 Found 15503 hits with Last Name = 'berg' and Initial = 'a'
Found 15503 hits with Last Name = 'berg' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50214385
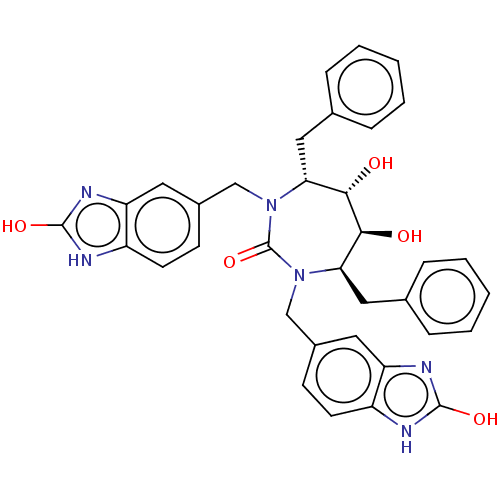
(CHEMBL316681)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]c(O)nc3c2)C(=O)N(Cc2ccc3[nH]c(O)nc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H34N6O5/c42-31-29(17-21-7-3-1-4-8-21)40(19-23-11-13-25-27(15-23)38-33(44)36-25)35(46)41(30(32(31)43)18-22-9-5-2-6-10-22)20-24-12-14-26-28(16-24)39-34(45)37-26/h1-16,29-32,42-43H,17-20H2,(H2,36,38,44)(H2,37,39,45)/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM161
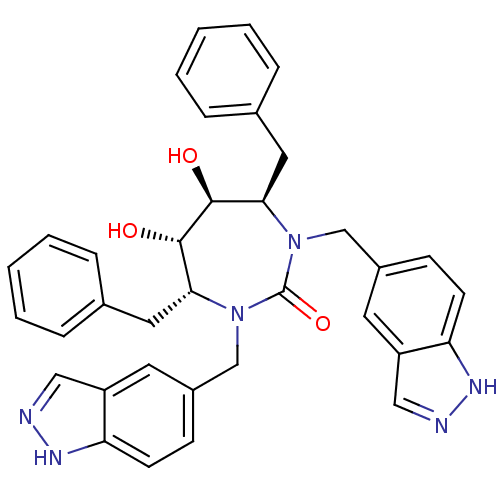
((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(1...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]ncc3c2)C(=O)N(Cc2ccc3[nH]ncc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H34N6O3/c42-33-31(17-23-7-3-1-4-8-23)40(21-25-11-13-29-27(15-25)19-36-38-29)35(44)41(22-26-12-14-30-28(16-26)20-37-39-30)32(34(33)43)18-24-9-5-2-6-10-24/h1-16,19-20,31-34,42-43H,17-18,21-22H2,(H,36,38)(H,37,39)/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288430

((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1,3-bis-(...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3c[nH]nc3c2)C(=O)N(Cc2ccc3c[nH]nc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H34N6O3/c42-33-31(17-23-7-3-1-4-8-23)40(21-25-11-13-27-19-36-38-29(27)15-25)35(44)41(22-26-12-14-28-20-37-39-30(28)16-26)32(34(33)43)18-24-9-5-2-6-10-24/h1-16,19-20,31-34,42-43H,17-18,21-22H2,(H,36,38)(H,37,39)/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50155838
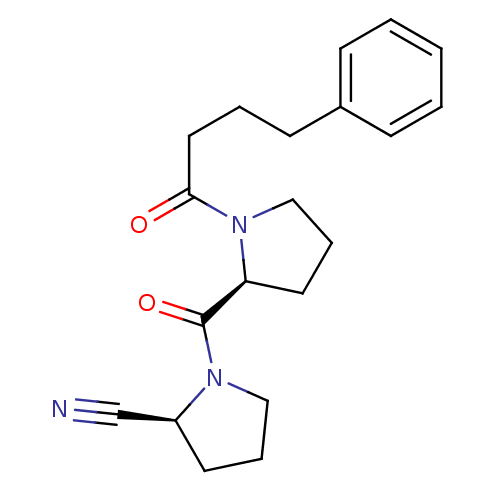
((S)-1-((S)-1-(4-phenylbutanoyl)pyrrolidine-2-carbo...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C#N Show InChI InChI=1S/C20H25N3O2/c21-15-17-10-5-13-22(17)20(25)18-11-6-14-23(18)19(24)12-4-9-16-7-2-1-3-8-16/h1-3,7-8,17-18H,4-6,9-14H2/t17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Inhibition of prolyl oligopeptidase (unknown origin) |
Eur J Med Chem 79: 436-45 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.014
BindingDB Entry DOI: 10.7270/Q2G162BW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50155307

(CHEMBL3781796)Show SMILES [H]C1(NC(=O)C(Cc2ccccc2)NC(=O)C([H])(NC(=O)C(CCCCN)NC(=O)C([H])(NC(=O)C(Cc2ccccc2)NC(=O)C(Cc2ccccc2)NC(=O)C(CC(N)=O)NC(=O)C(CCCCN)NC(=O)C(CSSCC(NC(=O)C(CO)NC1=O)C(O)=O)NC(=O)CNC(=O)C(C)NC(=O)C(CCCCN)NC(=O)C(CCCNC(N)=N)NC(=O)C(CCC(O)=O)NC(=O)C(CCCNC(N)=N)NC(=O)C1CCCN1C(=O)C(C)NC(=O)C(CCSC)NC(=O)C(C)NC(=O)C1CCCN1C(=O)C(CC(N)=O)NC(=O)C(CO)NC(=O)C(CC(N)=O)NC(=O)C(C)NC(=O)C(N)CO)C(C)O)C(C)O)C(C)O Show InChI InChI=1S/C130H204N40O40S3/c1-64(146-106(187)75(34-18-21-44-131)151-108(189)78(37-24-47-142-129(138)139)152-111(192)80(41-42-98(181)182)154-109(190)79(38-25-48-143-130(140)141)155-122(203)93-40-26-49-169(93)126(207)67(4)148-107(188)81(43-51-211-8)150-103(184)66(3)147-121(202)92-39-27-50-170(92)127(208)87(57-96(137)179)162-118(199)88(60-172)163-115(196)85(55-94(135)177)157-104(185)65(2)145-105(186)74(134)59-171)102(183)144-58-97(180)149-90-62-212-213-63-91(128(209)210)165-119(200)89(61-173)164-125(206)101(70(7)176)168-117(198)84(54-73-32-16-11-17-33-73)161-124(205)100(69(6)175)166-112(193)77(36-20-23-46-133)156-123(204)99(68(5)174)167-116(197)83(53-72-30-14-10-15-31-72)159-113(194)82(52-71-28-12-9-13-29-71)158-114(195)86(56-95(136)178)160-110(191)76(153-120(90)201)35-19-22-45-132/h9-17,28-33,64-70,74-93,99-101,171-176H,18-27,34-63,131-134H2,1-8H3,(H2,135,177)(H2,136,178)(H2,137,179)(H,144,183)(H,145,186)(H,146,187)(H,147,202)(H,148,188)(H,149,180)(H,150,184)(H,151,189)(H,152,192)(H,153,201)(H,154,190)(H,155,203)(H,156,204)(H,157,185)(H,158,195)(H,159,194)(H,160,191)(H,161,205)(H,162,199)(H,163,196)(H,164,206)(H,165,200)(H,166,193)(H,167,197)(H,168,198)(H,181,182)(H,209,210)(H4,138,139,142)(H4,140,141,143)/t64-,65-,66-,67-,68+,69+,70+,74-,75-,76+,77-,78-,79-,80-,81-,82-,83-,84-,85?,86-,87-,88-,89-,90-,91-,92-,93-,99-,100-,101-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gothenburg
Curated by ChEMBL
| Assay Description
Displacement of [125I]tyr11-SRIF from human sst2 receptor after 60 mins by liquid scintillation counting method |
Eur J Med Chem 114: 59-64 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.046
BindingDB Entry DOI: 10.7270/Q2WH2RWC |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50326055

