

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase 1 (Bacillus cereus) | BDBM50450973 (CHEMBL4209255) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Santiago de Compostela Curated by ChEMBL | Assay Description Inhibition of Bacillus cereus beta-lactamase AmpC using CENTA as substrate up to 30 mins by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 27: 4221-4228 (2017) Article DOI: 10.1016/j.bmcl.2017.08.027 BindingDB Entry DOI: 10.7270/Q2N0193Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase 1 (Bacillus cereus) | BDBM50450974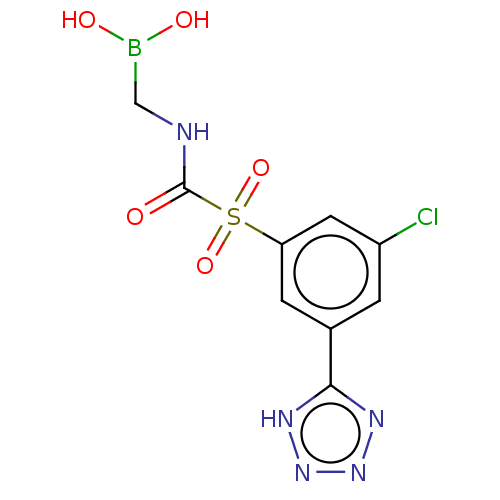 (CHEMBL4206040) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Santiago de Compostela Curated by ChEMBL | Assay Description Inhibition of Bacillus cereus beta-lactamase AmpC using CENTA as substrate up to 30 mins by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 27: 4221-4228 (2017) Article DOI: 10.1016/j.bmcl.2017.08.027 BindingDB Entry DOI: 10.7270/Q2N0193Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase 1 (Bacillus cereus) | BDBM50450969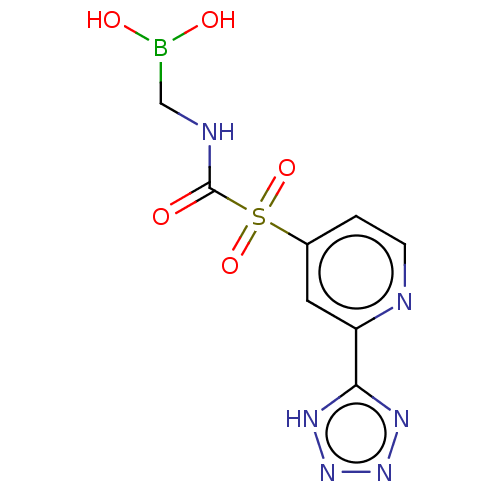 (CHEMBL4217629) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Santiago de Compostela Curated by ChEMBL | Assay Description Inhibition of Bacillus cereus beta-lactamase AmpC using CENTA as substrate up to 30 mins by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 27: 4221-4228 (2017) Article DOI: 10.1016/j.bmcl.2017.08.027 BindingDB Entry DOI: 10.7270/Q2N0193Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50114077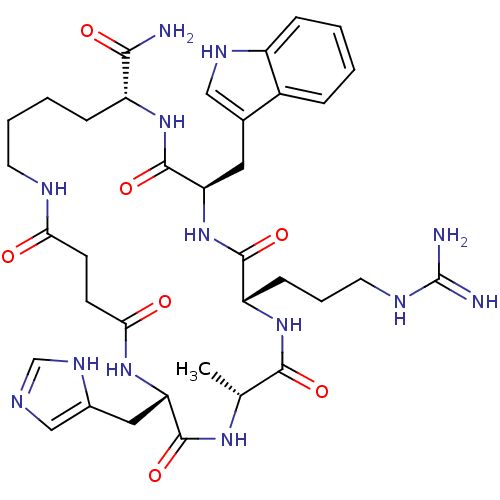 (6-(3-Guanidino-propyl)-12-(3H-imidazol-4-ylmethyl)...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity for human Melanocortin-4 receptor (hMC4R) | J Med Chem 45: 2644-50 (2002) BindingDB Entry DOI: 10.7270/Q2RB75B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50114077 (6-(3-Guanidino-propyl)-12-(3H-imidazol-4-ylmethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity for human Melanocortin-3 receptor (hMC3R) expressed in HEK293 cells | J Med Chem 45: 2644-50 (2002) BindingDB Entry DOI: 10.7270/Q2RB75B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50114083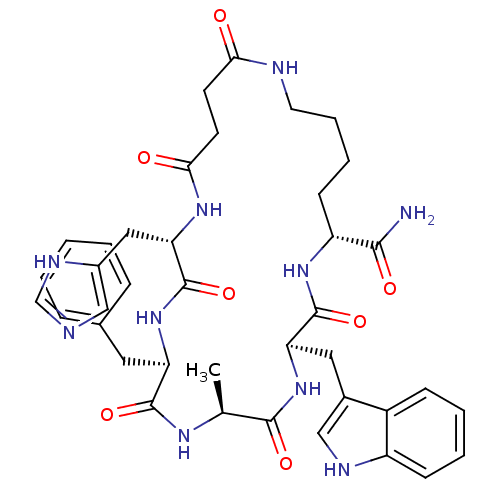 (9-Benzyl-12-(3H-imidazol-4-ylmethyl)-3-(1H-indol-3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity for human melanocortin 5 receptor (hMC5R) expressed in HEK293 cells | J Med Chem 45: 2644-50 (2002) BindingDB Entry DOI: 10.7270/Q2RB75B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50114088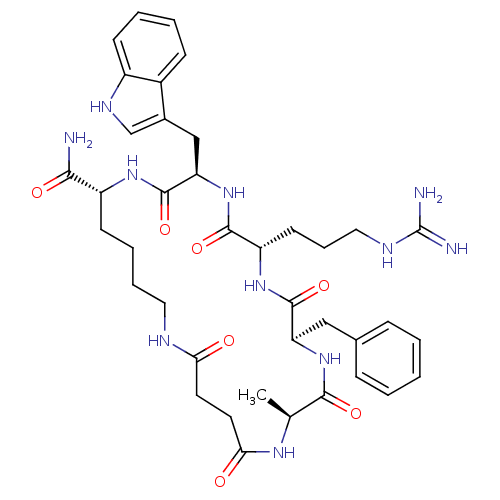 (9-Benzyl-6-(3-guanidino-propyl)-3-(1H-indol-3-ylme...