 Found 1356 hits with Last Name = 'cai' and Initial = 'y'
Found 1356 hits with Last Name = 'cai' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50149381
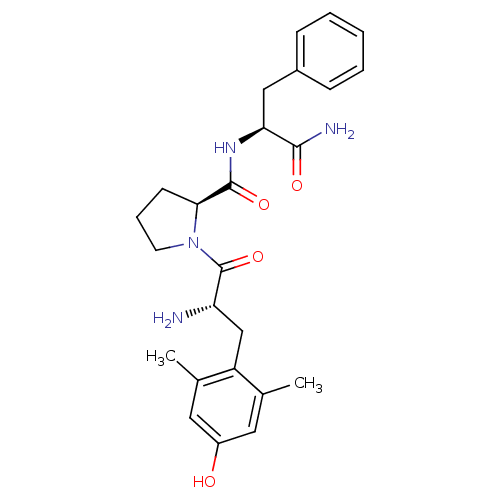
(1-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-p...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C25H32N4O4/c1-15-11-18(30)12-16(2)19(15)14-20(26)25(33)29-10-6-9-22(29)24(32)28-21(23(27)31)13-17-7-4-3-5-8-17/h3-5,7-8,11-12,20-22,30H,6,9-10,13-14,26H2,1-2H3,(H2,27,31)(H,28,32)/t20-,21-,22-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor (unknown origin) |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50009271

(CHEMBL3233014)Show SMILES Cc1cc(C)c(C[C@H](NC(=O)[C@@H]2CCCN2C(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(N)=O)c(C)c1 |r| Show InChI InChI=1S/C28H38N4O4/c1-15-9-16(2)22(17(3)10-15)14-24(26(30)34)31-27(35)25-7-6-8-32(25)28(36)23(29)13-21-18(4)11-20(33)12-19(21)5/h9-12,23-25,33H,6-8,13-14,29H2,1-5H3,(H2,30,34)(H,31,35)/t23-,24-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membranes |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50010483
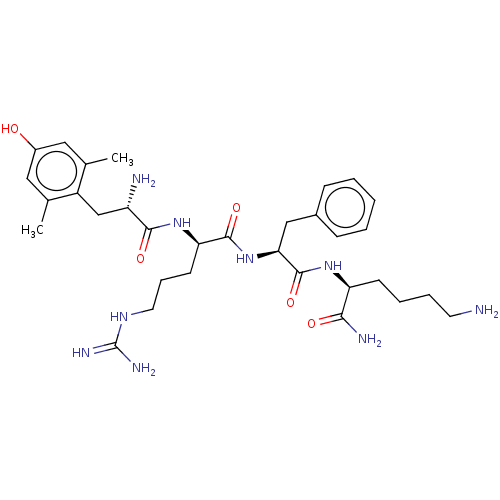
(CHEMBL2181202)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(N)=O |r| Show InChI InChI=1S/C32H49N9O5/c1-19-15-22(42)16-20(2)23(19)18-24(34)29(44)40-26(12-8-14-38-32(36)37)30(45)41-27(17-21-9-4-3-5-10-21)31(46)39-25(28(35)43)11-6-7-13-33/h3-5,9-10,15-16,24-27,42H,6-8,11-14,17-18,33-34H2,1-2H3,(H2,35,43)(H,39,46)(H,40,44)(H,41,45)(H4,36,37,38)/t24-,25-,26+,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-type opioid receptor in rat brain membranes incubated for 2 hrs |
Bioorg Med Chem Lett 26: 3629-31 (2016)
Article DOI: 10.1016/j.bmcl.2016.06.003
BindingDB Entry DOI: 10.7270/Q2ZW1NVQ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50009272
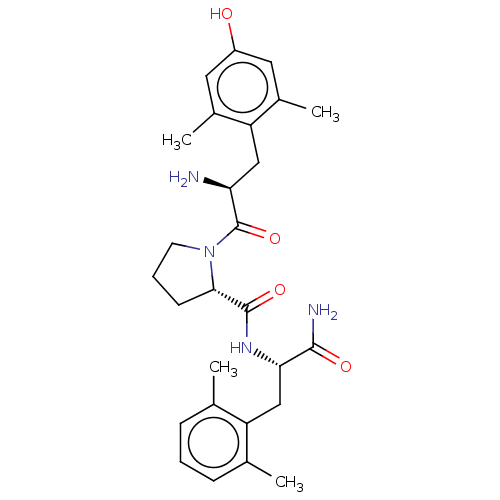
(CHEMBL3233200)Show SMILES Cc1cccc(C)c1C[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(N)=O |r| Show InChI InChI=1S/C27H36N4O4/c1-15-7-5-8-16(2)21(15)14-23(25(29)33)30-26(34)24-9-6-10-31(24)27(35)22(28)13-20-17(3)11-19(32)12-18(20)4/h5,7-8,11-12,22-24,32H,6,9-10,13-14,28H2,1-4H3,(H2,29,33)(H,30,34)/t22-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membranes |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50009268

(CHEMBL3233197)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r| Show InChI InChI=1S/C27H33N5O4/c1-15-10-18(33)11-16(2)20(15)13-21(28)27(36)32-9-5-8-24(32)26(35)31-23(25(29)34)12-17-14-30-22-7-4-3-6-19(17)22/h3-4,6-7,10-11,14,21,23-24,30,33H,5,8-9,12-13,28H2,1-2H3,(H2,29,34)(H,31,35)/t21-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membranes |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334730
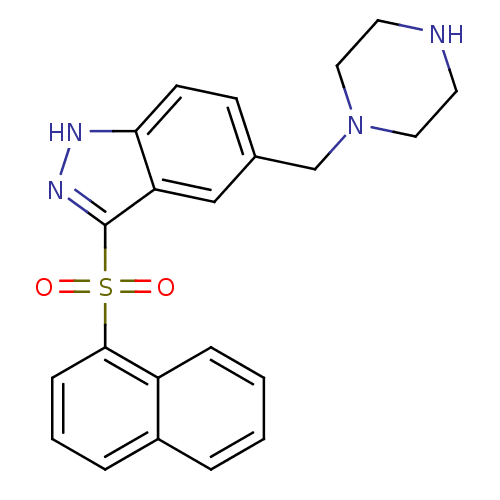
(3-(Naphthalen-1-ylsulfonyl)-5-(piperazin-1-ylmethy...)Show SMILES O=S(=O)(c1n[nH]c2ccc(CN3CCNCC3)cc12)c1cccc2ccccc12 Show InChI InChI=1S/C22H22N4O2S/c27-29(28,21-7-3-5-17-4-1-2-6-18(17)21)22-19-14-16(8-9-20(19)24-25-22)15-26-12-10-23-11-13-26/h1-9,14,23H,10-13,15H2,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50414600
