

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM227253 (US9328106, 204) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00100 | -68.5 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
GENENTECH, INC. US Patent | Assay Description LC3K assay: PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Ch... | US Patent US9328106 (2016) BindingDB Entry DOI: 10.7270/Q29W0DBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM227334 (US9328106, 285) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00100 | -68.5 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
GENENTECH, INC. US Patent | Assay Description LC3K assay: PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Ch... | US Patent US9328106 (2016) BindingDB Entry DOI: 10.7270/Q29W0DBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM144940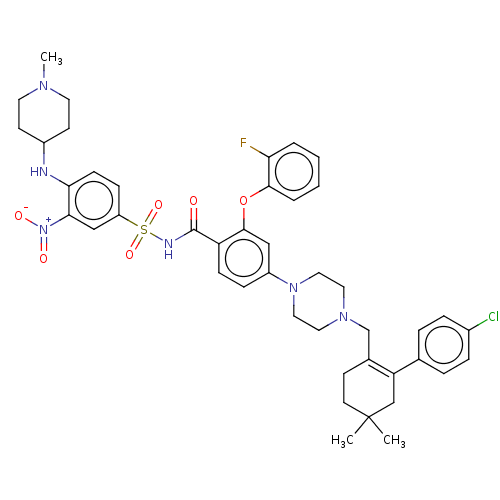 (US8952157, 122 | US9303025, 122) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AbbVie Inc. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 μM (2× starting concentration; 10% DMSO) and 10 μ... | US Patent US9303025 (2016) BindingDB Entry DOI: 10.7270/Q2XD10JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189797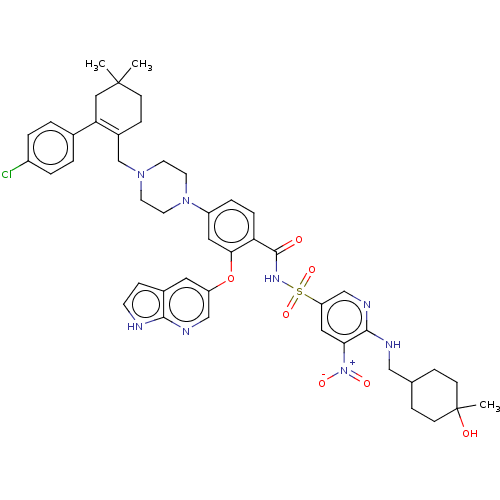 (US11369599, Compound 376 | US9174982, 371 | US9174...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9174982 (2015) BindingDB Entry DOI: 10.7270/Q2RB73D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189799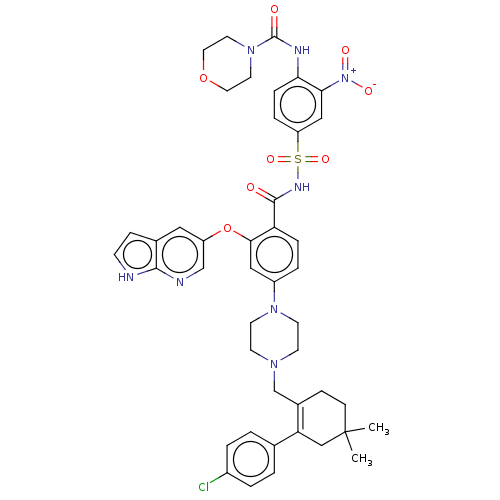 (US10213433, Compound 373 | US11369599, Compound 37...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9174982 (2015) BindingDB Entry DOI: 10.7270/Q2RB73D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189800 (US10213433, Compound 374 | US11369599, Compound 37...