

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244370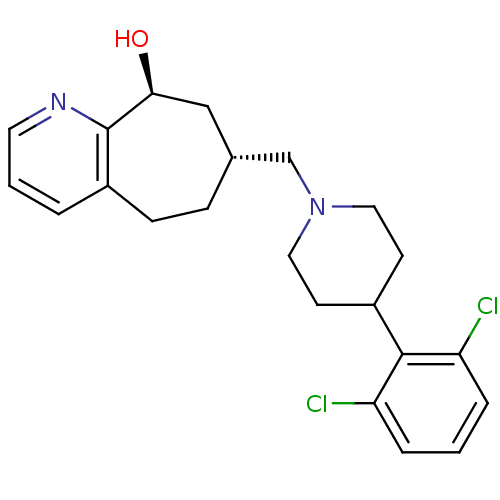 ((7R,9S)-7-((4-(2,6-dichlorophenyl)piperidin-1-yl)m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244371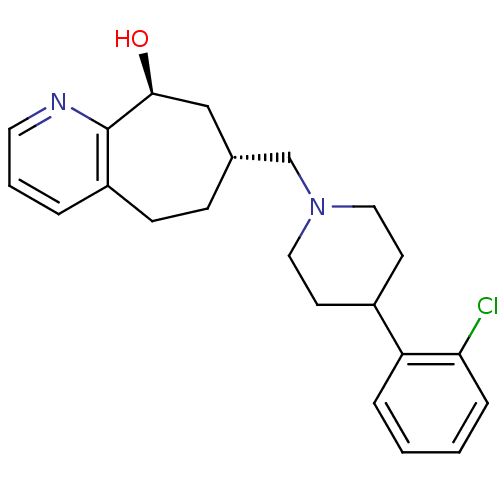 ((7R,9S)-7-((4-(2-chlorophenyl)piperidin-1-yl)methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127501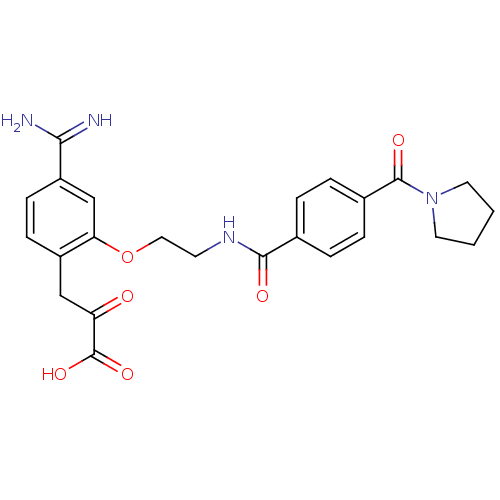 (3-(4-Carbamimidoyl-2-{2-[4-(pyrrolidine-1-carbonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243726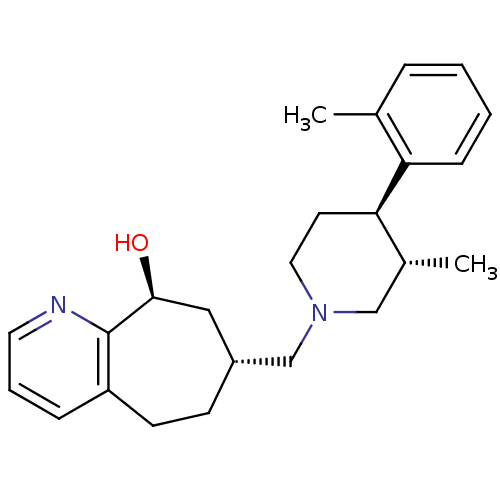 ((7R,9S)-7-(((3S,4R)-3-methyl-4-o-tolylpiperidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243729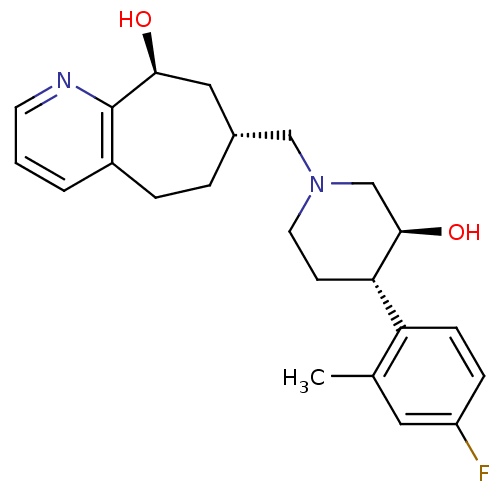 ((7R,9S)-7-(((3S,4S)-4-(4-fluoro-2-methylphenyl)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243727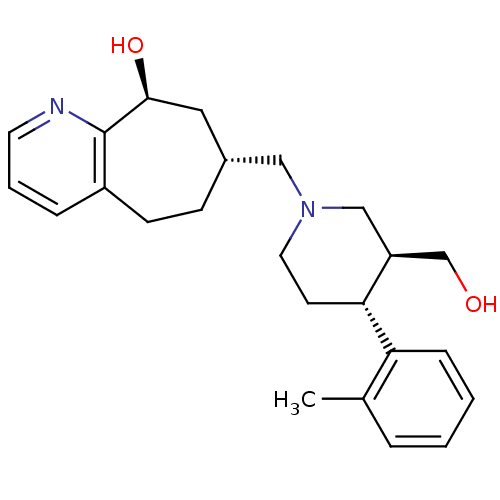 ((7R,9S)-7-(((3S,4R)-3-(hydroxymethyl)-4-o-tolylpip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243730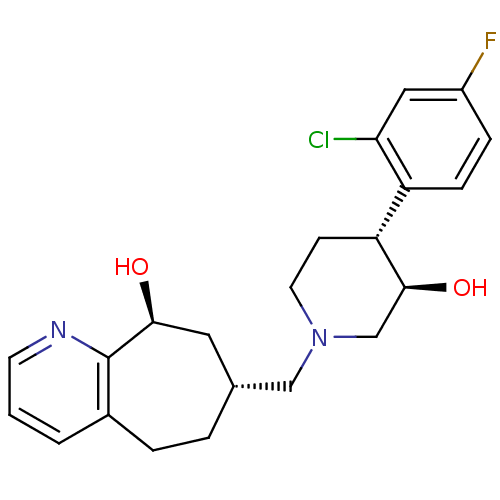 ((7R,9S)-7-(((3R,4R)-4-(2-chloro-4-fluorophenyl)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244297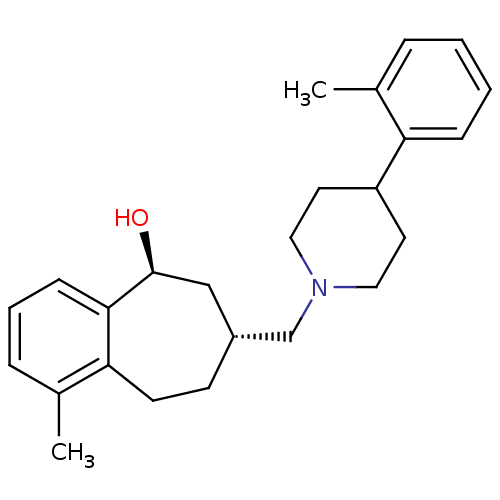 (CHEMBL513585 | cis-1-methyl-7-((4-o-tolylpiperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50296870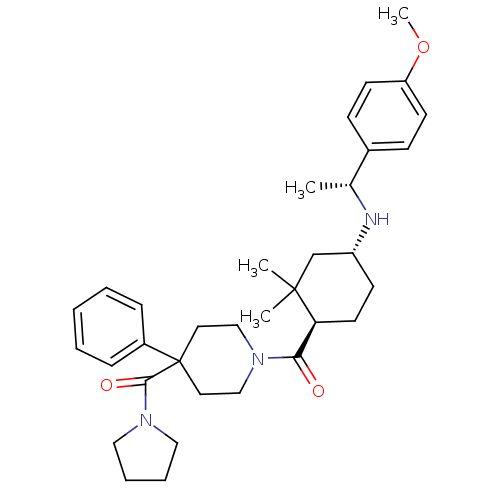 ((1-((1R,4R)-4-((R)-1-(4-methoxyphenyl)ethylamino)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y1 receptor expressed in CHO (NFAT-bla) cells by scintillation counting | Bioorg Med Chem Lett 