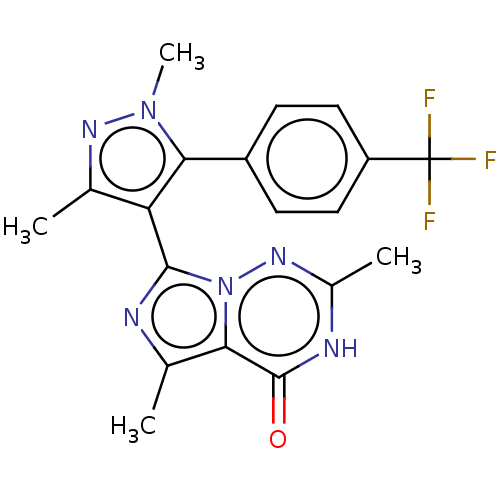
(CHEMBL4160171)Show SMILES Cc1nn(C)c(c1-c1nc(C)c2n1nc(C)[nH]c2=O)-c1ccc(cc1)C(F)(F)F |(18.8,-12.08,;20.34,-12.07,;21.23,-10.82,;22.7,-11.29,;23.94,-10.38,;22.71,-12.83,;21.25,-13.32,;20.78,-14.79,;21.69,-16.02,;20.8,-17.27,;21.28,-18.73,;19.33,-16.8,;19.33,-15.27,;18,-14.49,;16.67,-15.27,;15.33,-14.5,;16.67,-16.81,;18,-17.57,;18,-19.11,;23.95,-13.73,;23.8,-15.26,;25.05,-16.16,;26.45,-15.53,;26.6,-13.98,;25.35,-13.09,;27.7,-16.42,;27.55,-17.95,;29.11,-15.79,;29.03,-17.19,)| Show InChI InChI=1S/C42H56N4O4/c1-28-18-19-32(41(50)45-21-20-30-14-8-10-16-33(30)25-45)23-35(28)39(48)43-36(22-29-12-6-5-7-13-29)38(47)27-46-26-34-17-11-9-15-31(34)24-37(46)40(49)44-42(2,3)4/h5-8,10,12-14,16,18-19,23,30-31,33-34,36-38,47H,9,11,15,17,20-22,24-27H2,1-4H3,(H,43,48)(H,44,49)/t30?,31-,33?,34+,36-,37-,38+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PDE2A1 using 3',5'-[3H]cGMP as substrate measured after 30 mins by Yttrium silicate scintillation proximity assay |
ACS Med Chem Lett 9: 68-72 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00343
BindingDB Entry DOI: 10.7270/Q2MG7S23 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50452874
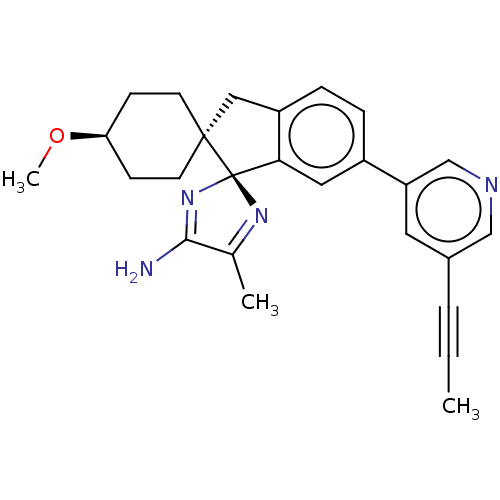
(CHEMBL4217620)Show SMILES CO[C@H]1CC[C@]2(Cc3ccc(cc3[C@@]22N=C(C)C(N)=N2)-c2cncc(c2)C#CC)CC1 |r,wU:13.15,5.4,wD:2.1,c:20,t:16,(42.8,-16.77,;41.71,-15.68,;40.22,-16.08,;39.13,-14.99,;37.64,-15.39,;37.25,-16.87,;37.1,-18.41,;35.59,-18.75,;34.81,-20.09,;33.28,-20.09,;32.51,-18.75,;33.27,-17.42,;34.81,-17.41,;35.84,-16.25,;34.46,-15.55,;34.7,-14.03,;33.6,-12.94,;36.22,-13.78,;36.92,-12.4,;36.93,-15.15,;30.97,-18.75,;30.21,-17.42,;28.66,-17.43,;27.9,-18.76,;28.67,-20.08,;30.21,-20.08,;27.9,-21.41,;27.12,-22.74,;26.34,-24.06,;38.34,-17.96,;39.82,-17.56,)| Show InChI InChI=1S/C26H28N4O/c1-4-5-18-12-21(16-28-15-18)19-6-7-20-14-25(10-8-22(31-3)9-11-25)26(23(20)13-19)29-17(2)24(27)30-26/h6-7,12-13,15-16,22H,8-11,14H2,1-3H3,(H2,27,30)/t22-,25+,26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 |
J Med Chem 61: 4476-4504 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00246
BindingDB Entry DOI: 10.7270/Q23J3GJ5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM312938