((1R,21S,24S)-21-tert-Butyl-N-((1R,2R)-1-{[(cyclopr...)Show SMILES CC(C)(C)[C@@H]1NC(=O)OCC(C)(C)CCCCc2cccc3CN(Cc23)C(=O)O[C@@H]2C[C@H](N(C2)C1=O)C(=O)N[C@@]1(C[C@H]1C=C)C(=O)NS(=O)(=O)C1CC1 |r| Show InChI InChI=1S/C38H53N5O9S/c1-7-25-18-38(25,33(46)41-53(49,50)27-14-15-27)40-31(44)29-17-26-20-43(29)32(45)30(36(2,3)4)39-34(47)51-22-37(5,6)16-9-8-11-23-12-10-13-24-19-42(21-28(23)24)35(48)52-26/h7,10,12-13,25-27,29-30H,1,8-9,11,14-22H2,2-6H3,(H,39,47)(H,40,44)(H,41,46)/t25-,26-,29+,30-,38-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay |
Bioorg Med Chem 22: 6595-615 (2015)
Article DOI: 10.1016/j.bmc.2014.10.010
BindingDB Entry DOI: 10.7270/Q2GX4D50 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50121507
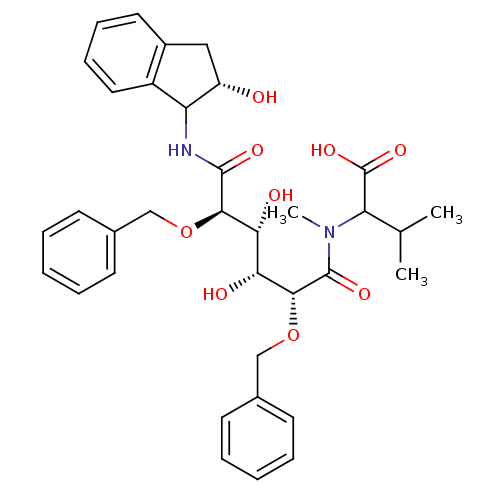
(2-{[2,5-Bis-benzyloxy-3,4-dihydroxy-5-(2-hydroxy-i...)Show SMILES CC(C)C(N(C)C(=O)[C@H](OCc1ccccc1)[C@H](O)[C@@H](O)[C@@H](OCc1ccccc1)C(=O)NC1[C@@H](O)Cc2ccccc12)C(O)=O Show InChI InChI=1S/C35H42N2O9/c1-21(2)28(35(43)44)37(3)34(42)32(46-20-23-14-8-5-9-15-23)30(40)29(39)31(45-19-22-12-6-4-7-13-22)33(41)36-27-25-17-11-10-16-24(25)18-26(27)38/h4-17,21,26-32,38-40H,18-20H2,1-3H3,(H,36,41)(H,43,44)/t26-,27?,28?,29+,30+,31+,32+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0540 | n/a | n/a | 4.42 | n/a | n/a | 5.97E+6 | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Association rate constant for the interaction between inhibitor and HIV-1 protease |
J Med Chem 45: 5430-9 (2002)
BindingDB Entry DOI: 10.7270/Q2GH9JP4 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(PIG) | BDBM78940

(METHIOTHEPIN | MLS000859918 | Methiothepin mesylat...)Show InChI InChI=1S/C20H24N2S2/c1-21-9-11-22(12-10-21)18-13-15-5-3-4-6-19(15)24-20-8-7-16(23-2)14-17(18)20/h3-8,14,18H,9-13H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by PDSP Ki Database
| |
Eur J Pharmacol 347: 301-9 (1998)
Article DOI: 10.1016/s0014-2999(98)00104-6
BindingDB Entry DOI: 10.7270/Q2GT5KQK |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 619-26 (2005)
Article DOI: 10.1124/jpet.104.075069
BindingDB Entry DOI: 10.7270/Q2BK19XS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM85818
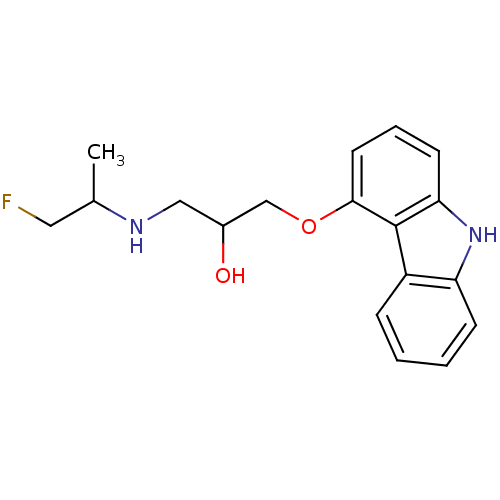
(Fluorocarazolol,(R) | Fluorocarazolol,(S))Show InChI InChI=1S/C18H21FN2O2/c1-12(9-19)20-10-13(22)11-23-17-8-4-7-16-18(17)14-5-2-3-6-15(14)21-16/h2-8,12-13,20-22H,9-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 157: 111-4 (2001)
Article DOI: 10.1007/s002130100844
BindingDB Entry DOI: 10.7270/Q22V2DPJ |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(PIG) | BDBM81444
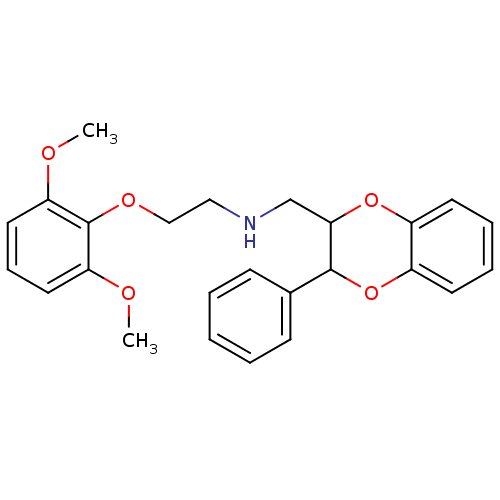
(CAS_185453 | NSC_185453 | WB 4101 | WB-4101)Show InChI InChI=1S/C25H27NO5/c1-27-21-13-8-14-22(28-2)25(21)29-16-15-26-17-23-24(18-9-4-3-5-10-18)31-20-12-7-6-11-19(20)30-23/h3-14,23-24,26H,15-17H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by PDSP Ki Database
| |
Eur J Pharmacol 347: 301-9 (1998)
Article DOI: 10.1016/s0014-2999(98)00104-6
BindingDB Entry DOI: 10.7270/Q2GT5KQK |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599]
(Human immunodeficiency virus type 1) | BDBM854
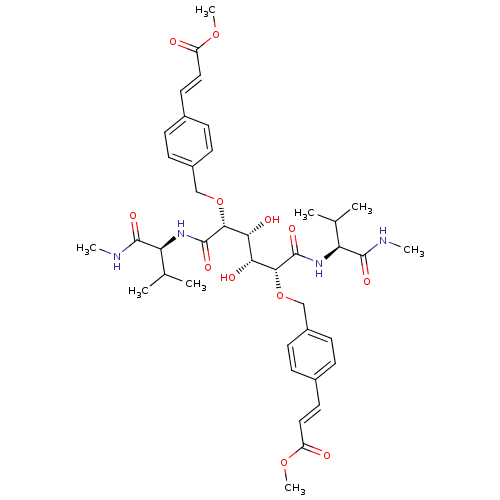
(C2-Symmetric inhibitor 12 | CHEMBL127045 | N1,N6-B...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](OCc1ccc(\C=C\C(=O)OC)cc1)[C@H](O)[C@@H](O)[C@@H](OCc1ccc(\C=C\C(=O)OC)cc1)C(=O)N[C@@H](C(C)C)C(=O)NC)C(C)C |r| Show InChI InChI=1S/C40H54N4O12/c1-23(2)31(37(49)41-5)43-39(51)35(55-21-27-13-9-25(10-14-27)17-19-29(45)53-7)33(47)34(48)36(40(52)44-32(24(3)4)38(50)42-6)56-22-28-15-11-26(12-16-28)18-20-30(46)54-8/h9-20,23-24,31-36,47-48H,21-22H2,1-8H3,(H,41,49)(H,42,50)(H,43,51)(H,44,52)/b19-17+,20-18+/t31-,32-,33+,34+,35+,36+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0900 | -58.3 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University
| Assay Description
Ki values were determined by using a fluorescent substrate (DABCYL-gamma-Abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS). All incubations were performed a... |
J Med Chem 42: 3835-44 (1999)
Article DOI: 10.1021/jm9910371
BindingDB Entry DOI: 10.7270/Q2T151VX |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288424