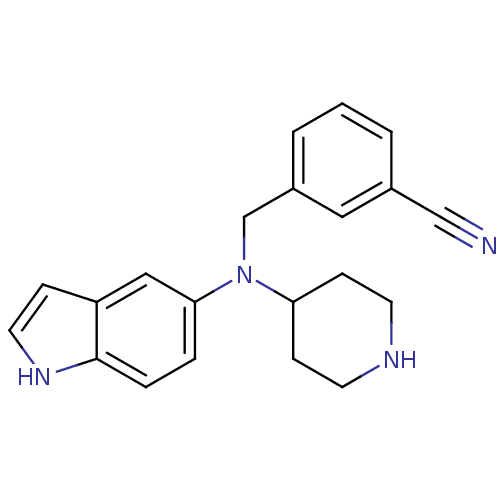
(CHEMBL570317)Show InChI InChI=1S/C21H22N4/c22-14-16-2-1-3-17(12-16)15-25(19-7-9-23-10-8-19)20-4-5-21-18(13-20)6-11-24-21/h1-6,11-13,19,23-24H,7-10,15H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of SERT |
Bioorg Med Chem Lett 19: 4630-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.076
BindingDB Entry DOI: 10.7270/Q2WH2R7V |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50009266
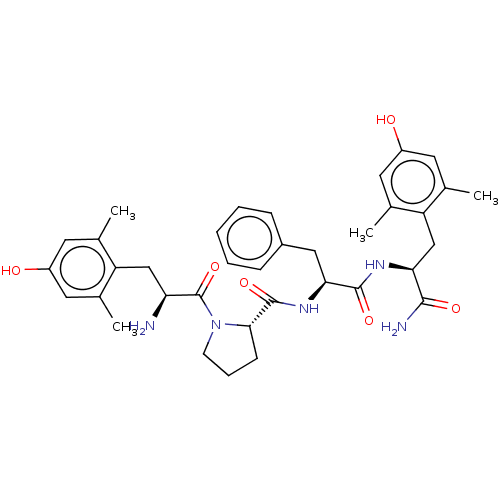
(CHEMBL3233195)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1c(C)cc(O)cc1C)C(N)=O |r| Show InChI InChI=1S/C36H45N5O6/c1-20-13-25(42)14-21(2)27(20)18-29(37)36(47)41-12-8-11-32(41)35(46)40-31(17-24-9-6-5-7-10-24)34(45)39-30(33(38)44)19-28-22(3)15-26(43)16-23(28)4/h5-7,9-10,13-16,29-32,42-43H,8,11-12,17-19,37H2,1-4H3,(H2,38,44)(H,39,45)(H,40,46)/t29-,30-,31-,32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membranes |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50009251

(CHEMBL3233191)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cccc2ccccc12)C(N)=O |r| Show InChI InChI=1S/C38H43N5O5/c1-23-18-28(44)19-24(2)30(23)22-31(39)38(48)43-17-9-16-34(43)37(47)42-33(20-25-10-4-3-5-11-25)36(46)41-32(35(40)45)21-27-14-8-13-26-12-6-7-15-29(26)27/h3-8,10-15,18-19,31-34,44H,9,16-17,20-22,39H2,1-2H3,(H2,40,45)(H,41,46)(H,42,47)/t31-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membranes |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50009265

(CHEMBL3233194)Show SMILES Cc1cccc(C)c1C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(N)=O |r| Show InChI InChI=1S/C36H45N5O5/c1-21-10-8-11-22(2)28(21)20-30(33(38)43)39-34(44)31(18-25-12-6-5-7-13-25)40-35(45)32-14-9-15-41(32)36(46)29(37)19-27-23(3)16-26(42)17-24(27)4/h5-8,10-13,16-17,29-32,42H,9,14-15,18-20,37H2,1-4H3,(H2,38,43)(H,39,44)(H,40,45)/t29-,30-,31-,32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membranes |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50166065
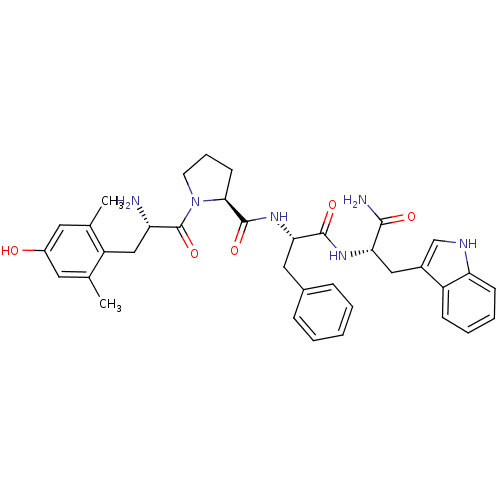
((S)-1-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-pheny...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O Show InChI InChI=1S/C36H42N6O5/c1-21-15-25(43)16-22(2)27(21)19-28(37)36(47)42-14-8-13-32(42)35(46)41-31(17-23-9-4-3-5-10-23)34(45)40-30(33(38)44)18-24-20-39-29-12-7-6-11-26(24)29/h3-7,9-12,15-16,20,28,30-32,39,43H,8,13-14,17-19,37H2,1-2H3,(H2,38,44)(H,40,45)(H,41,46)/t28-,30-,31-,32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membranes |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50185754

