

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prolyl endopeptidase (Rattus norvegicus) | BDBM50274983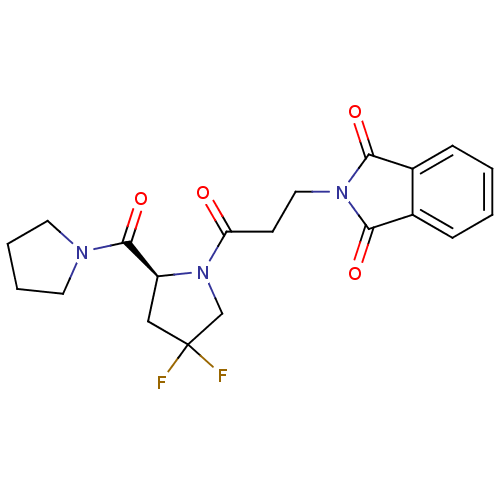 (2-{3-[(2S)-4,4-Difluoro-2-(pyrrolidinocarbonyl)pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
CHINOIN, Ltd Curated by ChEMBL | Assay Description Inhibition of POP in Sprague-Dawley rat brain homogenates | J Med Chem 51: 7514-22 (2009) Article DOI: 10.1021/jm800944x BindingDB Entry DOI: 10.7270/Q2NG4QFC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prolyl endopeptidase (Rattus norvegicus) | BDBM50274985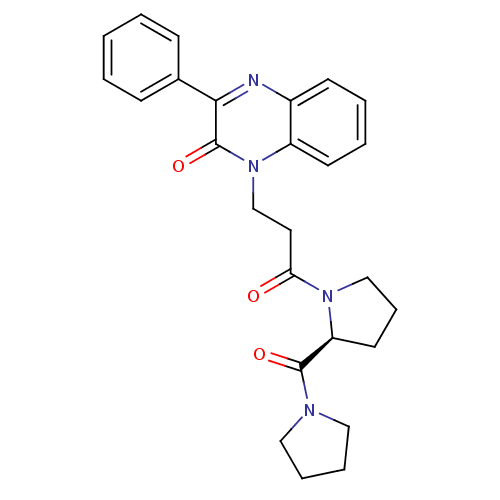 (1-{3-Oxo-3-[(2S)-2-(pyrrolidinocarbonyl)pyrrolidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
CHINOIN, Ltd Curated by ChEMBL | Assay Description Inhibition of POP in Sprague-Dawley rat brain homogenates | J Med Chem 51: 7514-22 (2009) Article DOI: 10.1021/jm800944x BindingDB Entry DOI: 10.7270/Q2NG4QFC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50352962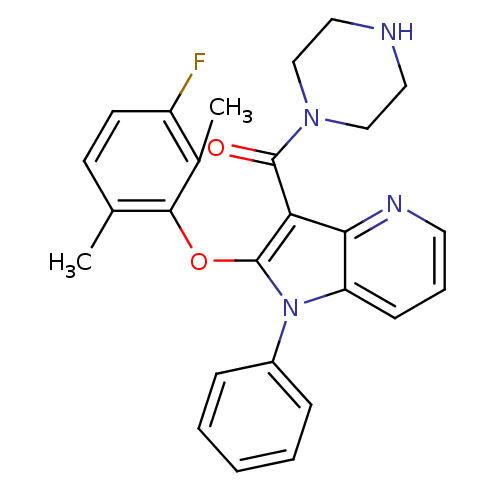 (CHEMBL1824936) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using dabcyl-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate preincubated for 30 mins afte... | Bioorg Med Chem Lett 21: 5480-6 (2011) Article DOI: 10.1016/j.bmcl.2011.06.114 BindingDB Entry DOI: 10.7270/Q2HH6KFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50352995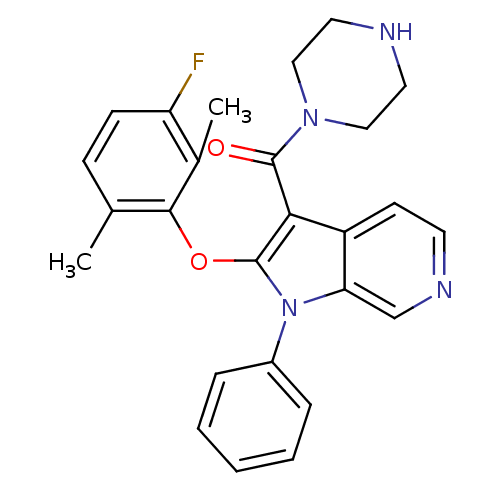 (CHEMBL1825181) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using dabcyl-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate preincubated for 30 mins afte... | Bioorg Med Chem Lett 21: 5480-6 (2011) Article DOI: 10.1016/j.bmcl.2011.06.114 BindingDB Entry DOI: 10.7270/Q2HH6KFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50329018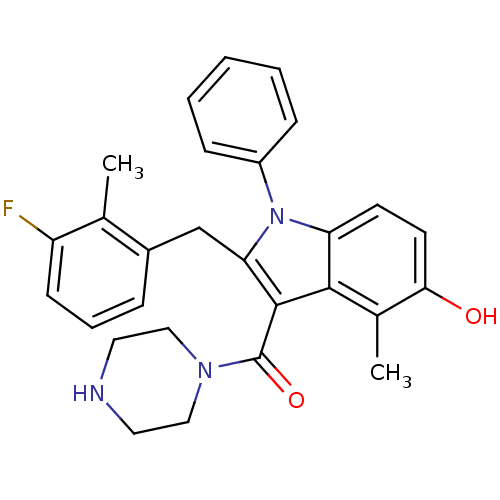 ((2-(3-fluoro-2-methylbenzyl)-5-hydroxy-4-methyl-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using flurorogenic substrate by microplate spectrofluorometer | Bioorg Med Chem Lett 20: 6268-72 (2010) Article DOI: 10.1016/j.bmcl.2010.08.092 BindingDB Entry DOI: 10.7270/Q2VH5P2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50329031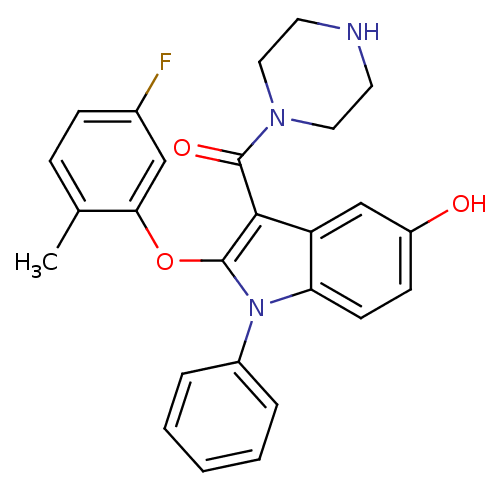 ((2-(5-fluoro-2-methylphenoxy)-5-hydroxy-1-phenyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using flurorogenic substrate by microplate spectrofluorometer | Bioorg Med Chem Lett 20: 