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9174982 (2015) BindingDB Entry DOI: 10.7270/Q2RB73D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189797 (US11369599, Compound 376 | US9174982, 371 | US9174...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9174982 (2015) BindingDB Entry DOI: 10.7270/Q2RB73D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189803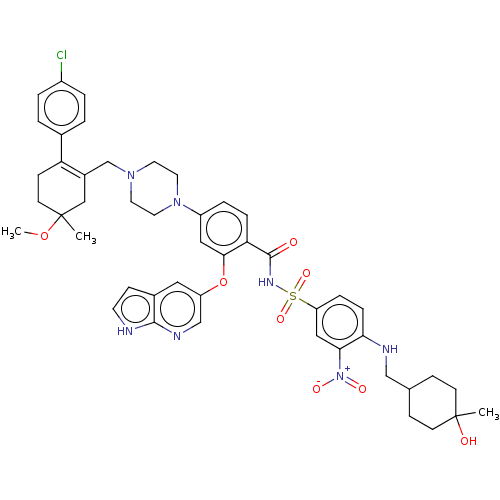 (US11369599, Compound 377 | US9174982, 377) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9174982 (2015) BindingDB Entry DOI: 10.7270/Q2RB73D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189804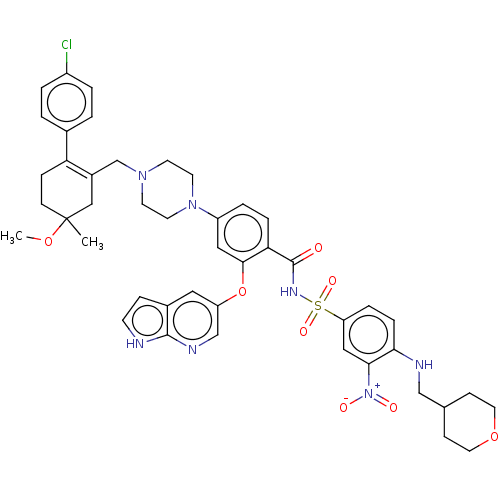 (US10213433, Compound 378 | US11369599, Compound 37...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9174982 (2015) BindingDB Entry DOI: 10.7270/Q2RB73D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178562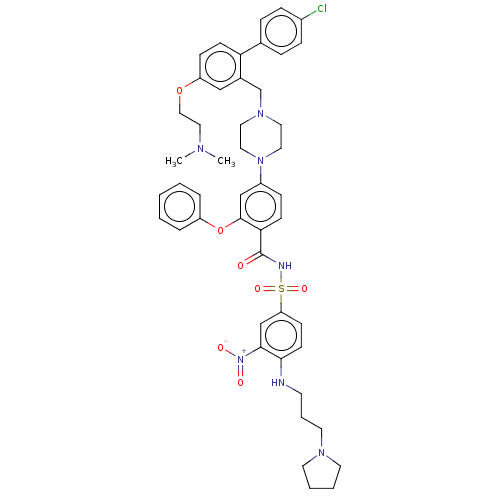 (US9125913, 121) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM356985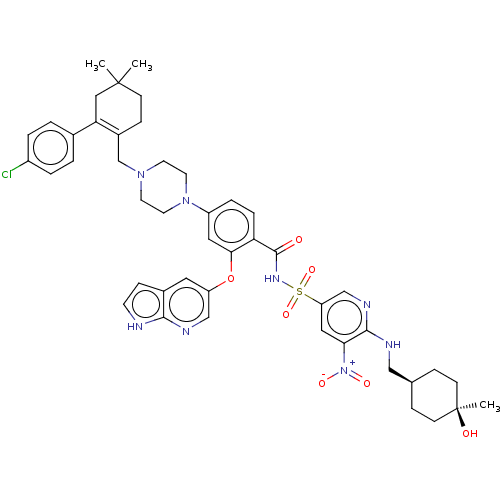 (4-(4-{[2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189799 (US10213433, Compound 373 | US11369599, Compound 37...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189800 (US10213433, Compound 374 | US11369599, Compound 37...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM356989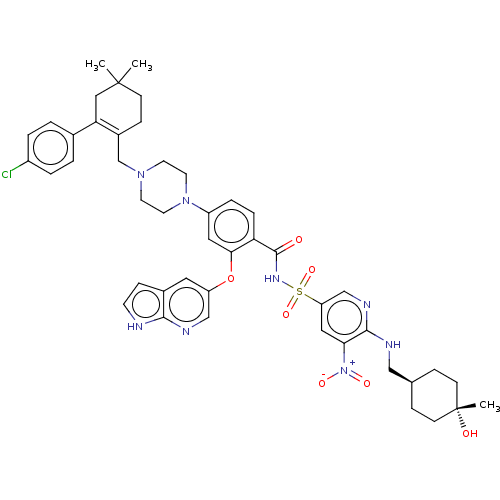 (4-(4-{[2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM356990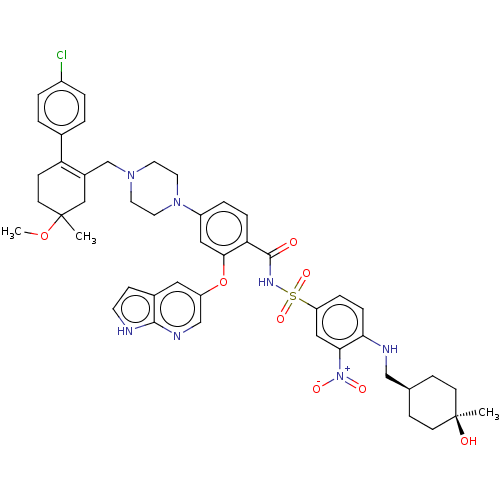 (4-(4-{[2-(4-chlorophenyl)-5-methoxy-5-methylcycloh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189804 (US10213433, Compound 378 | US11369599, Compound 37...