

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM50339708 ((S)-2-(5-chlorothiophen-2-yl)-N-(1-(5-fluoro-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1588-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.129 BindingDB Entry DOI: 10.7270/Q2RN385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50339718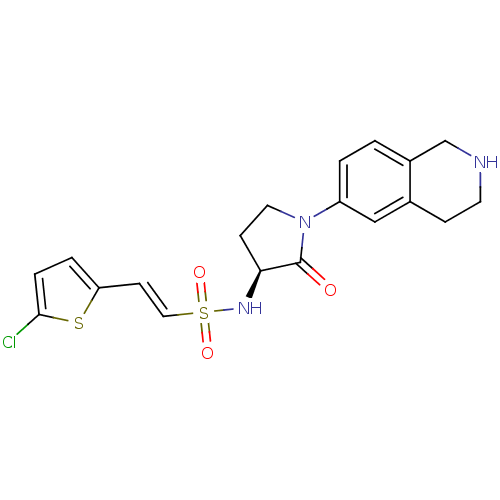 ((S)-2-(5-chlorothiophen-2-yl)-N-(2-oxo-1-(1,2,3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1588-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.129 BindingDB Entry DOI: 10.7270/Q2RN385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50339716 ((S)-6-chloro-N-(1-(6-fluoro-2,3,4,5-tetrahydro-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1588-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.129 BindingDB Entry DOI: 10.7270/Q2RN385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50339713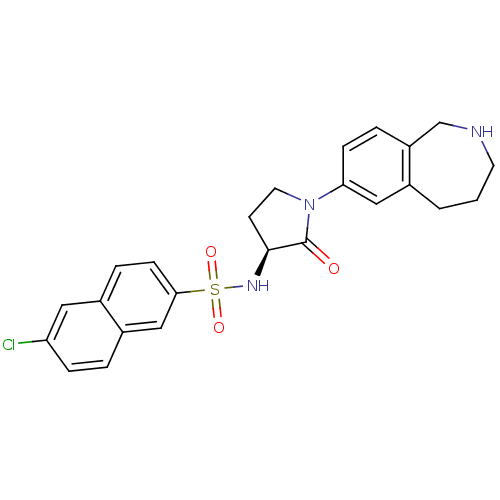 ((S)-6-chloro-N-(2-oxo-1-(2,3,4,5-tetrahydro-1H-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1588-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.129 BindingDB Entry DOI: 10.7270/Q2RN385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50339714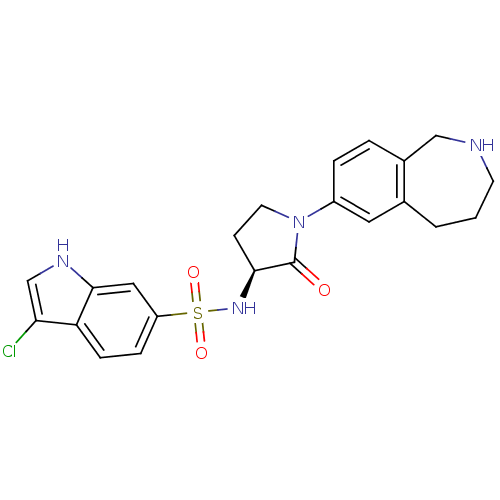 ((S)-3-chloro-N-(2-oxo-1-(2,3,4,5-tetrahydro-1H-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1588-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.129 BindingDB Entry DOI: 