(N-{2-[(4aR,6S,8aR)-2-amino-6-methyl-4,4a,5,6-tetra...)Show SMILES C[C@H]1C[C@H]2CSC(N)=N[C@]2(CO1)c1nc(NC(=O)c2ccn(n2)C(F)F)cs1 |c:7| Show InChI InChI=1S/C16H18F2N6O2S2/c1-8-4-9-5-28-15(19)22-16(9,7-26-8)13-21-11(6-27-13)20-12(25)10-2-3-24(23-10)14(17)18/h2-3,6,8-9,14H,4-5,7H2,1H3,(H2,19,22)(H,20,25)/t8-,9-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... |
J Med Chem 61: 4476-4504 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00246
BindingDB Entry DOI: 10.7270/Q23J3GJ5 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B
(Homo sapiens (Human)) | BDBM50390333

(CHEMBL2070733)Show SMILES Nc1nc(nc2n(C[C@H]3CN(Cc4nccs4)CCO3)nnc12)C1CC1 |r| Show InChI InChI=1S/C16H20N8OS/c17-14-13-16(20-15(19-14)10-1-2-10)24(22-21-13)8-11-7-23(4-5-25-11)9-12-18-3-6-26-12/h3,6,10-11H,1-2,4-5,7-9H2,(H2,17,19,20)/t11-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8B expressed in Sf9 insect cells |
Bioorg Med Chem Lett 22: 5721-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.079
BindingDB Entry DOI: 10.7270/Q2J67J0X |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM143218

(US8940748, 34 | US9029362, 34 | US9687494, 34)Show SMILES CN1C(=N)N[C@@](C)(CS1(=O)=O)c1cc(NC(=O)c2ccc(Cl)cn2)ccc1F |r| Show InChI InChI=1S/C17H17ClFN5O3S/c1-17(9-28(26,27)24(2)16(20)23-17)12-7-11(4-5-13(12)19)22-15(25)14-6-3-10(18)8-21-14/h3-8H,9H2,1-2H3,(H2,20,23)(H,22,25)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 |
J Med Chem 61: 4476-4504 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00246
BindingDB Entry DOI: 10.7270/Q23J3GJ5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50452874