(CHEMBL3822676)Show SMILES CCc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(N)=O |r| Show InChI InChI=1S/C33H51N9O5/c1-3-22-18-23(43)16-20(2)24(22)19-25(35)30(45)41-27(13-9-15-39-33(37)38)31(46)42-28(17-21-10-5-4-6-11-21)32(47)40-26(29(36)44)12-7-8-14-34/h4-6,10-11,16,18,25-28,43H,3,7-9,12-15,17,19,34-35H2,1-2H3,(H2,36,44)(H,40,47)(H,41,45)(H,42,46)(H4,37,38,39)/t25-,26-,27+,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.367 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-type opioid receptor in rat brain membranes incubated for 2 hrs |
Bioorg Med Chem Lett 26: 3629-31 (2016)
Article DOI: 10.1016/j.bmcl.2016.06.003
BindingDB Entry DOI: 10.7270/Q2ZW1NVQ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50009264
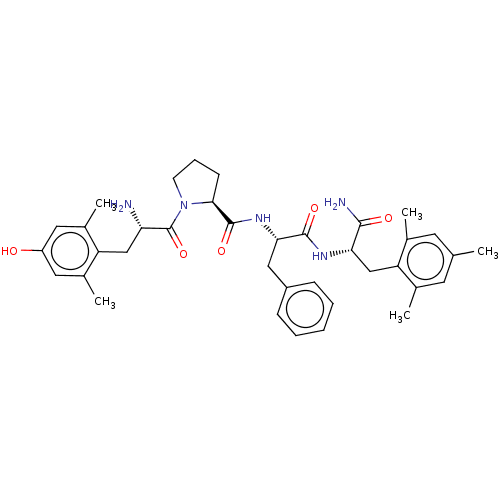
(CHEMBL3233193)Show SMILES Cc1cc(C)c(C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(N)=O)c(C)c1 |r| Show InChI InChI=1S/C37H47N5O5/c1-21-14-22(2)29(23(3)15-21)20-31(34(39)44)40-35(45)32(18-26-10-7-6-8-11-26)41-36(46)33-12-9-13-42(33)37(47)30(38)19-28-24(4)16-27(43)17-25(28)5/h6-8,10-11,14-17,30-33,43H,9,12-13,18-20,38H2,1-5H3,(H2,39,44)(H,40,45)(H,41,46)/t30-,31-,32-,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membranes |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50274953
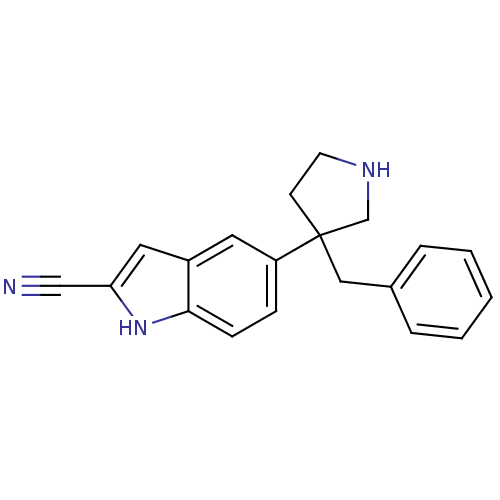
(5-(3-benzylpyrrolidin-3-yl)-1H-indole-2-carbonitri...)Show InChI InChI=1S/C20H19N3/c21-13-18-11-16-10-17(6-7-19(16)23-18)20(8-9-22-14-20)12-15-4-2-1-3-5-15/h1-7,10-11,22-23H,8-9,12,14H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of SERT (unknown origin) |
Bioorg Med Chem Lett 18: 6062-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.025
BindingDB Entry DOI: 10.7270/Q29886TF |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50185755
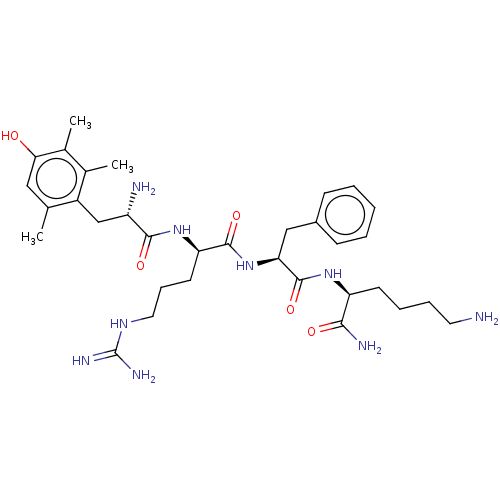
(CHEMBL3823054)Show SMILES Cc1cc(O)c(C)c(C)c1C[C@H](N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(N)=O |r| Show InChI InChI=1S/C33H51N9O5/c1-19-16-28(43)21(3)20(2)23(19)18-24(35)30(45)41-26(13-9-15-39-33(37)38)31(46)42-27(17-22-10-5-4-6-11-22)32(47)40-25(29(36)44)12-7-8-14-34/h4-6,10-11,16,24-27,43H,7-9,12-15,17-18,34-35H2,1-3H3,(H2,36,44)(H,40,47)(H,41,45)(H,42,46)(H4,37,38,39)/t24-,25-,26+,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.458 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-type opioid receptor in rat brain membranes incubated for 2 hrs |
Bioorg Med Chem Lett 26: 3629-31 (2016)
Article DOI: 10.1016/j.bmcl.2016.06.003
BindingDB Entry DOI: 10.7270/Q2ZW1NVQ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50009267
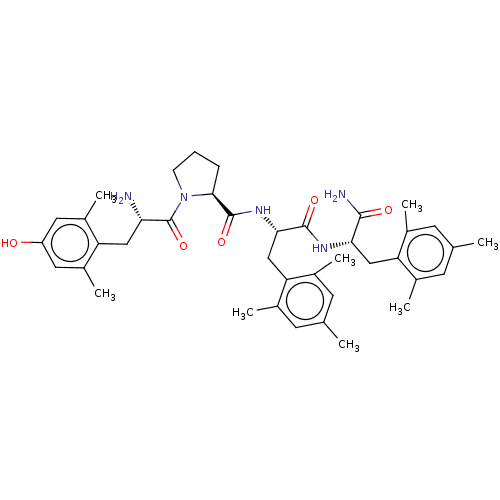
(CHEMBL3233196)Show SMILES Cc1cc(C)c(C[C@H](NC(=O)[C@H](Cc2c(C)cc(C)cc2C)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(N)=O)c(C)c1 |r| Show InChI InChI=1S/C40H53N5O5/c1-21-12-23(3)31(24(4)13-21)19-34(37(42)47)43-38(48)35(20-32-25(5)14-22(2)15-26(32)6)44-39(49)36-10-9-11-45(36)40(50)33(41)18-30-27(7)16-29(46)17-28(30)8/h12-17,33-36,46H,9-11,18-20,41H2,1-8H3,(H2,42,47)(H,43,48)(H,44,49)/t33-,34-,35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membranes |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM84745