10.7270/Q2RN385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50339720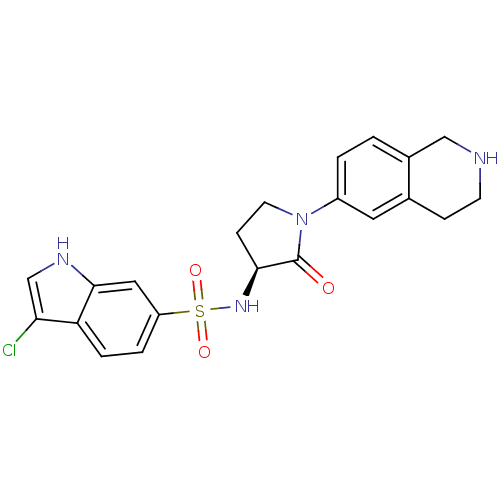 ((S)-3-chloro-N-(2-oxo-1-(1,2,3,4-tetrahydroisoquin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1588-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.129 BindingDB Entry DOI: 10.7270/Q2RN385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50339712 ((S,E)-2-(5-chlorothiophen-2-yl)-N-(2-oxo-1-(2,3,4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1588-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.129 BindingDB Entry DOI: 10.7270/Q2RN385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50339711 ((S)-3-chloro-N-(1-(7-fluoro-1,2,3,4-tetrahydroisoq...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1588-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.129 BindingDB Entry DOI: 10.7270/Q2RN385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50339717 ((S)-2-(5-chlorothiophen-2-yl)-N-(1-(2-methyl-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1588-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.129 BindingDB Entry DOI: 10.7270/Q2RN385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50339719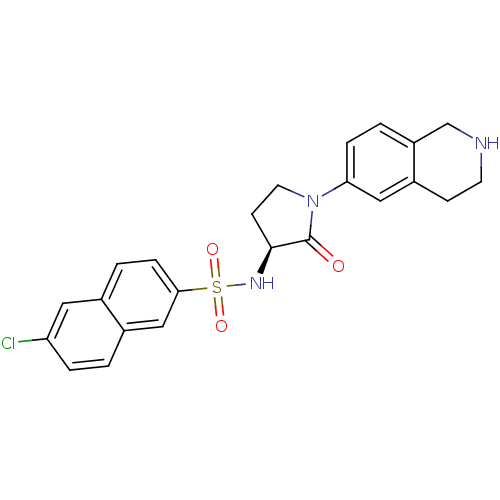 ((S)-6-chloro-N-(2-oxo-1-(1,2,3,4-tetrahydroisoquin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1588-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.129 BindingDB Entry DOI: 10.7270/Q2RN385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50339706 ((S)-6-chloro-N-(1-(5-fluoro-1,2,3,4-tetrahydroisoq...