19: 4781-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.050 BindingDB Entry DOI: 10.7270/Q2319VXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50296879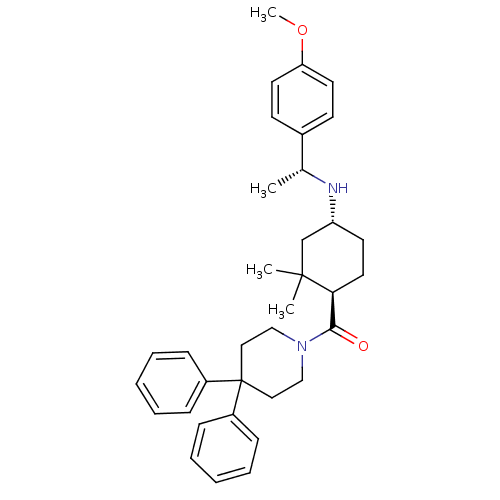 ((4,4-diphenylpiperidin-1-yl)((1R,4R)-4-((R)-1-(4-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y1 receptor expressed in CHO (NFAT-bla) cells by scintillation counting | Bioorg Med Chem Lett 19: 4781-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.050 BindingDB Entry DOI: 10.7270/Q2319VXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243728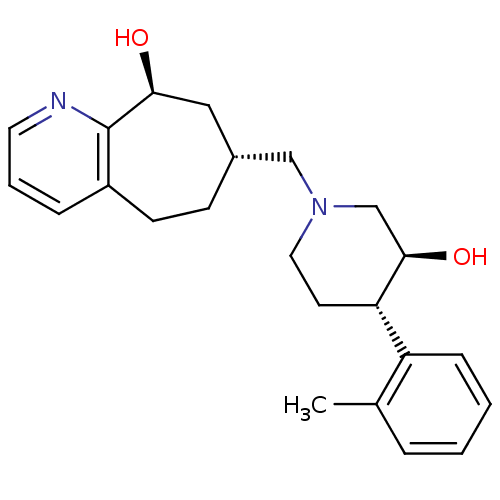 ((7R,9S)-7-(((3S,4S)-3-hydroxy-4-o-tolylpiperidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50296884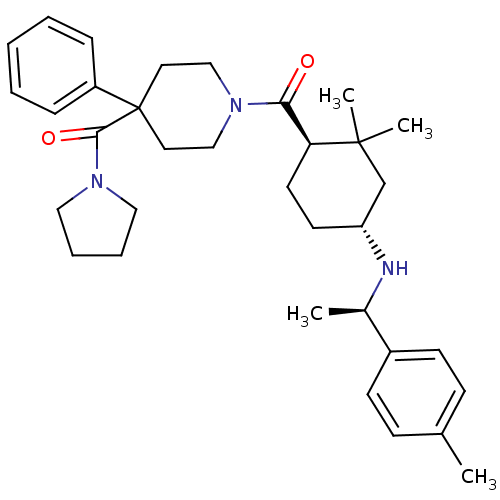 ((1-((1R,4R)-2,2-dimethyl-4-((R)-1-p-tolylethylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y1 receptor expressed in CHO (NFAT-bla) cells by scintillation counting | Bioorg Med Chem Lett 19: 4781-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.050 BindingDB Entry DOI: 10.7270/Q2319VXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127492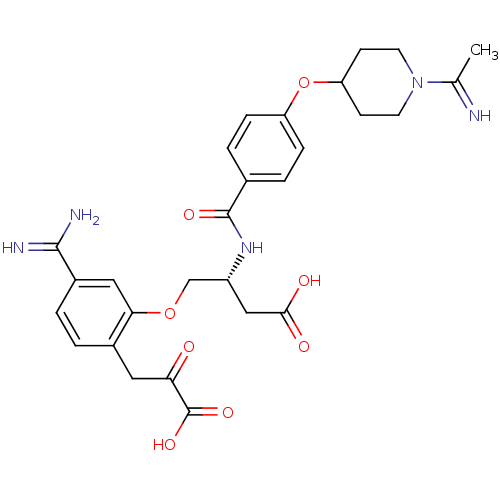 (4-[5-Carbamimidoyl-2-(2-carboxy-2-oxo-ethyl)-pheno...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243887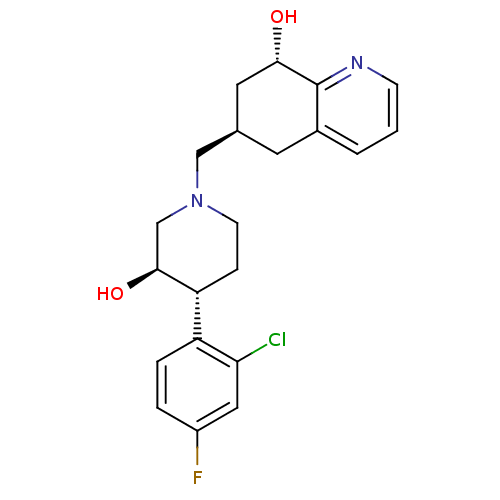 ((-)-(3R,4R)-4-(2-Chloro-4-fluorophenyl)-3-hydroxy-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244336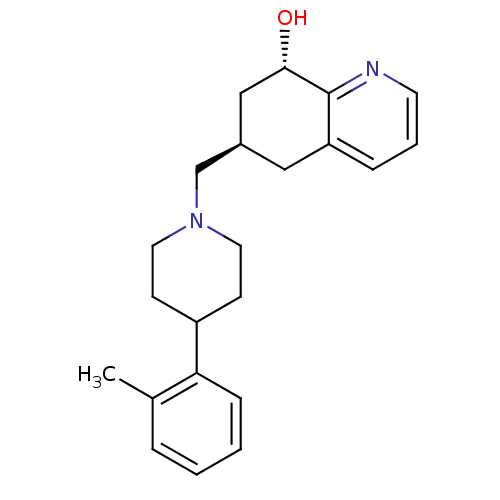 ((6R,8S)-6-((4-o-tolylpiperidin-1-yl)methyl)-5,6,7,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50296885 (1-((1R,4R)-4-((R)-1-(4-methoxyphenyl)ethylamino)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y1 receptor expressed in CHO (NFAT-bla) cells by scintillation counting | Bioorg Med Chem Lett 19: 4781-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.050 BindingDB Entry DOI: 10.7270/Q2319VXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244334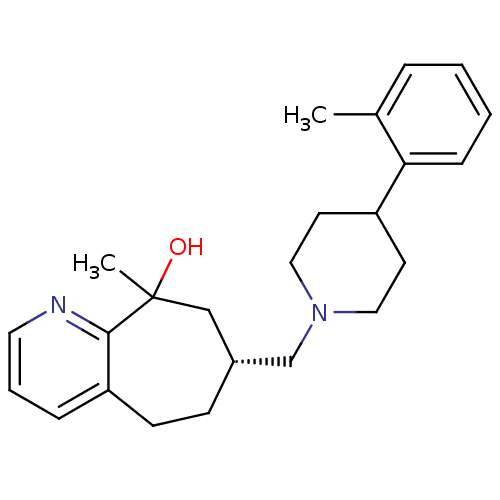 ((7R)-9-methyl-7-((4-o-tolylpiperidin-1-yl)methyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244334 ((7R)-9-methyl-7-((4-o-tolylpiperidin-1-yl)methyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244295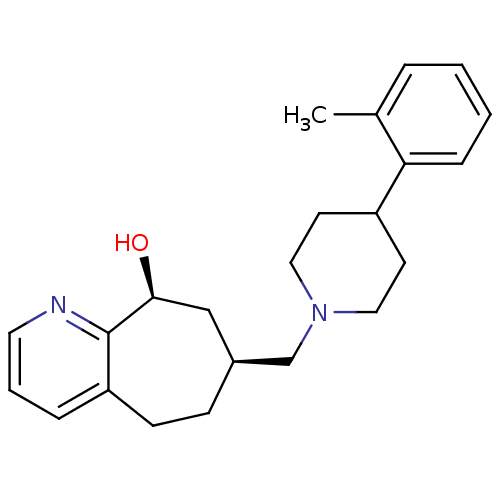 ((-)-7-((4-o-tolylpiperidin-1-yl)methyl)-6,7,8,9-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50296873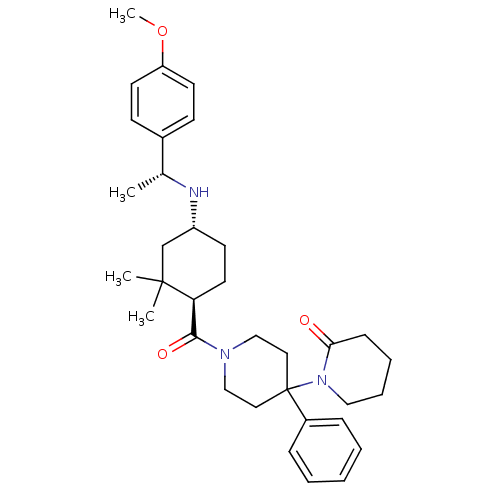 (1'-((1R,4R)-4-((R)-1-(4-methoxyphenyl)ethylamino)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y1 receptor expressed in CHO (NFAT-bla) cells by scintillation counting | Bioorg Med Chem Lett 19: 4781-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.050 BindingDB Entry DOI: 10.7270/Q2319VXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127502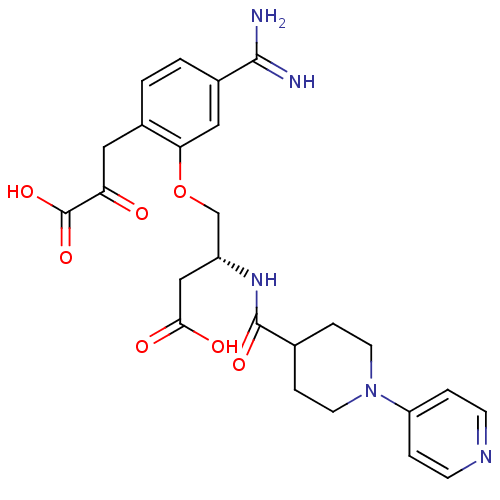 (4-[5-Carbamimidoyl-2-(2-carboxy-2-oxo-ethyl)-pheno...