(CAS_136434-34-9 | DULOXETINE | LY-248686 | LY24868...)Show InChI InChI=1S/C18H19NOS/c1-19-12-11-17(18-10-5-13-21-18)20-16-9-4-7-14-6-2-3-8-15(14)16/h2-10,13,17,19H,11-12H2,1H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of SERT |
Bioorg Med Chem Lett 19: 4630-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.076
BindingDB Entry DOI: 10.7270/Q2WH2R7V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334734
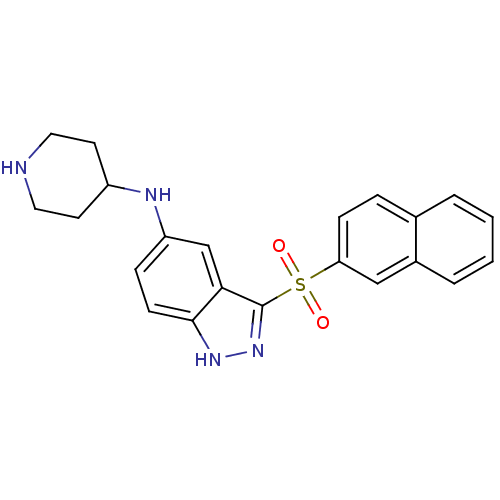
(3-(Naphthalen-2-ylsulfonyl)-N-(piperidin-4-yl)-1H-...)Show SMILES O=S(=O)(c1n[nH]c2ccc(NC3CCNCC3)cc12)c1ccc2ccccc2c1 Show InChI InChI=1S/C22H22N4O2S/c27-29(28,19-7-5-15-3-1-2-4-16(15)13-19)22-20-14-18(6-8-21(20)25-26-22)24-17-9-11-23-12-10-17/h1-8,13-14,17,23-24H,9-12H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334731
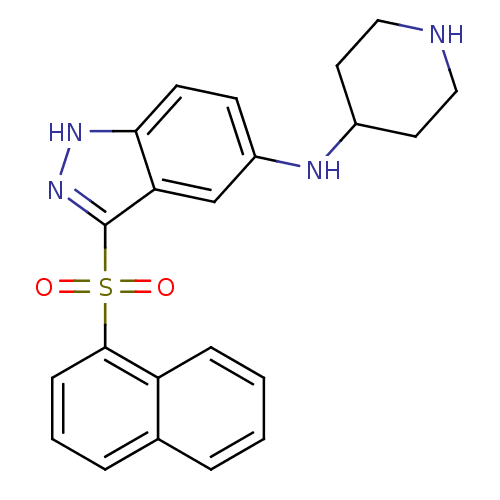
(CHEMBL1642851 | [3-(Naphthalen-1-sulfonyl)-1H-inda...)Show SMILES O=S(=O)(c1n[nH]c2ccc(NC3CCNCC3)cc12)c1cccc2ccccc12 Show InChI InChI=1S/C22H22N4O2S/c27-29(28,21-7-3-5-15-4-1-2-6-18(15)21)22-19-14-17(8-9-20(19)25-26-22)24-16-10-12-23-13-11-16/h1-9,14,16,23-24H,10-13H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50009269
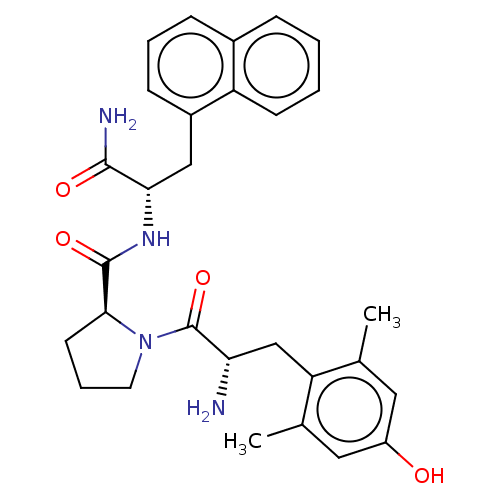
(CHEMBL3233198)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1cccc2ccccc12)C(N)=O |r| Show InChI InChI=1S/C29H34N4O4/c1-17-13-21(34)14-18(2)23(17)16-24(30)29(37)33-12-6-11-26(33)28(36)32-25(27(31)35)15-20-9-5-8-19-7-3-4-10-22(19)20/h3-5,7-10,13-14,24-26,34H,6,11-12,15-16,30H2,1-2H3,(H2,31,35)(H,32,36)/t24-,25-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membranes |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50414593
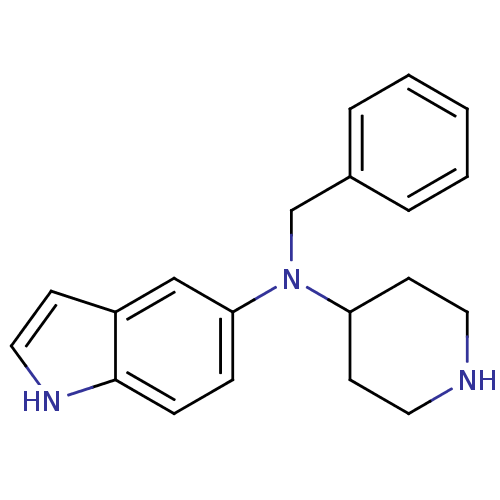
(CHEMBL560535)Show InChI InChI=1S/C20H23N3/c1-2-4-16(5-3-1)15-23(18-9-11-21-12-10-18)19-6-7-20-17(14-19)8-13-22-20/h1-8,13-14,18,21-22H,9-12,15H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of SERT |
Bioorg Med Chem Lett 19: 4630-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.076
BindingDB Entry DOI: 10.7270/Q2WH2R7V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334754
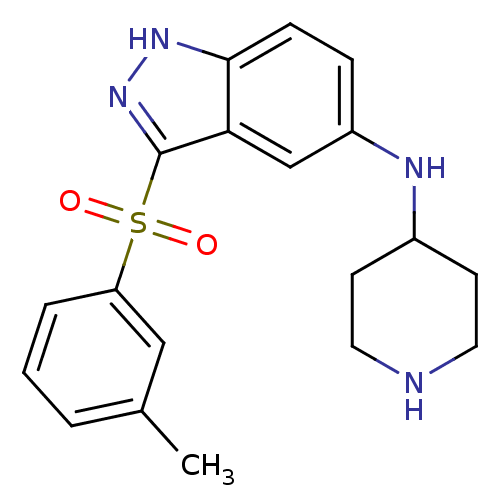
(CHEMBL1642883 | N-(Piperidin-4-yl)-3-(m-tolylsulfo...)Show SMILES Cc1cccc(c1)S(=O)(=O)c1n[nH]c2ccc(NC3CCNCC3)cc12 Show InChI InChI=1S/C19H22N4O2S/c1-13-3-2-4-16(11-13)26(24,25)19-17-12-15(5-6-18(17)22-23-19)21-14-7-9-20-10-8-14/h2-6,11-12,14,20-21H,7-10H2,1H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50009254

(CHEMBL3233192)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc2ccccc2c1)C(N)=O |r| Show InChI InChI=1S/C38H43N5O5/c1-23-17-29(44)18-24(2)30(23)22-31(39)38(48)43-16-8-13-34(43)37(47)42-33(20-25-9-4-3-5-10-25)36(46)41-32(35(40)45)21-26-14-15-27-11-6-7-12-28(27)19-26/h3-7,9-12,14-15,17-19,31-34,44H,8,13,16,20-22,39H2,1-2H3,(H2,40,45)(H,41,46)(H,42,47)/t31-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membranes |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50185752