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1588-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.129 BindingDB Entry DOI: 10.7270/Q2RN385P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50339715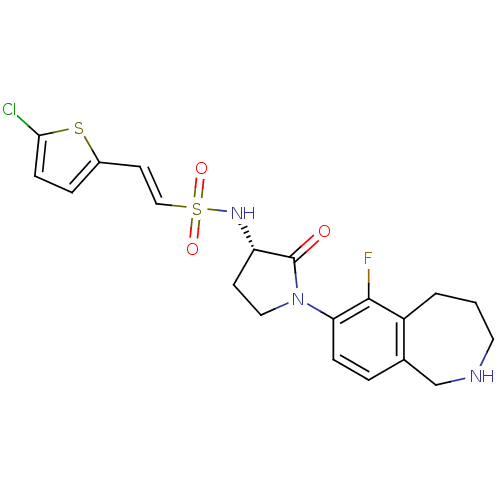 ((S)-2-(5-chlorothiophen-2-yl)-N-(1-(6-fluoro-2,3,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1588-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.129 BindingDB Entry DOI: 10.7270/Q2RN385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50339709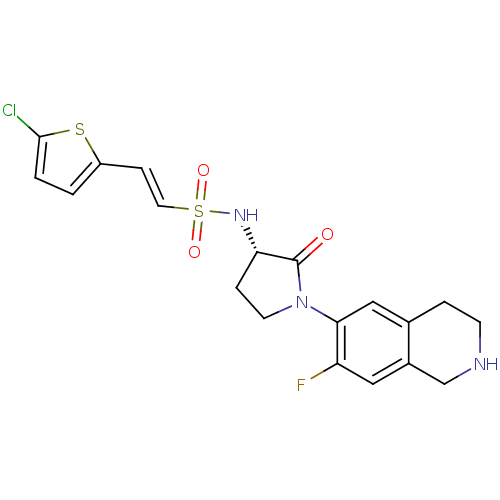 ((S)-2-(5-chlorothiophen-2-yl)-N-(1-(7-fluoro-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1588-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.129 BindingDB Entry DOI: 10.7270/Q2RN385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50339710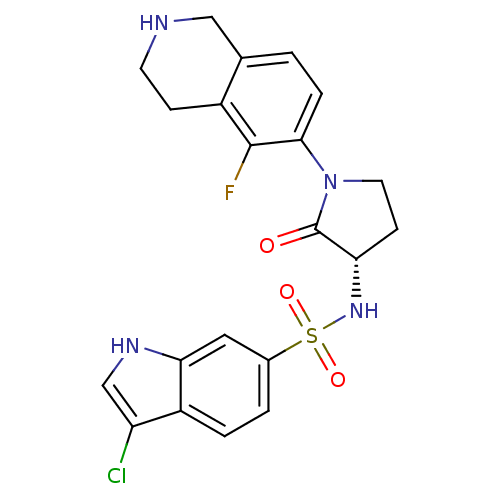 ((S)-3-chloro-N-(1-(5-fluoro-1,2,3,4-tetrahydroisoq...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1588-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.129 BindingDB Entry DOI: 10.7270/Q2RN385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50306134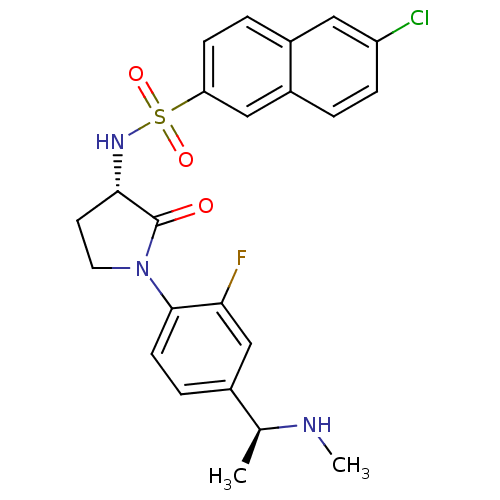 (6-chloro-N-((S)-1-(2-fluoro-4-((S)-1-(methylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1588-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.129 BindingDB Entry DOI: 10.7270/Q2RN385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50339707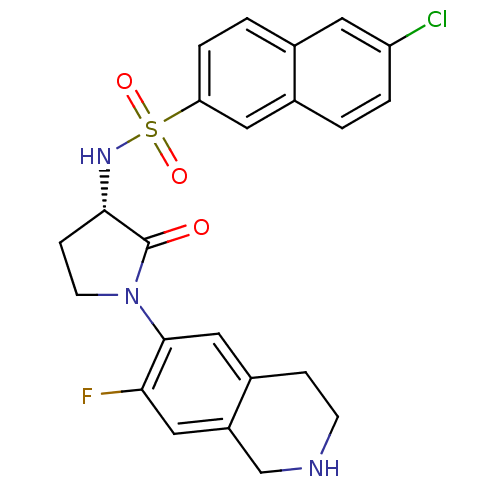 ((S)-6-chloro-N-(1-(7-fluoro-1,2,3,4-tetrahydroisoq...