((4R,5S,6S,7R)-1,3-Bis-(1H-benzotriazol-5-ylmethyl)...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3nn[nH]c3c2)C(=O)N(Cc2ccc3nn[nH]c3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C33H32N8O3/c42-31-29(17-21-7-3-1-4-8-21)40(19-23-11-13-25-27(15-23)36-38-34-25)33(44)41(20-24-12-14-26-28(16-24)37-39-35-26)30(32(31)43)18-22-9-5-2-6-10-22/h1-16,29-32,42-43H,17-20H2,(H,34,36,38)(H,35,37,39)/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM854

(C2-Symmetric inhibitor 12 | CHEMBL127045 | N1,N6-B...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](OCc1ccc(\C=C\C(=O)OC)cc1)[C@H](O)[C@@H](O)[C@@H](OCc1ccc(\C=C\C(=O)OC)cc1)C(=O)N[C@@H](C(C)C)C(=O)NC)C(C)C |r| Show InChI InChI=1S/C40H54N4O12/c1-23(2)31(37(49)41-5)43-39(51)35(55-21-27-13-9-25(10-14-27)17-19-29(45)53-7)33(47)34(48)36(40(52)44-32(24(3)4)38(50)42-6)56-22-28-15-11-26(12-16-28)18-20-30(46)54-8/h9-20,23-24,31-36,47-48H,21-22H2,1-8H3,(H,41,49)(H,42,50)(H,43,51)(H,44,52)/b19-17+,20-18+/t31-,32-,33+,34+,35+,36+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0900 | n/a | n/a | 1.95 | n/a | n/a | 2.04E+5 | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Association rate constant for the interaction between inhibitor and HIV-1 protease |
J Med Chem 45: 5430-9 (2002)
BindingDB Entry DOI: 10.7270/Q2GH9JP4 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-5
(RAT) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 619-26 (2005)
Article DOI: 10.1124/jpet.104.075069
BindingDB Entry DOI: 10.7270/Q2BK19XS |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50155838

((S)-1-((S)-1-(4-phenylbutanoyl)pyrrolidine-2-carbo...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C#N Show InChI InChI=1S/C20H25N3O2/c21-15-17-10-5-13-22(17)20(25)18-11-6-14-23(18)19(24)12-4-9-16-7-2-1-3-8-16/h1-3,7-8,17-18H,4-6,9-14H2/t17-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Inhibition of porcine prolyl oligopeptidase using Z-Gly-Pro-AMC as substrate after 60 mins by double-reciprocal plot analysis |
Eur J Med Chem 79: 436-45 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.014
BindingDB Entry DOI: 10.7270/Q2G162BW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM12218

((2R,3R,4R,5R)-N-benzyl-2,5-bis(benzyloxy)-3,4-dihy...)Show SMILES O[C@H]([C@@H](O)[C@@H](OCc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)[C@@H](OCc1ccccc1)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C36H38N2O7/c39-29-20-27-18-10-11-19-28(27)30(29)38-36(43)34(45-23-26-16-8-3-9-17-26)32(41)31(40)33(44-22-25-14-6-2-7-15-25)35(42)37-21-24-12-4-1-5-13-24/h1-19,29-34,39-41H,20-23H2,(H,37,42)(H,38,43)/t29-,30+,31-,32-,33-,34-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.100 | -58.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University
| Assay Description
A fluorimetric assay was used to determine the effects of the inhibitors on HIV-1 protease. This assay used an internally quenched fluorescent peptid... |
Eur J Biochem 270: 1746-58 (2003)
Article DOI: 10.1046/j.1432-1033.2003.03533.x
BindingDB Entry DOI: 10.7270/Q2NZ85WX |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM851
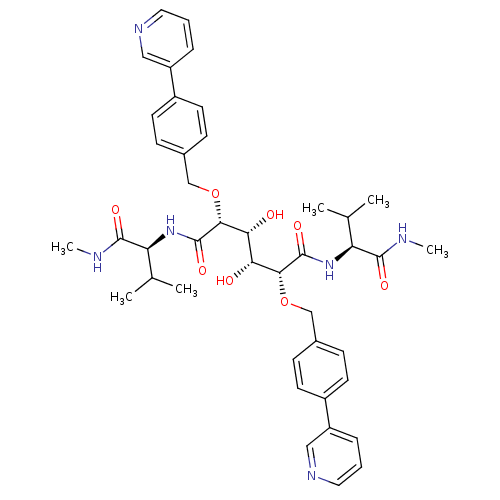
((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S)-2-methyl...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](OCc1ccc(cc1)-c1cccnc1)[C@H](O)[C@@H](O)[C@@H](OCc1ccc(cc1)-c1cccnc1)C(=O)N[C@@H](C(C)C)C(=O)NC)C(C)C |r| Show InChI InChI=1S/C42H52N6O8/c1-25(2)33(39(51)43-5)47-41(53)37(55-23-27-11-15-29(16-12-27)31-9-7-19-45-21-31)35(49)36(50)38(42(54)48-34(26(3)4)40(52)44-6)56-24-28-13-17-30(18-14-28)32-10-8-20-46-22-32/h7-22,25-26,33-38,49-50H,23-24H2,1-6H3,(H,43,51)(H,44,52)(H,47,53)(H,48,54)/t33-,34-,35+,36+,37+,38+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.100 | -58.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University
| Assay Description
A fluorimetric assay was used to determine the effects of the inhibitors on HIV-1 protease. This assay used an internally quenched fluorescent peptid... |
Eur J Biochem 270: 1746-58 (2003)
Article DOI: 10.1046/j.1432-1033.2003.03533.x
BindingDB Entry DOI: 10.7270/Q2NZ85WX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50121505
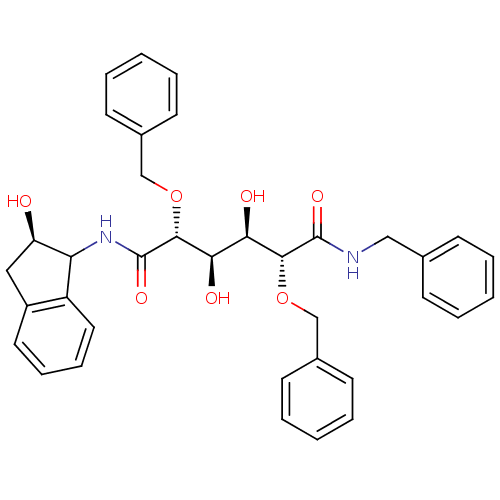
(2,5-Bis-benzyloxy-3,4-dihydroxy-hexanedioic acid b...)Show SMILES O[C@H]([C@@H](O)[C@@H](OCc1ccccc1)C(=O)NC1[C@H](O)Cc2ccccc12)[C@@H](OCc1ccccc1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C36H38N2O7/c39-29-20-27-18-10-11-19-28(27)30(29)38-36(43)34(45-23-26-16-8-3-9-17-26)32(41)31(40)33(44-22-25-14-6-2-7-15-25)35(42)37-21-24-12-4-1-5-13-24/h1-19,29-34,39-41H,20-23H2,(H,37,42)(H,38,43)/t29-,30?,31-,32-,33-,34-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | 70 | n/a | n/a | 1.01E+5 | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Association rate constant for the interaction between inhibitor and HIV-1 protease |
J Med Chem 45: 5430-9 (2002)
BindingDB Entry DOI: 10.7270/Q2GH9JP4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50067497
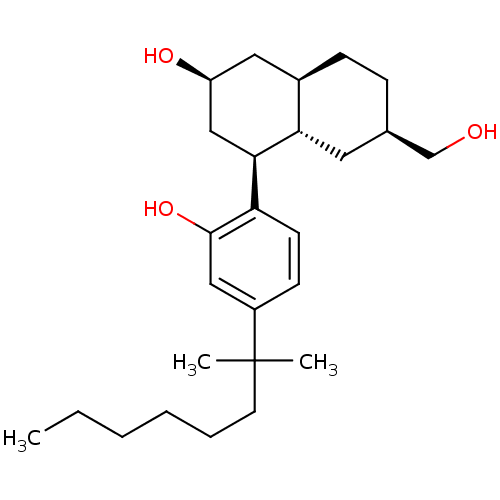
((2S,4S,4aS,6R,8aR)-4-[4-(1,1-Dimethyl-heptyl)-2-hy...)Show SMILES CCCCCCC(C)(C)c1ccc([C@H]2C[C@@H](O)C[C@H]3CC[C@@H](CO)C[C@H]23)c(O)c1 Show InChI InChI=1S/C26H42O3/c1-4-5-6-7-12-26(2,3)20-10-11-22(25(29)15-20)24-16-21(28)14-19-9-8-18(17-27)13-23(19)24/h10-11,15,18-19,21,23-24,27-29H,4-9,12-14,16-17H2,1-3H3/t18-,19-,21+,23+,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Louis University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 binding to Cannabinoid receptor 1 in rat brain membranes |
J Med Chem 41: 4207-15 (1998)
Article DOI: 10.1021/jm970239z
BindingDB Entry DOI: 10.7270/Q2C24VK6 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM85818