(CHEMBL4217620)Show SMILES CO[C@H]1CC[C@]2(Cc3ccc(cc3[C@@]22N=C(C)C(N)=N2)-c2cncc(c2)C#CC)CC1 |r,wU:13.15,5.4,wD:2.1,c:20,t:16,(42.8,-16.77,;41.71,-15.68,;40.22,-16.08,;39.13,-14.99,;37.64,-15.39,;37.25,-16.87,;37.1,-18.41,;35.59,-18.75,;34.81,-20.09,;33.28,-20.09,;32.51,-18.75,;33.27,-17.42,;34.81,-17.41,;35.84,-16.25,;34.46,-15.55,;34.7,-14.03,;33.6,-12.94,;36.22,-13.78,;36.92,-12.4,;36.93,-15.15,;30.97,-18.75,;30.21,-17.42,;28.66,-17.43,;27.9,-18.76,;28.67,-20.08,;30.21,-20.08,;27.9,-21.41,;27.12,-22.74,;26.34,-24.06,;38.34,-17.96,;39.82,-17.56,)| Show InChI InChI=1S/C26H28N4O/c1-4-5-18-12-21(16-28-15-18)19-6-7-20-14-25(10-8-22(31-3)9-11-25)26(23(20)13-19)29-17(2)24(27)30-26/h6-7,12-13,15-16,22H,8-11,14H2,1-3H3,(H2,27,30)/t22-,25+,26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human BACE2 |
J Med Chem 61: 4476-4504 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00246
BindingDB Entry DOI: 10.7270/Q23J3GJ5 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B
(Homo sapiens (Human)) | BDBM50344014
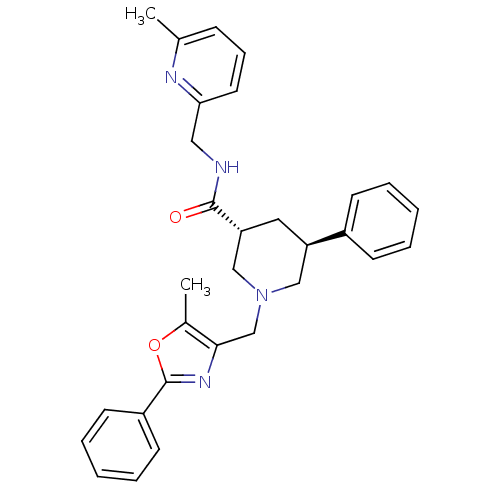
(CHEMBL1779987 | trans-(3R,5S)-1-((5-methyl-2-pheny...)Show SMILES Cc1oc(nc1CN1C[C@@H](C[C@H](C1)c1ccccc1)C(=O)NCc1cccc(C)n1)-c1ccccc1 |r| Show InChI InChI=1S/C30H32N4O2/c1-21-10-9-15-27(32-21)17-31-29(35)26-16-25(23-11-5-3-6-12-23)18-34(19-26)20-28-22(2)36-30(33-28)24-13-7-4-8-14-24/h3-15,25-26H,16-20H2,1-2H3,(H,31,35)/t25-,26-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8B |
Bioorg Med Chem Lett 21: 3095-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.022
BindingDB Entry DOI: 10.7270/Q20002F8 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B
(Homo sapiens (Human)) | BDBM50344015
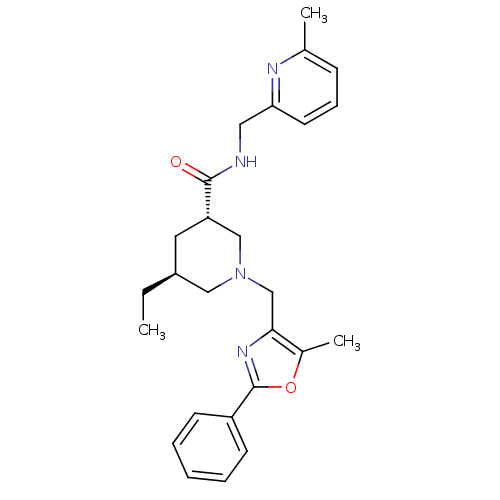
(CHEMBL1780096 | trans-(3S,5S)-5-ethyl-1-((5-methyl...)Show SMILES CC[C@H]1C[C@@H](CN(Cc2nc(oc2C)-c2ccccc2)C1)C(=O)NCc1cccc(C)n1 |r| Show InChI InChI=1S/C26H32N4O2/c1-4-20-13-22(25(31)27-14-23-12-8-9-18(2)28-23)16-30(15-20)17-24-19(3)32-26(29-24)21-10-6-5-7-11-21/h5-12,20,22H,4,13-17H2,1-3H3,(H,27,31)/t20-,22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.955 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8B |
Bioorg Med Chem Lett 21: 3095-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.022
BindingDB Entry DOI: 10.7270/Q20002F8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50226096
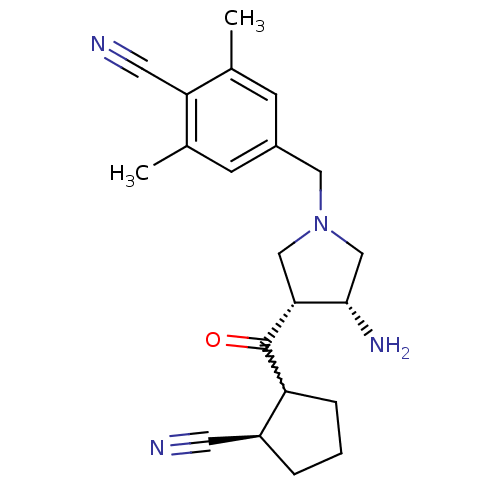
(4-(((3R,4R)-3-amino-4-((2R)-2-cyanocyclopentanecar...)Show SMILES Cc1cc(CN2C[C@H](N)[C@@H](C2)C(=O)C2CCC[C@H]2C#N)cc(C)c1C#N |w:13.13| Show InChI InChI=1S/C21H26N4O/c1-13-6-15(7-14(2)18(13)9-23)10-25-11-19(20(24)12-25)21(26)17-5-3-4-16(17)8-22/h6-7,16-17,19-20H,3-5,10-12,24H2,1-2H3/t16-,17?,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 17: 6707-13 (2007)
Article DOI: 10.1016/j.bmcl.2007.10.063
BindingDB Entry DOI: 10.7270/Q2ZS2W8B |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B
(Homo sapiens (Human)) | BDBM50390334