6268-72 (2010) Article DOI: 10.1016/j.bmcl.2010.08.092 BindingDB Entry DOI: 10.7270/Q2VH5P2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50352922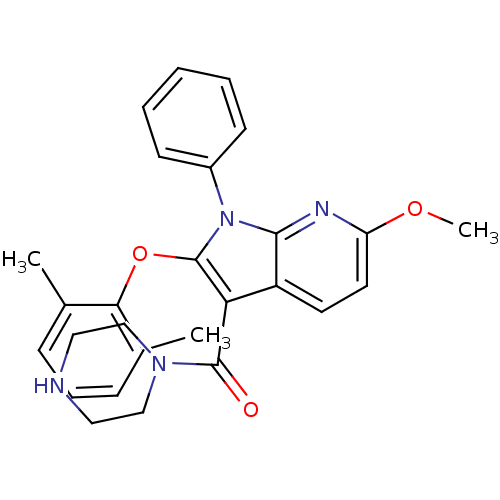 (CHEMBL1824630) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using Dabcyl-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate after 2 hrs by spectrofluorom... | Bioorg Med Chem Lett 21: 5487-92 (2011) Article DOI: 10.1016/j.bmcl.2011.06.112 BindingDB Entry DOI: 10.7270/Q2RX9CFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50352986 (CHEMBL1824961) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using dabcyl-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate preincubated for 30 mins afte... | Bioorg Med Chem Lett 21: 5480-6 (2011) Article DOI: 10.1016/j.bmcl.2011.06.114 BindingDB Entry DOI: 10.7270/Q2HH6KFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Rattus norvegicus) | BDBM50274959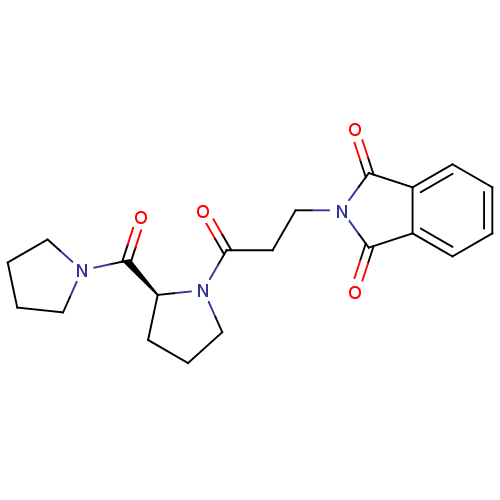 ((S)-2-(3-oxo-3-(2-(pyrrolidine-1-carbonyl)pyrrolid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHINOIN, Ltd Curated by ChEMBL | Assay Description Inhibition of POP in Sprague-Dawley rat brain homogenates | J Med Chem 51: 7514-22 (2009) Article DOI: 10.1021/jm800944x BindingDB Entry DOI: 10.7270/Q2NG4QFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50353000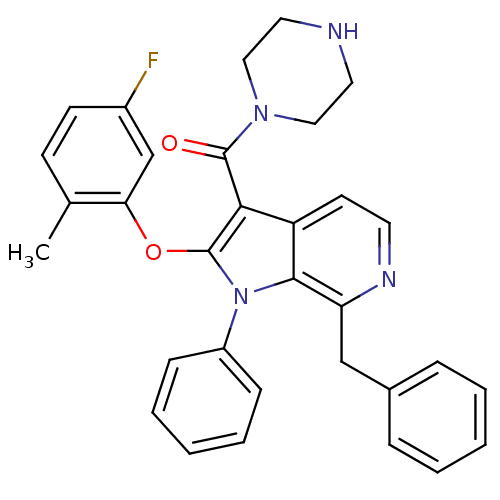 (CHEMBL1825187) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using dabcyl-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate preincubated for 30 mins afte... | Bioorg Med Chem Lett 21: 5480-6 (2011) Article DOI: 10.1016/j.bmcl.2011.06.114 BindingDB Entry DOI: 10.7270/Q2HH6KFK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50329017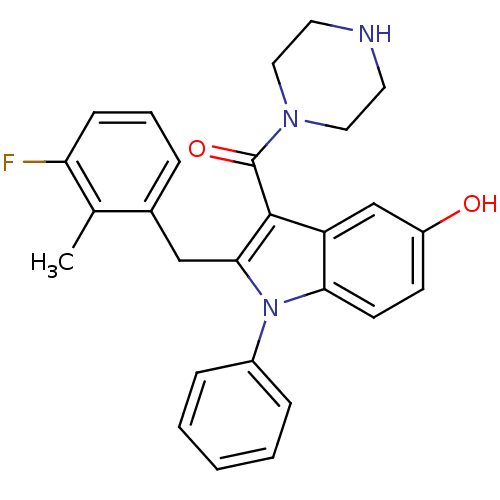 ((2-(3-fluoro-2-methylbenzyl)-5-hydroxy-1-phenyl-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using flurorogenic substrate by microplate spectrofluorometer | Bioorg Med Chem Lett 20: 6268-72 (2010) Article DOI: 10.1016/j.bmcl.2010.08.092 BindingDB Entry DOI: 10.7270/Q2VH5P2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50329016 ((2-(3-fluoro-2-methylbenzyl)-6-hydroxy-1-phenyl-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using flurorogenic substrate by microplate spectrofluorometer | Bioorg Med Chem Lett 20: 6268-72 (2010) Article DOI: 10.1016/j.bmcl.2010.08.092 BindingDB Entry DOI: 