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM451126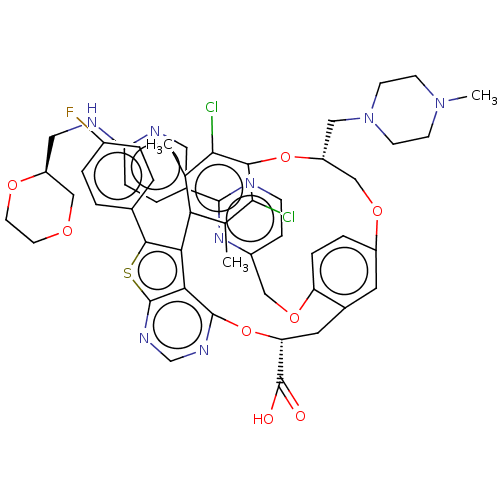 (US10676485, Example 36) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The ability of the exemplary MCL-1 inhibitors of Examples 1 through 151 to bind MCL-1 was demonstrated using the Time Resolved-Fluorescence Resonance... | US Patent US10676485 (2020) BindingDB Entry DOI: 10.7270/Q2PZ5CWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM227278 (US9328106, 229) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00100 | -68.5 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
GENENTECH, INC. US Patent | Assay Description LC3K assay: PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Ch... | US Patent US9328106 (2016) BindingDB Entry DOI: 10.7270/Q29W0DBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM172740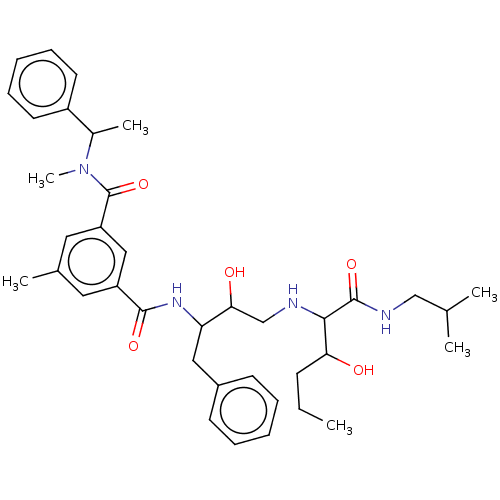 (US9096541, 113) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oklahoma Medical Research Foundation; Purdue Research Foundation US Patent | Assay Description Previously, one of the following compounds discussed below have been studied in memapsin 2 inhibition (Ghosh et al., 2008), which the others are prev... | US Patent US9096541 (2015) BindingDB Entry DOI: 10.7270/Q2TH8KF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50059997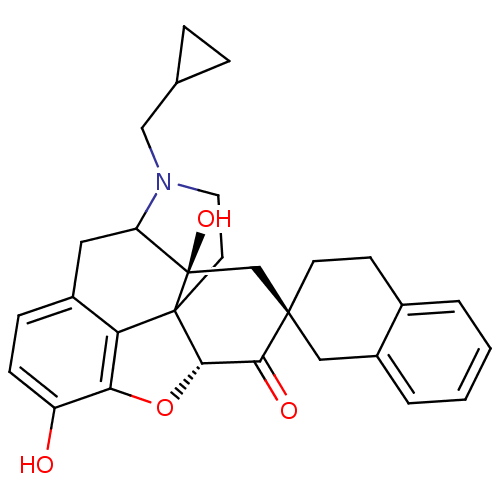 (7 beta-Spirobenzocyclohexylnaltrexone | CHEMBL1016...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of [3H]-NTI binding to Opioid receptor delta 1 from mouse brain membranes. | J Med Chem 40: 3064-70 (1997) Article DOI: 10.1021/jm970283e BindingDB Entry DOI: 10.7270/Q27D2T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM110797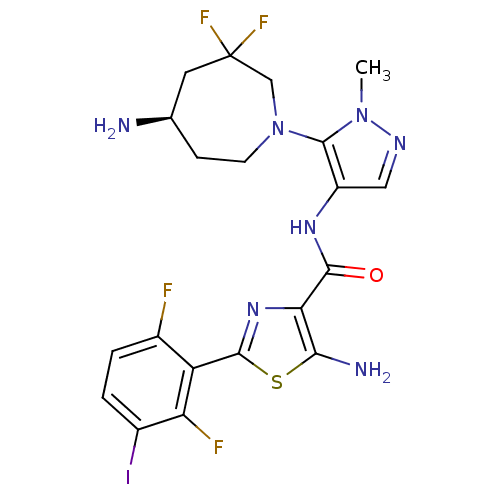 (US8614206, 346) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