(CHEMBL2070735)Show SMILES Cc1nc(N)c2nnn(C[C@H]3CN(Cc4nccs4)CCO3)c2n1 |r| Show InChI InChI=1S/C14H18N8OS/c1-9-17-13(15)12-14(18-9)22(20-19-12)7-10-6-21(3-4-23-10)8-11-16-2-5-24-11/h2,5,10H,3-4,6-8H2,1H3,(H2,15,17,18)/t10-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8B expressed in Sf9 insect cells |
Bioorg Med Chem Lett 22: 5721-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.079
BindingDB Entry DOI: 10.7270/Q2J67J0X |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50226064
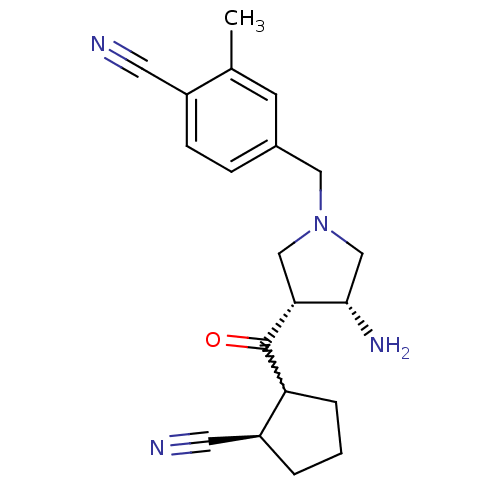
(4-(((3R,4R)-3-amino-4-((2R)-2-cyanocyclopentanecar...)Show SMILES Cc1cc(CN2C[C@H](N)[C@@H](C2)C(=O)C2CCC[C@H]2C#N)ccc1C#N |w:13.13| Show InChI InChI=1S/C20H24N4O/c1-13-7-14(5-6-15(13)8-21)10-24-11-18(19(23)12-24)20(25)17-4-2-3-16(17)9-22/h5-7,16-19H,2-4,10-12,23H2,1H3/t16-,17?,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 17: 6707-13 (2007)
Article DOI: 10.1016/j.bmcl.2007.10.063
BindingDB Entry DOI: 10.7270/Q2ZS2W8B |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B
(Homo sapiens (Human)) | BDBM50390332
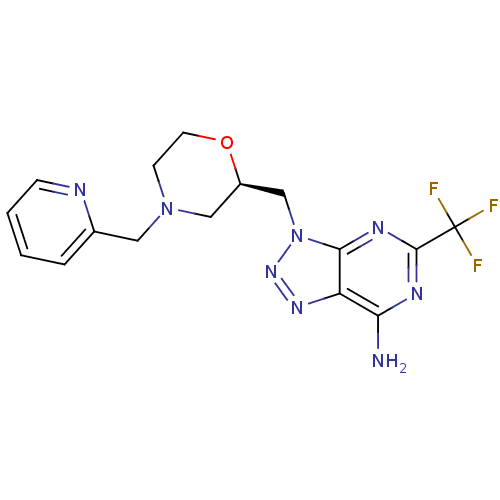
(CHEMBL2070732)Show SMILES Nc1nc(nc2n(C[C@H]3CN(Cc4ccccn4)CCO3)nnc12)C(F)(F)F |r| Show InChI InChI=1S/C16H17F3N8O/c17-16(18,19)15-22-13(20)12-14(23-15)27(25-24-12)9-11-8-26(5-6-28-11)7-10-3-1-2-4-21-10/h1-4,11H,5-9H2,(H2,20,22,23)/t11-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8B expressed in Sf9 insect cells |
Bioorg Med Chem Lett 22: 5721-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.079
BindingDB Entry DOI: 10.7270/Q2J67J0X |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50338507
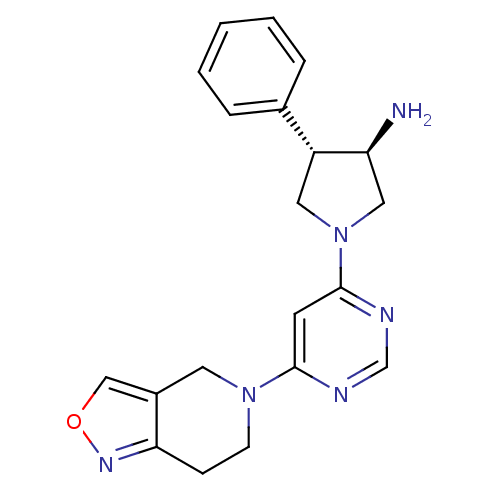
((3R,4S)-1-(6-(6,7-dihydroisoxazolo[4,3-c]pyridin-5...)Show SMILES N[C@H]1CN(C[C@@H]1c1ccccc1)c1cc(ncn1)N1CCc2nocc2C1 |r| Show InChI InChI=1S/C20H22N6O/c21-17-11-26(10-16(17)14-4-2-1-3-5-14)20-8-19(22-13-23-20)25-7-6-18-15(9-25)12-27-24-18/h1-5,8,12-13,16-17H,6-7,9-11,21H2/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 21: 1810-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.055
BindingDB Entry DOI: 10.7270/Q28W3DM7 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50439646