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1588-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.129 BindingDB Entry DOI: 10.7270/Q2RN385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50339721 ((S)-2-(5-chlorothiophen-2-yl)-N-(2-oxo-1-(1,2,3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1588-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.129 BindingDB Entry DOI: 10.7270/Q2RN385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50339722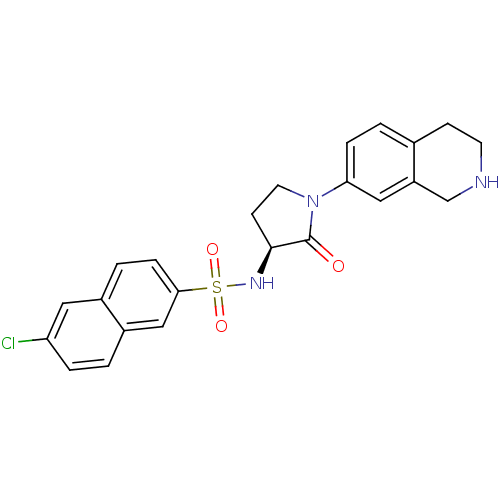 ((S)-6-chloro-N-(2-oxo-1-(1,2,3,4-tetrahydroisoquin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1588-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.129 BindingDB Entry DOI: 10.7270/Q2RN385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50339723 ((S)-3-chloro-N-(2-oxo-1-(1,2,3,4-tetrahydroisoquin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1588-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.129 BindingDB Entry DOI: 10.7270/Q2RN385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096484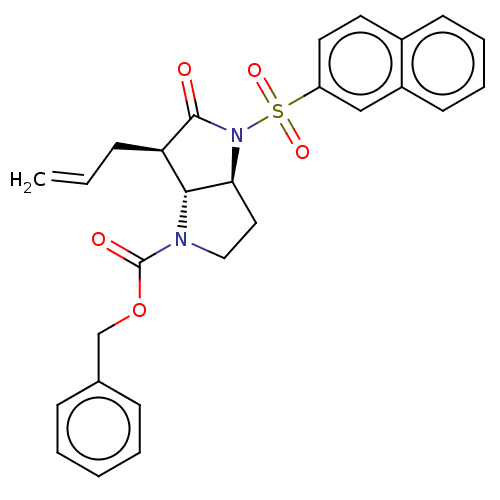 ((4S,6R)-6-Allyl-4-(naphthalene-2-sulfonyl)-5-oxo-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50066997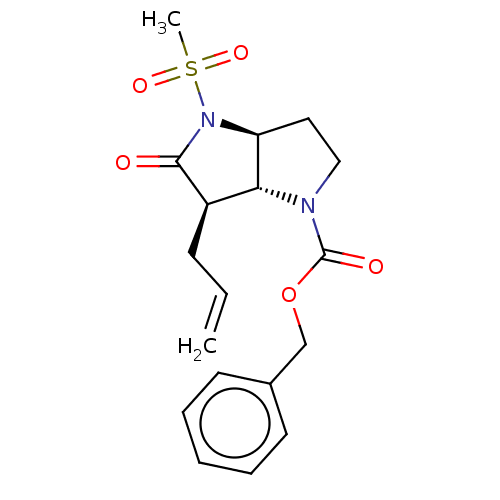 ((3aS,6R)-6-Allyl-4-methanesulfonyl-5-oxo-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000293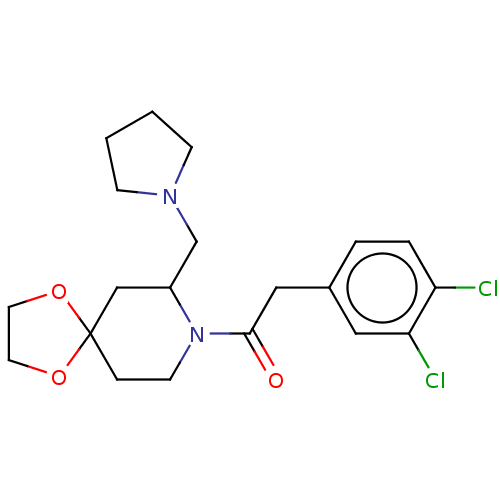 (2-(3,4-Dichloro-phenyl)-1-(7-pyrrolidin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency was determined in vitro using rabbit vas deferens (LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000288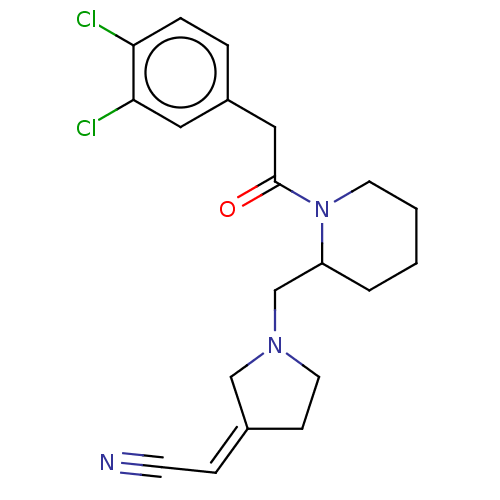 ((1-{1-[2-(3,4-Dichloro-phenyl)-acetyl]-piperidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency of the compound was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000271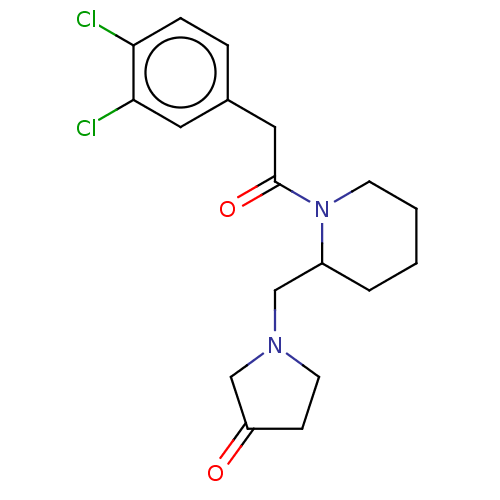 (1-{1-[2-(3,4-Dichloro-phenyl)-acetyl]-piperidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency of the compound was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50066999 ((S)-3,3-Diethyl-2-[4-(4-methyl-piperazine-1-carbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity in human whole blood (HWB) elastase at a concentration of 10 | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096486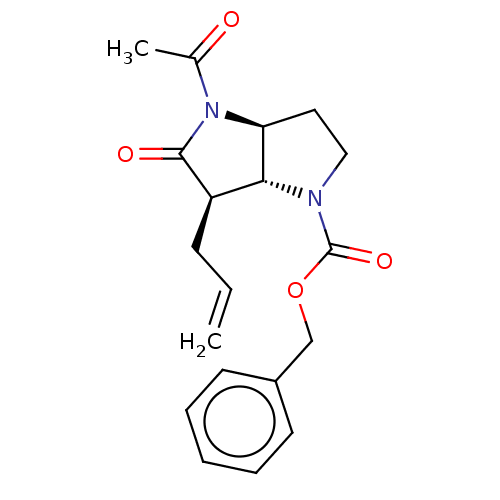 (CHEMBL2367646 | benzyl (3aS,6aR)-4-acetyl-6-allyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000260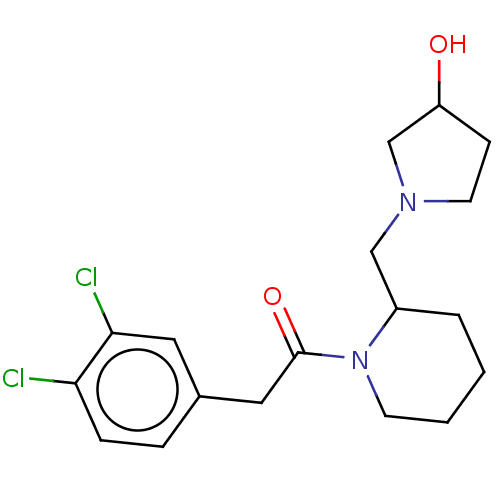 (2-(3,4-Dichloro-phenyl)-1-[2-(3-hydroxy-pyrrolidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096488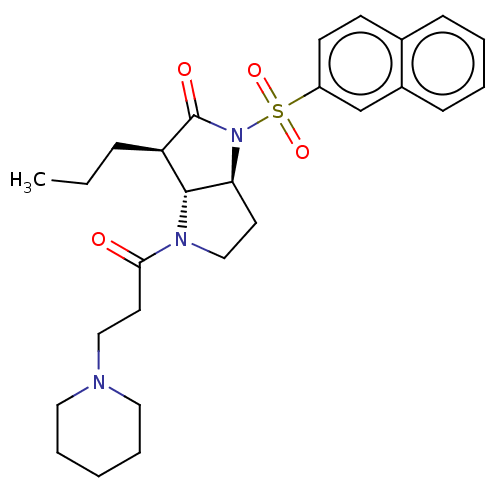 ((1S,3R)-1-(Naphthalene-2-sulfonyl)-4-(3-piperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity for dopamine