F. Hoffmann-La Roche AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US8614206 (2013) BindingDB Entry DOI: 10.7270/Q2W66JFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM110798 (US8614206, 347) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
F. Hoffmann-La Roche AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US8614206 (2013) BindingDB Entry DOI: 10.7270/Q2W66JFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM227169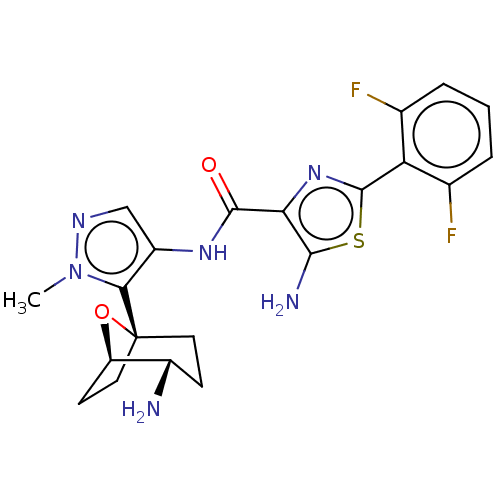 (US9328106, 117) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00200 | -66.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
GENENTECH, INC. US Patent | Assay Description LC3K assay: PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Ch... | US Patent US9328106 (2016) BindingDB Entry DOI: 10.7270/Q29W0DBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189567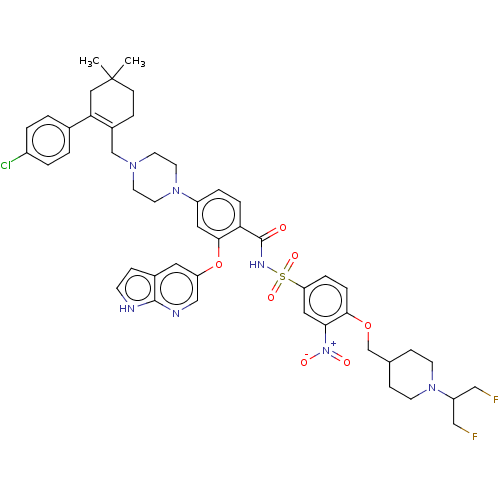 (US10213433, Compound 129 | US11369599, Compound 12...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00200 | -66.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9174982 (2015) BindingDB Entry DOI: 10.7270/Q2RB73D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178836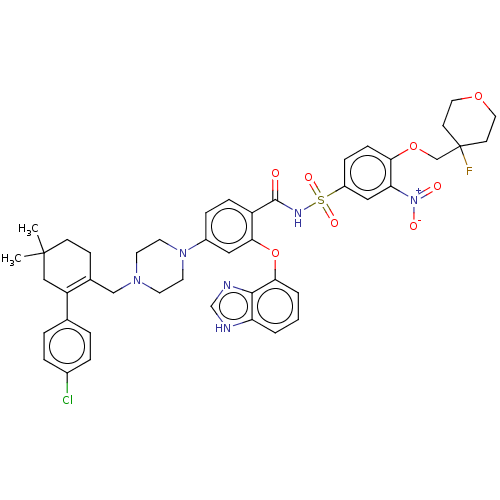 (US9125913, 408) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM227362 (US9328106, 313) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00200 | -66.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
