 Found 2498 hits with Last Name = 'bauer' and Initial = 'm'
Found 2498 hits with Last Name = 'bauer' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50193861
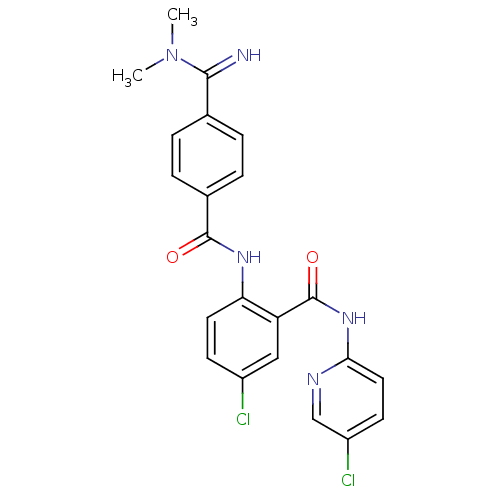
(5-chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(N,N-dimet...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C22H19Cl2N5O2/c1-29(2)20(25)13-3-5-14(6-4-13)21(30)27-18-9-7-15(23)11-17(18)22(31)28-19-10-8-16(24)12-26-19/h3-12,25H,1-2H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249120
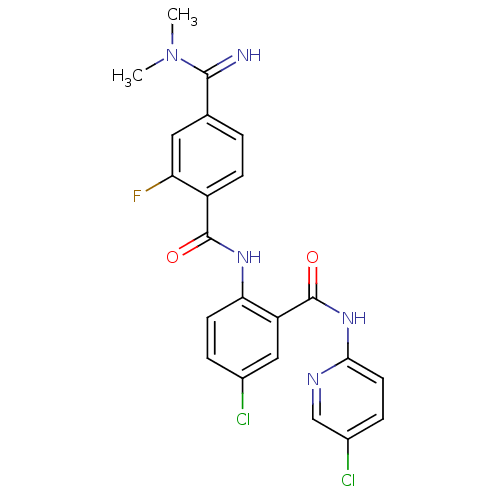
(CHEMBL472967 | N-(4-chloro-2-(5-chloropyridin-2-yl...)Show SMILES CN(C)C(=N)c1ccc(C(=O)Nc2ccc(Cl)cc2C(=O)Nc2ccc(Cl)cn2)c(F)c1 Show InChI InChI=1S/C22H18Cl2FN5O2/c1-30(2)20(26)12-3-6-15(17(25)9-12)21(31)28-18-7-4-13(23)10-16(18)22(32)29-19-8-5-14(24)11-27-19/h3-11,26H,1-2H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM19023

(1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-y...)Show SMILES COc1ccc(cc1)-n1nc(C(N)=O)c2CCN(C(=O)c12)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C25H25N5O4/c1-34-19-11-9-18(10-12-19)30-23-20(22(27-30)24(26)32)13-15-29(25(23)33)17-7-5-16(6-8-17)28-14-3-2-4-21(28)31/h5-12H,2-4,13-15H2,1H3,(H2,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249423
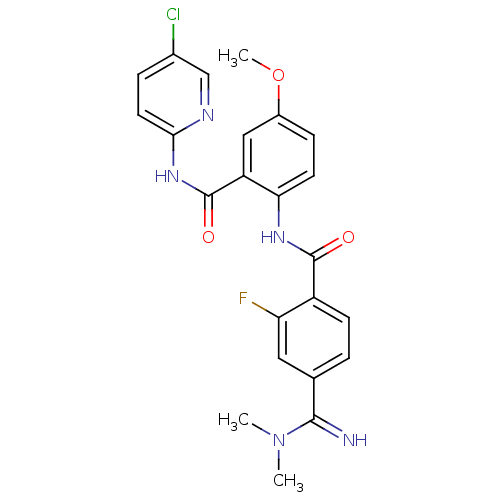
(CHEMBL515919 | N-(2-(5-chloropyridin-2-ylcarbamoyl...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2F)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H21ClFN5O3/c1-30(2)21(26)13-4-7-16(18(25)10-13)22(31)28-19-8-6-15(33-3)11-17(19)23(32)29-20-9-5-14(24)12-27-20/h4-12,26H,1-3H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249298
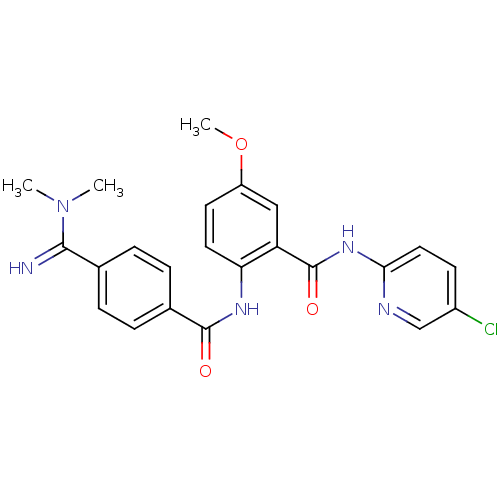
(BEVYXXA | CHEMBL512351 | N-(5-chloropyridin-2-yl)-...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H22ClN5O3/c1-29(2)21(25)14-4-6-15(7-5-14)22(30)27-19-10-9-17(32-3)12-18(19)23(31)28-20-11-8-16(24)13-26-20/h4-13,25H,1-3H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM7840
(RIVAROXABAN | US8822458, 44 | US8822458, 97)Show SMILES Clc1ccc(s1)C(=O)NC[C@H]1CN(C(=O)O1)c1ccc(cc1)N1CCOCC1=O |r| Show InChI InChI=1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249295
(CHEMBL471725 | N-(5-chloropyridin-2-yl)-2-(4-(N,N-...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)Nc1ccccc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C22H20ClN5O2/c1-28(2)20(24)14-7-9-15(10-8-14)21(29)26-18-6-4-3-5-17(18)22(30)27-19-12-11-16(23)13-25-19/h3-13,24H,1-2H3,(H,26,29)(H,25,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144092
(CHEMBL294121 | [4-(5-Chloro-1H-indole-2-sulfonyl)-...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 |c:4| Show InChI InChI=1S/C23H24ClN5O3S/c1-27-9-8-25-22(27)16-2-4-17(5-3-16)23(30)28-10-12-29(13-11-28)33(31,32)21-15-18-14-19(24)6-7-20(18)26-21/h2-7,14-15,26H,8-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17298
(4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...)Show SMILES Cn1c(CNc2ccc(cc2)C(N)=N)nc2cc(ccc12)C1(CC1)C(=O)N1CCCC1 Show InChI InChI=1S/C24H28N6O/c1-29-20-9-6-17(24(10-11-24)23(31)30-12-2-3-13-30)14-19(20)28-21(29)15-27-18-7-4-16(5-8-18)22(25)26/h4-9,14,27H,2-3,10-13,15H2,1H3,(H3,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | -46.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG
| Assay Description
For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... |
Structure 9: 29-37 (2001)
Article DOI: 10.1016/s0969-2126(00)00551-7
BindingDB Entry DOI: 10.7270/Q2028PTC |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM17298