(CHEMBL3822822)Show SMILES CCc1cc(O)cc(CC)c1C[C@H](N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(N)=O |r| Show InChI InChI=1S/C34H53N9O5/c1-3-22-18-24(44)19-23(4-2)25(22)20-26(36)31(46)42-28(14-10-16-40-34(38)39)32(47)43-29(17-21-11-6-5-7-12-21)33(48)41-27(30(37)45)13-8-9-15-35/h5-7,11-12,18-19,26-29,44H,3-4,8-10,13-17,20,35-36H2,1-2H3,(H2,37,45)(H,41,48)(H,42,46)(H,43,47)(H4,38,39,40)/t26-,27-,28+,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.875 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-type opioid receptor in rat brain membranes incubated for 2 hrs |
Bioorg Med Chem Lett 26: 3629-31 (2016)
Article DOI: 10.1016/j.bmcl.2016.06.003
BindingDB Entry DOI: 10.7270/Q2ZW1NVQ |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50274951
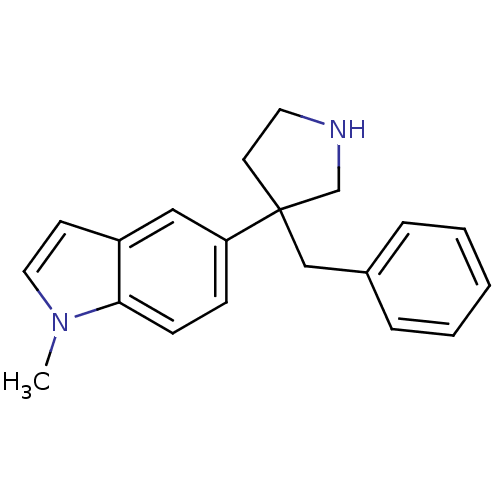
(5-(3-benzylpyrrolidin-3-yl)-1-methyl-1H-indole | C...)Show InChI InChI=1S/C20H22N2/c1-22-12-9-17-13-18(7-8-19(17)22)20(10-11-21-15-20)14-16-5-3-2-4-6-16/h2-9,12-13,21H,10-11,14-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of NET (unknown origin) |
Bioorg Med Chem Lett 18: 6062-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.025
BindingDB Entry DOI: 10.7270/Q29886TF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50275439
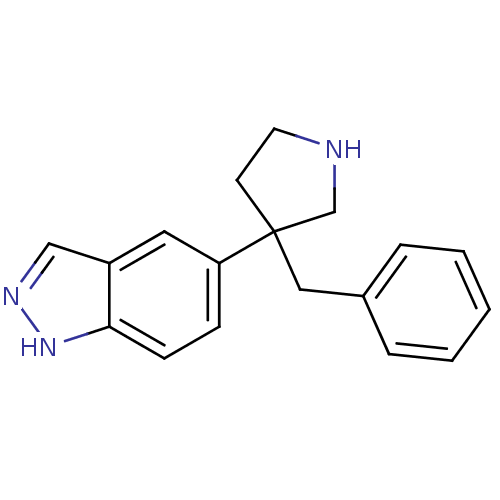
(5-(3-benzylpyrrolidin-3-yl)-1H-indazole | CHEMBL52...)Show InChI InChI=1S/C18H19N3/c1-2-4-14(5-3-1)11-18(8-9-19-13-18)16-6-7-17-15(10-16)12-20-21-17/h1-7,10,12,19H,8-9,11,13H2,(H,20,21) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of SERT (unknown origin) |
Bioorg Med Chem Lett 18: 6062-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.025
BindingDB Entry DOI: 10.7270/Q29886TF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334732
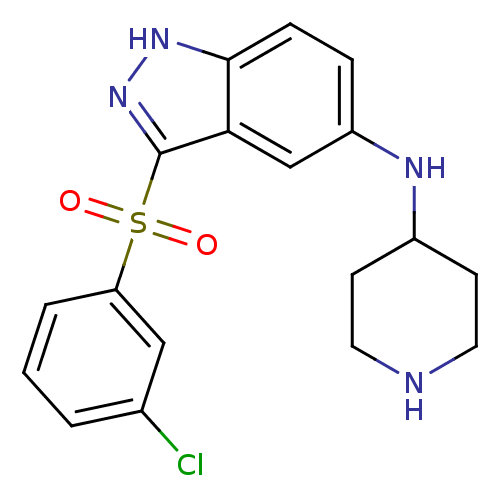
(3-(3-Chlorophenylsulfonyl)-N-(piperidin-4-yl)-1H-i...)Show SMILES Clc1cccc(c1)S(=O)(=O)c1n[nH]c2ccc(NC3CCNCC3)cc12 Show InChI InChI=1S/C18H19ClN4O2S/c19-12-2-1-3-15(10-12)26(24,25)18-16-11-14(4-5-17(16)22-23-18)21-13-6-8-20-9-7-13/h1-5,10-11,13,20-21H,6-9H2,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334733
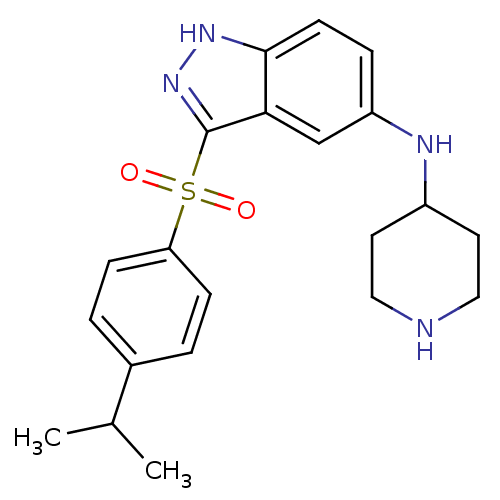
(3-(4-Isopropylphenylsulfonyl)-N-(piperidin-4-yl)-1...)Show SMILES CC(C)c1ccc(cc1)S(=O)(=O)c1n[nH]c2ccc(NC3CCNCC3)cc12 Show InChI InChI=1S/C21H26N4O2S/c1-14(2)15-3-6-18(7-4-15)28(26,27)21-19-13-17(5-8-20(19)24-25-21)23-16-9-11-22-12-10-16/h3-8,13-14,16,22-23H,9-12H2,1-2H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334725
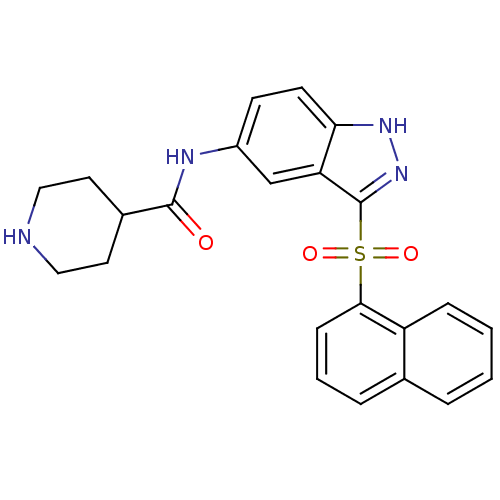
(CHEMBL1642866 | N-(3-(Naphthalen-1-ylsulfonyl)-1H-...)Show SMILES O=C(Nc1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12)C1CCNCC1 Show InChI InChI=1S/C23H22N4O3S/c28-22(16-10-12-24-13-11-16)25-17-8-9-20-19(14-17)23(27-26-20)31(29,30)21-7-3-5-15-4-1-2-6-18(15)21/h1-9,14,16,24H,10-13H2,(H,25,28)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50414583
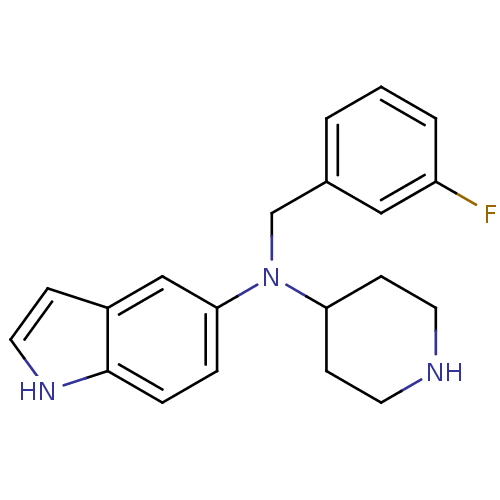
(CHEMBL564094)Show InChI InChI=1S/C20H22FN3/c21-17-3-1-2-15(12-17)14-24(18-7-9-22-10-8-18)19-4-5-20-16(13-19)6-11-23-20/h1-6,11-13,18,22-23H,7-10,14H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of SERT |