GENENTECH, INC. US Patent | Assay Description LC3K assay: PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Ch... | US Patent US9328106 (2016) BindingDB Entry DOI: 10.7270/Q29W0DBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178855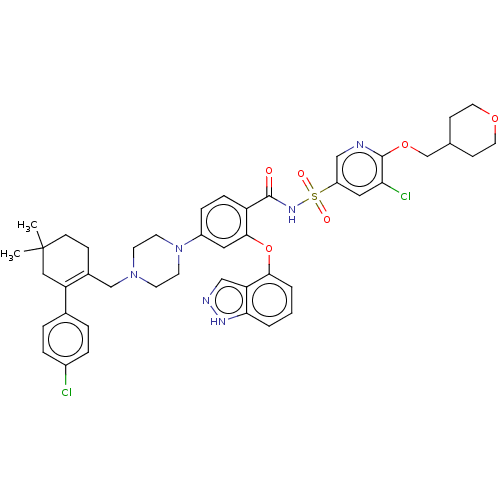 (US9125913, 427) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM110726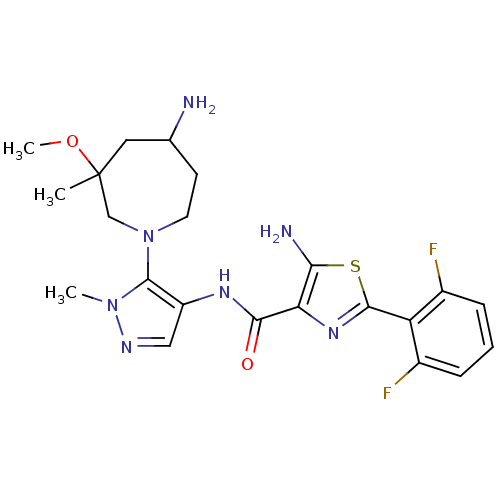 (US8614206, 252 | US8614206, 254 | US8614206, 352 |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
F. Hoffmann-La Roche AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US8614206 (2013) BindingDB Entry DOI: 10.7270/Q2W66JFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189567 (US10213433, Compound 129 | US11369599, Compound 12...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM393298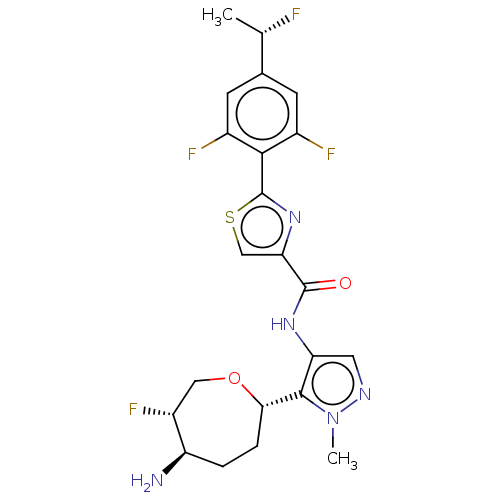 (N-(5-((2S,5R,6S)-5-amino-6- fluorooxepan-2-yl)-1-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description Determination of the Pim kinase activity of a Formula I compound is possible by a number of direct and indirect detection methods. Certain exemplary ... | Chem Biol Drug Des 71: 131-9 (2008) BindingDB Entry DOI: 10.7270/Q22B91B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM227443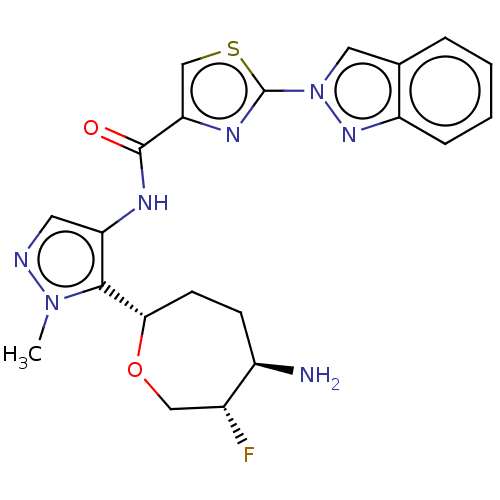 (US9328106, 396) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00200 | -66.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
GENENTECH, INC. US Patent | Assay Description LC3K assay: PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Ch... | US Patent US9328106 (2016) BindingDB Entry DOI: 10.7270/Q29W0DBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178843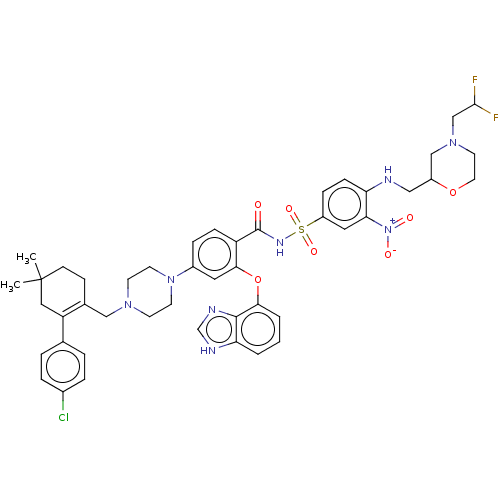 (US9125913, 415) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM227267 (US9328106, 218) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00224 | -66.5 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