10.7270/Q2VH5P2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50352970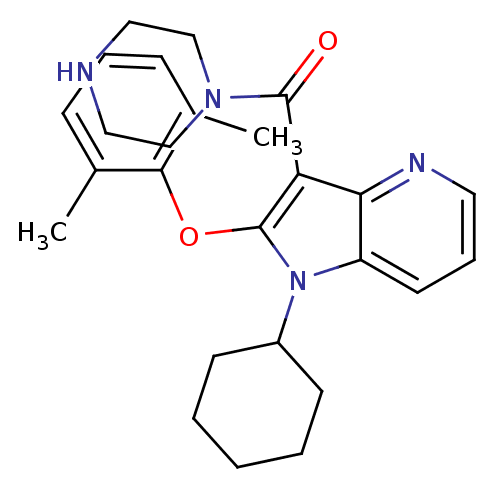 (CHEMBL1824944) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using dabcyl-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate preincubated for 30 mins afte... | Bioorg Med Chem Lett 21: 5480-6 (2011) Article DOI: 10.1016/j.bmcl.2011.06.114 BindingDB Entry DOI: 10.7270/Q2HH6KFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50329043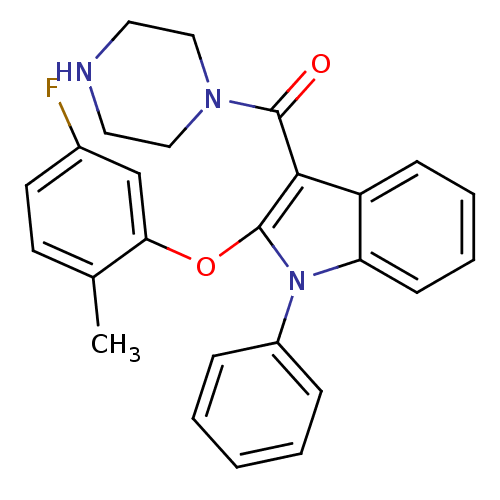 ((2-(5-fluoro-2-methylphenoxy)-1-phenyl-1H-indol-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using flurorogenic substrate by microplate spectrofluorometer | Bioorg Med Chem Lett 20: 6268-72 (2010) Article DOI: 10.1016/j.bmcl.2010.08.092 BindingDB Entry DOI: 10.7270/Q2VH5P2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50353002 (CHEMBL1825189) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using dabcyl-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate preincubated for 30 mins afte... | Bioorg Med Chem Lett 21: 5480-6 (2011) Article DOI: 10.1016/j.bmcl.2011.06.114 BindingDB Entry DOI: 10.7270/Q2HH6KFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50352893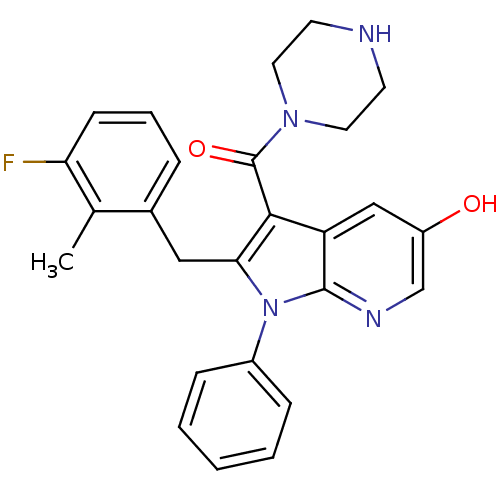 (CHEMBL1824627) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using Dabcyl-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate after 2 hrs by spectrofluorom... | Bioorg Med Chem Lett 21: 5487-92 (2011) Article DOI: 10.1016/j.bmcl.2011.06.112 BindingDB Entry DOI: 10.7270/Q2RX9CFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50352894 (CHEMBL1824626) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using Dabcyl-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate after 2 hrs by spectrofluorom... | Bioorg Med Chem Lett 21: 5487-92 (2011) Article DOI: 10.1016/j.bmcl.2011.06.112 BindingDB Entry DOI: 10.7270/Q2RX9CFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50329032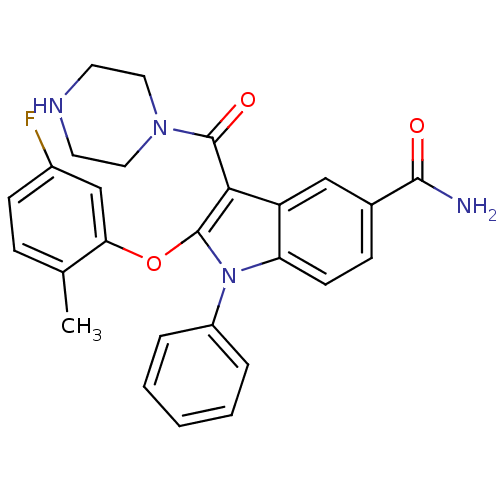 (2-(5-fluoro-2-methylphenoxy)-1-phenyl-3-(piperazin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using flurorogenic substrate by microplate spectrofluorometer | Bioorg Med Chem Lett 20: 6268-72 (2010) Article DOI: 10.1016/j.bmcl.2010.08.092 BindingDB Entry DOI: 10.7270/Q2VH5P2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50352891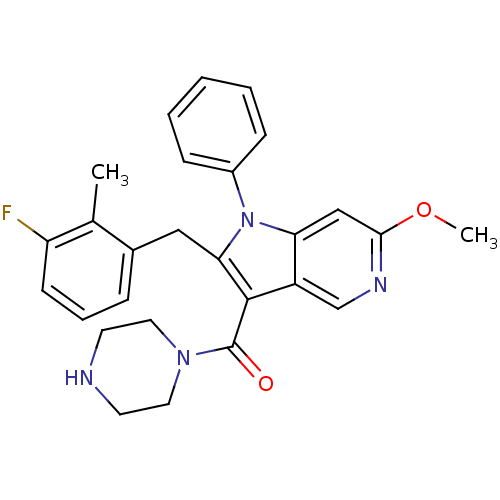 (CHEMBL1824645) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using Dabcyl-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate after 2 hrs by spectrofluorom... | Bioorg Med Chem Lett 21: 5487-92 (2011) Article DOI: 10.1016/j.bmcl.2011.06.112 BindingDB Entry DOI: 10.7270/Q2RX9CFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50352892 (CHEMBL1824642) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using Dabcyl-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate after 2 hrs by spectrofluorom... | Bioorg Med Chem Lett 21: 5487-92 (2011) Article DOI: 10.1016/j.bmcl.2011.06.112 BindingDB Entry DOI: 10.7270/Q2RX9CFJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50352989 (CHEMBL1824964) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using dabcyl-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate preincubated for 30 mins afte... | Bioorg Med Chem Lett 21: 5480-6 (2011) Article DOI: 10.1016/j.bmcl.2011.06.114 BindingDB Entry DOI: 10.7270/Q2HH6KFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50329047 ((2-(3-fluoro-2-methylbenzyl)-1-phenyl-1H-indol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using flurorogenic substrate by microplate spectrofluorometer | Bioorg Med Chem Lett 20: 6268-72 (2010) Article DOI: 10.1016/j.bmcl.2010.08.092 BindingDB Entry DOI: 10.7270/Q2VH5P2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Prolyl endopeptidase (Rattus norvegicus) | BDBM50240495 ((R)-1-(4-(pyrrolidine-1-carbonyl)thiazolidin-3-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
CHINOIN, Ltd Curated by ChEMBL | Assay Description Inhibition of POP in Sprague-Dawley rat brain homogenates | J Med Chem 51: 7514-22 (2009) Article DOI: 10.1021/jm800944x BindingDB Entry DOI: 10.7270/Q2NG4QFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50352901 (CHEMBL1824897) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using Dabcyl-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate after 2 hrs by spectrofluorom... | Bioorg Med Chem Lett 21: 5487-92 (2011) Article DOI: 10.1016/j.bmcl.2011.06.112 BindingDB Entry DOI: 10.7270/Q2RX9CFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50329045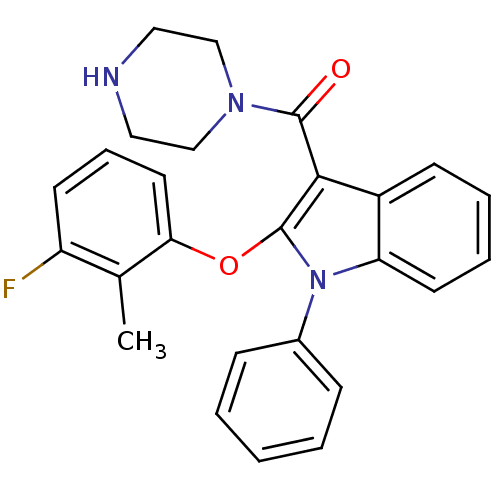 ((2-(3-fluoro-2-methylphenoxy)-1-phenyl-1H-indol-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using flurorogenic substrate by microplate spectrofluorometer | Bioorg Med Chem Lett 20: 6268-72 (2010) Article