(4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...)Show SMILES Cn1c(CNc2ccc(cc2)C(N)=N)nc2cc(ccc12)C1(CC1)C(=O)N1CCCC1 Show InChI InChI=1S/C24H28N6O/c1-29-20-9-6-17(24(10-11-24)23(31)30-12-2-3-13-30)14-19(20)28-21(29)15-27-18-7-4-16(5-8-18)22(25)26/h4-9,14,27H,2-3,10-13,15H2,1H3,(H3,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | -45.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG
| Assay Description
For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... |
Structure 9: 29-37 (2001)
Article DOI: 10.1016/s0969-2126(00)00551-7
BindingDB Entry DOI: 10.7270/Q2028PTC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17297
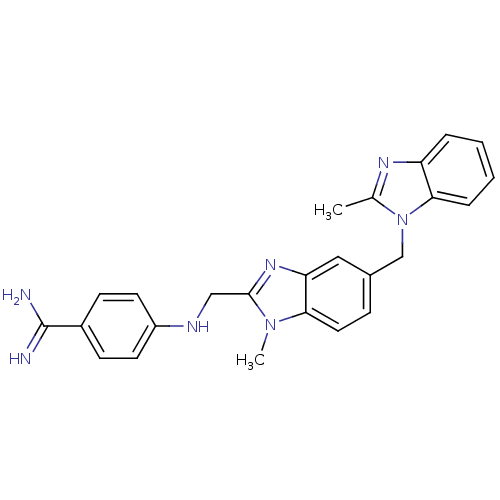
(4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...)Show SMILES Cc1nc2ccccc2n1Cc1ccc2n(C)c(CNc3ccc(cc3)C(N)=N)nc2c1 Show InChI InChI=1S/C25H25N7/c1-16-29-20-5-3-4-6-23(20)32(16)15-17-7-12-22-21(13-17)30-24(31(22)2)14-28-19-10-8-18(9-11-19)25(26)27/h3-13,28H,14-15H2,1-2H3,(H3,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 40 | -43.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG
| Assay Description
For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... |
Structure 9: 29-37 (2001)
Article DOI: 10.1016/s0969-2126(00)00551-7
BindingDB Entry DOI: 10.7270/Q2028PTC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17295
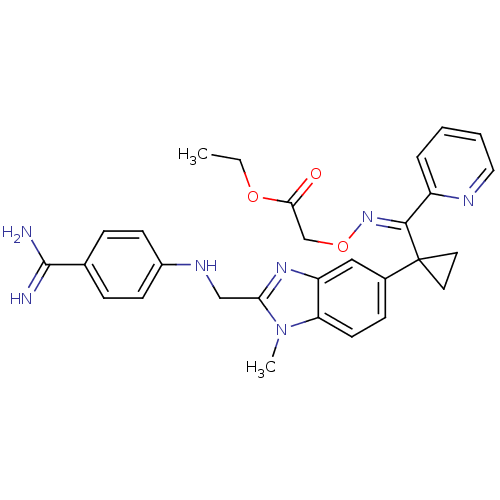
(BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...)Show SMILES CCOC(=O)CO\N=C(\c1ccccn1)C1(CC1)c1ccc2n(C)c(CNc3ccc(cc3)C(N)=N)nc2c1 Show InChI InChI=1S/C29H31N7O3/c1-3-38-26(37)18-39-35-27(22-6-4-5-15-32-22)29(13-14-29)20-9-12-24-23(16-20)34-25(36(24)2)17-33-21-10-7-19(8-11-21)28(30)31/h4-12,15-16,33H,3,13-14,17-18H2,1-2H3,(H3,30,31)/b35-27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 57 | -43.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG
| Assay Description
For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... |
Structure 9: 29-37 (2001)
Article DOI: 10.1016/s0969-2126(00)00551-7
BindingDB Entry DOI: 10.7270/Q2028PTC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine protease 1
(Bos taurus (bovine)) | BDBM17297

(4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...)Show SMILES Cc1nc2ccccc2n1Cc1ccc2n(C)c(CNc3ccc(cc3)C(N)=N)nc2c1 Show InChI InChI=1S/C25H25N7/c1-16-29-20-5-3-4-6-23(20)32(16)15-17-7-12-22-21(13-17)30-24(31(22)2)14-28-19-10-8-18(9-11-19)25(26)27/h3-13,28H,14-15H2,1-2H3,(H3,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
MMDB
PDB
Article
PubMed
| 67 | -42.6 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG
| Assay Description
For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... |
Structure 9: 29-37 (2001)
Article DOI: 10.1016/s0969-2126(00)00551-7
BindingDB Entry DOI: 10.7270/Q2028PTC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine protease 1
(Bos taurus (bovine)) | BDBM17298