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity for human melanocortin 5 receptor (hMC5R) expressed in HEK293 cells | J Med Chem 45: 2644-50 (2002) BindingDB Entry DOI: 10.7270/Q2RB75B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50304627 ((2S,3S,4R,5R,6S)-6-methylpiperidine-2,3,4,5-tetrao...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of bovine kidney alpha-L-fucosidase by para-nitrophenolate release assay | Bioorg Med Chem 17: 8020-6 (2009) Article DOI: 10.1016/j.bmc.2009.10.010 BindingDB Entry DOI: 10.7270/Q2FB5307 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50114077 (6-(3-Guanidino-propyl)-12-(3H-imidazol-4-ylmethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity for human melanocortin 5 receptor (hMC5R) expressed in HEK293 cells | J Med Chem 45: 2644-50 (2002) BindingDB Entry DOI: 10.7270/Q2RB75B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50114085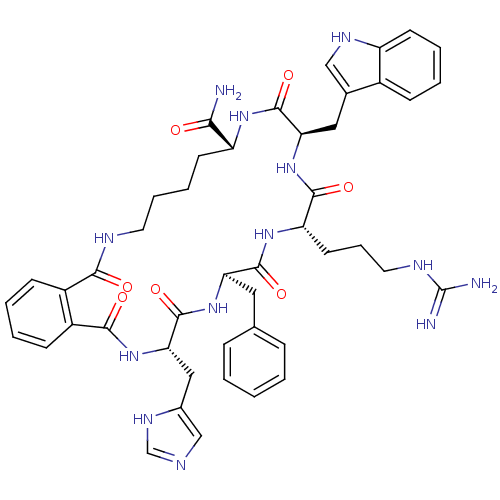 ((O)C-(C6H4)-C(O)-c[His-D-Phe-Arg-Trp-Lys]-NH2) (MK...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity for human melanocortin 5 receptor (hMC5R) expressed in HEK293 cells | J Med Chem 45: 2644-50 (2002) BindingDB Entry DOI: 10.7270/Q2RB75B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50114083 (9-Benzyl-12-(3H-imidazol-4-ylmethyl)-3-(1H-indol-3...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity for human Melanocortin-4 receptor (hMC4R) | J Med Chem 45: 2644-50 (2002) BindingDB Entry DOI: 10.7270/Q2RB75B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50114079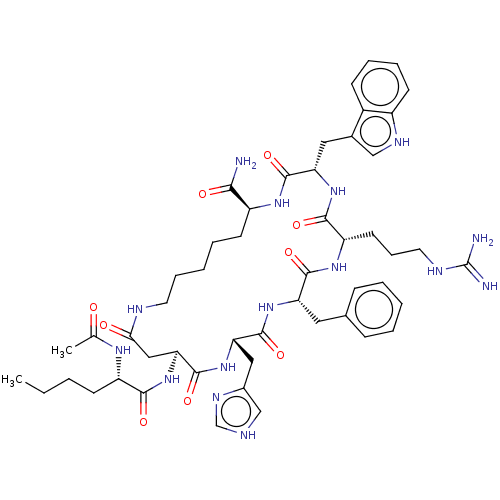 (15-(2-Acetylamino-hexanoylamino)-9-benzyl-6-(3-gua...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity for human Melanocortin-4 receptor (hMC4R) | J Med Chem 45: 2644-50 (2002) BindingDB Entry DOI: 10.7270/Q2RB75B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50114079 (15-(2-Acetylamino-hexanoylamino)-9-benzyl-6-(3-gua...