DOI: 10.1016/j.bmcl.2010.08.092 BindingDB Entry DOI: 10.7270/Q2VH5P2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50329015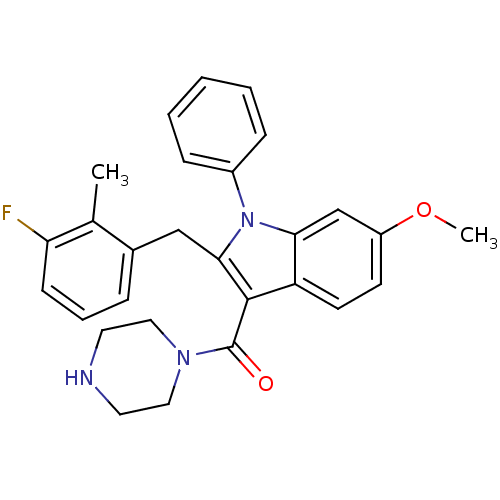 ((2-(3-fluoro-2-methylbenzyl)-6-methoxy-1-phenyl-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using flurorogenic substrate by microplate spectrofluorometer | Bioorg Med Chem Lett 20: 6268-72 (2010) Article DOI: 10.1016/j.bmcl.2010.08.092 BindingDB Entry DOI: 10.7270/Q2VH5P2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50352969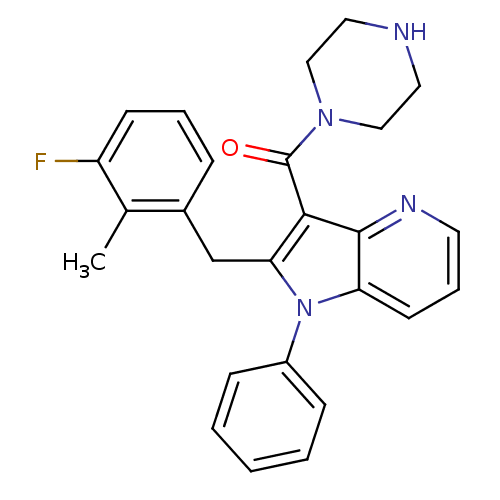 (CHEMBL1824943) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using dabcyl-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate preincubated for 30 mins afte... | Bioorg Med Chem Lett 21: 5480-6 (2011) Article DOI: 10.1016/j.bmcl.2011.06.114 BindingDB Entry DOI: 10.7270/Q2HH6KFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50352961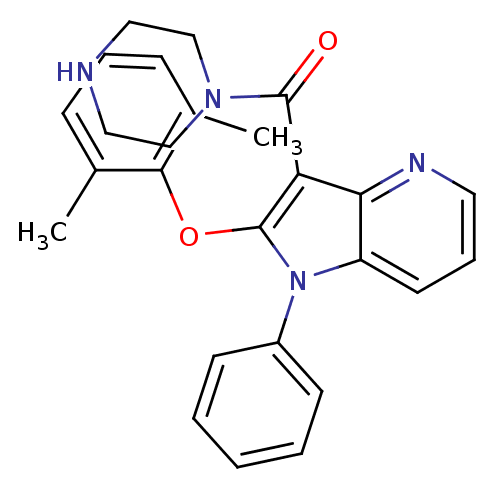 (CHEMBL1824935) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using dabcyl-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate preincubated for 30 mins afte... | Bioorg Med Chem Lett 21: 5480-6 (2011) Article DOI: 10.1016/j.bmcl.2011.06.114 BindingDB Entry DOI: 10.7270/Q2HH6KFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50352985 (CHEMBL1824960) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using dabcyl-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate preincubated for 30 mins afte... | Bioorg Med Chem Lett 21: 5480-6 (2011) Article DOI: 10.1016/j.bmcl.2011.06.114 BindingDB Entry DOI: 10.7270/Q2HH6KFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50353003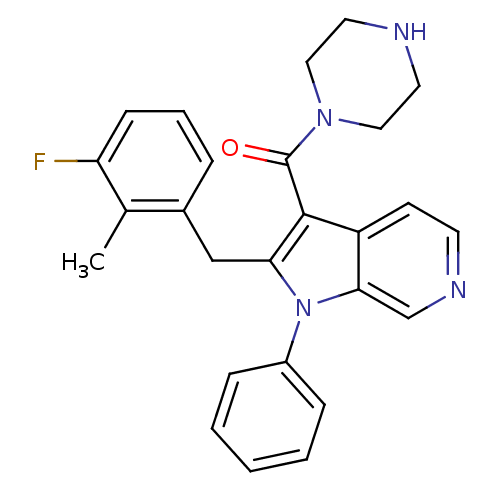 (CHEMBL1825183) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using dabcyl-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate preincubated for 30 mins afte... | Bioorg Med Chem Lett 21: 5480-6 (2011) Article DOI: 10.1016/j.bmcl.2011.06.114 BindingDB Entry DOI: 10.7270/Q2HH6KFK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50352961 (CHEMBL1824935) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using dabcyl-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate preincubated for 30 mins afte... | Bioorg Med Chem Lett 21: 5480-6 (2011) Article DOI: 10.1016/j.bmcl.2011.06.114 BindingDB Entry DOI: 10.7270/Q2HH6KFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50352923 (CHEMBL1824629) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using Dabcyl-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate after 2 hrs by spectrofluorom... | Bioorg Med Chem Lett 21: 5487-92 (2011) Article DOI: 10.1016/j.bmcl.2011.06.112 BindingDB Entry DOI: 10.7270/Q2RX9CFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50329044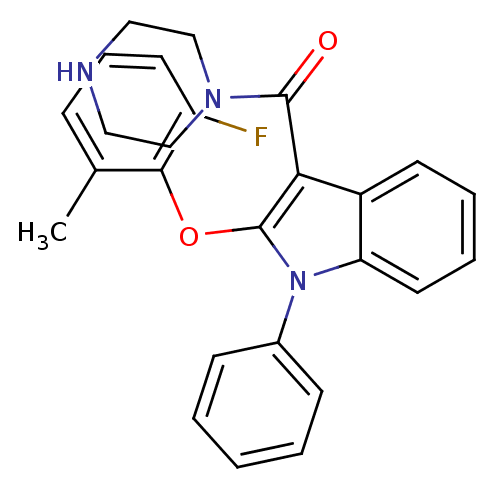 ((2-(2-fluoro-6-methylphenoxy)-1-phenyl-1H-indol-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using flurorogenic substrate by microplate spectrofluorometer | Bioorg Med Chem Lett 20: 6268-72 (2010) Article DOI: 10.1016/j.bmcl.2010.08.092 BindingDB Entry DOI: 10.7270/Q2VH5P2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50329048 ((2-(2-chloro-6-fluorobenzyl)-1-phenyl-1H-indol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using flurorogenic substrate by microplate spectrofluorometer | Bioorg Med Chem Lett 20: 6268-72 (2010) Article DOI: 10.1016/j.bmcl.2010.08.092 BindingDB Entry DOI: 10.7270/Q2VH5P2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50329025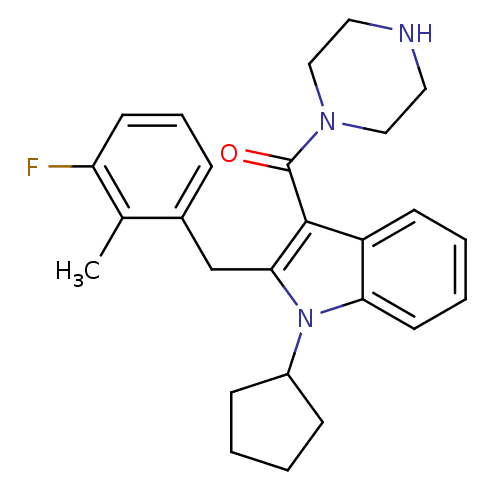 ((1-cyclopentyl-2-(3-fluoro-2-methylbenzyl)-1H-indo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using flurorogenic substrate by microplate spectrofluorometer | Bioorg Med Chem Lett 20: 6268-72 (2010) Article DOI: 10.1016/j.bmcl.2010.08.092 BindingDB Entry DOI: 10.7270/Q2VH5P2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50329038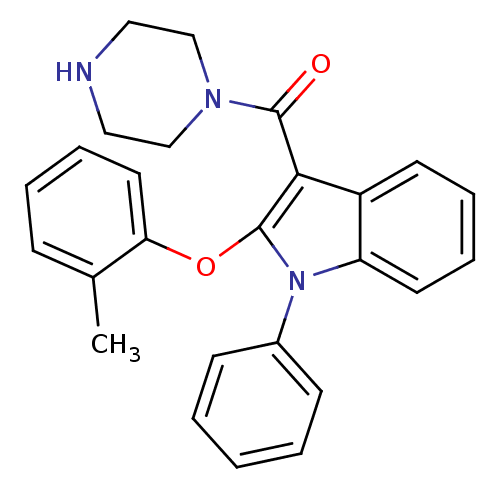 ((1-phenyl-2-(o-tolyloxy)-1H-indol-3-yl)(piperazin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using flurorogenic substrate by microplate spectrofluorometer | Bioorg Med Chem Lett 20: 6268-72 (2010) Article DOI: 10.1016/j.bmcl.2010.08.092 BindingDB Entry DOI: 10.7270/Q2VH5P2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50329009 ((2-(3-fluoro-2-methylbenzyl)-6-(2-phenoxyethoxy)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using flurorogenic substrate by microplate spectrofluorometer | Bioorg Med Chem Lett 20: 6268-72 (2010) Article DOI: 10.1016/j.bmcl.2010.08.092 BindingDB Entry DOI: 10.7270/Q2VH5P2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50352907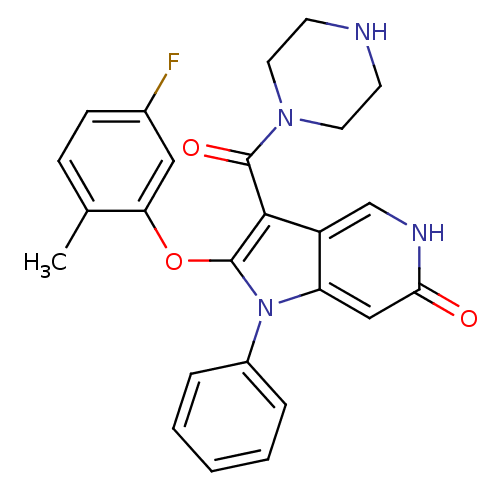 (CHEMBL1824647) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using Dabcyl-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate after 