Bioorg Med Chem Lett 19: 4630-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.076
BindingDB Entry DOI: 10.7270/Q2WH2R7V |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50414590
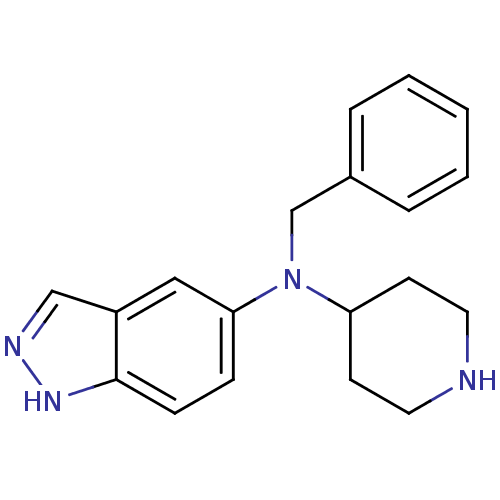
(CHEMBL561598)Show InChI InChI=1S/C19H22N4/c1-2-4-15(5-3-1)14-23(17-8-10-20-11-9-17)18-6-7-19-16(12-18)13-21-22-19/h1-7,12-13,17,20H,8-11,14H2,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of SERT |
Bioorg Med Chem Lett 19: 4630-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.076
BindingDB Entry DOI: 10.7270/Q2WH2R7V |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50139013

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C32H37N5O5/c33-25(18-23-13-15-24(38)16-14-23)32(42)37-17-7-12-28(37)31(41)36-27(20-22-10-5-2-6-11-22)30(40)35-26(29(34)39)19-21-8-3-1-4-9-21/h1-6,8-11,13-16,25-28,38H,7,12,17-20,33H2,(H2,34,39)(H,35,40)(H,36,41)/t25-,26-,27-,28-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Binding affinity to mu opioid receptor (unknown origin) |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334747
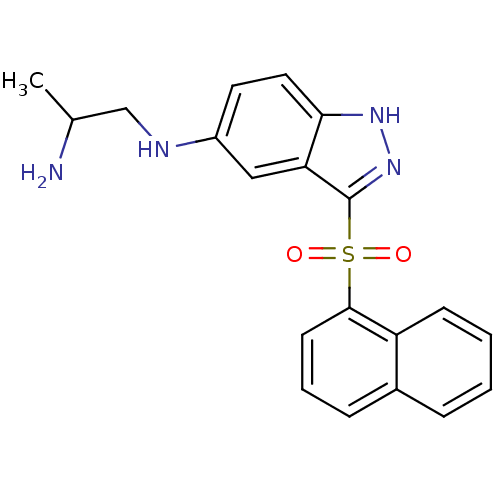
(CHEMBL1642849 | N1-(3-(Naphthalen-1-ylsulfonyl)-1H...)Show SMILES CC(N)CNc1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C20H20N4O2S/c1-13(21)12-22-15-9-10-18-17(11-15)20(24-23-18)27(25,26)19-8-4-6-14-5-2-3-7-16(14)19/h2-11,13,22H,12,21H2,1H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334720
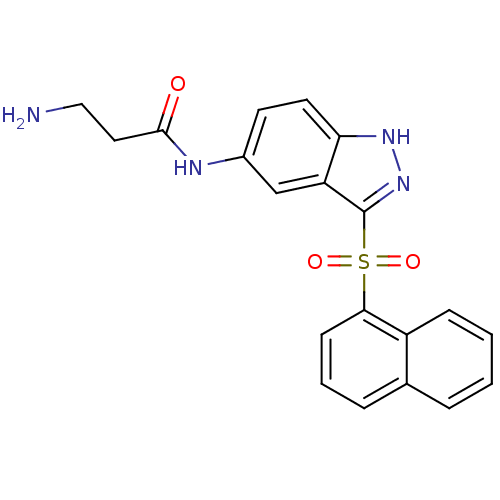
(3-Amino-N-[3-(naphthalen-1-sulfonyl)-1H-indazol-5-...)Show SMILES NCCC(=O)Nc1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C20H18N4O3S/c21-11-10-19(25)22-14-8-9-17-16(12-14)20(24-23-17)28(26,27)18-7-3-5-13-4-1-2-6-15(13)18/h1-9,12H,10-11,21H2,(H,22,25)(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334757
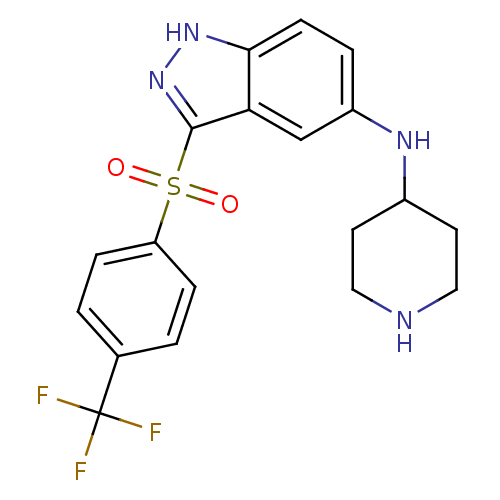
(CHEMBL1642887 | N-(Piperidin-4-yl)-3-(4-(trifluoro...)Show SMILES FC(F)(F)c1ccc(cc1)S(=O)(=O)c1n[nH]c2ccc(NC3CCNCC3)cc12 Show InChI InChI=1S/C19H19F3N4O2S/c20-19(21,22)12-1-4-15(5-2-12)29(27,28)18-16-11-14(3-6-17(16)25-26-18)24-13-7-9-23-10-8-13/h1-6,11,13,23-24H,7-10H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50414597
(CHEMBL550870)Show InChI InChI=1S/C21H25N3O/c1-25-20-4-2-3-16(13-20)15-24(18-8-10-22-11-9-18)19-5-6-21-17(14-19)7-12-23-21/h2-7,12-14,18,22-23H,8-11,15H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of SERT |
Bioorg Med Chem Lett 19: 4630-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.076
BindingDB Entry DOI: 10.7270/Q2WH2R7V |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50009267

(CHEMBL3233196)Show SMILES Cc1cc(C)c(C[C@H](NC(=O)[C@H](Cc2c(C)cc(C)cc2C)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(N)=O)c(C)c1 |r| Show InChI InChI=1S/C40H53N5O5/c1-21-12-23(3)31(24(4)13-21)19-34(37(42)47)43-38(48)35(20-32-25(5)14-22(2)15-26(32)6)44-39(49)36-10-9-11-45(36)40(50)33(41)18-30-27(7)16-29(46)17-28(30)8/h12-17,33-36,46H,9-11,18-20,41H2,1-8H3,(H2,42,47)(H,43,48)(H,44,49)/t33-,34-,35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]deltorphin-2 from delta opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membranes |