(CHEMBL2419600 | US8993586, 110)Show SMILES CCCNc1ccc2ccc(cc2n1)C(=O)N1CCC2(CC1)Cc1cn(nc1C(=O)N2)C(C)(C)C Show InChI InChI=1S/C27H34N6O2/c1-5-12-28-22-9-8-18-6-7-19(15-21(18)29-22)25(35)32-13-10-27(11-14-32)16-20-17-33(26(2,3)4)31-23(20)24(34)30-27/h6-9,15,17H,5,10-14,16H2,1-4H3,(H,28,29)(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... |
J Med Chem 56: 7110-9 (2013)
Article DOI: 10.1021/jm401033t
BindingDB Entry DOI: 10.7270/Q2JW8G9D |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50344018
(CHEMBL1779984 | trans-(3R,5R)-5-ethyl-1-((5-methyl...)Show SMILES CC[C@@H]1C[C@H](CN(Cc2nc(oc2C)-c2ccccc2)C1)C(=O)NCc1cccc(C)n1 |r| Show InChI InChI=1S/C26H32N4O2/c1-4-20-13-22(25(31)27-14-23-12-8-9-18(2)28-23)16-30(15-20)17-24-19(3)32-26(29-24)21-10-6-5-7-11-21/h5-12,20,22H,4,13-17H2,1-3H3,(H,27,31)/t20-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8A |
Bioorg Med Chem Lett 21: 3095-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.022
BindingDB Entry DOI: 10.7270/Q20002F8 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50344017

(1-((5-methyl-2-phenyloxazol-4-yl)methyl)-N-((6-met...)Show SMILES Cc1oc(nc1CN1CCCC(C1)C(=O)NCc1cccc(C)n1)-c1ccccc1 Show InChI InChI=1S/C24H28N4O2/c1-17-8-6-12-21(26-17)14-25-23(29)20-11-7-13-28(15-20)16-22-18(2)30-24(27-22)19-9-4-3-5-10-19/h3-6,8-10,12,20H,7,11,13-16H2,1-2H3,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8A |
Bioorg Med Chem Lett 21: 3095-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.022
BindingDB Entry DOI: 10.7270/Q20002F8 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50344016

(2-((5-methyl-2-phenyloxazol-4-yl)methyl)-N-((6-met...)Show SMILES Cc1oc(nc1CN1CC(C(=O)NCc2cccc(C)n2)c2ccccc2C1)-c1ccccc1 Show InChI InChI=1S/C28H28N4O2/c1-19-9-8-13-23(30-19)15-29-27(33)25-17-32(16-22-12-6-7-14-24(22)25)18-26-20(2)34-28(31-26)21-10-4-3-5-11-21/h3-14,25H,15-18H2,1-2H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8A |
Bioorg Med Chem Lett 21: 3095-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.022
BindingDB Entry DOI: 10.7270/Q20002F8 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50439646

(CHEMBL2419600 | US8993586, 110)Show SMILES CCCNc1ccc2ccc(cc2n1)C(=O)N1CCC2(CC1)Cc1cn(nc1C(=O)N2)C(C)(C)C Show InChI InChI=1S/C27H34N6O2/c1-5-12-28-22-9-8-18-6-7-19(15-21(18)29-22)25(35)32-13-10-27(11-14-32)16-20-17-33(26(2,3)4)31-23(20)24(34)30-27/h6-9,15,17H,5,10-14,16H2,1-4H3,(H,28,29)(H,30,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... |
J Med Chem 56: 7110-9 (2013)
Article DOI: 10.1021/jm401033t
BindingDB Entry DOI: 10.7270/Q2JW8G9D |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50390334