2 hrs by spectrofluorom... | Bioorg Med Chem Lett 21: 5487-92 (2011) Article DOI: 10.1016/j.bmcl.2011.06.112 BindingDB Entry DOI: 10.7270/Q2RX9CFJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50329029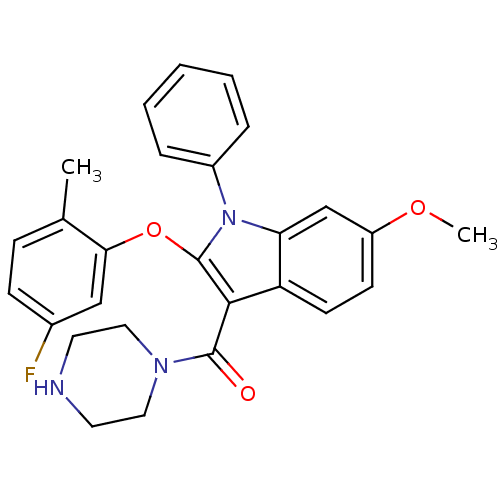 ((2-(5-fluoro-2-methylphenoxy)-6-methoxy-1-phenyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using flurorogenic substrate by microplate spectrofluorometer | Bioorg Med Chem Lett 20: 6268-72 (2010) Article DOI: 10.1016/j.bmcl.2010.08.092 BindingDB Entry DOI: 10.7270/Q2VH5P2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50352957 (CHEMBL1824931) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using dabcyl-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate preincubated for 30 mins afte... | Bioorg Med Chem Lett 21: 5480-6 (2011) Article DOI: 10.1016/j.bmcl.2011.06.114 BindingDB Entry DOI: 10.7270/Q2HH6KFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50353003 (CHEMBL1825183) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using dabcyl-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate preincubated for 30 mins afte... | Bioorg Med Chem Lett 21: 5480-6 (2011) Article DOI: 10.1016/j.bmcl.2011.06.114 BindingDB Entry DOI: 10.7270/Q2HH6KFK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50353003 (CHEMBL1825183) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using dabcyl-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate preincubated for 30 mins afte... | Bioorg Med Chem Lett 21: 5480-6 (2011) Article DOI: 10.1016/j.bmcl.2011.06.114 BindingDB Entry DOI: 10.7270/Q2HH6KFK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50352993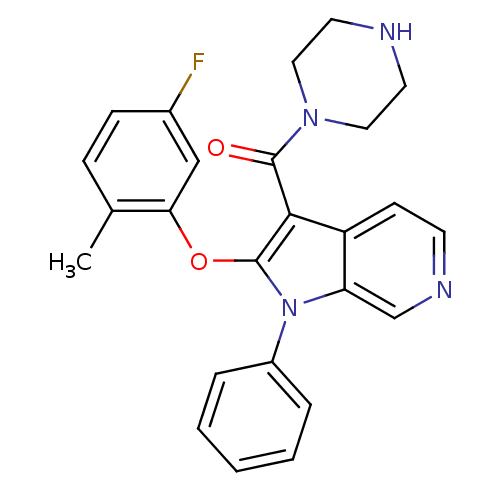 (CHEMBL1825179) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using dabcyl-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate preincubated for 30 mins afte... | Bioorg Med Chem Lett 21: 5480-6 (2011) Article DOI: 10.1016/j.bmcl.2011.06.114 BindingDB Entry DOI: 10.7270/Q2HH6KFK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50329013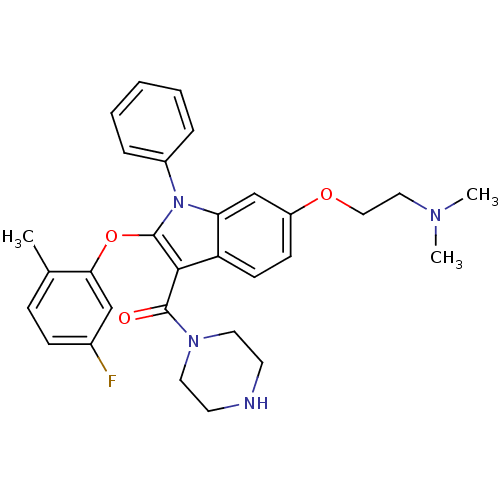 ((6-(2-(dimethylamino)ethoxy)-2-(5-fluoro-2-methylp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using flurorogenic substrate by microplate spectrofluorometer | Bioorg Med Chem Lett 20: 6268-72 (2010) Article DOI: 10.1016/j.bmcl.2010.08.092 BindingDB Entry DOI: 10.7270/Q2VH5P2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Rattus