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244295 ((-)-7-((4-o-tolylpiperidin-1-yl)methyl)-6,7,8,9-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127495 (3-[4-Carbamimidoyl-2-(2-{4-[1-(1-imino-ethyl)-pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127504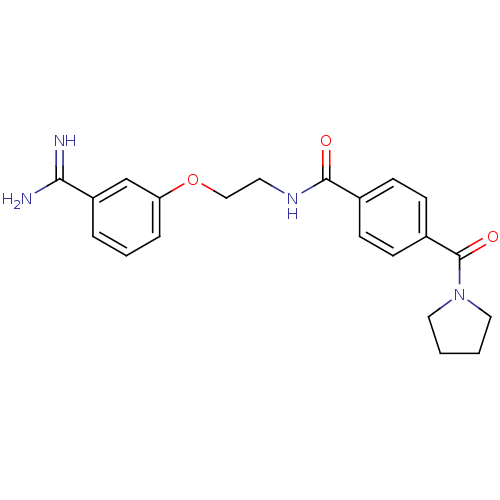 (CHEMBL55770 | N-[2-(3-Carbamimidoyl-phenoxy)-ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244372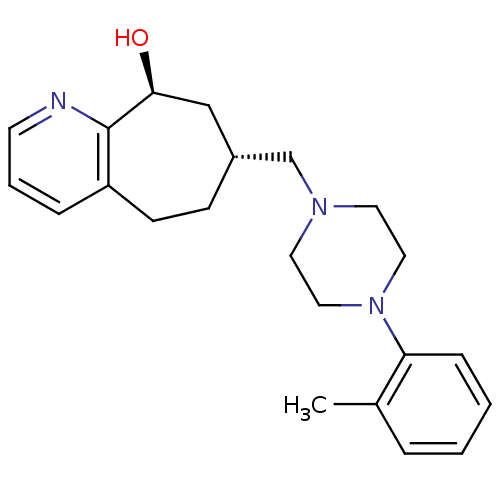 ((7R,9S)-7-((4-o-tolylpiperazin-1-yl)methyl)-6,7,8,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127494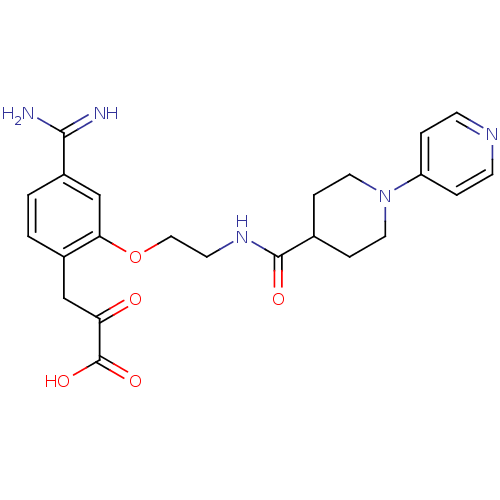 (3-(4-Carbamimidoyl-2-{2-[(3,4,5,6-tetrahydro-2H-[1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243886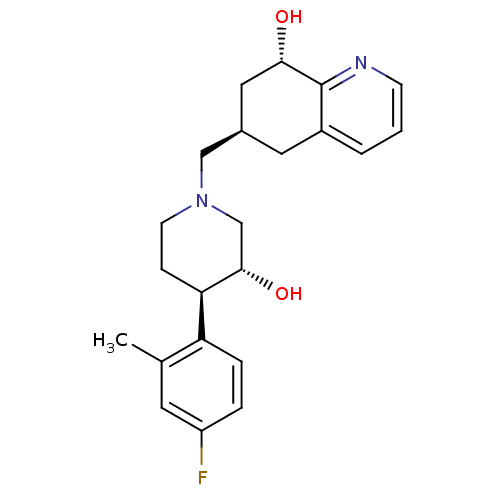 ((6R,8S)-6-(((3R,4R)-4-(4-fluoro-2-methylphenyl)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50296881 ((1-((1R,4R)-4-((S)-2,2-difluoro-1-(4-methoxyphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y1 receptor expressed in CHO (NFAT-bla) cells by scintillation counting | Bioorg Med Chem Lett 19: 4781-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.050 BindingDB Entry DOI: 10.7270/Q2319VXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50296877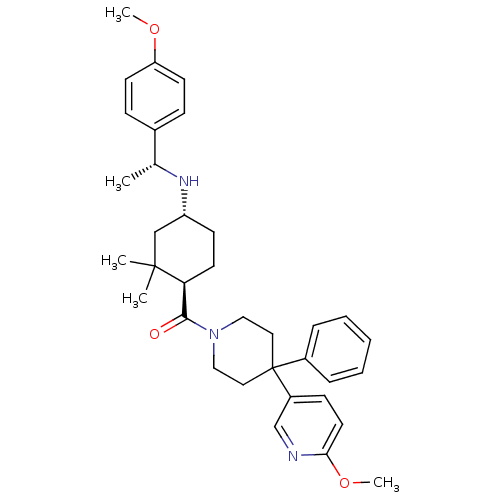 (((1R,4R)-4-((R)-1-(4-methoxyphenyl)ethylamino)-2,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y1 receptor expressed in CHO (NFAT-bla) cells by scintillation counting | Bioorg Med Chem Lett 19: 4781-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.050 BindingDB Entry DOI: 10.7270/Q2319VXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244335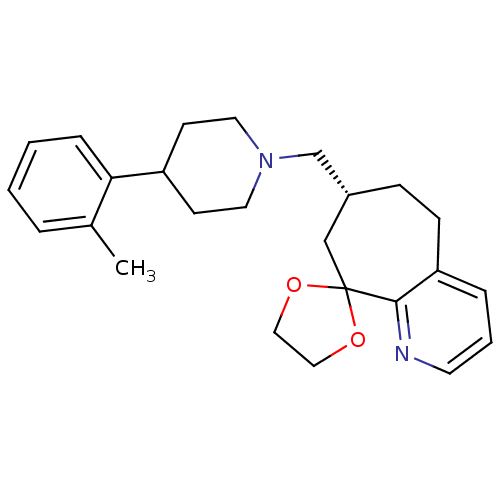 ((3R)-3-{[4-(2-methylphenyl)piperidin-1-yl]methyl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127498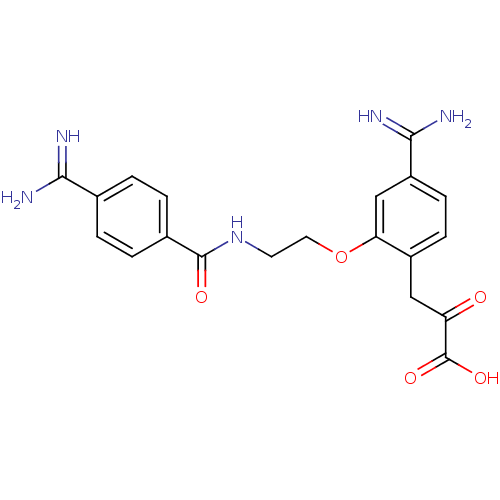 (3-{4-Carbamimidoyl-2-[2-(4-carbamimidoyl-benzoylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50296883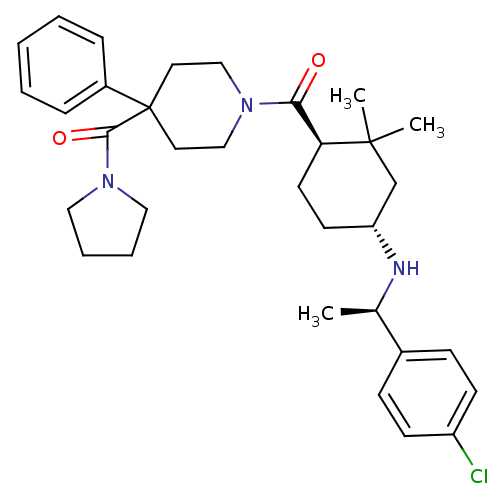 ((1-((1R,4R)-4-((R)-1-(4-chlorophenyl)ethylamino)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y1 receptor expressed in CHO (NFAT-bla) cells by scintillation counting | Bioorg Med Chem Lett 19: 4781-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.050 BindingDB Entry DOI: 10.7270/Q2319VXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127503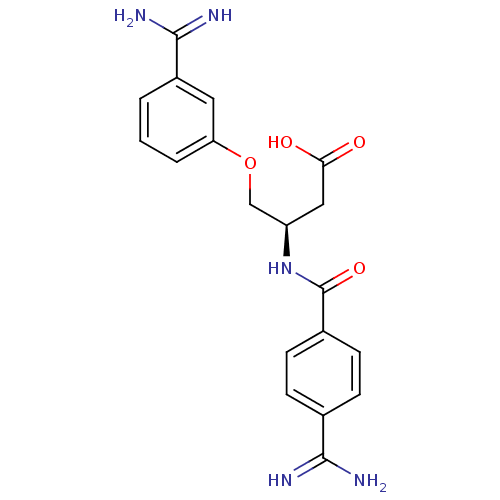 (3-(4-Carbamimidoyl-benzoylamino)-4-(3-carbamimidoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50296878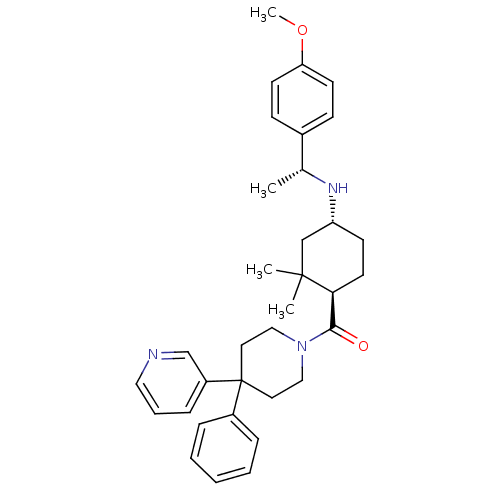 (((1R,4R)-4-((R)-1-(4-methoxyphenyl)ethylamino)-2,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y1 receptor expressed in CHO (NFAT-bla) cells by scintillation counting | Bioorg Med Chem Lett 19: 4781-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.050 BindingDB Entry DOI: 10.7270/Q2319VXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244373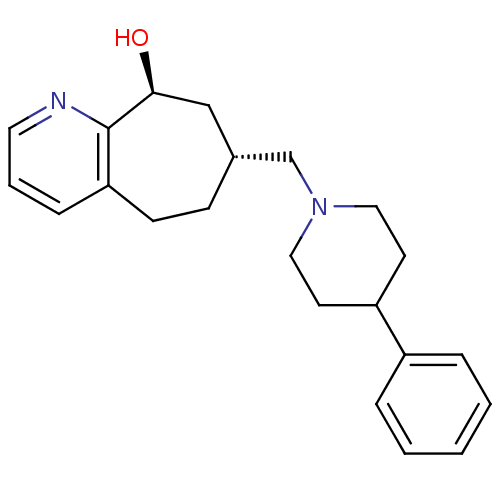 ((7R,9S)-7-((4-phenylpiperidin-1-yl)methyl)-6,7,8,9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50296876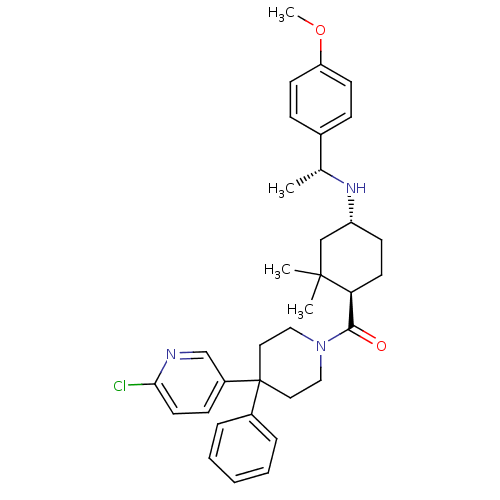 ((4-(6-chloropyridin-3-yl)-4-phenylpiperidin-1-yl)(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y1 