(CHEMBL2070735)Show SMILES Cc1nc(N)c2nnn(C[C@H]3CN(Cc4nccs4)CCO3)c2n1 |r| Show InChI InChI=1S/C14H18N8OS/c1-9-17-13(15)12-14(18-9)22(20-19-12)7-10-6-21(3-4-23-10)8-11-16-2-5-24-11/h2,5,10H,3-4,6-8H2,1H3,(H2,15,17,18)/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8A |
Bioorg Med Chem Lett 22: 5721-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.079
BindingDB Entry DOI: 10.7270/Q2J67J0X |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B
(Homo sapiens (Human)) | BDBM50344019
(CHEMBL1780085 | trans-(3R,5R)-5-(methoxymethyl)-1-...)Show SMILES COC[C@@H]1C[C@H](CN(Cc2nc(oc2C)-c2ccccc2)C1)C(=O)NCC1CCOCC1 |r| Show InChI InChI=1S/C25H35N3O4/c1-18-23(27-25(32-18)21-6-4-3-5-7-21)16-28-14-20(17-30-2)12-22(15-28)24(29)26-13-19-8-10-31-11-9-19/h3-7,19-20,22H,8-17H2,1-2H3,(H,26,29)/t20-,22-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.96 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8B |
Bioorg Med Chem Lett 21: 3095-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.022
BindingDB Entry DOI: 10.7270/Q20002F8 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50277775
((3R,4R)-3-(4-(3,5-dimethylphenoxy)phenyl)-1-oxo-2-...)Show SMILES CCCN1[C@H]([C@H](C(O)=O)c2ccccc2C1=O)c1ccc(Oc2cc(C)cc(C)c2)cc1 |r| Show InChI InChI=1S/C27H27NO4/c1-4-13-28-25(24(27(30)31)22-7-5-6-8-23(22)26(28)29)19-9-11-20(12-10-19)32-21-15-17(2)14-18(3)16-21/h5-12,14-16,24-25H,4,13H2,1-3H3,(H,30,31)/t24-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D
Curated by ChEMBL
| Assay Description
Antagonist activity at human GPR40 expressed HEK293 cells assessed as effect on intracellular calcium concentration by FLIPR assay |
Bioorg Med Chem Lett 19: 2400-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.082
BindingDB Entry DOI: 10.7270/Q23N238P |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM47353

(BDBM143220 | US9029362, 173 | US9687494, 25)Show SMILES CN1C(=N)N[C@@](C)(CS1(=O)=O)c1cc(NC(=O)c2ccc(F)cn2)ccc1F |r| Show InChI InChI=1S/C17H17F2N5O3S/c1-17(9-28(26,27)24(2)16(20)23-17)12-7-11(4-5-13(12)19)22-15(25)14-6-3-10(18)8-21-14/h3-8H,9H2,1-2H3,(H2,20,23)(H,22,25)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 |
J Med Chem 61: 4476-4504 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00246
BindingDB Entry DOI: 10.7270/Q23J3GJ5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM143218

(US8940748, 34 | US9029362, 34 | US9687494, 34)Show SMILES CN1C(=N)N[C@@](C)(CS1(=O)=O)c1cc(NC(=O)c2ccc(Cl)cn2)ccc1F |r| Show InChI InChI=1S/C17H17ClFN5O3S/c1-17(9-28(26,27)24(2)16(20)23-17)12-7-11(4-5-13(12)19)22-15(25)14-6-3-10(18)8-21-14/h3-8H,9H2,1-2H3,(H2,20,23)(H,22,25)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization assay |
J Med Chem 61: 4476-4504 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00246
BindingDB Entry DOI: 10.7270/Q23J3GJ5 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50439642
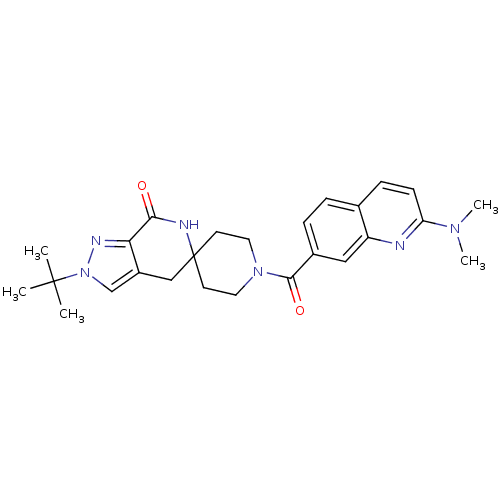
(CHEMBL2419589 | US8993586, 105)Show SMILES CN(C)c1ccc2ccc(cc2n1)C(=O)N1CCC2(CC1)Cc1cn(nc1C(=O)N2)C(C)(C)C Show InChI InChI=1S/C26H32N6O2/c1-25(2,3)32-16-19-15-26(28-23(33)22(19)29-32)10-12-31(13-11-26)24(34)18-7-6-17-8-9-21(30(4)5)27-20(17)14-18/h6-9,14,16H,10-13,15H2,1-5H3,(H,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... |
J Med Chem 56: 7110-9 (2013)
Article DOI: 10.1021/jm401033t
BindingDB Entry DOI: 10.7270/Q2JW8G9D |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50226086