D4-like receptor labelled with [3H]YM-09151-2 in retina | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096488 ((1S,3R)-1-(Naphthalene-2-sulfonyl)-4-(3-piperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000284 (2-(3,4-Dichloro-phenyl)-1-(2-methyl-6-pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096487 ((3R,6aS)-3-Allyl-2-oxo-hexahydro-pyrrolo[3,2-b]pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096483 ((3R,6aS)-1-Methanesulfonyl-4-(3-piperidin-1-yl-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity in human whole blood (HWB) elastase at a concentration of 10 uM | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000292 (2-(3,4-Dichloro-phenyl)-1-[2-(2,5-dihydro-pyrrol-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency of the compound was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000274 ((1-{1-[2-(3,4-Dichloro-phenyl)-acetyl]-piperidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000286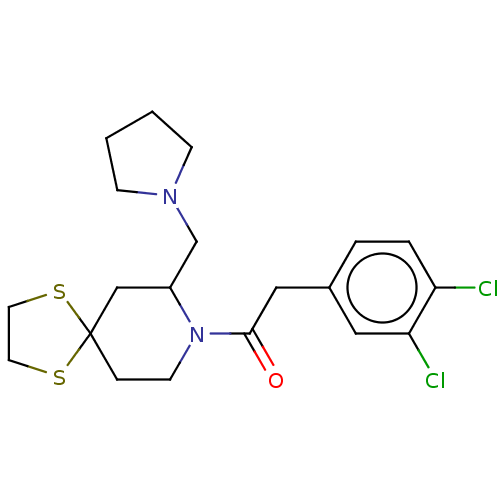 (2-(3,4-Dichloro-phenyl)-1-(7-pyrrolidin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000297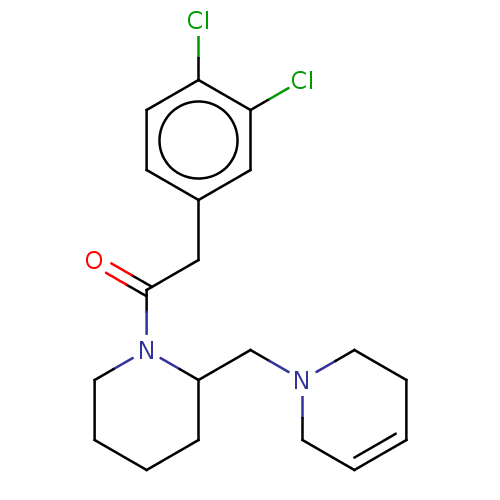 (2-(3,4-Dichloro-phenyl)-1-[2-(3,6-dihydro-2H-pyrid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency was determined in vitro using rabbit vas deferens (LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50061024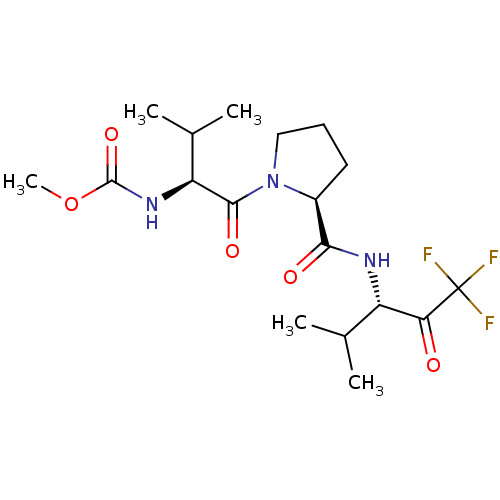 ((2-Methyl-1-{(S)-oxo-[(S)-2-((S)-3,3,3-trifluoro-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity in human whole blood (HWB) elastase | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000287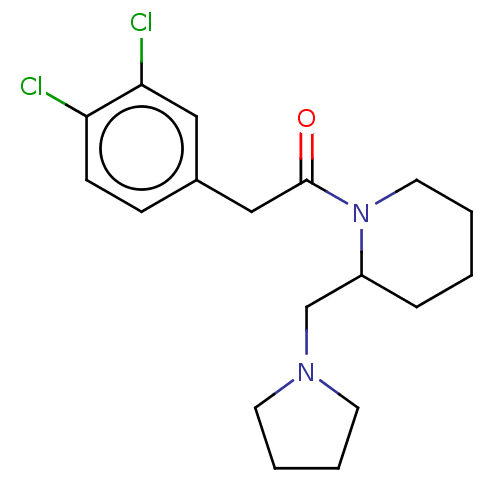 (2-(3,4-Dichloro-phenyl)-1-(2-pyrrolidin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Kappa-opioid receptor agonist potency of the compound was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000285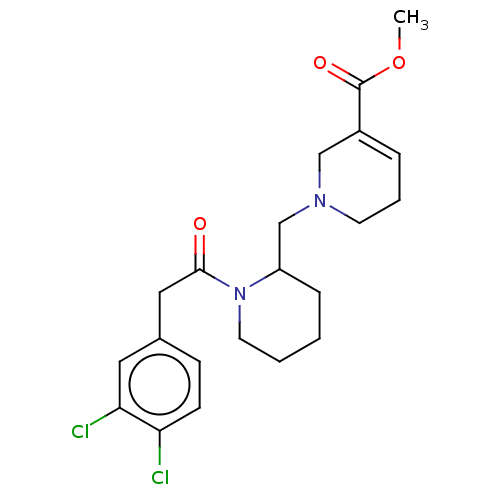 (1-{1-[2-(3,4-Dichloro-phenyl)-acetyl]-piperidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency was determined in vitro using rabbit vas deferens (LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50066999 ((S)-3,3-Diethyl-2-[4-(4-methyl-piperazine-1-carbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of human neutrophil elastase enzyme with a preincubation time of 0 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000273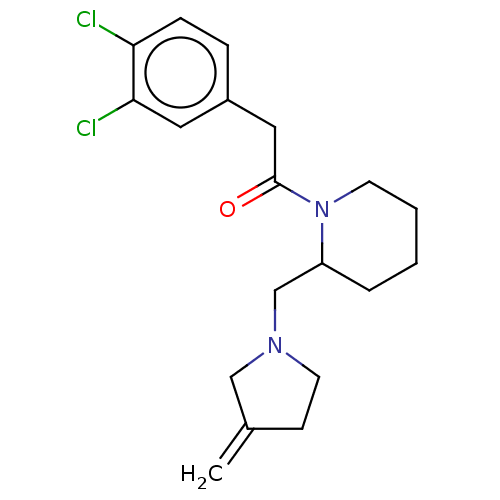 (2-(3,4-Dichloro-phenyl)-1-[2-(3-methylene-pyrrolid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096490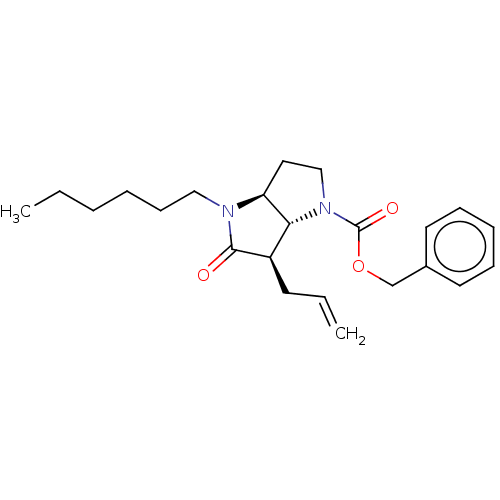 ((3aS,6R)-6-Allyl-4-hexyl-5-oxo-hexahydro-pyrrolo[3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096485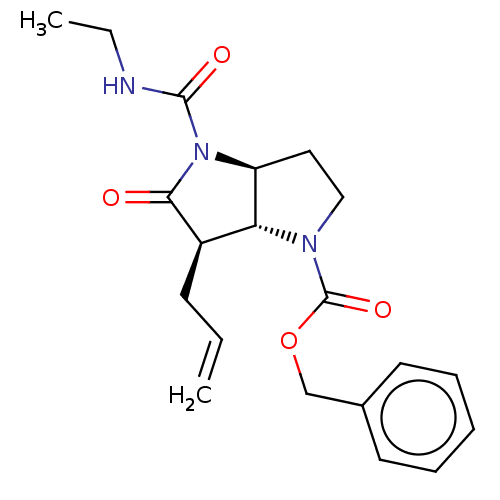 ((3aS,6R)-6-Allyl-4-ethylcarbamoyl-5-oxo-hexahydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50066999 ((S)-3,3-Diethyl-2-[4-(4-methyl-piperazine-1-carbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of