

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301603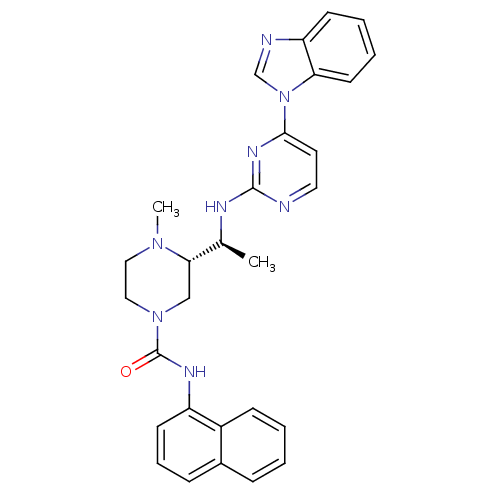 ((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301619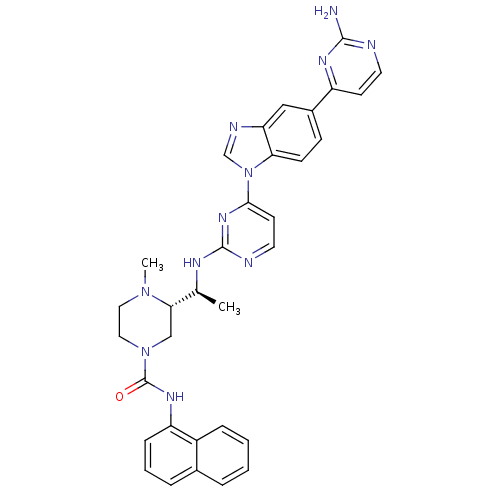 ((S)-3-((S)-1-(4-(5-(2-aminopyrimidin-4-yl)-1H-benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301604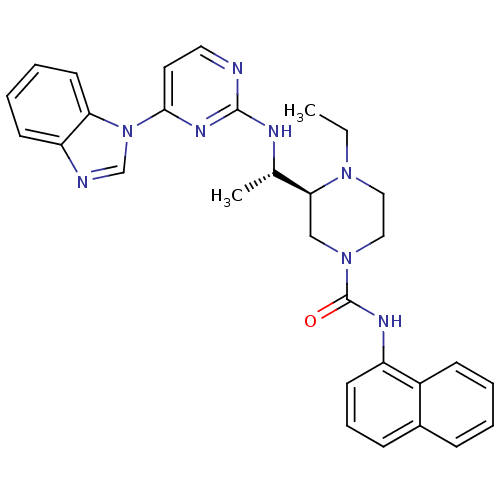 ((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301624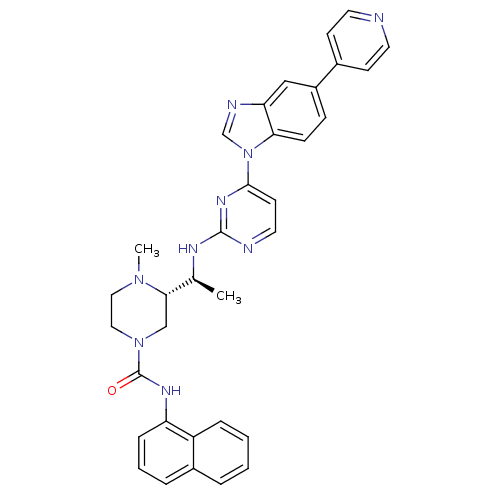 ((S)-4-methyl-N(S)-4-methyl-N-(naphthalen-1-yl)-3-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301605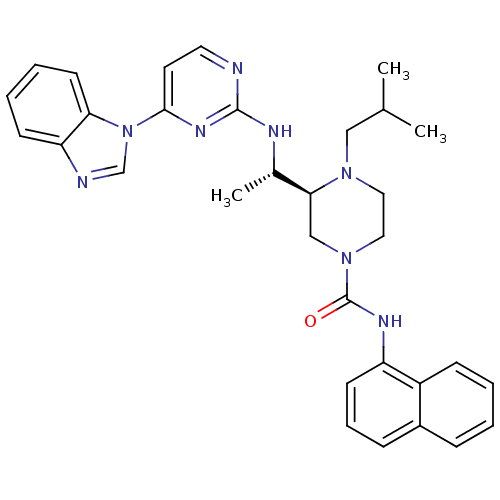 ((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301607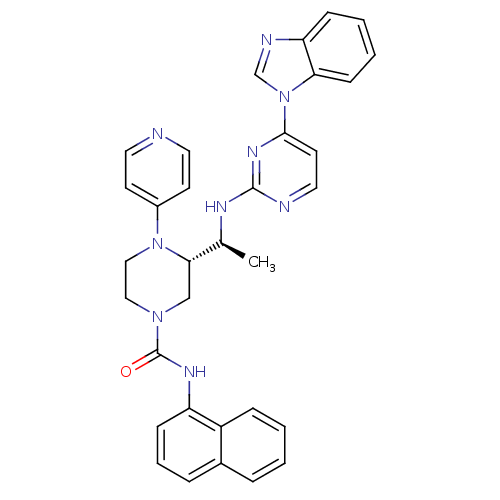 ((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301594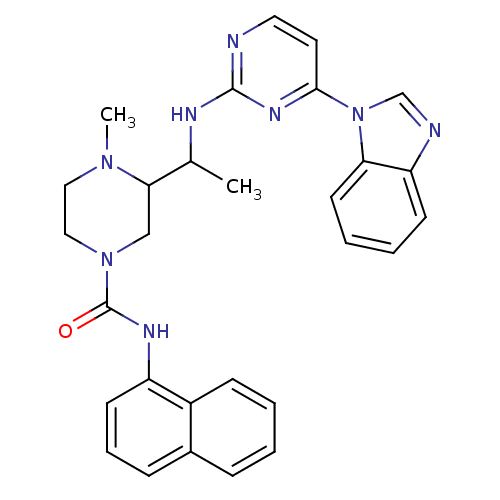 (3-(1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidin-2-ylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301594 (3-(1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidin-2-ylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301588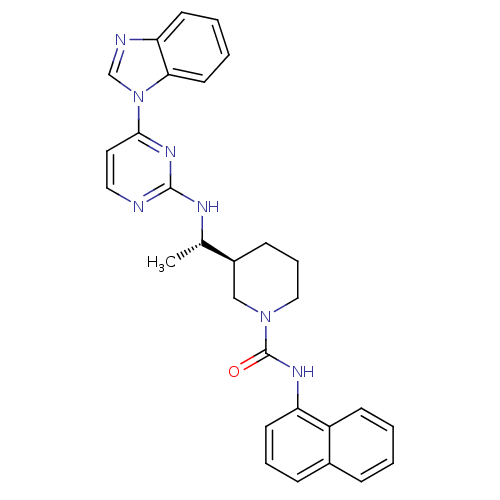 ((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301618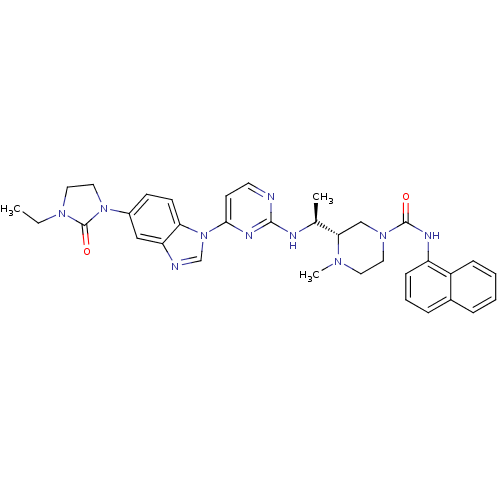 ((S)-3-((S)-1-(4-(5-(3-ethyl-2-oxoimidazolidin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301608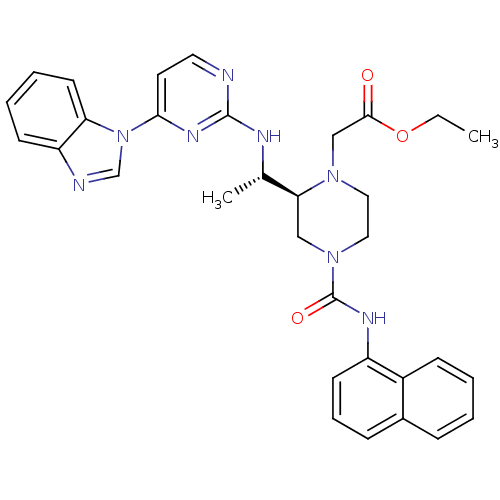 (CHEMBL566507 | ethyl 2-((S)-2-((S)-1-(4-(1H-benzo[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332524 (4-(1-(2-chloro-6- (trifluoromethyl)benzoyl)-6- (3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM332524 (4-(1-(2-chloro-6- (trifluoromethyl)benzoyl)-6- (3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10196354 (2019) BindingDB Entry DOI: 10.7270/Q2M90BSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM235032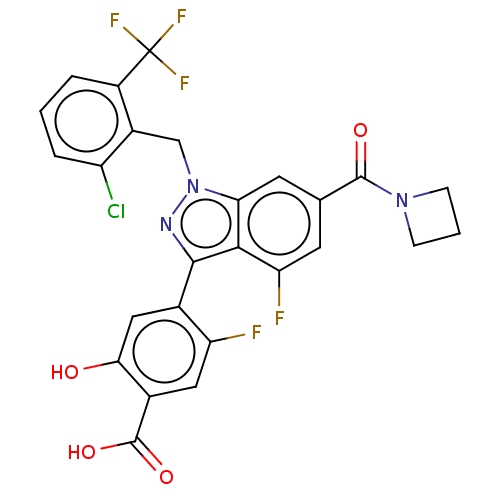 (US9556168, 10B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US9556168 (2017) BindingDB Entry DOI: 10.7270/Q2D220MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301621 ((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301587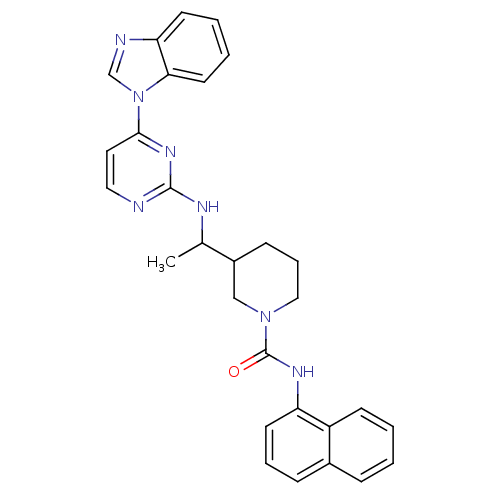 (CHEMBL567885 | rac 3-(1-(4-(1H-benzo[d]imidazol-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301617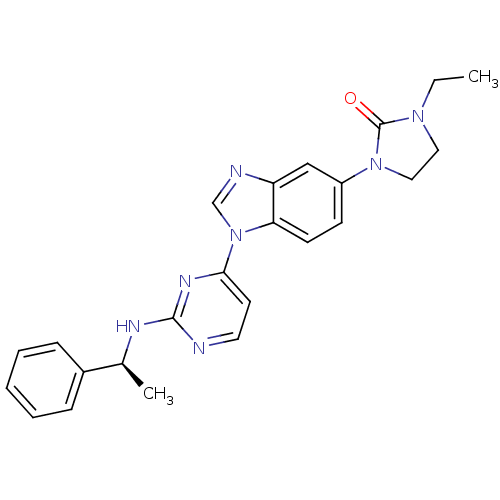 ((S)-1-ethyl-3-(1-(2-(1-phenylethylamino)pyrimidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301616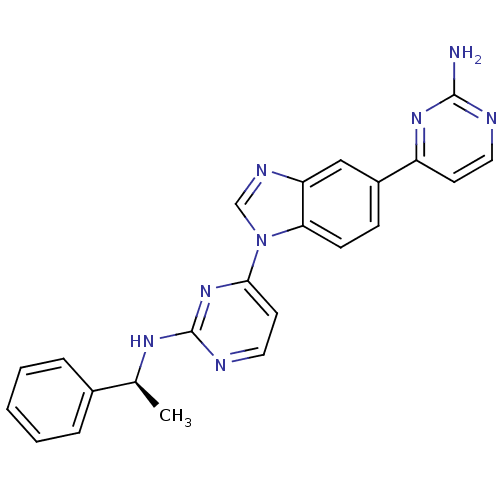 ((S)-4-(5-(2-aminopyrimidin-4-yl)-1H-benzo[d]imidaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301606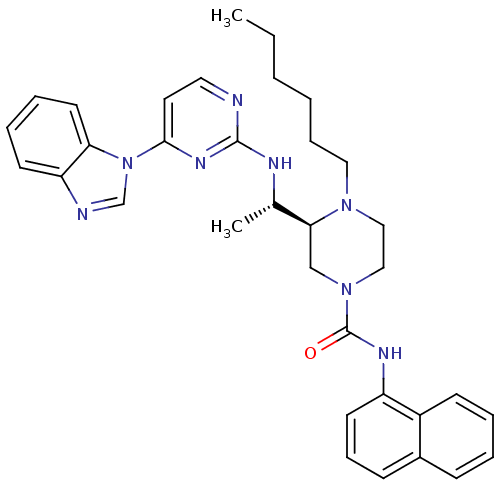 ((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332523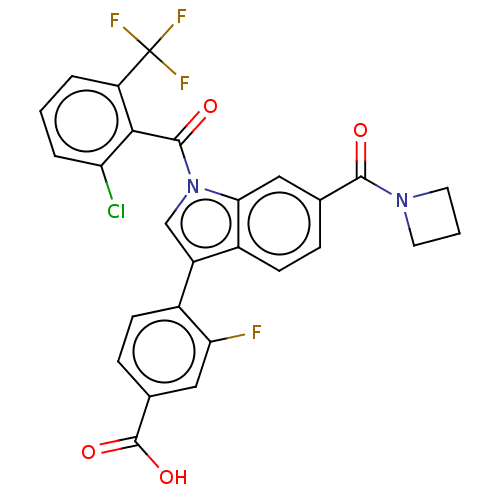 (4-(6-(azetidine-1-carbonyl)-1-(2-chloro-6-(trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM332523 (4-(6-(azetidine-1-carbonyl)-1-(2-chloro-6-(trifluo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10196354 (2019) BindingDB Entry DOI: 10.7270/Q2M90BSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332431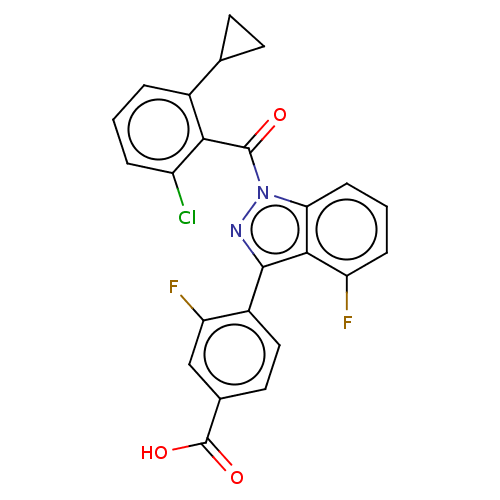 (4-(1-(2- chloro-6- cyclopropyl- benzoyl)-4- fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332433 (4-(1-(2- chloro-6- cyclopropyl- benzoyl)-1H- indaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM329723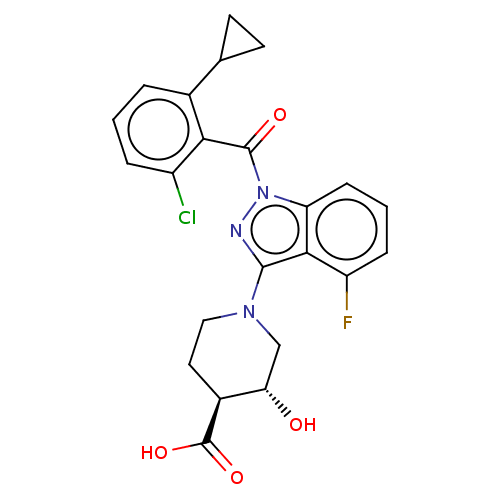 ((3R,4S)-1-(1-(2-chloro-6-cyclopropylbenzoyl)-4-flu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US9663522 (2017) BindingDB Entry DOI: 10.7270/Q2S46V33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM329725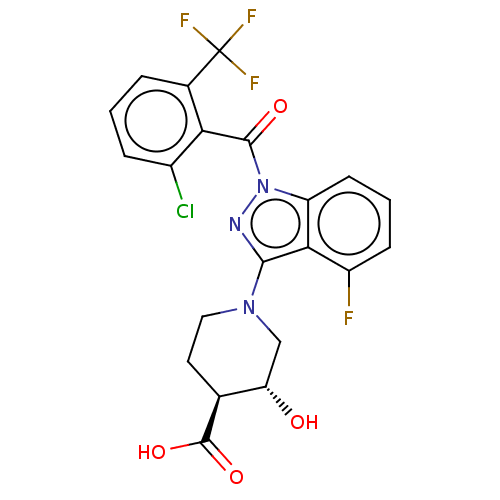 ((3R,4S)-1-(1-(2- chloro-6- (trifluoromethyl)benzoy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US9663522 (2017) BindingDB Entry DOI: 10.7270/Q2S46V33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM329729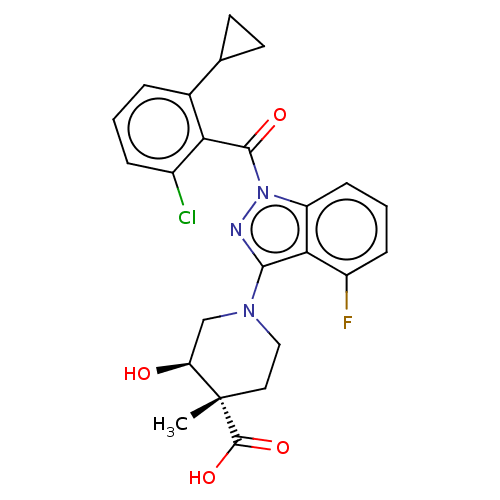 ((3R,4S)-1-(1-(2-chloro-6-cyclopropyl benzoyl)-4-fl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US9663522 (2017) BindingDB Entry DOI: 10.7270/Q2S46V33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM332431 (4-(1-(2- chloro-6- cyclopropyl- benzoyl)-4- fluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10196354 (2019) BindingDB Entry DOI: 10.7270/Q2M90BSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM332433 (4-(1-(2- chloro-6- cyclopropyl- benzoyl)-1H- indaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10196354 (2019) BindingDB Entry DOI: 10.7270/Q2M90BSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 