(4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...)Show SMILES Cn1c(CNc2ccc(cc2)C(N)=N)nc2cc(ccc12)C1(CC1)C(=O)N1CCCC1 Show InChI InChI=1S/C24H28N6O/c1-29-20-9-6-17(24(10-11-24)23(31)30-12-2-3-13-30)14-19(20)28-21(29)15-27-18-7-4-16(5-8-18)22(25)26/h4-9,14,27H,2-3,10-13,15H2,1H3,(H3,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 102 | -41.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG
| Assay Description
For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... |
Structure 9: 29-37 (2001)
Article DOI: 10.1016/s0969-2126(00)00551-7
BindingDB Entry DOI: 10.7270/Q2028PTC |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM17295

(BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...)Show SMILES CCOC(=O)CO\N=C(\c1ccccn1)C1(CC1)c1ccc2n(C)c(CNc3ccc(cc3)C(N)=N)nc2c1 Show InChI InChI=1S/C29H31N7O3/c1-3-38-26(37)18-39-35-27(22-6-4-5-15-32-22)29(13-14-29)20-9-12-24-23(16-20)34-25(36(24)2)17-33-21-10-7-19(8-11-21)28(30)31/h4-12,15-16,33H,3,13-14,17-18H2,1-2H3,(H3,30,31)/b35-27- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| DrugBank
MMDB
PDB
Article
PubMed
| 110 | -41.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG
| Assay Description
For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... |
Structure 9: 29-37 (2001)
Article DOI: 10.1016/s0969-2126(00)00551-7
BindingDB Entry DOI: 10.7270/Q2028PTC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50193861

(5-chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(N,N-dimet...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C22H19Cl2N5O2/c1-29(2)20(25)13-3-5-14(6-4-13)21(30)27-18-9-7-15(23)11-17(18)22(31)28-19-10-8-16(24)12-26-19/h3-12,25H,1-2H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM17295

(BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...)Show SMILES CCOC(=O)CO\N=C(\c1ccccn1)C1(CC1)c1ccc2n(C)c(CNc3ccc(cc3)C(N)=N)nc2c1 Show InChI InChI=1S/C29H31N7O3/c1-3-38-26(37)18-39-35-27(22-6-4-5-15-32-22)29(13-14-29)20-9-12-24-23(16-20)34-25(36(24)2)17-33-21-10-7-19(8-11-21)28(30)31/h4-12,15-16,33H,3,13-14,17-18H2,1-2H3,(H3,30,31)/b35-27- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 140 | -40.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG
| Assay Description
For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... |
Structure 9: 29-37 (2001)
Article DOI: 10.1016/s0969-2126(00)00551-7
BindingDB Entry DOI: 10.7270/Q2028PTC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50193849

(CHEMBL219106 | N-(2-(5-chloropyridin-2-ylcarbamoyl...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2N2CCCCC2)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C28H31ClN6O3/c1-34(2)26(30)18-7-10-21(24(15-18)35-13-5-4-6-14-35)27(36)32-23-11-9-20(38-3)16-22(23)28(37)33-25-12-8-19(29)17-31-25/h7-12,15-17,30H,4-6,13-14H2,1-3H3,(H,32,36)(H,31,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50249120

(CHEMBL472967 | N-(4-chloro-2-(5-chloropyridin-2-yl...)Show SMILES CN(C)C(=N)c1ccc(C(=O)Nc2ccc(Cl)cc2C(=O)Nc2ccc(Cl)cn2)c(F)c1 Show InChI InChI=1S/C22H18Cl2FN5O2/c1-30(2)20(26)12-3-6-15(17(25)9-12)21(31)28-18-7-4-13(23)10-16(18)22(32)29-19-8-5-14(24)11-27-19/h3-11,26H,1-2H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM17297

(4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...)Show SMILES Cc1nc2ccccc2n1Cc1ccc2n(C)c(CNc3ccc(cc3)C(N)=N)nc2c1 Show InChI InChI=1S/C25H25N7/c1-16-29-20-5-3-4-6-23(20)32(16)15-17-7-12-22-21(13-17)30-24(31(22)2)14-28-19-10-8-18(9-11-19)25(26)27/h3-13,28H,14-15H2,1-2H3,(H3,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 780 | -36.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG
| Assay Description
For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... |
Structure 9: 29-37 (2001)
Article DOI: 10.1016/s0969-2126(00)00551-7
BindingDB Entry DOI: 10.7270/Q2028PTC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50249298

(BEVYXXA | CHEMBL512351 | N-(5-chloropyridin-2-yl)-...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H22ClN5O3/c1-29(2)21(25)14-4-6-15(7-5-14)22(30)27-19-10-9-17(32-3)12-18(19)23(31)28-20-11-8-16(24)13-26-20/h4-13,25H,1-3H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50249423

(CHEMBL515919 | N-(2-(5-chloropyridin-2-ylcarbamoyl...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2F)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H21ClFN5O3/c1-30(2)21(26)13-4-7-16(18(25)10-13)22(31)28-19-8-6-15(33-3)11-17(19)23(32)29-20-9-5-14(24)12-27-20/h4-12,26H,1-3H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM17297

(4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...)Show SMILES Cc1nc2ccccc2n1Cc1ccc2n(C)c(CNc3ccc(cc3)C(N)=N)nc2c1 Show InChI InChI=1S/C25H25N7/c1-16-29-20-5-3-4-6-23(20)32(16)15-17-7-12-22-21(13-17)30-24(31(22)2)14-28-19-10-8-18(9-11-19)25(26)27/h3-13,28H,14-15H2,1-2H3,(H3,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 4.10E+3 | -32.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG
| Assay Description
For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... |
Structure 9: 29-37 (2001)
Article DOI: 10.1016/s0969-2126(00)00551-7
BindingDB Entry DOI: 10.7270/Q2028PTC |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM17298