human neutrophil elastase enzyme with a preincubation time of 40 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000275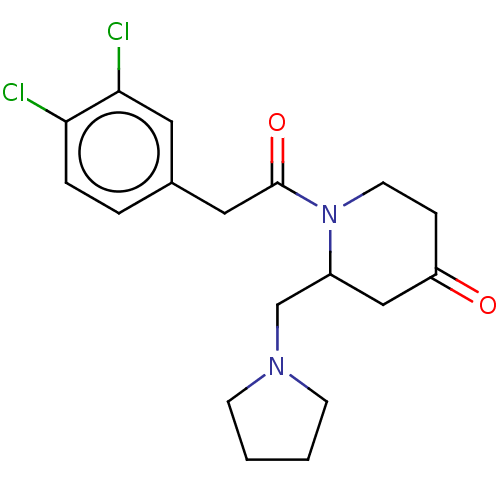 (1-[2-(3,4-Dichloro-phenyl)-acetyl]-2-pyrrolidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50067002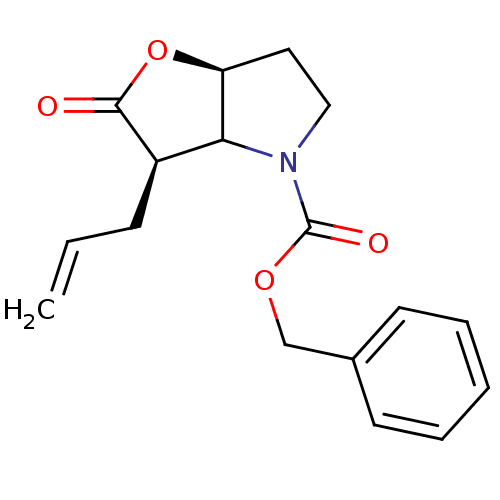 ((3R,6aS)-3-Allyl-2-oxo-hexahydro-furo[3,2-b]pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of human neutrophil elastase enzyme with a preincubation time of 40 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50066997 ((3aS,6R)-6-Allyl-4-methanesulfonyl-5-oxo-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of human neutrophil elastase enzyme with a preincubation time of 40 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000272 (2-(3,4-Dichloro-phenyl)-1-(2-methyl-6-pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50066998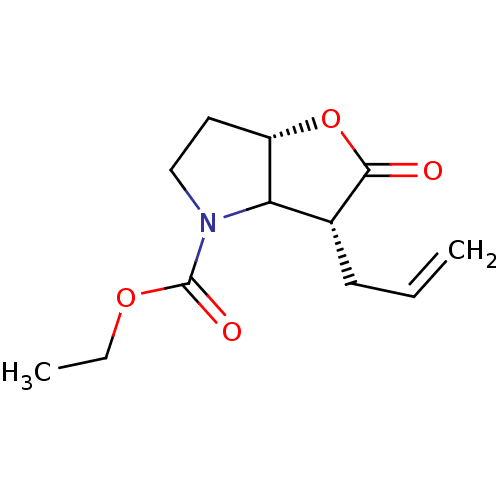 ((3R,6aS)-3-Allyl-2-oxo-hexahydro-furo[3,2-b]pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of human neutrophil elastase enzyme with a preincubation time of 15 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000282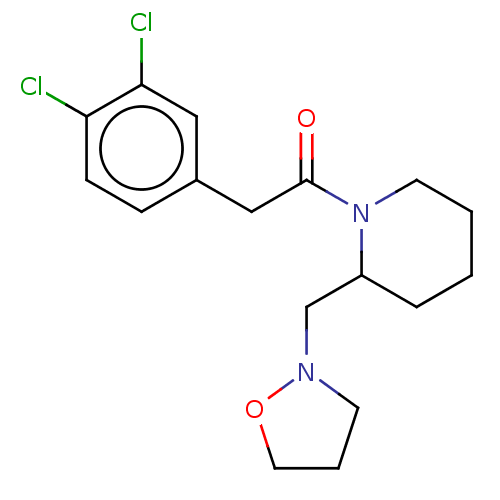 (2-(3,4-Dichloro-phenyl)-1-(2-isoxazolidin-2-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency was determined in vitro using rabbit vas deferens (LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 102 total ) | Next | Last >> |