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity for human Melanocortin-3 receptor (hMC3R) expressed in HEK293 cells | J Med Chem 45: 2644-50 (2002) BindingDB Entry DOI: 10.7270/Q2RB75B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase 1 (Bacillus cereus) | BDBM50450975 (CHEMBL4204887) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Santiago de Compostela Curated by ChEMBL | Assay Description Inhibition of Bacillus cereus beta-lactamase AmpC using CENTA as substrate up to 30 mins by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 27: 4221-4228 (2017) Article DOI: 10.1016/j.bmcl.2017.08.027 BindingDB Entry DOI: 10.7270/Q2N0193Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50065258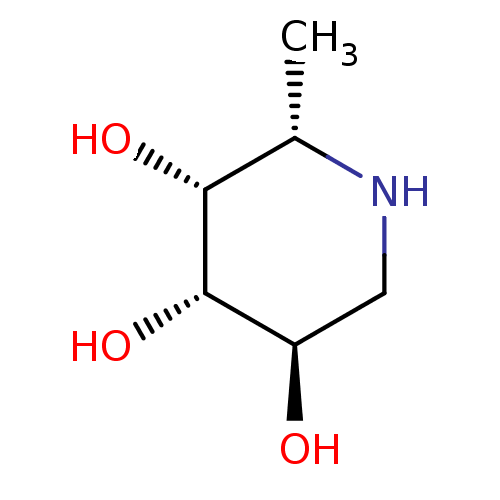 ((2S,3R,4S,5R)-2-Methyl-piperidine-3,4,5-triol | (2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of bovine kidney alpha-L-fucosidase by para-nitrophenolate release assay | Bioorg Med Chem 17: 8020-6 (2009) Article DOI: 10.1016/j.bmc.2009.10.010 BindingDB Entry DOI: 10.7270/Q2FB5307 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50114087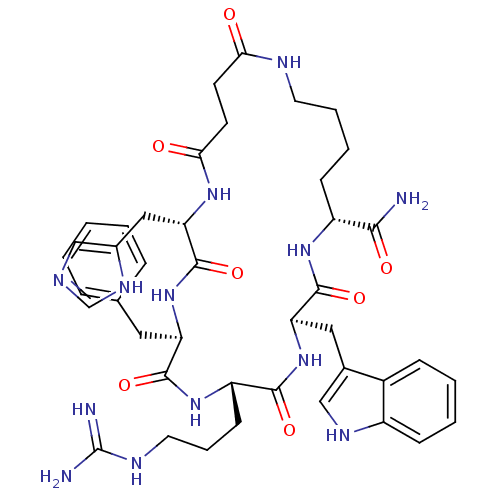 (9-Benzyl-6-(3-guanidino-propyl)-12-(3H-imidazol-4-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity for human Melanocortin-4 receptor (hMC4R) | J Med Chem 45: 2644-50 (2002) BindingDB Entry DOI: 10.7270/Q2RB75B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50114078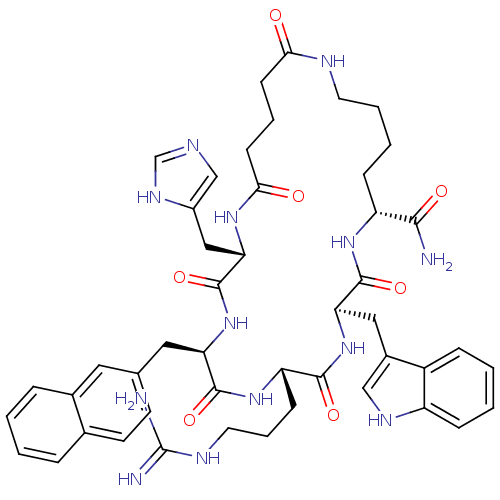 (8-(3-Guanidino-propyl)-2-(3H-imidazol-4-ylmethyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity for human Melanocortin-3 receptor (hMC3R) expressed in HEK293 cells | J Med Chem 45: 2644-50 (2002) BindingDB Entry DOI: 10.7270/Q2RB75B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50114079 (15-(2-Acetylamino-hexanoylamino)-9-benzyl-6-(3-gua...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity for human melanocortin 5 receptor (hMC5R) expressed in HEK293 cells | J Med Chem 45: 2644-50 (2002) BindingDB Entry DOI: 10.7270/Q2RB75B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50114084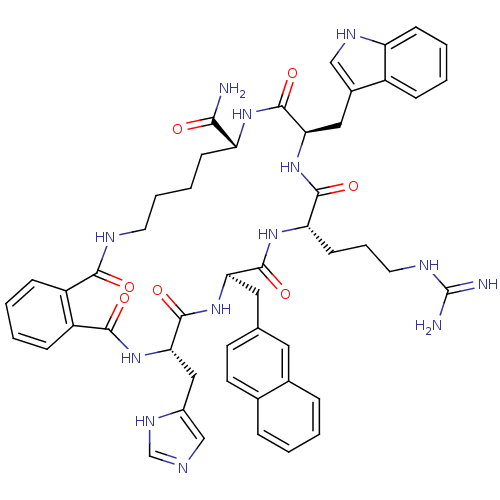 ((O)C-(C6H4)-C(O)-c[His-D-Nal(2')-Arg-Trp-Lys]-NH2)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity for human Melanocortin-3 receptor (hMC3R) expressed in HEK293 cells | J Med Chem 45: 2644-50 (2002) BindingDB Entry DOI: 10.7270/Q2RB75B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50304628 ((2S,3R,4S)-2-methyl-3,4-dihydro-2H-pyrrole-3,4-dio...