((S)-1-((3R,4R)-1-(4-cyanobenzyl)-3-aminopyrrolidin...)Show SMILES N[C@H]1CN(Cc2ccc(cc2)C#N)C[C@H]1C(=O)N1CCC[C@H]1C#N Show InChI InChI=1S/C18H21N5O/c19-8-13-3-5-14(6-4-13)10-22-11-16(17(21)12-22)18(24)23-7-1-2-15(23)9-20/h3-6,15-17H,1-2,7,10-12,21H2/t15-,16+,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 17: 6707-13 (2007)
Article DOI: 10.1016/j.bmcl.2007.10.063
BindingDB Entry DOI: 10.7270/Q2ZS2W8B |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM223400
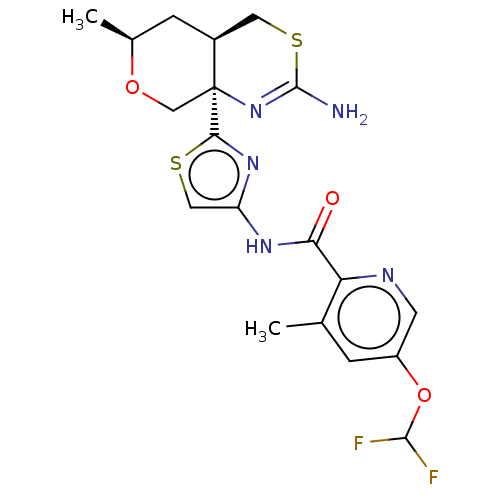
(US9315520, 45 | US9315520, Example 45 | US9605007,...)Show SMILES C[C@H]1C[C@H]2CSC(N)=N[C@]2(CO1)c1nc(NC(=O)c2ncc(OC(F)F)cc2C)cs1 |r,c:7| Show InChI InChI=1S/C19H21F2N5O3S2/c1-9-3-12(29-17(20)21)5-23-14(9)15(27)24-13-7-30-16(25-13)19-8-28-10(2)4-11(19)6-31-18(22)26-19/h3,5,7,10-11,17H,4,6,8H2,1-2H3,(H2,22,26)(H,24,27)/t10-,11-,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... |
J Med Chem 61: 4476-4504 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00246
BindingDB Entry DOI: 10.7270/Q23J3GJ5 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B
(Homo sapiens (Human)) | BDBM50344020
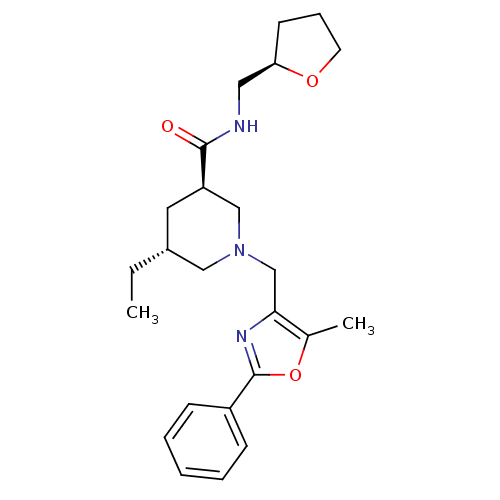
(CHEMBL1780116 | trans-(3R,5R)-5-ethyl-1-((5-methyl...)Show SMILES CC[C@@H]1C[C@H](CN(Cc2nc(oc2C)-c2ccccc2)C1)C(=O)NC[C@H]1CCCO1 |r| Show InChI InChI=1S/C24H33N3O3/c1-3-18-12-20(23(28)25-13-21-10-7-11-29-21)15-27(14-18)16-22-17(2)30-24(26-22)19-8-5-4-6-9-19/h4-6,8-9,18,20-21H,3,7,10-16H2,1-2H3,(H,25,28)/t18-,20-,21-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.91 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8B |
Bioorg Med Chem Lett 21: 3095-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.022
BindingDB Entry DOI: 10.7270/Q20002F8 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM136576
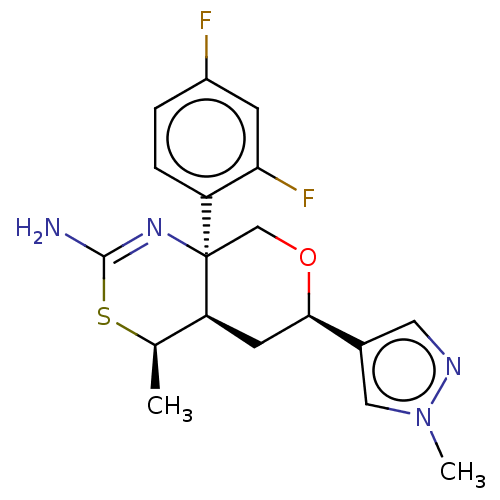
(US8865706, 16)Show SMILES C[C@H]1SC(N)=N[C@]2(CO[C@H](C[C@@H]12)c1cnn(C)c1)c1ccc(F)cc1F |c:4| Show InChI InChI=1S/C18H20F2N4OS/c1-10-14-6-16(11-7-22-24(2)8-11)25-9-18(14,23-17(21)26-10)13-4-3-12(19)5-15(13)20/h3-5,7-8,10,14,16H,6,9H2,1-2H3,(H2,21,23)/t10-,14+,16-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50081645