(4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...)Show SMILES Cn1c(CNc2ccc(cc2)C(N)=N)nc2cc(ccc12)C1(CC1)C(=O)N1CCCC1 Show InChI InChI=1S/C24H28N6O/c1-29-20-9-6-17(24(10-11-24)23(31)30-12-2-3-13-30)14-19(20)28-21(29)15-27-18-7-4-16(5-8-18)22(25)26/h4-9,14,27H,2-3,10-13,15H2,1H3,(H3,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.50E+3 | -30.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG
| Assay Description
For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... |
Structure 9: 29-37 (2001)
Article DOI: 10.1016/s0969-2126(00)00551-7
BindingDB Entry DOI: 10.7270/Q2028PTC |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM17295

(BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...)Show SMILES CCOC(=O)CO\N=C(\c1ccccn1)C1(CC1)c1ccc2n(C)c(CNc3ccc(cc3)C(N)=N)nc2c1 Show InChI InChI=1S/C29H31N7O3/c1-3-38-26(37)18-39-35-27(22-6-4-5-15-32-22)29(13-14-29)20-9-12-24-23(16-20)34-25(36(24)2)17-33-21-10-7-19(8-11-21)28(30)31/h4-12,15-16,33H,3,13-14,17-18H2,1-2H3,(H3,30,31)/b35-27- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 6.80E+3 | -30.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG
| Assay Description
For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... |
Structure 9: 29-37 (2001)
Article DOI: 10.1016/s0969-2126(00)00551-7
BindingDB Entry DOI: 10.7270/Q2028PTC |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM17298

(4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...)Show SMILES Cn1c(CNc2ccc(cc2)C(N)=N)nc2cc(ccc12)C1(CC1)C(=O)N1CCCC1 Show InChI InChI=1S/C24H28N6O/c1-29-20-9-6-17(24(10-11-24)23(31)30-12-2-3-13-30)14-19(20)28-21(29)15-27-18-7-4-16(5-8-18)22(25)26/h4-9,14,27H,2-3,10-13,15H2,1H3,(H3,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.20E+3 | -30.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG
| Assay Description
For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... |
Structure 9: 29-37 (2001)
Article DOI: 10.1016/s0969-2126(00)00551-7
BindingDB Entry DOI: 10.7270/Q2028PTC |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM17295

(BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...)Show SMILES CCOC(=O)CO\N=C(\c1ccccn1)C1(CC1)c1ccc2n(C)c(CNc3ccc(cc3)C(N)=N)nc2c1 Show InChI InChI=1S/C29H31N7O3/c1-3-38-26(37)18-39-35-27(22-6-4-5-15-32-22)29(13-14-29)20-9-12-24-23(16-20)34-25(36(24)2)17-33-21-10-7-19(8-11-21)28(30)31/h4-12,15-16,33H,3,13-14,17-18H2,1-2H3,(H3,30,31)/b35-27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 9.00E+3 | -30.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG
| Assay Description
For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... |
Structure 9: 29-37 (2001)
Article DOI: 10.1016/s0969-2126(00)00551-7
BindingDB Entry DOI: 10.7270/Q2028PTC |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM17297

(4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...)Show SMILES Cc1nc2ccccc2n1Cc1ccc2n(C)c(CNc3ccc(cc3)C(N)=N)nc2c1 Show InChI InChI=1S/C25H25N7/c1-16-29-20-5-3-4-6-23(20)32(16)15-17-7-12-22-21(13-17)30-24(31(22)2)14-28-19-10-8-18(9-11-19)25(26)27/h3-13,28H,14-15H2,1-2H3,(H3,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 9.20E+3 | -29.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG
| Assay Description
For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... |
Structure 9: 29-37 (2001)
Article DOI: 10.1016/s0969-2126(00)00551-7
BindingDB Entry DOI: 10.7270/Q2028PTC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50193850
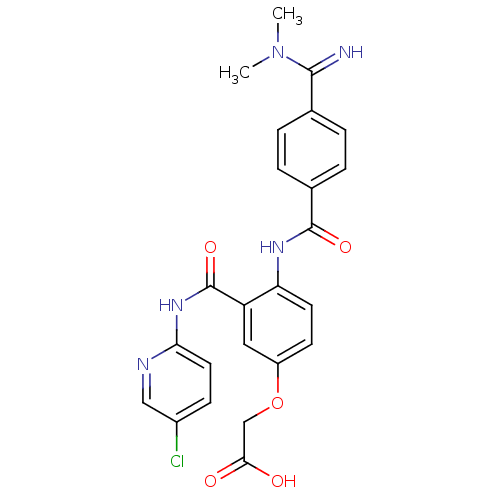
(2-(3-(5-chloropyridin-2-ylcarbamoyl)-4-(4-(N,N-dim...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)Nc1ccc(OCC(O)=O)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C24H22ClN5O5/c1-30(2)22(26)14-3-5-15(6-4-14)23(33)28-19-9-8-17(35-13-21(31)32)11-18(19)24(34)29-20-10-7-16(25)12-27-20/h3-12,26H,13H2,1-2H3,(H,28,33)(H,31,32)(H,27,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50193842
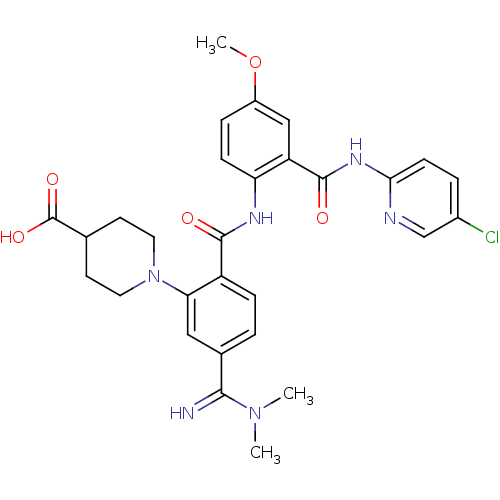
(1-(2-(2-(5-chloropyridin-2-ylcarbamoyl)-4-methoxyp...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2N2CCC(CC2)C(O)=O)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C29H31ClN6O5/c1-35(2)26(31)18-4-7-21(24(14-18)36-12-10-17(11-13-36)29(39)40)27(37)33-23-8-6-20(41-3)15-22(23)28(38)34-25-9-5-19(30)16-32-25/h4-9,14-17,31H,10-13H2,1-3H3,(H,33,37)(H,39,40)(H,32,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM17298