(Fluorocarazolol,(R) | Fluorocarazolol,(S))Show InChI InChI=1S/C18H21FN2O2/c1-12(9-19)20-10-13(22)11-23-17-8-4-7-16-18(17)14-5-2-3-6-15(14)21-16/h2-8,12-13,20-22H,9-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 157: 111-4 (2001)
Article DOI: 10.1007/s002130100844
BindingDB Entry DOI: 10.7270/Q22V2DPJ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM85818

(Fluorocarazolol,(R) | Fluorocarazolol,(S))Show InChI InChI=1S/C18H21FN2O2/c1-12(9-19)20-10-13(22)11-23-17-8-4-7-16-18(17)14-5-2-3-6-15(14)21-16/h2-8,12-13,20-22H,9-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 157: 111-4 (2001)
Article DOI: 10.1007/s002130100844
BindingDB Entry DOI: 10.7270/Q22V2DPJ |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030815
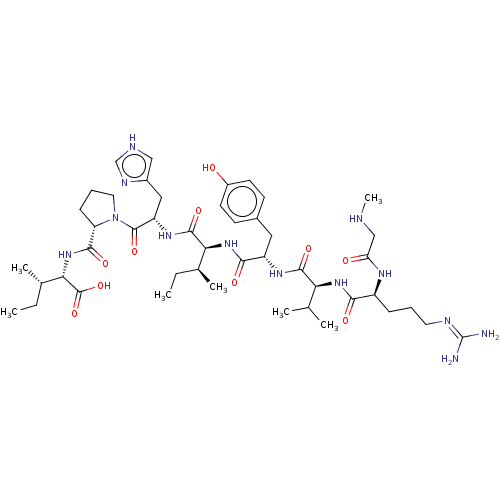
(CHEMBL404594)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)[C@@H](C)CC)C(O)=O |wU:8.7,25.27,wD:41.44,4.4,15.16,29.31,45.48,2.2,62.65,(8.44,-18.06,;9.36,-17.24,;9.05,-15.73,;7.88,-15.34,;10.19,-14.7,;9.88,-13.19,;11.03,-12.16,;12.2,-12.55,;10.71,-10.66,;11.74,-9.51,;10.97,-8.18,;9.46,-8.5,;9.33,-10.02,;7.99,-10.79,;7.99,-12.02,;6.66,-10.02,;5.32,-10.79,;5.32,-12.33,;4.07,-13.22,;4.55,-14.68,;6.09,-14.68,;6.56,-13.21,;6.66,-8.48,;5.33,-7.71,;4.43,-8.22,;5.33,-6.17,;3.99,-5.39,;4,-3.85,;5.06,-3.24,;2.66,-3.08,;1.33,-3.85,;-.01,-3.08,;-1.34,-3.84,;-2.67,-3.07,;-2.67,-1.53,;-3.73,-.91,;-1.33,-.76,;,-1.54,;2.67,-1.54,;4,-.77,;5.07,-1.39,;4.01,.77,;5.35,1.53,;5.36,3.07,;4.29,3.69,;6.69,3.84,;6.7,5.37,;8.04,6.14,;8.04,7.68,;9.38,8.44,;9.39,9.99,;10.45,10.6,;8.32,10.61,;8.03,3.06,;8.02,1.52,;6.94,.91,;9.35,.74,;9.34,-.8,;10.22,-1.32,;2.68,1.54,;1.61,.93,;2.69,2.78,;6.66,-5.4,;7.73,-6.01,;6.66,-3.86,;7.73,-3.24,;11.66,-15.17,;11.91,-16.38,;12.58,-14.35,)| Show InChI InChI=1S/C46H73N13O10/c1-8-26(5)37(43(66)55-33(21-29-22-50-24-52-29)44(67)59-19-11-13-34(59)41(64)58-38(45(68)69)27(6)9-2)57-40(63)32(20-28-14-16-30(60)17-15-28)54-42(65)36(25(3)4)56-39(62)31(53-35(61)23-49-7)12-10-18-51-46(47)48/h14-17,22,24-27,31-34,36-38,49,60H,8-13,18-21,23H2,1-7H3,(H,50,52)(H,53,61)(H,54,65)(H,55,66)(H,56,62)(H,57,63)(H,58,64)(H,68,69)(H4,47,48,51)/t26-,27-,31-,32-,33-,34-,36-,37-,38-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity to type-2 angiotensin-2 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 1355-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.10.084
BindingDB Entry DOI: 10.7270/Q2SN0BT6 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288420