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of bovine kidney alpha-L-fucosidase by para-nitrophenolate release assay | Bioorg Med Chem 17: 8020-6 (2009) Article DOI: 10.1016/j.bmc.2009.10.010 BindingDB Entry DOI: 10.7270/Q2FB5307 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50114080 (6-(3-Guanidino-propyl)-12-(3H-imidazol-4-ylmethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity for human Melanocortin-3 receptor (hMC3R) expressed in HEK293 cells | J Med Chem 45: 2644-50 (2002) BindingDB Entry DOI: 10.7270/Q2RB75B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50114078 (8-(3-Guanidino-propyl)-2-(3H-imidazol-4-ylmethyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity for human melanocortin 5 receptor (hMC5R) expressed in HEK293 cells | J Med Chem 45: 2644-50 (2002) BindingDB Entry DOI: 10.7270/Q2RB75B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Mycobacterium tuberculosis) | BDBM50303445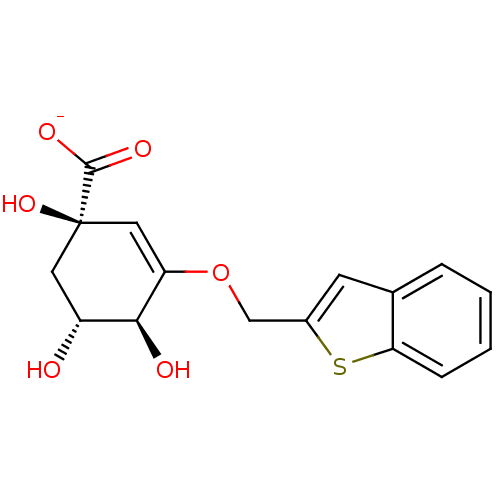 (CHEMBL567563 | Sodium(1R,4S,5R)-1,4,5-Trihydroxy-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis DHQ2 | J Med Chem 54: 6063-84 (2011) Article DOI: 10.1021/jm2006063 BindingDB Entry DOI: 10.7270/Q2XS5VSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Mycobacterium tuberculosis) | BDBM50303446 (CHEMBL576247 | Sodium(1R,4S,5R)-1,4,5-Trihydroxy-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis DHQ2 | J Med Chem 54: 6063-84 (2011) Article DOI: 10.1021/jm2006063 BindingDB Entry DOI: 10.7270/Q2XS5VSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Mycobacterium tuberculosis) | BDBM50352031 (CHEMBL1823560) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis DHQ2 | J Med Chem 54: 6063-84 (2011) Article DOI: 10.1021/jm2006063 BindingDB Entry DOI: 10.7270/Q2XS5VSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Mycobacterium tuberculosis) | BDBM50352032 (CHEMBL1823561) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis DHQ2 | J Med Chem 54: 6063-84 (2011) Article DOI: 10.1021/jm2006063 BindingDB Entry DOI: 10.7270/Q2XS5VSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Mycobacterium tuberculosis) | BDBM50352030 (CHEMBL1823559) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis DHQ2 | J Med Chem 54: 6063-84 (2011) Article DOI: 10.1021/jm2006063 BindingDB Entry DOI: 10.7270/Q2XS5VSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Mycobacterium tuberculosis) | BDBM50352033 (CHEMBL1823562) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis DHQ2 | J Med Chem 54: 6063-84 (2011) Article DOI: 10.1021/jm2006063 BindingDB Entry DOI: 