(CHEMBL3422237)Show SMILES [H][C@]12CSC(N)=N[C@]1(CO[C@H](C2)c1cc(C)no1)c1ccc(F)cc1F |r,c:5| Show InChI InChI=1S/C17H17F2N3O2S/c1-9-4-15(24-22-9)14-5-10-7-25-16(20)21-17(10,8-23-14)12-3-2-11(18)6-13(12)19/h2-4,6,10,14H,5,7-8H2,1H3,(H2,20,21)/t10-,14+,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50439644
(CHEMBL2419593 | US8993586, 86)Show SMILES CC(C)(C)n1cc2CC3(CCN(CC3)C(=O)c3ccc4c(Cl)c[nH]c4c3)NC(=O)c2n1 Show InChI InChI=1S/C23H26ClN5O2/c1-22(2,3)29-13-15-11-23(26-20(30)19(15)27-29)6-8-28(9-7-23)21(31)14-4-5-16-17(24)12-25-18(16)10-14/h4-5,10,12-13,25H,6-9,11H2,1-3H3,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... |
J Med Chem 56: 7110-9 (2013)
Article DOI: 10.1021/jm401033t
BindingDB Entry DOI: 10.7270/Q2JW8G9D |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50277776
((3R,4R)-3-(4-(3,4-dimethylphenoxy)phenyl)-1-oxo-2-...)Show SMILES CCCN1[C@H]([C@H](C(O)=O)c2ccccc2C1=O)c1ccc(Oc2ccc(C)c(C)c2)cc1 |r| Show InChI InChI=1S/C27H27NO4/c1-4-15-28-25(24(27(30)31)22-7-5-6-8-23(22)26(28)29)19-10-13-20(14-11-19)32-21-12-9-17(2)18(3)16-21/h5-14,16,24-25H,4,15H2,1-3H3,(H,30,31)/t24-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D
Curated by ChEMBL
| Assay Description
Antagonist activity at human GPR40 expressed HEK293 cells assessed as effect on intracellular calcium concentration by FLIPR assay |
Bioorg Med Chem Lett 19: 2400-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.082
BindingDB Entry DOI: 10.7270/Q23N238P |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50226093

((1R)-2-((3R,4R)-1-(1-(4-(1H-1,2,4-triazol-1-yl)phe...)Show SMILES CC(N1C[C@H](N)[C@@H](C1)C(=O)C1CCC[C@H]1C#N)c1ccc(cc1)-n1cncn1 |w:10.10,1.0| Show InChI InChI=1S/C21H26N6O/c1-14(15-5-7-17(8-6-15)27-13-24-12-25-27)26-10-19(20(23)11-26)21(28)18-4-2-3-16(18)9-22/h5-8,12-14,16,18-20H,2-4,10-11,23H2,1H3/t14?,16-,18?,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem Lett 17: 6707-13 (2007)
Article DOI: 10.1016/j.bmcl.2007.10.063
BindingDB Entry DOI: 10.7270/Q2ZS2W8B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data