(4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...)Show SMILES Cn1c(CNc2ccc(cc2)C(N)=N)nc2cc(ccc12)C1(CC1)C(=O)N1CCCC1 Show InChI InChI=1S/C24H28N6O/c1-29-20-9-6-17(24(10-11-24)23(31)30-12-2-3-13-30)14-19(20)28-21(29)15-27-18-7-4-16(5-8-18)22(25)26/h4-9,14,27H,2-3,10-13,15H2,1H3,(H3,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30E+4 | -29.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG
| Assay Description
For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... |
Structure 9: 29-37 (2001)
Article DOI: 10.1016/s0969-2126(00)00551-7
BindingDB Entry DOI: 10.7270/Q2028PTC |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM17297

(4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...)Show SMILES Cc1nc2ccccc2n1Cc1ccc2n(C)c(CNc3ccc(cc3)C(N)=N)nc2c1 Show InChI InChI=1S/C25H25N7/c1-16-29-20-5-3-4-6-23(20)32(16)15-17-7-12-22-21(13-17)30-24(31(22)2)14-28-19-10-8-18(9-11-19)25(26)27/h3-13,28H,14-15H2,1-2H3,(H3,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 1.60E+4 | -28.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG
| Assay Description
For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... |
Structure 9: 29-37 (2001)
Article DOI: 10.1016/s0969-2126(00)00551-7
BindingDB Entry DOI: 10.7270/Q2028PTC |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM17298

(4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...)Show SMILES Cn1c(CNc2ccc(cc2)C(N)=N)nc2cc(ccc12)C1(CC1)C(=O)N1CCCC1 Show InChI InChI=1S/C24H28N6O/c1-29-20-9-6-17(24(10-11-24)23(31)30-12-2-3-13-30)14-19(20)28-21(29)15-27-18-7-4-16(5-8-18)22(25)26/h4-9,14,27H,2-3,10-13,15H2,1H3,(H3,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >3.00E+4 | >-26.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG
| Assay Description
For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... |
Structure 9: 29-37 (2001)
Article DOI: 10.1016/s0969-2126(00)00551-7
BindingDB Entry DOI: 10.7270/Q2028PTC |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM17298

(4-[({1-methyl-5-[1-(pyrrolidin-1-ylcarbonyl)cyclop...)Show SMILES Cn1c(CNc2ccc(cc2)C(N)=N)nc2cc(ccc12)C1(CC1)C(=O)N1CCCC1 Show InChI InChI=1S/C24H28N6O/c1-29-20-9-6-17(24(10-11-24)23(31)30-12-2-3-13-30)14-19(20)28-21(29)15-27-18-7-4-16(5-8-18)22(25)26/h4-9,14,27H,2-3,10-13,15H2,1H3,(H3,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >4.00E+4 | >-26.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG
| Assay Description
For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... |
Structure 9: 29-37 (2001)
Article DOI: 10.1016/s0969-2126(00)00551-7
BindingDB Entry DOI: 10.7270/Q2028PTC |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM17297

(4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...)Show SMILES Cc1nc2ccccc2n1Cc1ccc2n(C)c(CNc3ccc(cc3)C(N)=N)nc2c1 Show InChI InChI=1S/C25H25N7/c1-16-29-20-5-3-4-6-23(20)32(16)15-17-7-12-22-21(13-17)30-24(31(22)2)14-28-19-10-8-18(9-11-19)25(26)27/h3-13,28H,14-15H2,1-2H3,(H3,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| >4.00E+4 | >-26.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG
| Assay Description
For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... |
Structure 9: 29-37 (2001)
Article DOI: 10.1016/s0969-2126(00)00551-7
BindingDB Entry DOI: 10.7270/Q2028PTC |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM17295

(BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...)Show SMILES CCOC(=O)CO\N=C(\c1ccccn1)C1(CC1)c1ccc2n(C)c(CNc3ccc(cc3)C(N)=N)nc2c1 Show InChI InChI=1S/C29H31N7O3/c1-3-38-26(37)18-39-35-27(22-6-4-5-15-32-22)29(13-14-29)20-9-12-24-23(16-20)34-25(36(24)2)17-33-21-10-7-19(8-11-21)28(30)31/h4-12,15-16,33H,3,13-14,17-18H2,1-2H3,(H3,30,31)/b35-27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >4.00E+4 | >-26.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG
| Assay Description
For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... |
Structure 9: 29-37 (2001)
Article DOI: 10.1016/s0969-2126(00)00551-7
BindingDB Entry DOI: 10.7270/Q2028PTC |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM17295

(BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...)Show SMILES CCOC(=O)CO\N=C(\c1ccccn1)C1(CC1)c1ccc2n(C)c(CNc3ccc(cc3)C(N)=N)nc2c1 Show InChI InChI=1S/C29H31N7O3/c1-3-38-26(37)18-39-35-27(22-6-4-5-15-32-22)29(13-14-29)20-9-12-24-23(16-20)34-25(36(24)2)17-33-21-10-7-19(8-11-21)28(30)31/h4-12,15-16,33H,3,13-14,17-18H2,1-2H3,(H3,30,31)/b35-27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 4.40E+4 | -25.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG
| Assay Description
For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... |
Structure 9: 29-37 (2001)
Article DOI: 10.1016/s0969-2126(00)00551-7
BindingDB Entry DOI: 10.7270/Q2028PTC |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM17297