10.7270/Q2XS5VSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Mycobacterium tuberculosis) | BDBM50303444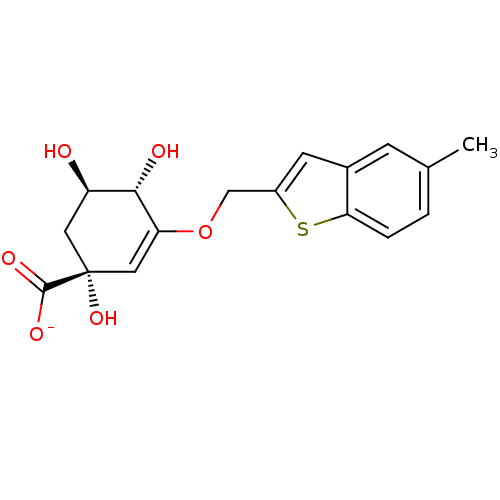 (CHEMBL567564 | Sodium(1R,4S,5R)-1,4,5-Trihydroxy-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis DHQ2 | J Med Chem 54: 6063-84 (2011) Article DOI: 10.1021/jm2006063 BindingDB Entry DOI: 10.7270/Q2XS5VSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Helicobacter pylori) | BDBM50352020 (CHEMBL1823563) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Competitive inhibition of Helicobacter pylori DHQ2 | J Med Chem 54: 6063-84 (2011) Article DOI: 10.1021/jm2006063 BindingDB Entry DOI: 10.7270/Q2XS5VSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Mycobacterium tuberculosis) | BDBM50170809 ((1R,4R,5R)-1,4,5-Trihydroxy-3-(3-nitro-phenyl)-cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis DHQ2 | J Med Chem 54: 6063-84 (2011) Article DOI: 10.1021/jm2006063 BindingDB Entry DOI: 10.7270/Q2XS5VSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Mycobacterium tuberculosis) | BDBM50170809 ((1R,4R,5R)-1,4,5-Trihydroxy-3-(3-nitro-phenyl)-cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | 8.2 | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis type II dehydroquinase at 25 degree C pH 8.2 | J Med Chem 48: 4871-81 (2005) Article DOI: 10.1021/jm0501836 BindingDB Entry DOI: 10.7270/Q2RX9BMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50114084 ((O)C-(C6H4)-C(O)-c[His-D-Nal(2')-Arg-Trp-Lys]-NH2)...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity for human Melanocortin-4 receptor (hMC4R) | J Med Chem 45: 2644-50 (2002) BindingDB Entry DOI: 10.7270/Q2RB75B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50114085 ((O)C-(C6H4)-C(O)-c[His-D-Phe-Arg-Trp-Lys]-NH2) (MK...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity for human Melanocortin-4 receptor (hMC4R) | J Med Chem 45: 2644-50 (2002) BindingDB Entry DOI: 10.7270/Q2RB75B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Mycobacterium tuberculosis) | BDBM50352027 (CHEMBL1823571) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis DHQ2 | J Med Chem 54: 6063-84 (2011) Article DOI: 10.1021/jm2006063 BindingDB Entry DOI: 10.7270/Q2XS5VSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50114087 (9-Benzyl-6-(3-guanidino-propyl)-12-(3H-imidazol-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity for human Melanocortin-3 receptor (hMC3R) expressed in HEK293 cells | J Med Chem 45: 2644-50 (2002) BindingDB Entry DOI: 10.7270/Q2RB75B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50114080 (6-(3-Guanidino-propyl)-12-(3H-imidazol-4-ylmethyl)...