(5-[4,7-dibenzyl-5,6-dihydroxy-2-oxo-3-(2-oxo-2,3-d...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3NC(=O)Cc3c2)C(=O)N(Cc2ccc3NC(=O)Cc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C37H36N4O5/c42-33-19-27-15-25(11-13-29(27)38-33)21-40-31(17-23-7-3-1-4-8-23)35(44)36(45)32(18-24-9-5-2-6-10-24)41(37(40)46)22-26-12-14-30-28(16-26)20-34(43)39-30/h1-16,31-32,35-36,44-45H,17-22H2,(H,38,42)(H,39,43)/t31-,32-,35+,36+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-quinpirole from Dopamine receptor D2 |
J Med Chem 39: 4421-9 (1996)
Article DOI: 10.1021/jm960350p
BindingDB Entry DOI: 10.7270/Q21G0KCQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50370684
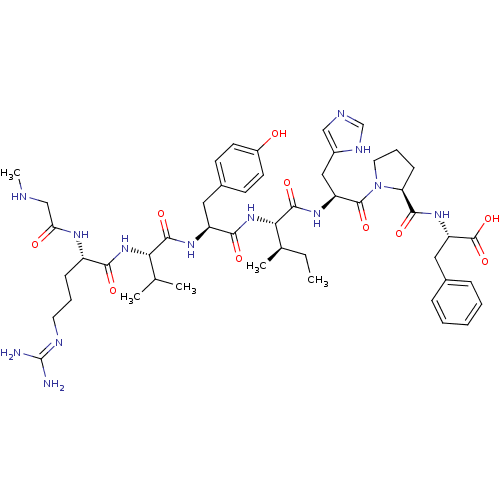
(CHEMBL1791349)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C49H71N13O10/c1-6-29(4)41(46(69)58-36(24-32-25-53-27-55-32)47(70)62-21-11-15-38(62)44(67)59-37(48(71)72)23-30-12-8-7-9-13-30)61-43(66)35(22-31-16-18-33(63)19-17-31)57-45(68)40(28(2)3)60-42(65)34(56-39(64)26-52-5)14-10-20-54-49(50)51/h7-9,12-13,16-19,25,27-29,34-38,40-41,52,63H,6,10-11,14-15,20-24,26H2,1-5H3,(H,53,55)(H,56,64)(H,57,68)(H,58,69)(H,59,67)(H,60,65)(H,61,66)(H,71,72)(H4,50,51,54)/t29-,34+,35+,36+,37+,38+,40+,41+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity for angiotensin II receptor, type 1 in rat liver membrane using [125I]-Ang II as radioligand, in pH 7.4 Tris-HCl buffer for 2 hr at ... |
J Med Chem 48: 6620-31 (2005)
Article DOI: 10.1021/jm050280z
BindingDB Entry DOI: 10.7270/Q2HX1DG6 |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(PIG) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by PDSP Ki Database
| |
Eur J Pharmacol 347: 301-9 (1998)
Article DOI: 10.1016/s0014-2999(98)00104-6
BindingDB Entry DOI: 10.7270/Q2GT5KQK |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-quinpirole from human dopamine D2A receptors expressed in LtK cells |
Bioorg Med Chem Lett 9: 2167-72 (1999)
BindingDB Entry DOI: 10.7270/Q2CF9P97 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288421
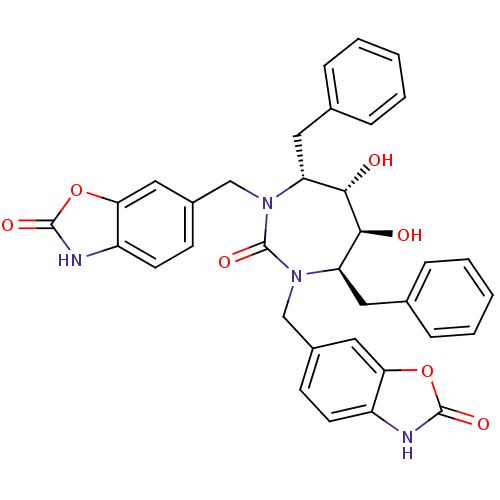
(6-[4,7-dibenzyl-5,6-dihydroxy-2-oxo-3-(2-oxo-2,3-d...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]c(=O)oc3c2)C(=O)N(Cc2ccc3[nH]c(=O)oc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H32N4O7/c40-31-27(15-21-7-3-1-4-8-21)38(19-23-11-13-25-29(17-23)45-33(42)36-25)35(44)39(28(32(31)41)16-22-9-5-2-6-10-22)20-24-12-14-26-30(18-24)46-34(43)37-26/h1-14,17-18,27-28,31-32,40-41H,15-16,19-20H2,(H,36,42)(H,37,43)/t27-,28-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50370684

(CHEMBL1791349)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C49H71N13O10/c1-6-29(4)41(46(69)58-36(24-32-25-53-27-55-32)47(70)62-21-11-15-38(62)44(67)59-37(48(71)72)23-30-12-8-7-9-13-30)61-43(66)35(22-31-16-18-33(63)19-17-31)57-45(68)40(28(2)3)60-42(65)34(56-39(64)26-52-5)14-10-20-54-49(50)51/h7-9,12-13,16-19,25,27-29,34-38,40-41,52,63H,6,10-11,14-15,20-24,26H2,1-5H3,(H,53,55)(H,56,64)(H,57,68)(H,58,69)(H,59,67)(H,60,65)(H,61,66)(H,71,72)(H4,50,51,54)/t29-,34+,35+,36+,37+,38+,40+,41+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity for angiotensin II receptor, type 2 in pig uterus myometrium using [125I]-Ang II as radioligand, in pH 7.4 Tris-HCl buffer for 1.5 h... |
J Med Chem 48: 6620-31 (2005)
Article DOI: 10.1021/jm050280z
BindingDB Entry DOI: 10.7270/Q2HX1DG6 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288434
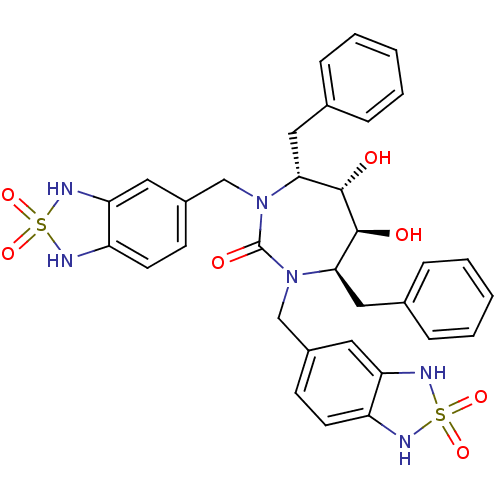
((4R,5S,6S,7R)-4,7-Dibenzyl-1,3-bis-(2,2-dioxo-2,3-...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3NS(=O)(=O)Nc3c2)C(=O)N(Cc2ccc3NS(=O)(=O)Nc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C33H34N6O7S2/c40-31-29(17-21-7-3-1-4-8-21)38(19-23-11-13-25-27(15-23)36-47(43,44)34-25)33(42)39(30(32(31)41)18-22-9-5-2-6-10-22)20-24-12-14-26-28(16-24)37-48(45,46)35-26/h1-16,29-32,34-37,40-41H,17-20H2/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50472361
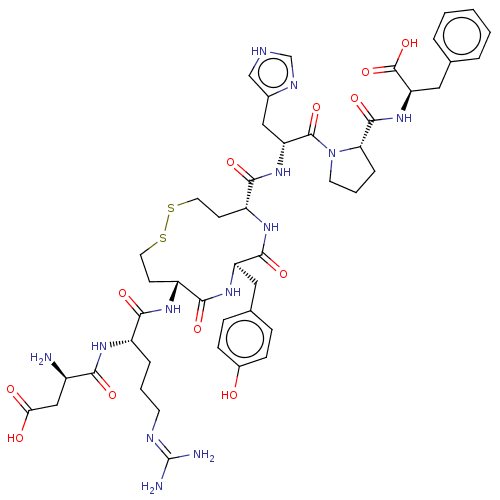
(CHEMBL406349)Show SMILES N[C@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H]1CCSSCC[C@@H](NC(=O)[C@@H](Cc2ccc(O)cc2)NC1=O)C(=O)N[C@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@H](Cc1ccccc1)C(O)=O |wU:59.63,46.47,9.8,31.31,wD:20.19,27.44,63.66,1.0,(-2.49,-6.28,;-2.47,-4.62,;-3.84,-3.93,;-5.11,-4.77,;-5.13,-6.21,;-6.53,-4.07,;-1.11,-3.93,;-1.11,-2.41,;.19,-4.74,;1.56,-4.01,;1.59,-2.47,;2.96,-1.73,;2.99,-.19,;4.35,.53,;4.39,2.07,;5.75,2.8,;3.09,2.89,;2.87,-4.81,;2.68,-3.3,;4.21,-5.54,;5.55,-4.77,;5.54,-3.23,;6.63,-2.13,;7.96,-1.36,;11.63,-.89,;13.17,-.89,;14.26,-1.99,;13.54,-3.36,;12.02,-3.43,;10.48,-3.48,;9.64,-2.18,;9.76,-4.85,;10.66,-6.02,;9.85,-7.26,;10.53,-8.56,;9.71,-9.78,;8.24,-9.71,;7.43,-10.95,;7.57,-8.41,;8.36,-7.17,;8.22,-4.77,;6.89,-5.54,;6.89,-7.08,;14.37,-4.67,;14.36,-6.19,;15.85,-4.25,;17.23,-4.78,;17.41,-6.19,;16.4,-7.33,;15.13,-6.82,;14.1,-7.96,;14.75,-9.19,;16.19,-8.82,;18.23,-3.64,;17.74,-2.18,;19.74,-3.95,;19.74,-2.41,;21.22,-1.94,;22.12,-3.13,;21.21,-4.39,;21.7,-5.86,;20.68,-7.01,;23.2,-6.16,;24.74,-6.17,;25.76,-4.98,;24.97,-3.64,;23.43,-3.64,;22.64,-2.29,;23.43,-.94,;24.97,-.94,;25.76,-2.29,;25.25,-7.63,;24.37,-8.87,;26.67,-7.65,)| Show InChI InChI=1S/C47H63N13O12S2/c48-30(23-38(62)63)39(64)54-31(8-4-16-52-47(49)50)40(65)55-32-14-18-73-74-19-15-33(56-43(68)34(57-41(32)66)20-27-10-12-29(61)13-11-27)42(67)58-35(22-28-24-51-25-53-28)45(70)60-17-5-9-37(60)44(69)59-36(46(71)72)21-26-6-2-1-3-7-26/h1-3,6-7,10-13,24-25,30-37,61H,4-5,8-9,14-23,48H2,(H,51,53)(H,54,64)(H,55,65)(H,56,68)(H,57,66)(H,58,67)(H,59,69)(H,62,63)(H,71,72)(H4,49,50,52)/t30-,31+,32-,33-,34-,35-,36-,37+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Angiotensin II receptor, type 1 from rat liver membranes |
J Med Chem 42: 4524-37 (1999)
Article DOI: 10.1021/jm991089q
BindingDB Entry DOI: 10.7270/Q2PN98C1 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM12216