(4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl...)Show SMILES Cc1nc2ccccc2n1Cc1ccc2n(C)c(CNc3ccc(cc3)C(N)=N)nc2c1 Show InChI InChI=1S/C25H25N7/c1-16-29-20-5-3-4-6-23(20)32(16)15-17-7-12-22-21(13-17)30-24(31(22)2)14-28-19-10-8-18(9-11-19)25(26)27/h3-13,28H,14-15H2,1-2H3,(H3,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| >5.00E+4 | >-25.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG
| Assay Description
For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... |
Structure 9: 29-37 (2001)
Article DOI: 10.1016/s0969-2126(00)00551-7
BindingDB Entry DOI: 10.7270/Q2028PTC |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM17295

(BIBT0871 | ethyl 2-{[(E)-{[1-(2-{[(4-carbamimidoyl...)Show SMILES CCOC(=O)CO\N=C(\c1ccccn1)C1(CC1)c1ccc2n(C)c(CNc3ccc(cc3)C(N)=N)nc2c1 Show InChI InChI=1S/C29H31N7O3/c1-3-38-26(37)18-39-35-27(22-6-4-5-15-32-22)29(13-14-29)20-9-12-24-23(16-20)34-25(36(24)2)17-33-21-10-7-19(8-11-21)28(30)31/h4-12,15-16,33H,3,13-14,17-18H2,1-2H3,(H3,30,31)/b35-27- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >5.00E+4 | >-25.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Boehringer Ingelheim Pharma KG
| Assay Description
For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con... |
Structure 9: 29-37 (2001)
Article DOI: 10.1016/s0969-2126(00)00551-7
BindingDB Entry DOI: 10.7270/Q2028PTC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50241077
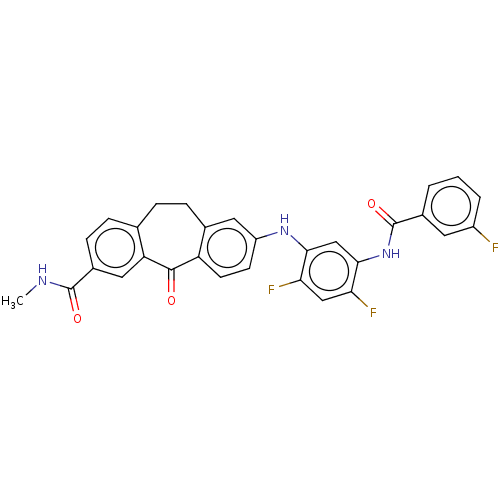
(CHEMBL4060815)Show SMILES CNC(=O)c1ccc2CCc3cc(Nc4cc(NC(=O)c5cccc(F)c5)c(F)cc4F)ccc3C(=O)c2c1 Show InChI InChI=1S/C30H22F3N3O3/c1-34-29(38)19-8-6-16-5-7-17-12-21(9-10-22(17)28(37)23(16)13-19)35-26-15-27(25(33)14-24(26)32)36-30(39)18-3-2-4-20(31)11-18/h2-4,6,8-15,35H,5,7H2,1H3,(H,34,38)(H,36,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-Universitaet Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK (unknown origin) by ELISA |
J Med Chem 60: 8027-8054 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00745
BindingDB Entry DOI: 10.7270/Q2RV0QVZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM202553
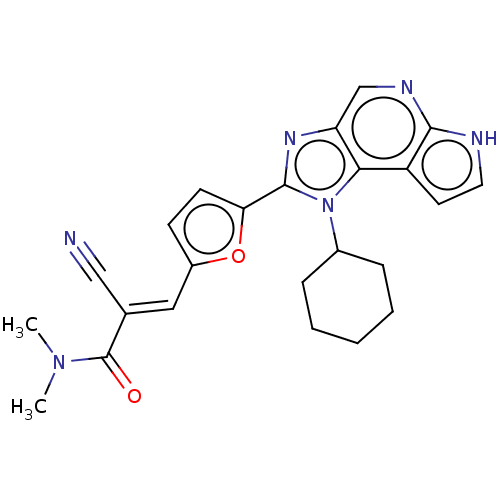
((E/Z)-2-cyano-3-(5-(1-cyclohexyl-1,6-dihydroimidaz...)Show SMILES CN(C)C(=O)C(=C\c1ccc(o1)-c1nc2cnc3[nH]ccc3c2n1C1CCCCC1)\C#N Show InChI InChI=1S/C24H24N6O2/c1-29(2)24(31)15(13-25)12-17-8-9-20(32-17)23-28-19-14-27-22-18(10-11-26-22)21(19)30(23)16-6-4-3-5-7-16/h8-12,14,16H,3-7H2,1-2H3,(H,26,27)/b15-12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.127 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
| Assay Description
A commercial assay by Reaction Biology Corp. |
Cell Chem Biol 23: 1335-1340 (2016)
Article DOI: 10.1016/j.chembiol.2016.10.008
BindingDB Entry DOI: 10.7270/Q2WS8S22 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM202554
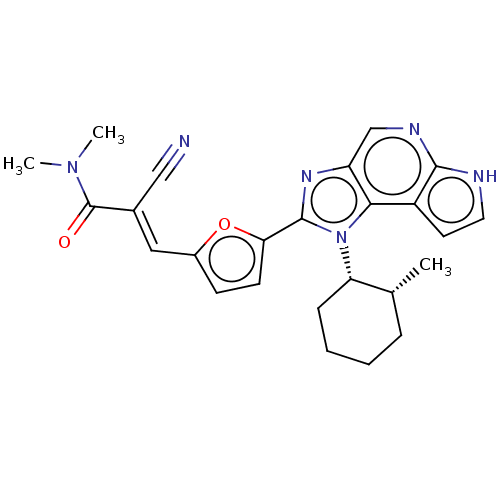
((E/Z)-2-cyano-N,N-dimethyl-3-(5-(1-((1S,2R)-2-meth...)Show SMILES C[C@@H]1CCCC[C@@H]1n1c(nc2cnc3[nH]ccc3c12)-c1ccc(\C=C(/C#N)C(=O)N(C)C)o1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.154 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