receptor expressed in CHO (NFAT-bla) cells by scintillation counting | Bioorg Med Chem Lett 19: 4781-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.050 BindingDB Entry DOI: 10.7270/Q2319VXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50296872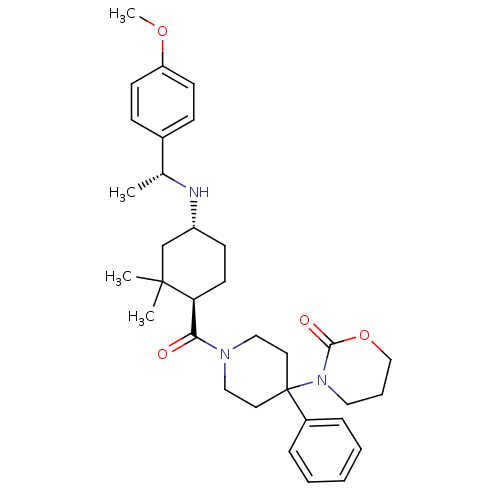 (3-(1-((1R,4R)-4-((R)-1-(4-methoxyphenyl)ethylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y1 receptor expressed in CHO (NFAT-bla) cells by scintillation counting | Bioorg Med Chem Lett 19: 4781-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.050 BindingDB Entry DOI: 10.7270/Q2319VXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127493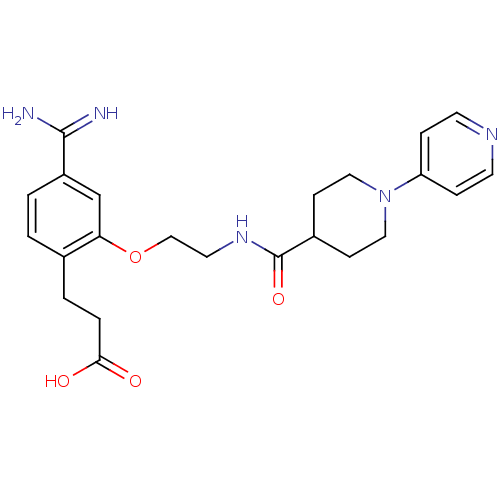 (3-(4-Carbamimidoyl-2-{2-[(3,4,5,6-tetrahydro-2H-[1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50296880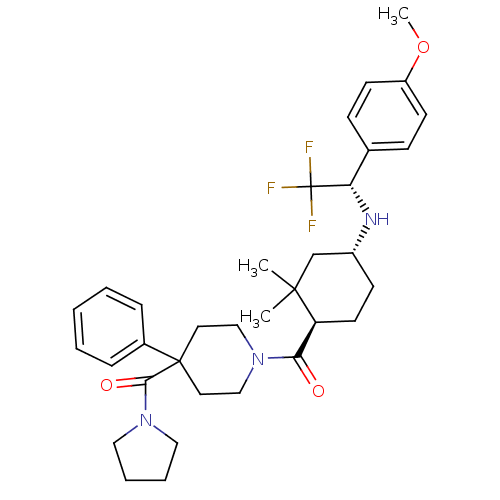 ((1-((1R,4R)-2,2-dimethyl-4-((S)-2,2,2-trifluoro-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y1 receptor expressed in CHO (NFAT-bla) cells by scintillation counting | Bioorg Med Chem Lett 19: 4781-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.050 BindingDB Entry DOI: 10.7270/Q2319VXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50296882 ((1-((1R,4R)-4-((R)-1-(4-fluorophenyl)ethylamino)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y1 receptor expressed in CHO (NFAT-bla) cells by scintillation counting | Bioorg Med Chem Lett 19: 4781-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.050 