GENENTECH, INC. US Patent | Assay Description LC3K assay: PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Ch... | US Patent US9328106 (2016) BindingDB Entry DOI: 10.7270/Q29W0DBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM14361 ((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.00230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM110712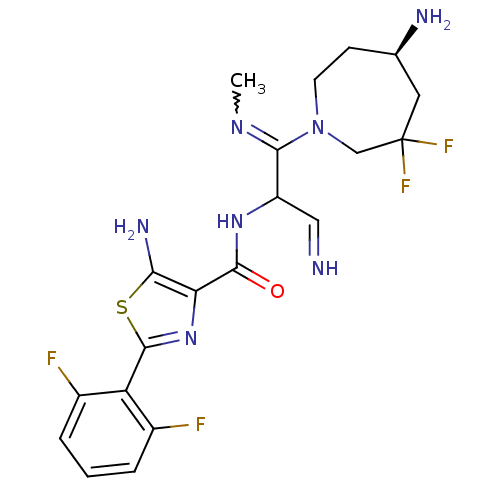 (US8614206, 167) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
F. Hoffmann-La Roche AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US8614206 (2013) BindingDB Entry DOI: 10.7270/Q2W66JFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50053685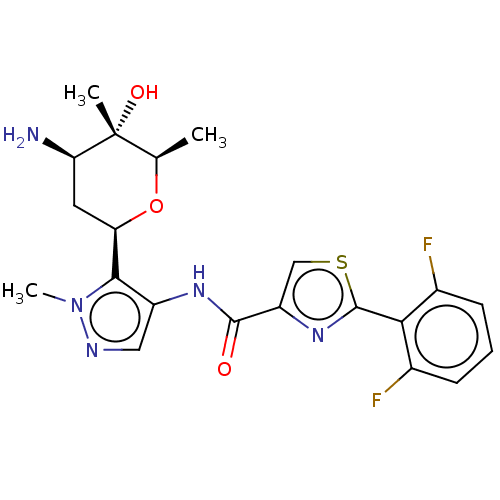 (CHEMBL3318855 | US9328106, 341) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.00300 | -65.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
GENENTECH, INC. US Patent | Assay Description LC3K assay: PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Ch... | US Patent US9328106 (2016) BindingDB Entry DOI: 10.7270/Q29W0DBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM227173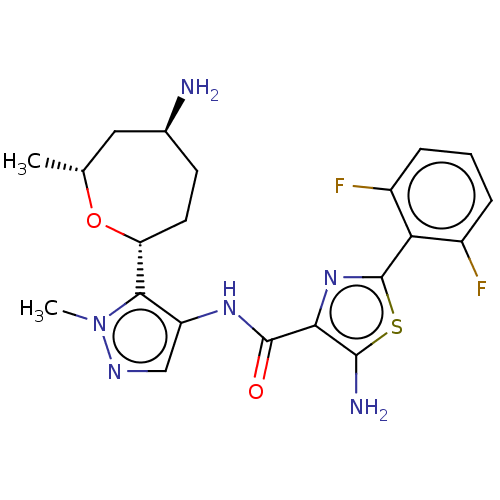 (US9328106, 121) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00300 | -65.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
GENENTECH, INC. US Patent | Assay Description LC3K assay: PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Ch... | US Patent US9328106 (2016) BindingDB Entry DOI: 10.7270/Q29W0DBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178831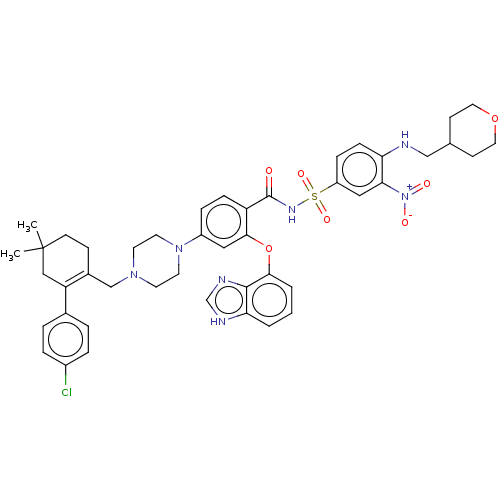 (US9125913, 403) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178839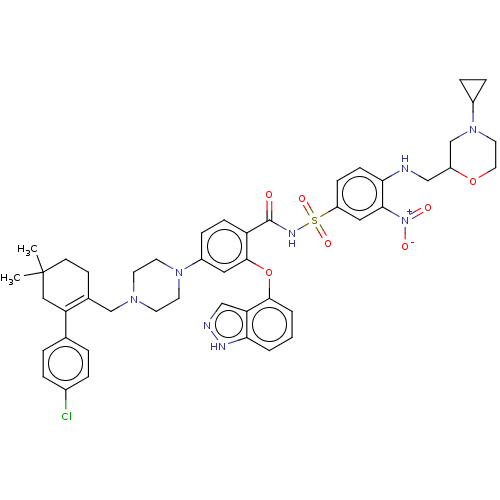 (US9125913, 411) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178853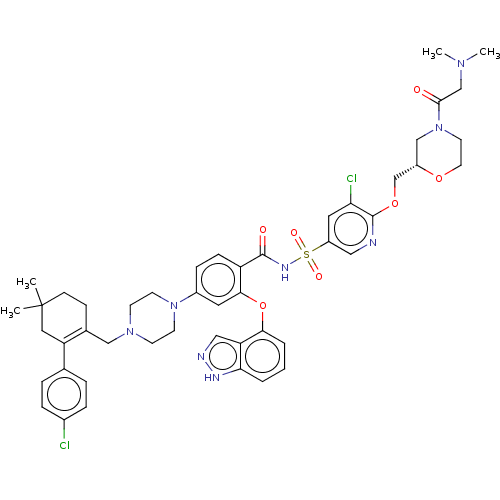 (US9125913, 425) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178856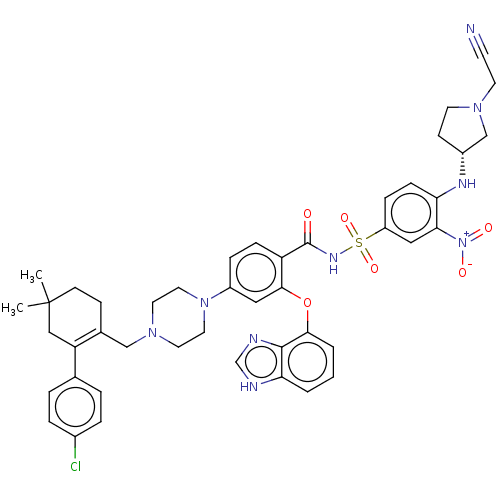 (US9125913, 428) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178566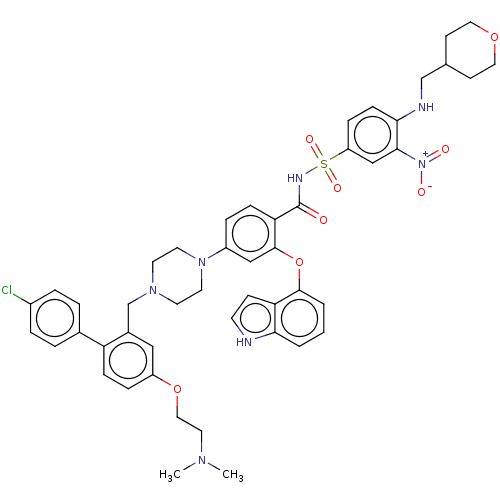 (US9125913, 125) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM110938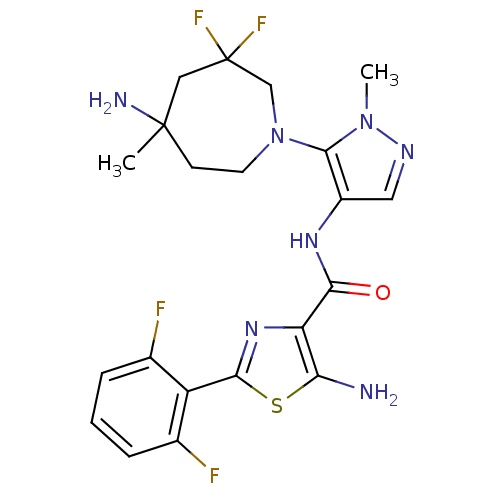 (US8614206, 495) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
F. Hoffmann-La Roche AG US Patent | Assay Description PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J. F., a... | US Patent US8614206 (2013) BindingDB Entry DOI: 10.7270/Q2W66JFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM451115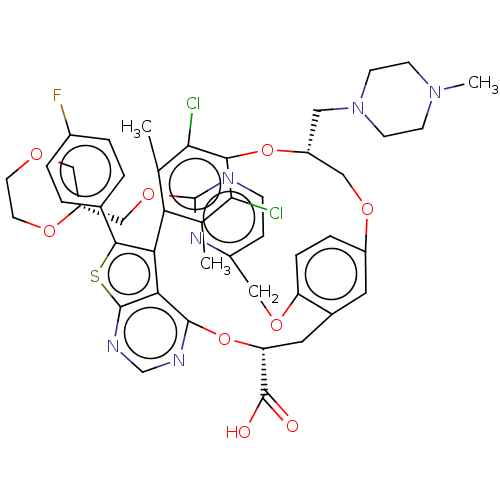 (US10676485, Example 25) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The ability of the exemplary MCL-1 inhibitors of Examples 1 through 151 to bind MCL-1 was demonstrated using the Time Resolved-Fluorescence Resonance... | US Patent US10676485 (2020) BindingDB Entry DOI: 10.7270/Q2PZ5CWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM110961 (US8614206, 518) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50434655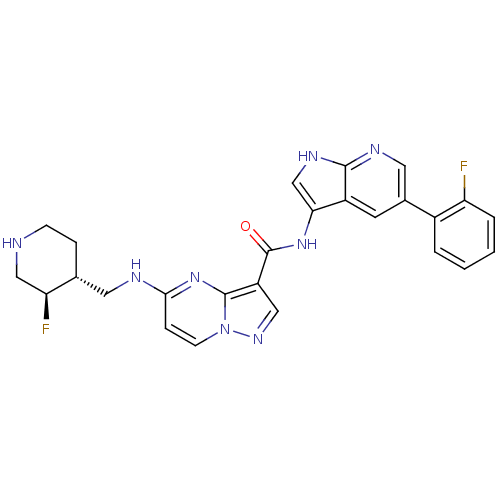 (CHEMBL2387464) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) using FAM-pimtide as substrate after 90 mins by spectrophotometry in presence of ATP | Bioorg Med Chem Lett 23: 3149-53 (2013) Article DOI: 10.1016/j.bmcl.2013.04.020 BindingDB Entry DOI: 10.7270/Q2PC33S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM227440 (US9328106, 393) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00300 | -65.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