| Assay Description
A commercial assay by Reaction Biology Corp. |
Cell Chem Biol 23: 1335-1340 (2016)
Article DOI: 10.1016/j.chembiol.2016.10.008
BindingDB Entry DOI: 10.7270/Q2WS8S22 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50241059
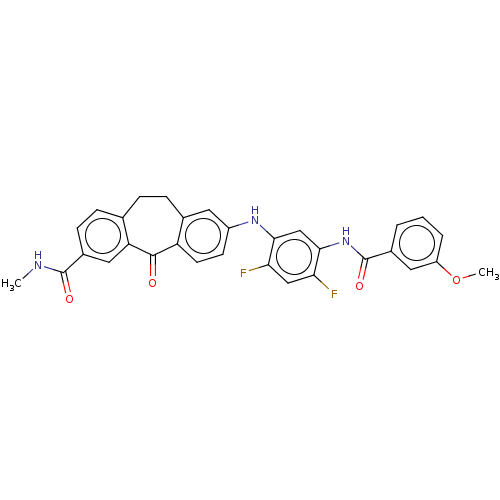
(CHEMBL4069496)Show SMILES CNC(=O)c1ccc2CCc3cc(Nc4cc(NC(=O)c5cccc(OC)c5)c(F)cc4F)ccc3C(=O)c2c1 Show InChI InChI=1S/C31H25F2N3O4/c1-34-30(38)20-9-7-17-6-8-18-12-21(10-11-23(18)29(37)24(17)14-20)35-27-16-28(26(33)15-25(27)32)36-31(39)19-4-3-5-22(13-19)40-2/h3-5,7,9-16,35H,6,8H2,1-2H3,(H,34,38)(H,36,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-Universitaet Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK (unknown origin) by ELISA |
J Med Chem 60: 8027-8054 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00745
BindingDB Entry DOI: 10.7270/Q2RV0QVZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50241058
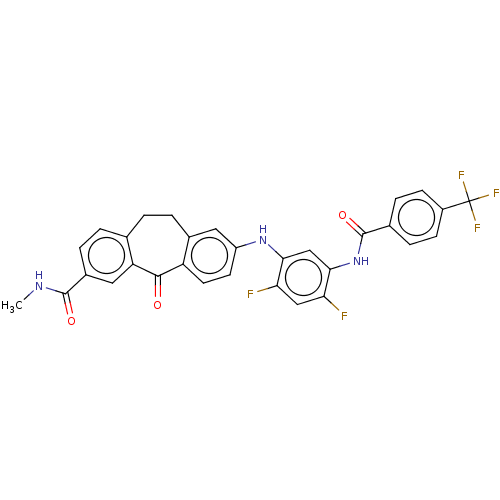
(CHEMBL4104440)Show SMILES CNC(=O)c1ccc2CCc3cc(Nc4cc(NC(=O)c5ccc(cc5)C(F)(F)F)c(F)cc4F)ccc3C(=O)c2c1 Show InChI InChI=1S/C31H22F5N3O3/c1-37-29(41)19-5-3-16-2-4-18-12-21(10-11-22(18)28(40)23(16)13-19)38-26-15-27(25(33)14-24(26)32)39-30(42)17-6-8-20(9-7-17)31(34,35)36/h3,5-15,38H,2,4H2,1H3,(H,37,41)(H,39,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-Universitaet Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK (unknown origin) by ELISA |
J Med Chem 60: 8027-8054 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00745
BindingDB Entry DOI: 10.7270/Q2RV0QVZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50425360
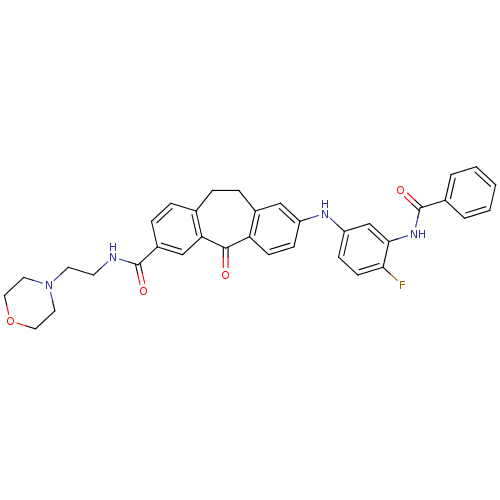
(CHEMBL2316207)Show SMILES Fc1ccc(Nc2ccc3c(CCc4ccc(cc4C3=O)C(=O)NCCN3CCOCC3)c2)cc1NC(=O)c1ccccc1 Show InChI InChI=1S/C35H33FN4O4/c36-31-13-11-28(22-32(31)39-35(43)24-4-2-1-3-5-24)38-27-10-12-29-25(20-27)8-6-23-7-9-26(21-30(23)33(29)41)34(42)37-14-15-40-16-18-44-19-17-40/h1-5,7,9-13,20-22,38H,6,8,14-19H2,(H,37,42)(H,39,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of T£bingen
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase (unknown origin) by ELISA |
J Med Chem 56: 241-53 (2013)
Article DOI: 10.1021/jm301539x
BindingDB Entry DOI: 10.7270/Q2TB187B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50241013
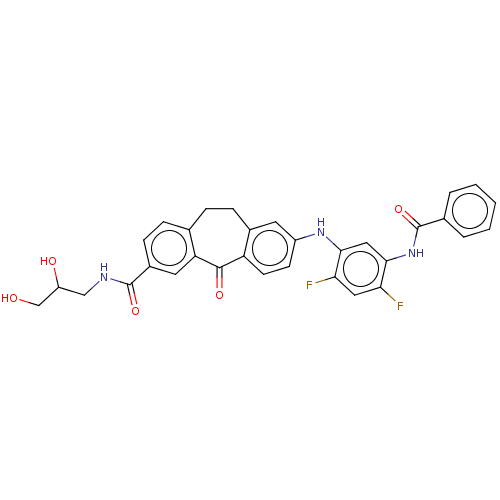
(CHEMBL4066023)Show SMILES OCC(O)CNC(=O)c1ccc2CCc3cc(Nc4cc(NC(=O)c5ccccc5)c(F)cc4F)ccc3C(=O)c2c1 Show InChI InChI=1S/C32H27F2N3O5/c33-26-14-27(34)29(37-32(42)19-4-2-1-3-5-19)15-28(26)36-22-10-11-24-20(12-22)8-6-18-7-9-21(13-25(18)30(24)40)31(41)35-16-23(39)17-38/h1-5,7,9-15,23,36,38-39H,6,8,16-17H2,(H,35,41)(H,37,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-Universitaet Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK (unknown origin) by ELISA |
J Med Chem 60: 8027-8054 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00745
BindingDB Entry DOI: 10.7270/Q2RV0QVZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50425360