BindingDB Entry DOI: 10.7270/Q2319VXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50296871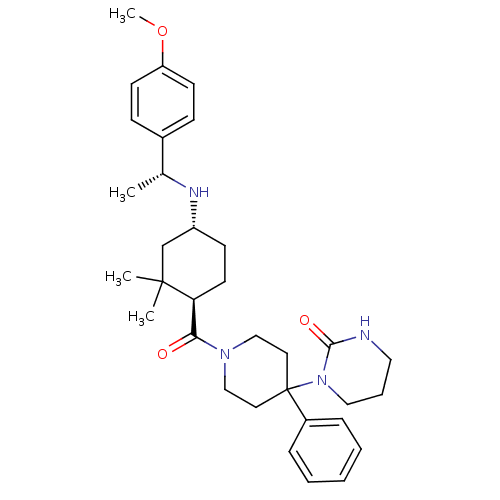 (1-(1-((1R,4R)-4-((R)-1-(4-methoxyphenyl)ethylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y1 receptor expressed in CHO (NFAT-bla) cells by scintillation counting | Bioorg Med Chem Lett 19: 4781-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.050 BindingDB Entry DOI: 10.7270/Q2319VXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50296875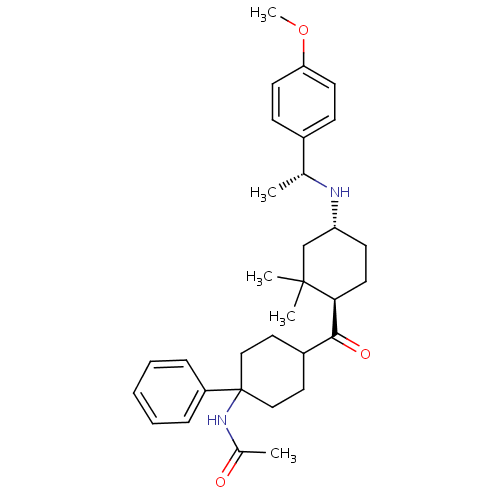 (CHEMBL556915 | N-(4-((1R,4R)-4-((R)-1-(4-methoxyph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y1 receptor expressed in CHO (NFAT-bla) cells by scintillation counting | Bioorg Med Chem Lett 19: 4781-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.050 BindingDB Entry DOI: 10.7270/Q2319VXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244333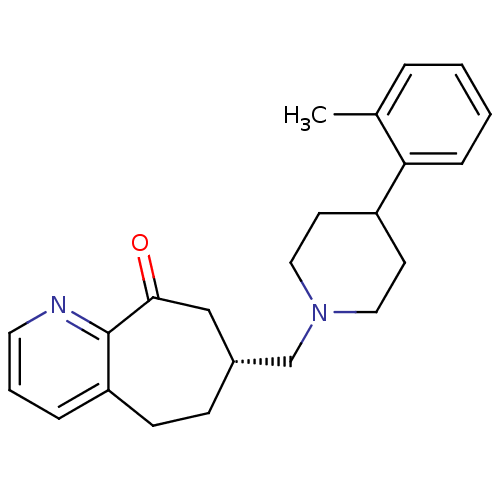 ((R)-7-((4-o-tolylpiperidin-1-yl)methyl)-5,6,7,8-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50296874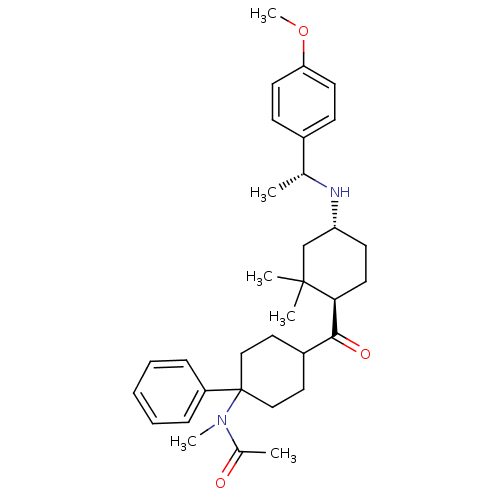 (CHEMBL561193 | N-(4-((1R,4R)-4-((R)-1-(4-methoxyph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y1 receptor expressed in CHO (NFAT-bla) cells by scintillation counting | Bioorg Med Chem Lett 19: 4781-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.050 BindingDB Entry DOI: 10.7270/Q2319VXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243797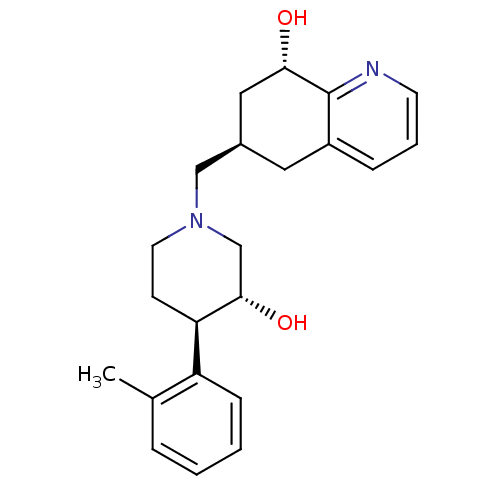 ((6R,8S)-6-(((3R,4R)-3-hydroxy-4-o-tolylpiperidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244374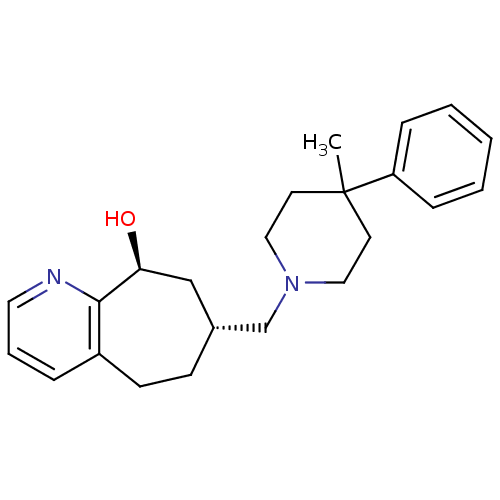 ((7R,9S)-7-((4-methyl-4-phenylpiperidin-1-yl)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50296894 (((1R,4R)-4-((R)-1-(4-methoxyphenyl)ethylamino)-2,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y1 receptor expressed in CHO (NFAT-bla) cells by scintillation counting | Bioorg Med Chem Lett 19: 4781-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.050 BindingDB Entry DOI: 10.7270/Q2319VXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50296891 ((1-((1R,4R)-4-((R)-1-(4-methoxyphenyl)ethylamino)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y1 receptor expressed in CHO (NFAT-bla) cells by scintillation counting | Bioorg Med Chem Lett 19: 4781-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.050 BindingDB Entry DOI: 10.7270/Q2319VXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50296888 ((1-((1R,4R)-2,2-dimethyl-4-((R)-1-phenylethylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y1 receptor expressed in CHO (NFAT-bla) cells by scintillation counting | Bioorg Med Chem Lett 19: 4781-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.050 BindingDB Entry DOI: 10.7270/Q2319VXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127496 (CHEMBL51796 | N-[2-(3-Carbamimidoyl-phenoxy)-ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 650 total ) | Next | Last >> |