GENENTECH, INC. US Patent | Assay Description LC3K assay: PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Ch... | US Patent US9328106 (2016) BindingDB Entry DOI: 10.7270/Q29W0DBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM227361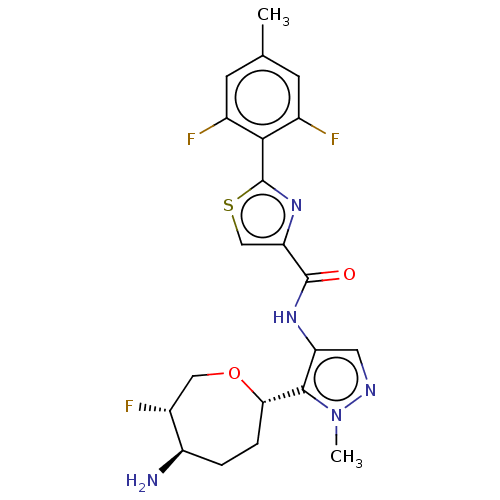 (US9328106, 312) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00300 | -65.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
GENENTECH, INC. US Patent | Assay Description LC3K assay: PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Ch... | US Patent US9328106 (2016) BindingDB Entry DOI: 10.7270/Q29W0DBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM227381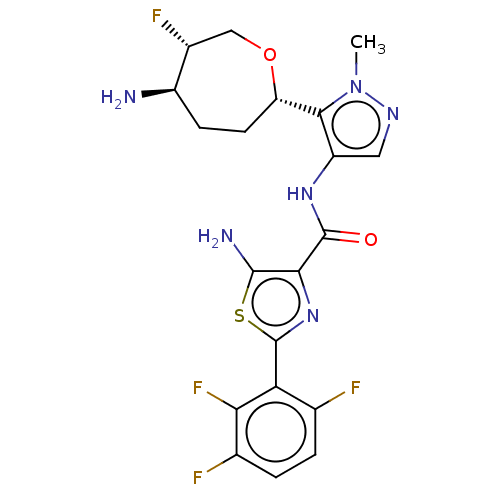 (US9328106, 332) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00300 | -65.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
GENENTECH, INC. US Patent | Assay Description LC3K assay: PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Ch... | US Patent US9328106 (2016) BindingDB Entry DOI: 10.7270/Q29W0DBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM227266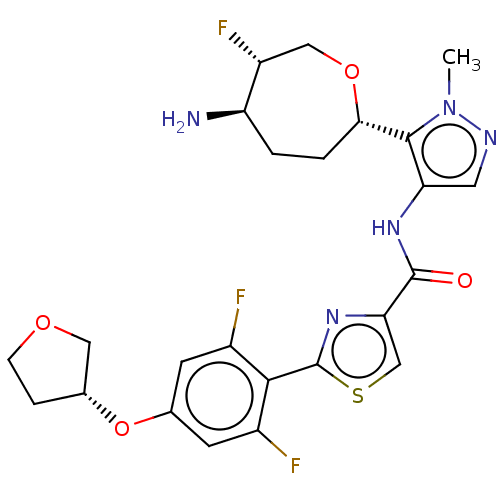 (US9328106, 217) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00300 | -65.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
GENENTECH, INC. US Patent | Assay Description LC3K assay: PIM-1, -2, and -3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Ch... | US Patent US9328106 (2016) BindingDB Entry DOI: 10.7270/Q29W0DBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 131555 total ) | Next | Last >> |