(CHEMBL2316207)Show SMILES Fc1ccc(Nc2ccc3c(CCc4ccc(cc4C3=O)C(=O)NCCN3CCOCC3)c2)cc1NC(=O)c1ccccc1 Show InChI InChI=1S/C35H33FN4O4/c36-31-13-11-28(22-32(31)39-35(43)24-4-2-1-3-5-24)38-27-10-12-29-25(20-27)8-6-23-7-9-26(21-30(23)33(29)41)34(42)37-14-15-40-16-18-44-19-17-40/h1-5,7,9-13,20-22,38H,6,8,14-19H2,(H,37,42)(H,39,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-Universitaet Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK (unknown origin) by ELISA |
J Med Chem 60: 8027-8054 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00745
BindingDB Entry DOI: 10.7270/Q2RV0QVZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50241078
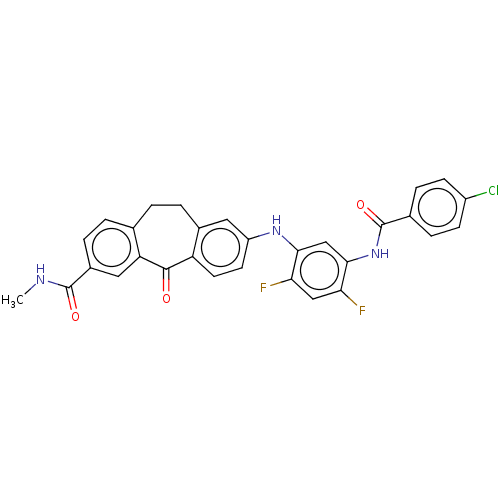
(CHEMBL4073561)Show SMILES CNC(=O)c1ccc2CCc3cc(Nc4cc(NC(=O)c5ccc(Cl)cc5)c(F)cc4F)ccc3C(=O)c2c1 Show InChI InChI=1S/C30H22ClF2N3O3/c1-34-29(38)19-5-3-16-2-4-18-12-21(10-11-22(18)28(37)23(16)13-19)35-26-15-27(25(33)14-24(26)32)36-30(39)17-6-8-20(31)9-7-17/h3,5-15,35H,2,4H2,1H3,(H,34,38)(H,36,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-Universitaet Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK (unknown origin) by ELISA |
J Med Chem 60: 8027-8054 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00745
BindingDB Entry DOI: 10.7270/Q2RV0QVZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50241010
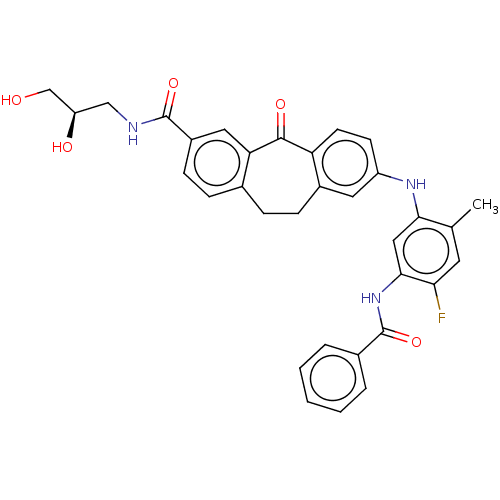
(CHEMBL4074237)Show SMILES Cc1cc(F)c(NC(=O)c2ccccc2)cc1Nc1ccc2c(CCc3ccc(cc3C2=O)C(=O)NC[C@@H](O)CO)c1 |r| Show InChI InChI=1S/C33H30FN3O5/c1-19-13-28(34)30(37-33(42)21-5-3-2-4-6-21)16-29(19)36-24-11-12-26-22(14-24)9-7-20-8-10-23(15-27(20)31(26)40)32(41)35-17-25(39)18-38/h2-6,8,10-16,25,36,38-39H,7,9,17-18H2,1H3,(H,35,41)(H,37,42)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-Universitaet Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK in human whole blood assessed as reduction in TNF-alpha release after 10 mins by ELISA |
J Med Chem 60: 8027-8054 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00745
BindingDB Entry DOI: 10.7270/Q2RV0QVZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.292 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
| Assay Description
A commercial assay by Reaction Biology Corp. |
Cell Chem Biol 23: 1335-1340 (2016)
Article DOI: 10.1016/j.chembiol.2016.10.008
BindingDB Entry DOI: 10.7270/Q2WS8S22 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data