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity for human Melanocortin-4 receptor (hMC4R) | J Med Chem 45: 2644-50 (2002) BindingDB Entry DOI: 10.7270/Q2RB75B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Helicobacter pylori) | BDBM50352033 (CHEMBL1823562) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Competitive inhibition of Helicobacter pylori DHQ2 | J Med Chem 54: 6063-84 (2011) Article DOI: 10.1021/jm2006063 BindingDB Entry DOI: 10.7270/Q2XS5VSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Helicobacter pylori) | BDBM50352027 (CHEMBL1823571) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Competitive inhibition of Helicobacter pylori DHQ2 | J Med Chem 54: 6063-84 (2011) Article DOI: 10.1021/jm2006063 BindingDB Entry DOI: 10.7270/Q2XS5VSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50114082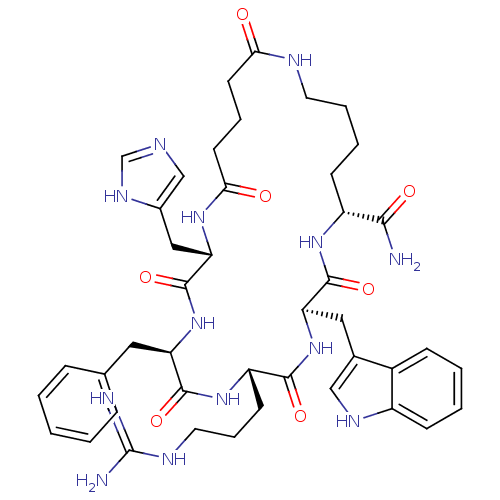 (5-Benzyl-8-(3-guanidino-propyl)-2-(3H-imidazol-4-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity for human Melanocortin-3 receptor (hMC3R) expressed in HEK293 cells | J Med Chem 45: 2644-50 (2002) BindingDB Entry DOI: 10.7270/Q2RB75B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50526862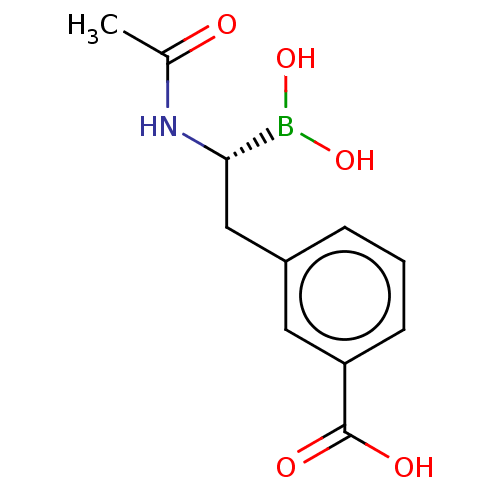 (CHEMBL1231367) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Santiago de Compostela Curated by ChEMBL | Assay Description Inhibition of Escherichia coli TEM-1 beta-lactamase | J Med Chem 63: 1859-1881 (2020) Article DOI: 10.1021/acs.jmedchem.9b01279 BindingDB Entry DOI: 10.7270/Q27P92V4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 3-dehydroquinate dehydratase (Mycobacterium tuberculosis) | BDBM50352029 (CHEMBL1823558) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis DHQ2 | J Med Chem 54: 6063-84 (2011) Article DOI: 10.1021/jm2006063 BindingDB Entry DOI: 10.7270/Q2XS5VSG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| 3-dehydroquinate dehydratase (Helicobacter pylori) | BDBM50303444 (CHEMBL567564 | Sodium(1R,4S,5R)-1,4,5-Trihydroxy-3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Inhibition of Helicobacter pylori DHQ2 by UV spectroscopy | J Med Chem 53: 191-200 (2010) Article