Bioorg Med Chem 22: 2208-19 (2014)
Article DOI: 10.1016/j.bmc.2014.02.015
BindingDB Entry DOI: 10.7270/Q29Z96DB |
More data for this
Ligand-Target Pair | |
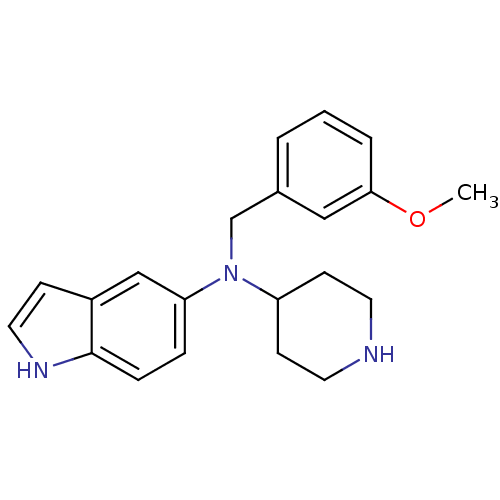
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334716
(3-(Naphthalen-1-ylsulfonyl)-N-(piperidin-5-yl)-1H-...)Show SMILES O=S(=O)(c1n[nH]c2ccc(NC3CCCNC3)cc12)c1cccc2ccccc12 Show InChI InChI=1S/C22H22N4O2S/c27-29(28,21-9-3-6-15-5-1-2-8-18(15)21)22-19-13-16(10-11-20(19)25-26-22)24-17-7-4-12-23-14-17/h1-3,5-6,8-11,13,17,23-24H,4,7,12,14H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50016867
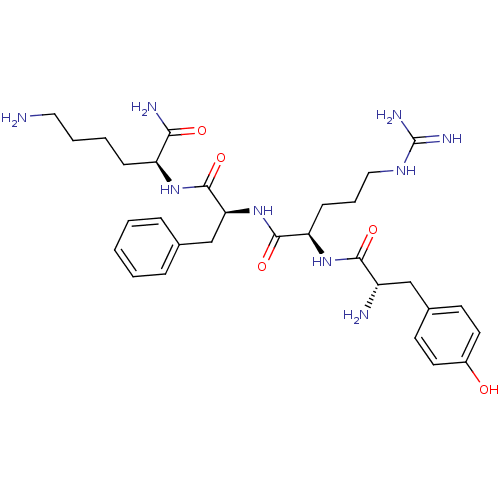
((S)-6-Amino-2-((S)-2-{(R)-2-[(S)-2-amino-3-(4-hydr...)Show SMILES NCCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C30H45N9O5/c31-15-5-4-9-23(26(33)41)37-29(44)25(18-19-7-2-1-3-8-19)39-28(43)24(10-6-16-36-30(34)35)38-27(42)22(32)17-20-11-13-21(40)14-12-20/h1-3,7-8,11-14,22-25,40H,4-6,9-10,15-18,31-32H2,(H2,33,41)(H,37,44)(H,38,42)(H,39,43)(H4,34,35,36)/t22-,23-,24+,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-type opioid receptor in rat brain membranes incubated for 2 hrs |
Bioorg Med Chem Lett 26: 3629-31 (2016)
Article DOI: 10.1016/j.bmcl.2016.06.003
BindingDB Entry DOI: 10.7270/Q2ZW1NVQ |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50048866

(1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...)Show InChI InChI=1S/C24H38N2O/c1-27-24-14-6-12-22-20(8-5-13-23(22)24)9-7-15-25-16-18-26(19-17-25)21-10-3-2-4-11-21/h6,12,14,20-21H,2-5,7-11,13,15-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pentazocin from the Sigma1 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50414602
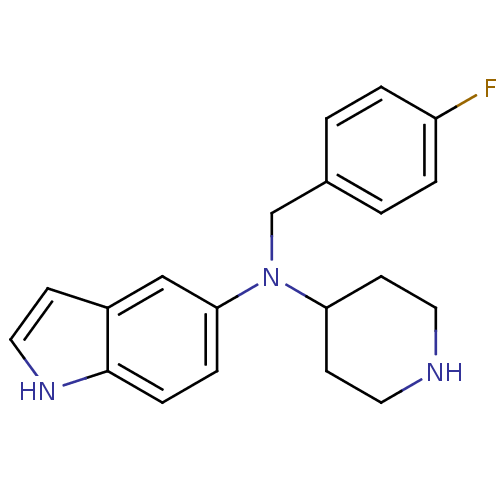
(CHEMBL551818)Show InChI InChI=1S/C20H22FN3/c21-17-3-1-15(2-4-17)14-24(18-8-10-22-11-9-18)19-5-6-20-16(13-19)7-12-23-20/h1-7,12-13,18,22-23H,8-11,14H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of SERT |
Bioorg Med Chem Lett 19: 4630-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.076
BindingDB Entry DOI: 10.7270/Q2WH2R7V |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50251208
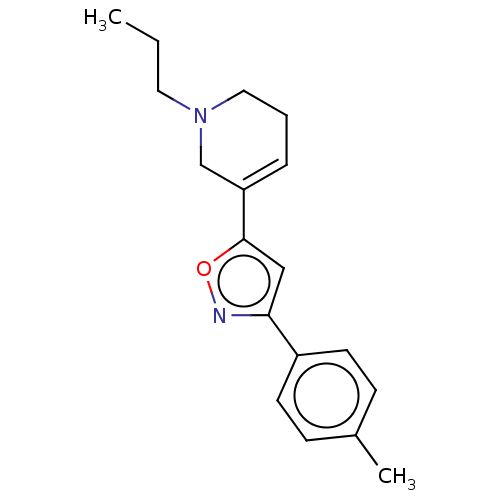
(CHEMBL4088272)Show InChI InChI=1S/C18H22N2O/c1-3-10-20-11-4-5-16(13-20)18-12-17(19-21-18)15-8-6-14(2)7-9-15/h5-9,12H,3-4,10-11,13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pentazocin from the Sigma1 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50274954
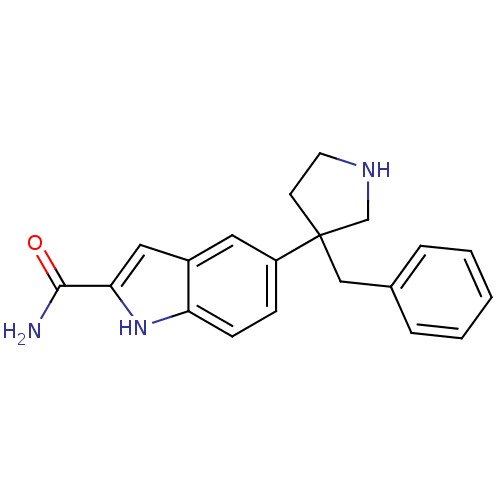
(5-(3-benzylpyrrolidin-3-yl)-1H-indole-2-carboxamid...)Show InChI InChI=1S/C20H21N3O/c21-19(24)18-11-15-10-16(6-7-17(15)23-18)20(8-9-22-13-20)12-14-4-2-1-3-5-14/h1-7,10-11,22-23H,8-9,12-13H2,(H2,21,24) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of SERT (unknown origin) |