norvegicus) | BDBM50051539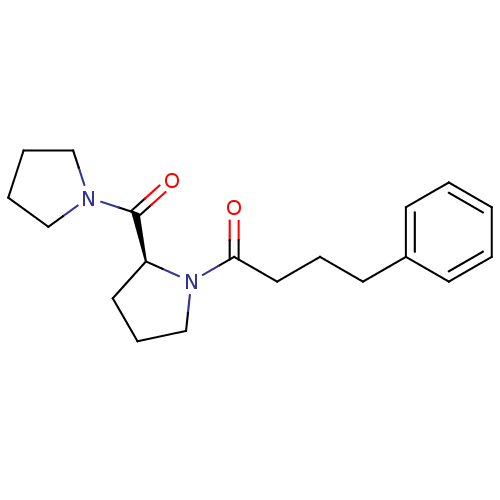 ((S)-4-phenyl-1-(2-(pyrrolidine-1-carbonyl)pyrrolid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CHINOIN, Ltd Curated by ChEMBL | Assay Description Inhibition of POP in Sprague-Dawley rat brain homogenates | J Med Chem 51: 7514-22 (2009) Article DOI: 10.1021/jm800944x BindingDB Entry DOI: 10.7270/Q2NG4QFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50352961 (CHEMBL1824935) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using dabcyl-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate preincubated for 30 mins afte... | Bioorg Med Chem Lett 21: 5480-6 (2011) Article DOI: 10.1016/j.bmcl.2011.06.114 BindingDB Entry DOI: 10.7270/Q2HH6KFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50329010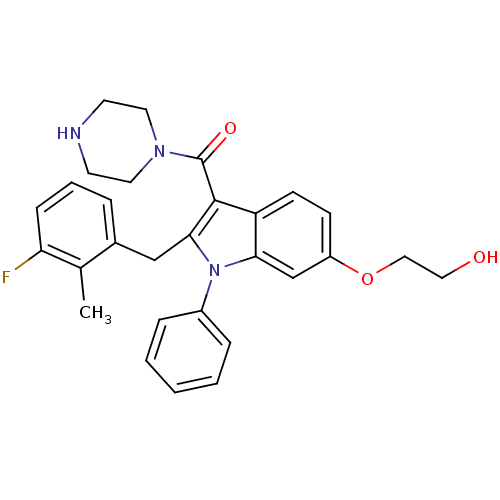 ((2-(3-fluoro-2-methylbenzyl)-6-(2-hydroxyethoxy)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using flurorogenic substrate by microplate spectrofluorometer | Bioorg Med Chem Lett 20: 6268-72 (2010) Article DOI: 10.1016/j.bmcl.2010.08.092 BindingDB Entry DOI: 10.7270/Q2VH5P2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50329024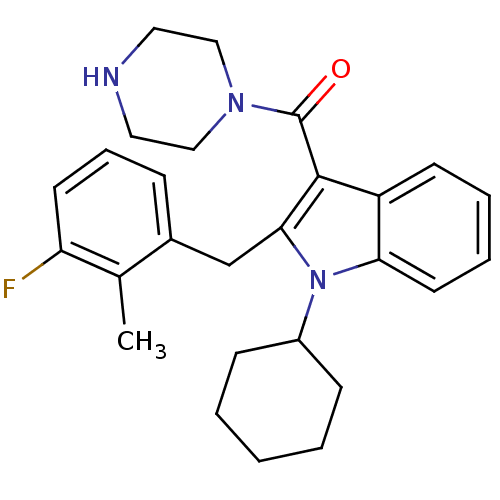 ((1-cyclohexyl-2-(3-fluoro-2-methylbenzyl)-1H-indol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using flurorogenic substrate by microplate spectrofluorometer | Bioorg Med Chem Lett 20: 6268-72 (2010) Article DOI: 10.1016/j.bmcl.2010.08.092 BindingDB Entry DOI: 10.7270/Q2VH5P2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50352962 (CHEMBL1824936) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using dabcyl-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate preincubated for 30 mins afte... | Bioorg Med Chem Lett 21: 5480-6 (2011) Article DOI: 10.1016/j.bmcl.2011.06.114 BindingDB Entry DOI: 10.7270/Q2HH6KFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50352900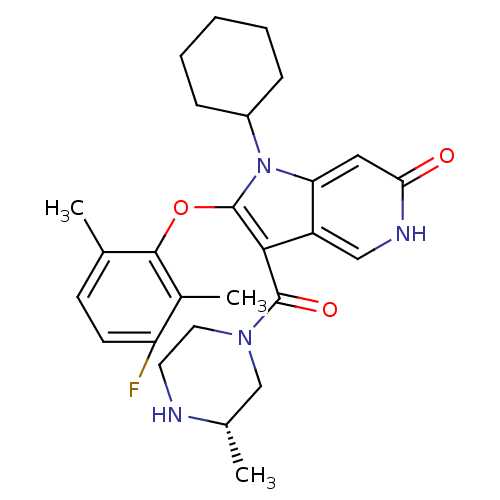 (CHEMBL1824898) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using Dabcyl-gamma-Abu-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-EDANS as substrate after 2 hrs by spectrofluorom... | Bioorg Med Chem Lett 21: 5487-92 (2011) Article DOI: 10.1016/j.bmcl.2011.06.112 BindingDB Entry DOI: 10.7270/Q2RX9CFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 167 total ) | Next | Last >> |