DOI: 10.1021/jm9010466 BindingDB Entry DOI: 10.7270/Q2Q52PQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Helicobacter pylori) | BDBM50303445 (CHEMBL567563 | Sodium(1R,4S,5R)-1,4,5-Trihydroxy-3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Inhibition of Helicobacter pylori DHQ2 by UV spectroscopy | J Med Chem 53: 191-200 (2010) Article DOI: 10.1021/jm9010466 BindingDB Entry DOI: 10.7270/Q2Q52PQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Helicobacter pylori) | BDBM50352021 (CHEMBL1823565) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Competitive inhibition of Helicobacter pylori DHQ2 | J Med Chem 54: 6063-84 (2011) Article DOI: 10.1021/jm2006063 BindingDB Entry DOI: 10.7270/Q2XS5VSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50114086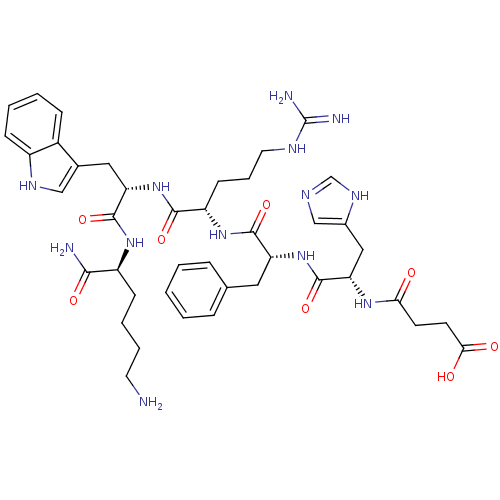 (CHEMBL433413 | MK-11 | N-[1-(1-{1-[1-(5-Amino-1-ca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity for human Melanocortin-3 receptor (hMC3R) expressed in HEK293 cells | J Med Chem 45: 2644-50 (2002) BindingDB Entry DOI: 10.7270/Q2RB75B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50114080 (6-(3-Guanidino-propyl)-12-(3H-imidazol-4-ylmethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity for human melanocortin 5 receptor (hMC5R) expressed in HEK293 cells | J Med Chem 45: 2644-50 (2002) BindingDB Entry DOI: 10.7270/Q2RB75B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Helicobacter pylori) | BDBM50303446 (CHEMBL576247 | Sodium(1R,4S,5R)-1,4,5-Trihydroxy-3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Inhibition of Helicobacter pylori DHQ2 by UV spectroscopy | J Med Chem 53: 191-200 (2010) Article DOI: 10.1021/jm9010466 BindingDB Entry DOI: 10.7270/Q2Q52PQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Mycobacterium tuberculosis) | BDBM50352020 (CHEMBL1823563) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis DHQ2 | J Med Chem 54: 6063-84 (2011) Article DOI: 10.1021/jm2006063 BindingDB Entry DOI: 10.7270/Q2XS5VSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50114081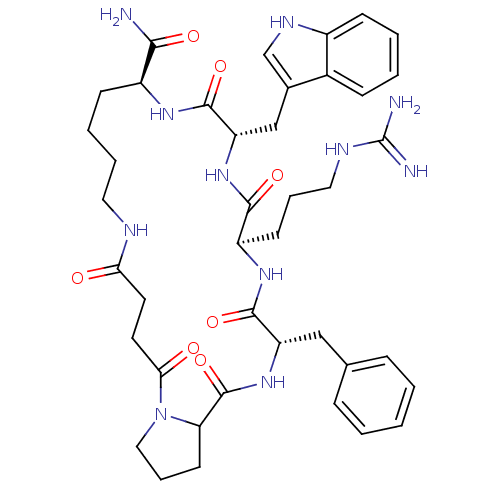 (22-Benzyl-19-(3-guanidino-propyl)-16-(1H-indol-3-y...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity for human Melanocortin-4 receptor (hMC4R) | J Med Chem 45: 2644-50 (2002) BindingDB Entry DOI: 10.7270/Q2RB75B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 613 total ) | Next | Last >> |