((2R,3R,4R,5R)-2,5-bis(benzyloxy)-3,4-dihydroxy-N-[...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](OCc1ccccc1)[C@H](O)[C@@H](O)[C@@H](OCc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)C(C)C |r| Show InChI InChI=1S/C35H43N3O8/c1-21(2)27(33(42)36-3)37-34(43)31(45-19-22-12-6-4-7-13-22)29(40)30(41)32(46-20-23-14-8-5-9-15-23)35(44)38-28-25-17-11-10-16-24(25)18-26(28)39/h4-17,21,26-32,39-41H,18-20H2,1-3H3,(H,36,42)(H,37,43)(H,38,44)/t26-,27+,28+,29-,30-,31-,32-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stockholm University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
Eur J Med Chem 45: 160-70 (2010)
Article DOI: 10.1016/j.ejmech.2009.09.038
BindingDB Entry DOI: 10.7270/Q2KD21QM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM12216

((2R,3R,4R,5R)-2,5-bis(benzyloxy)-3,4-dihydroxy-N-[...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](OCc1ccccc1)[C@H](O)[C@@H](O)[C@@H](OCc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)C(C)C |r| Show InChI InChI=1S/C35H43N3O8/c1-21(2)27(33(42)36-3)37-34(43)31(45-19-22-12-6-4-7-13-22)29(40)30(41)32(46-20-23-14-8-5-9-15-23)35(44)38-28-25-17-11-10-16-24(25)18-26(28)39/h4-17,21,26-32,39-41H,18-20H2,1-3H3,(H,36,42)(H,37,43)(H,38,44)/t26-,27+,28+,29-,30-,31-,32-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stockholm University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 recombinant protease by fluorometric assay |
Eur J Med Chem 45: 160-70 (2010)
Article DOI: 10.1016/j.ejmech.2009.09.038
BindingDB Entry DOI: 10.7270/Q2KD21QM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50213021
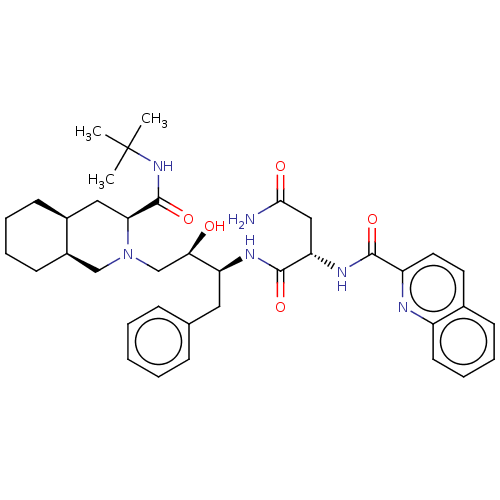
(CHEBI:63621 | Fortovase | Invirase | Ro-31-8959 | ...)Show SMILES [H][C@@]12CCCC[C@]1([H])CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc3ccccc3n1)[C@@H](C2)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C38H50N6O5/c1-38(2,3)43-37(49)32-20-26-14-7-8-15-27(26)22-44(32)23-33(45)30(19-24-11-5-4-6-12-24)41-36(48)31(21-34(39)46)42-35(47)29-18-17-25-13-9-10-16-28(25)40-29/h4-6,9-13,16-18,26-27,30-33,45H,7-8,14-15,19-23H2,1-3H3,(H2,39,46)(H,41,48)(H,42,47)(H,43,49)/t26-,27+,30-,31-,32-,33+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay |
J Med Chem 53: 607-15 (2010)
Article DOI: 10.1021/jm901165g
BindingDB Entry DOI: 10.7270/Q29Z97R7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288431

((4R,5S,6S,7R)-1,3-Bis-(2-amino-benzooxazol-6-ylmet...)Show SMILES Nc1nc2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4ccc5nc(N)oc5c4)C3=O)cc2o1 Show InChI InChI=1S/C35H34N6O5/c36-33-38-25-13-11-23(17-29(25)45-33)19-40-27(15-21-7-3-1-4-8-21)31(42)32(43)28(16-22-9-5-2-6-10-22)41(35(40)44)20-24-12-14-26-30(18-24)46-34(37)39-26/h1-14,17-18,27-28,31-32,42-43H,15-16,19-20H2,(H2,36,38)(H2,37,39)/t27-,28-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86311
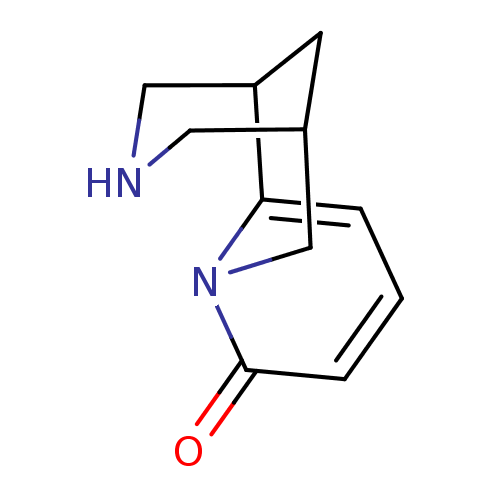
(CAS_485-35-8 | Cytisine | Cytisine-(-) | NSC_22407...)Show InChI InChI=1S/C11H14N2O/c14-11-3-1-2-10-9-4-8(5-12-6-9)7-13(10)11/h1-3,8-9,12H,4-7H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 619-26 (2005)
Article DOI: 10.1124/jpet.104.075069
BindingDB Entry DOI: 10.7270/Q2BK19XS |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM358