Bioorg Med Chem Lett 18: 6062-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.025
BindingDB Entry DOI: 10.7270/Q29886TF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334756
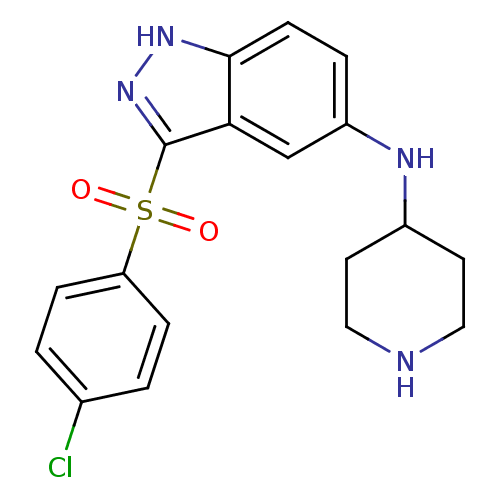
(3-(4-Chlorophenylsulfonyl)-N-(piperidin-4-yl)-1H-i...)Show SMILES Clc1ccc(cc1)S(=O)(=O)c1n[nH]c2ccc(NC3CCNCC3)cc12 Show InChI InChI=1S/C18H19ClN4O2S/c19-12-1-4-15(5-2-12)26(24,25)18-16-11-14(3-6-17(16)22-23-18)21-13-7-9-20-10-8-13/h1-6,11,13,20-21H,7-10H2,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50048866

(1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...)Show InChI InChI=1S/C24H38N2O/c1-27-24-14-6-12-22-20(8-5-13-23(22)24)9-7-15-25-16-18-26(19-17-25)21-10-3-2-4-11-21/h6,12,14,20-21H,2-5,7-11,13,15-19H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DTG from the Sigma2 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50414599
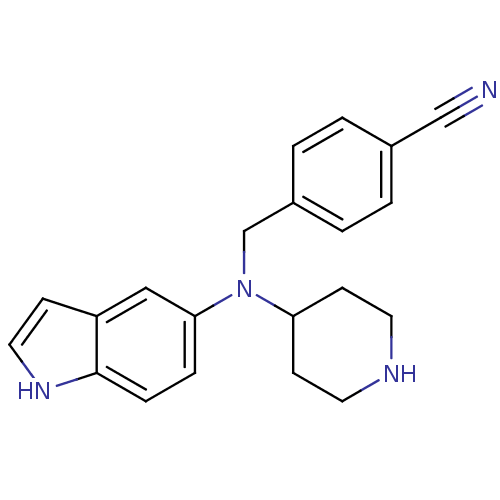
(CHEMBL564285)Show InChI InChI=1S/C21H22N4/c22-14-16-1-3-17(4-2-16)15-25(19-8-10-23-11-9-19)20-5-6-21-18(13-20)7-12-24-21/h1-7,12-13,19,23-24H,8-11,15H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of SERT |
Bioorg Med Chem Lett 19: 4630-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.076
BindingDB Entry DOI: 10.7270/Q2WH2R7V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334751
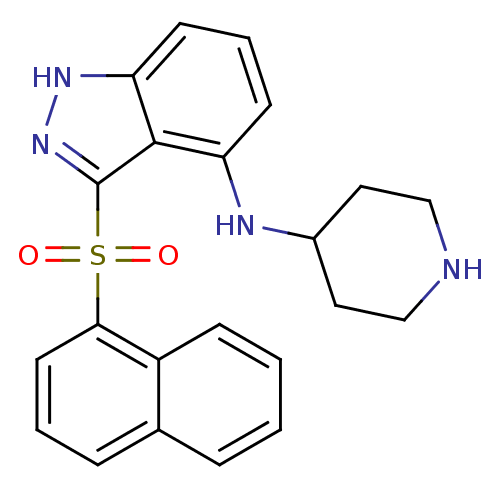
(3-(Naphthalen-1-ylsulfonyl)-N-(piperidin-4-yl)-1H-...)Show SMILES O=S(=O)(c1n[nH]c2cccc(NC3CCNCC3)c12)c1cccc2ccccc12 Show InChI InChI=1S/C22H22N4O2S/c27-29(28,20-10-3-6-15-5-1-2-7-17(15)20)22-21-18(8-4-9-19(21)25-26-22)24-16-11-13-23-14-12-16/h1-10,16,23-24H,11-14H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334727
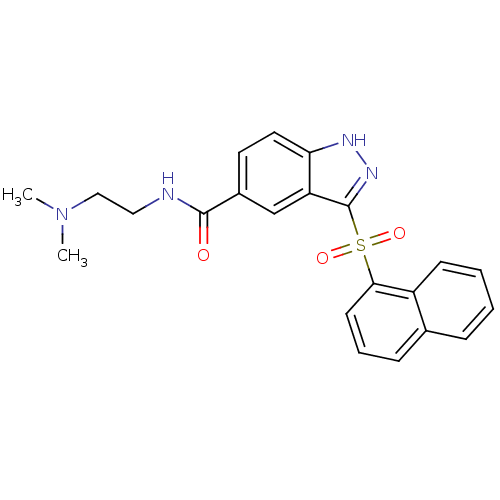
(CHEMBL1642878 | N-[2-(Dimethylamino)ethyl]-3-(1-na...)Show SMILES CN(C)CCNC(=O)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C22H22N4O3S/c1-26(2)13-12-23-21(27)16-10-11-19-18(14-16)22(25-24-19)30(28,29)20-9-5-7-15-6-3-4-8-17(15)20/h3-11,14H,12-13H2,1-2H3,(H,23,27)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50275440
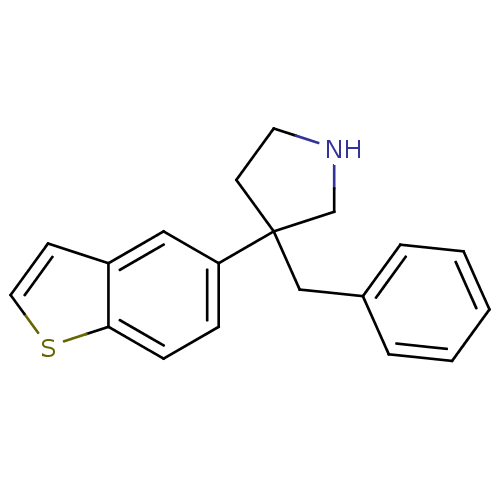
(3-(benzo[b]thiophen-5-yl)-3-benzylpyrrolidine | CH...)Show InChI InChI=1S/C19H19NS/c1-2-4-15(5-3-1)13-19(9-10-20-14-19)17-6-7-18-16(12-17)8-11-21-18/h1-8,11-12,20H,9-10,13-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of NET (unknown origin) |
Bioorg Med Chem Lett 18: 6062-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.025
BindingDB Entry DOI: 10.7270/Q29886TF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334752
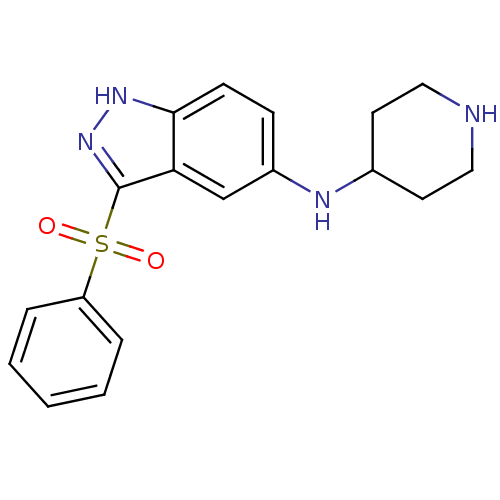
(3-(Phenylsulfonyl)-N-(piperidin-4-yl)-1H-indazol-5...)Show SMILES O=S(=O)(c1n[nH]c2ccc(NC3CCNCC3)cc12)c1ccccc1 Show InChI InChI=1S/C18H20N4O2S/c23-25(24,15-4-2-1-3-5-15)18-16-12-14(6-7-17(16)21-22-18)20-13-8-10-19-11-9-13/h1-7,12-13,19-20H,8-11H2,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data