2 of Nuclear receptor ROR-gamma (RORgT) (Homo sapiens (Human)) | BDBM256349 (US9487490, 2A) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9487490 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 2 of Nuclear receptor ROR-gamma (RORgT) (Homo sapiens (Human)) | BDBM256393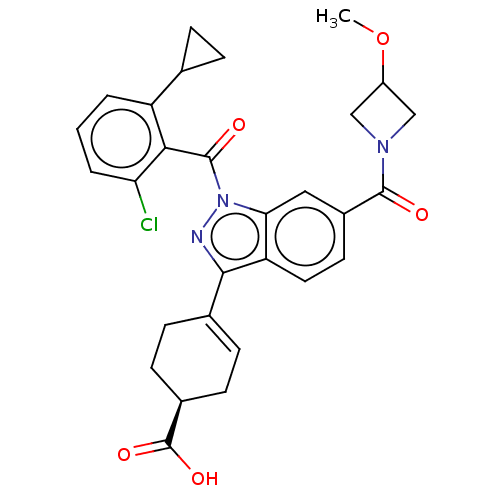 (US9487490, 2B) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9487490 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 2 of Nuclear receptor ROR-gamma (RORgT) (Homo sapiens (Human)) | BDBM256357 (US9487490, 4E) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9487490 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 2 of Nuclear receptor ROR-gamma (RORgT) (Homo sapiens (Human)) | BDBM256382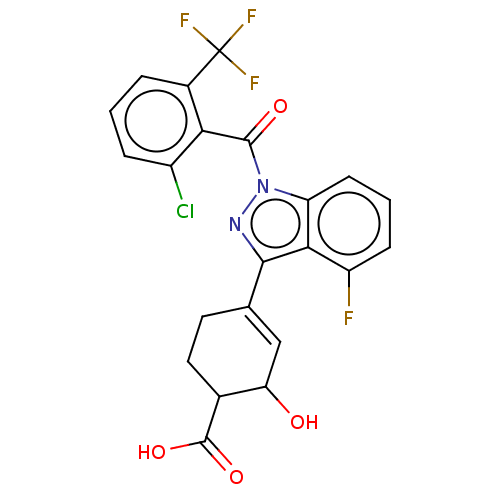 (US9487490, 6A) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9487490 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301595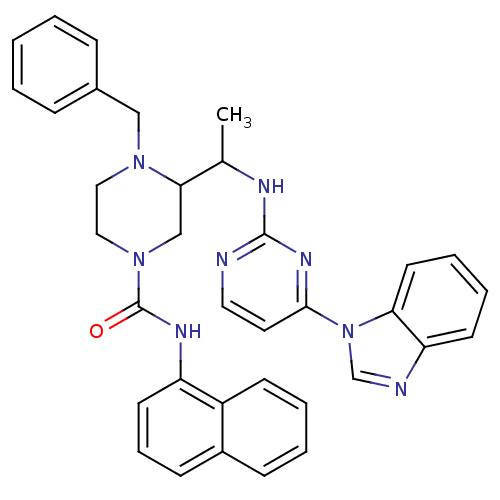 (3-(1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidin-2-ylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332520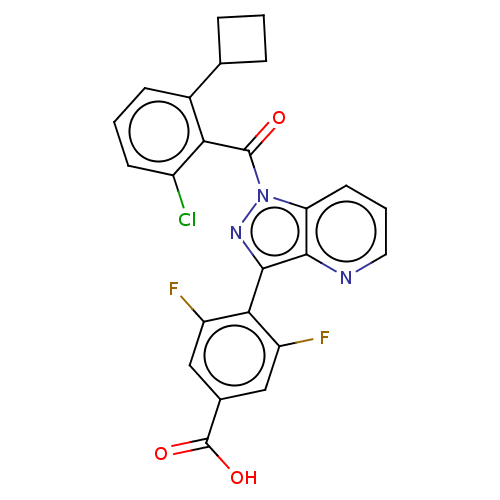 (4-(1-(2-chloro-6-cyclobutylbenzoyl)-1H-pyrazolo[4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332522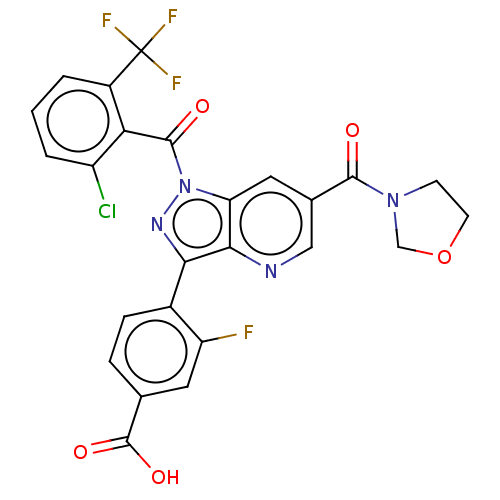 (4-(1-(2-chloro-6- (trifluoromethyl)benzoyl)- 6-(ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM235033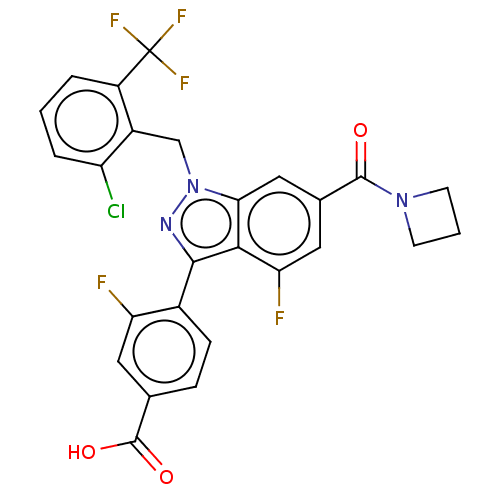 (US9556168, 10C) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US9556168 (2017) BindingDB Entry DOI: 10.7270/Q2D220MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM332520 (4-(1-(2-chloro-6-cyclobutylbenzoyl)-1H-pyrazolo[4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10196354 (2019) BindingDB Entry DOI: 10.7270/Q2M90BSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM332522 (4-(1-(2-chloro-6- (trifluoromethyl)benzoyl)- 6-(ox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10196354 (2019) BindingDB Entry DOI: 10.7270/Q2M90BSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332432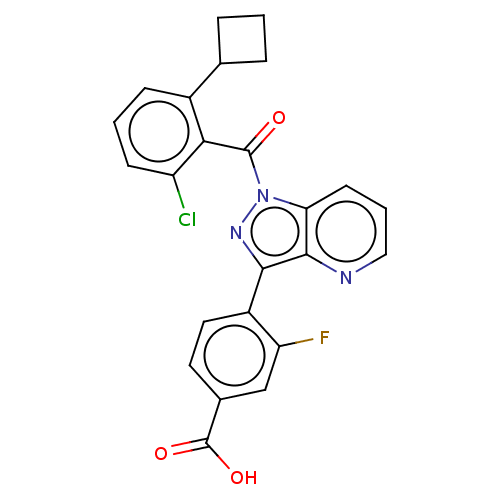 (4-(1-(2- chloro-6- cyclobutyl- benzoyl)-1H- pyrazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301623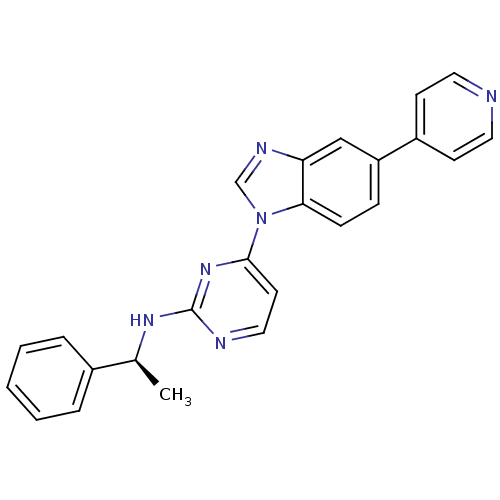 ((S)-N-(1-phenylethyl)-4-(5-(pyridin-4-yl)-1H-benzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM332432 (4-(1-(2- chloro-6- cyclobutyl- benzoyl)-1H- pyrazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10196354 (2019) BindingDB Entry DOI: 10.7270/Q2M90BSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 2 of Nuclear receptor ROR-gamma (RORgT) (Homo sapiens (Human)) | BDBM256361 (US9487490, 4I) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9487490 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 2 of Nuclear receptor ROR-gamma (RORgT) (Homo sapiens (Human)) | BDBM256366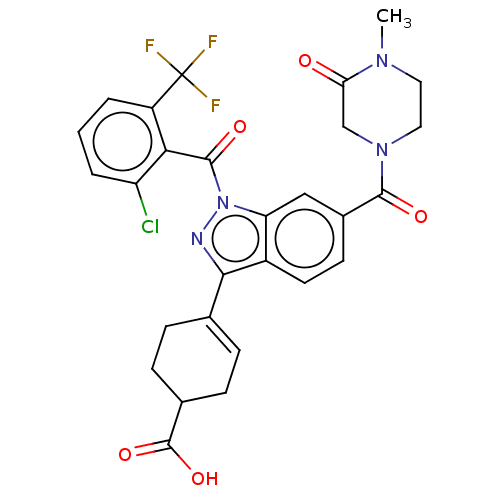 (US9487490, 4N) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9487490 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 2 of Nuclear receptor ROR-gamma (RORgT) (Homo sapiens (Human)) | BDBM256375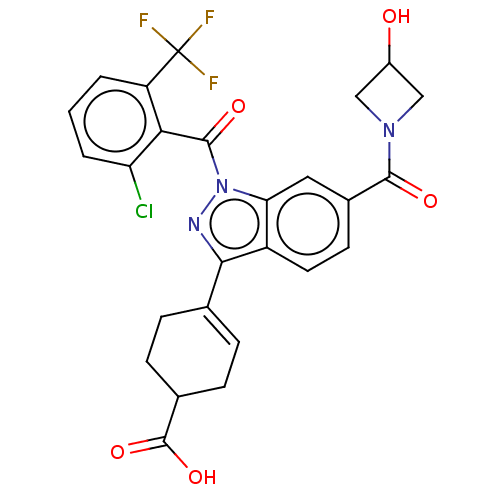 (US9487490, 4W) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9487490 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 2 of Nuclear receptor ROR-gamma (RORgT) (Homo sapiens (Human)) | BDBM256376 (US9487490, 4X) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9487490 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM329716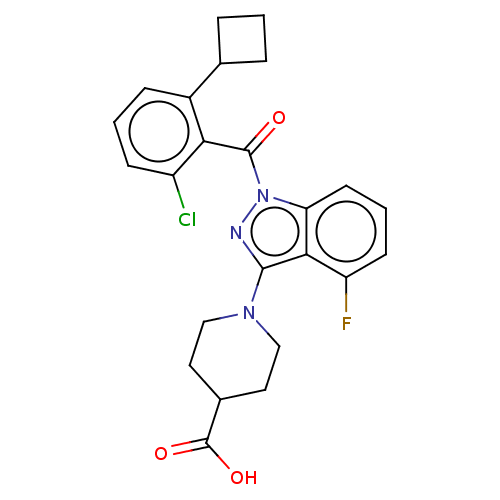 (1-(1-(2-chloro-6- cyclobutylbenzoyl)- 4-fluoro-1H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US9663522 (2017) BindingDB Entry DOI: 10.7270/Q2S46V33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 2 of Nuclear receptor ROR-gamma (RORgT) (Homo sapiens (Human)) | BDBM256381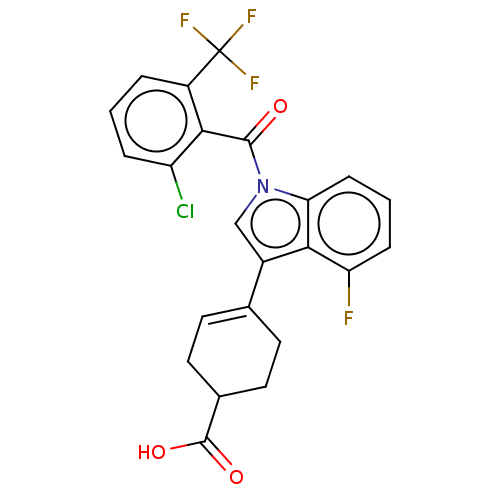 (US9487490, 5A) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9487490 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332430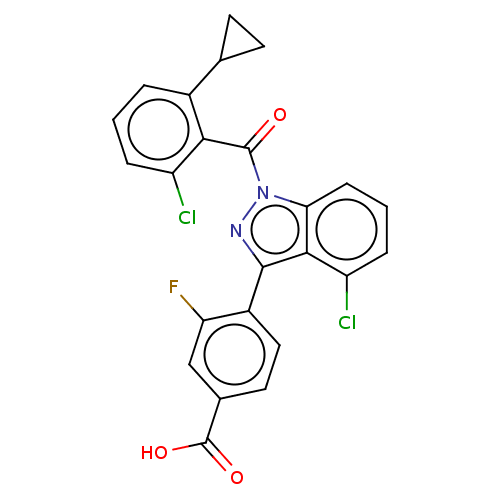 (4-(4-chloro-1- (2-chloro-6- cyclopropyl- benzoyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332437 (4-(1-(2-chloro- 6-(trifluoro- methyl)benzoyl)- 6-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM235000 (US9556168, 1AA) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US9556168 (2017) BindingDB Entry DOI: 10.7270/Q2D220MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 614 total ) | Next | Last >> |