((2R,3R,4R,5R)-2,5-bis(benzyloxy)-3,4-dihydroxy-N,N...)Show SMILES O[C@H]([C@@H](O)[C@@H](OCc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)[C@@H](OCc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C38H40N2O8/c41-29-19-25-15-7-9-17-27(25)31(29)39-37(45)35(47-21-23-11-3-1-4-12-23)33(43)34(44)36(48-22-24-13-5-2-6-14-24)38(46)40-32-28-18-10-8-16-26(28)20-30(32)42/h1-18,29-36,41-44H,19-22H2,(H,39,45)(H,40,46)/t29-,30-,31+,32+,33-,34-,35-,36-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.200 | -56.3 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University
| Assay Description
A fluorimetric assay was used to determine the effects of the inhibitors on HIV-1 protease. This assay used an internally quenched fluorescent peptid... |
Eur J Biochem 270: 1746-58 (2003)
Article DOI: 10.1046/j.1432-1033.2003.03533.x
BindingDB Entry DOI: 10.7270/Q2NZ85WX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50236697

(5-L-isoleucineangiotensin II | 5-isoleucine-angiot...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:4.4,47.47,60.63,35.36,20.21,wD:2.2,24.32,8.17,64.66,(24.13,1.24,;24.1,-.31,;25.42,-1.09,;26.77,-.35,;25.4,-2.63,;24.05,-3.38,;22.73,-2.59,;22.75,-1.05,;21.38,-3.34,;21.36,-4.88,;22.68,-5.66,;24.01,-4.93,;25.33,-5.71,;25.32,-7.24,;26.64,-8.05,;23.97,-8.01,;22.65,-7.22,;20.06,-2.55,;18.71,-3.29,;18.68,-4.83,;17.38,-2.5,;16.04,-3.25,;14.72,-2.46,;14.74,-.92,;13.37,-3.2,;13.34,-4.74,;14.66,-5.53,;14.64,-7.08,;15.96,-7.87,;15.93,-9.4,;14.59,-10.16,;17.25,-10.21,;12.05,-2.42,;10.69,-3.15,;10.67,-4.69,;9.37,-2.36,;8.03,-3.1,;9.4,-.82,;10.75,-.07,;12.07,-.88,;10.78,1.47,;17.41,-.96,;18.76,-.21,;16.1,-.17,;26.72,-3.43,;26.69,-4.97,;28.07,-2.68,;29.38,-3.48,;30.73,-2.73,;30.75,-1.19,;29.53,-.27,;30.03,1.18,;31.57,1.17,;32.02,-.31,;29.35,-5.02,;28.01,-5.76,;30.54,-5.79,;31.99,-5.25,;32.95,-6.46,;32.1,-7.74,;30.62,-7.33,;29.33,-8.36,;27.89,-7.82,;29.58,-9.88,;28.39,-10.86,;26.95,-10.32,;25.76,-11.3,;26.02,-12.81,;24.84,-13.79,;23.4,-13.26,;23.14,-11.74,;24.32,-10.75,;28.64,-12.38,;27.45,-13.36,;30.08,-12.92,)| Show InChI InChI=1S/C50H71N13O12/c1-5-28(4)41(47(72)59-36(23-31-25-54-26-56-31)48(73)63-20-10-14-38(63)45(70)60-37(49(74)75)22-29-11-7-6-8-12-29)62-44(69)35(21-30-15-17-32(64)18-16-30)58-46(71)40(27(2)3)61-43(68)34(13-9-19-55-50(52)53)57-42(67)33(51)24-39(65)66/h6-8,11-12,15-18,25-28,33-38,40-41,64H,5,9-10,13-14,19-24,51H2,1-4H3,(H,54,56)(H,57,67)(H,58,71)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,65,66)(H,74,75)(H4,52,53,55)/t28-,33-,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of CDK1/Cyclin B (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... |
Bioorg Med Chem Lett 26: 1355-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.10.084
BindingDB Entry DOI: 10.7270/Q2SN0BT6 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50066918

((2R,3R,4R,5R)-2,5-Bis-benzyloxy-3,4-dihydroxy-hexa...)Show SMILES O[C@H]([C@@H](O)[C@@H](OCc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)[C@@H](OCc1ccccc1)C(=O)N[C@H]1[C@@H](O)Cc2ccccc12 Show InChI InChI=1S/C38H40N2O8/c41-29-19-25-15-7-9-17-27(25)31(29)39-37(45)35(47-21-23-11-3-1-4-12-23)33(43)34(44)36(48-22-24-13-5-2-6-14-24)38(46)40-32-28-18-10-8-16-26(28)20-30(32)42/h1-18,29-36,41-44H,19-22H2,(H,39,45)(H,40,46)/t29-,30+,31+,32-,33-,34-,35-,36-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified HIV-1 protease expressed in E. coli in sensitive fluorometric assay |
J Med Chem 41: 3782-92 (1998)
Article DOI: 10.1021/jm970777b
BindingDB Entry DOI: 10.7270/Q2PK0F93 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2 [A65S,N67Q,E69T,I91L,F131V,K170E,L204A]
(Homo sapiens (Human)) | BDBM210938
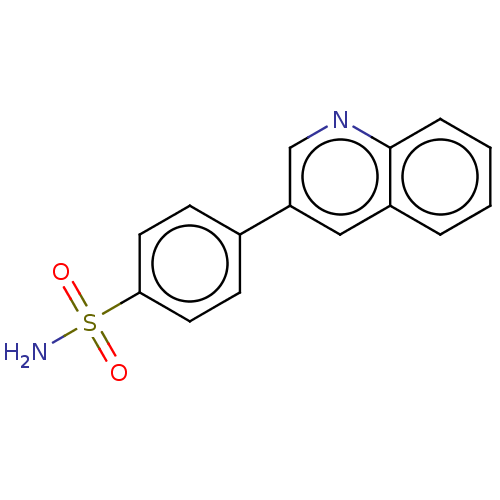
(4-(3-quinolinyl)-benzenesulfonamide (4p))Show InChI InChI=1S/C15H12N2O2S/c16-20(18,19)14-7-5-11(6-8-14)13-9-12-3-1-2-4-15(12)17-10-13/h1-10H,(H2,16,18,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
| Assay Description
CA-catalyzed CO2 hydration activity methods were published previously by Cornelio et al. as part of a larger series of benzenesulfonamide-based inhib... |
Chembiochem 18: 213-222 (2017)
Article DOI: 10.1002/cbic.201600513
BindingDB Entry DOI: 10.7270/Q27H1HFC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2 [A65S,N67Q,E69T,I91L,F131V,K170E,L204A]
(Homo sapiens (Human)) | BDBM210935
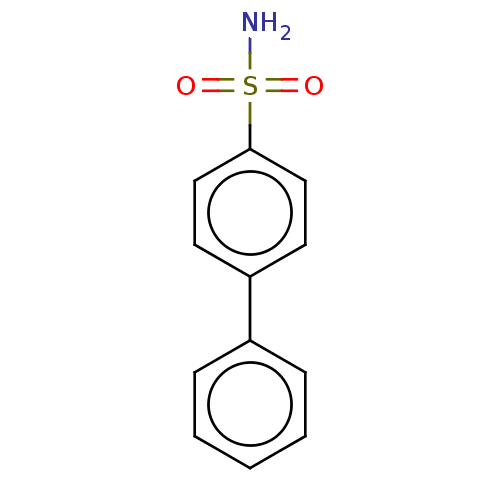
(4-(phenyl)-bezenesulfonamide (4a))Show InChI InChI=1S/C12H11NO2S/c13-16(14,15)12-8-6-11(7-9-12)10-4-2-1-3-5-10/h1-9H,(H2,13,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
| Assay Description
CA-catalyzed CO2 hydration activity methods were published previously by Cornelio et al. as part of a larger series of benzenesulfonamide-based inhib... |
Chembiochem 18: 213-222 (2017)
Article DOI: 10.1002/cbic.201600513
BindingDB Entry DOI: 10.7270/Q27H1HFC |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50579806
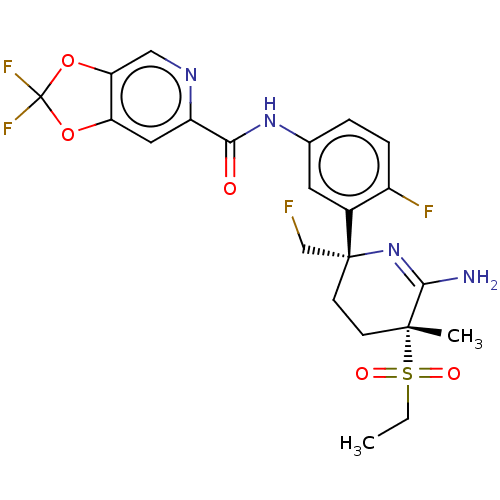
(CHEMBL5092328)Show SMILES CCS(=O)(=O)[C@]1(C)CC[C@](CF)(N=C1N)c1cc(NC(=O)c2cc3OC(F)(F)Oc3cn2)ccc1F |r,c:12| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-JNJ962 from BACE1 (unknown origin) expressed in HEK293 cell membrane assessed as inhibition constant by scintillation counting a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00935
BindingDB Entry DOI: 10.7270/Q2V69PFZ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50067499

((6aR,10aR)-3(1,1-dimethylheptyl)-9-hydroxymethyl)-...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(CO)=CC[C@H]3C(C)(C)Oc2c1 |r,c:18| Show InChI InChI=1S/C25H38O3/c1-6-7-8-9-12-24(2,3)18-14-21(27)23-19-13-17(16-26)10-11-20(19)25(4,5)28-22(23)15-18/h10,14-15,19-20,26-27H,6-9,11-13,16H2,1-5H3/t19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Louis University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 binding to Cannabinoid receptor 1 in rat brain membranes |
J Med Chem 41: 4207-15 (1998)
Article DOI: 10.1021/jm970239z
BindingDB Entry DOI: 10.7270/Q2C24VK6 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50015662
(3-Amino-N-{1-[5-[2-[2-(1-carboxy-2-phenyl-ethylcar...)Show SMILES N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H]1CCSSCC[C@H](NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C47H63N13O12S2/c48-30(23-38(62)63)39(64)54-31(8-4-16-52-47(49)50)40(65)55-32-14-18-73-74-19-15-33(56-43(68)34(57-41(32)66)20-27-10-12-29(61)13-11-27)42(67)58-35(22-28-24-51-25-53-28)45(70)60-17-5-9-37(60)44(69)59-36(46(71)72)21-26-6-2-1-3-7-26/h1-3,6-7,10-13,24-25,30-37,61H,4-5,8-9,14-23,48H2,(H,51,53)(H,54,64)(H,55,65)(H,56,68)(H,57,66)(H,58,67)(H,59,69)(H,62,63)(H,71,72)(H4,49,50,52)/t30-,31-,32-,33-,34-,35-,36-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
In vitro binding affinity at rat liver Angiotensin II receptor, type 1 was determined based on displacement of [125I]-Ang II |
J Med Chem 45: 1767-77 (2002)
BindingDB Entry DOI: 10.7270/Q2QV3N7W |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50121499
(5,6-Dihydroxy-1,3-bis-(4-hydroxymethyl-benzyl)-4,7...)Show SMILES OCc1ccc(CN2C(COc3ccccc3)[C@H](O)[C@@H](O)C(COc3ccccc3)N(Cc3ccc(CO)cc3)C2=O)cc1 Show InChI InChI=1S/C35H38N2O7/c38-21-27-15-11-25(12-16-27)19-36-31(23-43-29-7-3-1-4-8-29)33(40)34(41)32(24-44-30-9-5-2-6-10-30)37(35(36)42)20-26-13-17-28(22-39)18-14-26/h1-18,31-34,38-41H,19-24H2/t31?,32?,33-,34-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.230 | n/a | n/a | 7 | n/a | n/a | 7.06E+9 | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Association rate constant for the interaction between inhibitor and HIV-1 protease |
J Med Chem 45: 5430-9 (2002)
BindingDB Entry DOI: 10.7270/Q2GH9JP4 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM519

((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...)Show SMILES [H][C@@]12CCCC[C@]1([H])CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc3ccccc3n1)[C@@H](C2)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C38H50N6O5/c1-38(2,3)43-37(49)32-20-26-14-7-8-15-27(26)22-44(32)23-33(45)30(19-24-11-5-4-6-12-24)41-36(48)31(21-34(39)46)42-35(47)29-18-17-25-13-9-10-16-28(25)40-29/h4-6,9-13,16-18,26-27,30-33,45H,7-8,14-15,19-23H2,1-3H3,(H2,39,46)(H,41,48)(H,42,47)(H,43,49)/t26-,27+,30-,31-,32-,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.230 | n/a | n/a | 0.315 | n/a | n/a | 8.17E+5 | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Association rate constant for the interaction between inhibitor and HIV-1 protease |
J Med Chem 45: 5430-9 (2002)
BindingDB Entry DOI: 10.7270/Q2GH9JP4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM18137

(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H14N5O7P/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(22-10)1-21-23(18,19)20/h2-4,6-7,10,16-17H,1H2,(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.230 | n/a | n/a | 1.13 | n/a | n/a | 4.43E+6 | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Association rate constant for the interaction between inhibitor and HIV-1 protease |
J Med Chem 45: 5430-9 (2002)
BindingDB Entry DOI: 10.7270/Q2GH9JP4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50054342

(8-{4-[((R)-5-Fluoro-1,2,3,4-tetrahydro-naphthalen-...)Show SMILES CCCN(CCCCN1C(=O)CC2(CCCC2)CC1=O)[C@@H]1CCc2c(F)cccc2C1 Show InChI InChI=1S/C26H37FN2O2/c1-2-14-28(21-10-11-22-20(17-21)8-7-9-23(22)27)15-5-6-16-29-24(30)18-26(19-25(29)31)12-3-4-13-26/h7-9,21H,2-6,10-19H2,1H3/t21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.246 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor |
J Med Chem 39: 4421-9 (1996)
Article DOI: 10.1021/jm960350p
BindingDB Entry DOI: 10.7270/Q21G0KCQ |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(PIG) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by PDSP Ki Database
| |
Eur J Pharmacol 347: 301-9 (1998)
Article DOI: 10.1016/s0014-2999(98)00104-6
BindingDB Entry DOI: 10.7270/Q2GT5KQK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data