

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM35870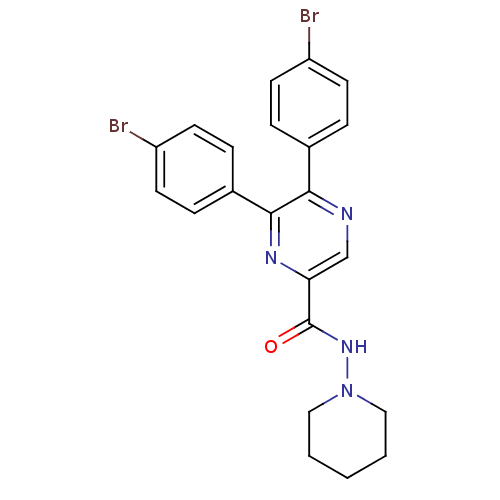 (5,6-bis-(4-bromo-phenyl)-pyrazine-2-carboxylicacid...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 30 |
AstraZeneca | Assay Description A GTP-gamma-S CB1 functional assay was used to assess the potency of the pyrazine derivatives. [35S]GTP-gamma-S binding assays were performed at 30 d... | Bioorg Med Chem 15: 4077-84 (2007) Article DOI: 10.1016/j.bmc.2007.03.075 BindingDB Entry DOI: 10.7270/Q2028PW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
AstraZeneca | Assay Description A GTP-gamma-S CB1 functional assay was used to assess the potency of the pyrazine derivatives. [35S]GTP-gamma-S binding assays were performed at 30 d... | Bioorg Med Chem 15: 4077-84 (2007) Article DOI: 10.1016/j.bmc.2007.03.075 BindingDB Entry DOI: 10.7270/Q2028PW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM35873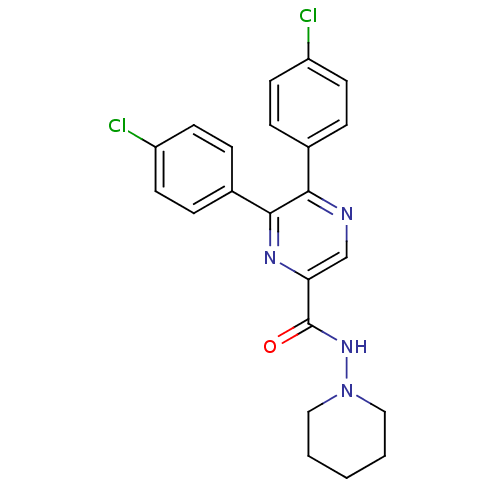 (5,6-bis(4-chlorophenyl)-N-(piperidin-1-yl)pyrazine...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 30 |
AstraZeneca | Assay Description A GTP-gamma-S CB1 functional assay was used to assess the potency of the pyrazine derivatives. [35S]GTP-gamma-S binding assays were performed at 30 d... | Bioorg Med Chem 15: 4077-84 (2007) Article DOI: 10.1016/j.bmc.2007.03.075 BindingDB Entry DOI: 10.7270/Q2028PW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM35871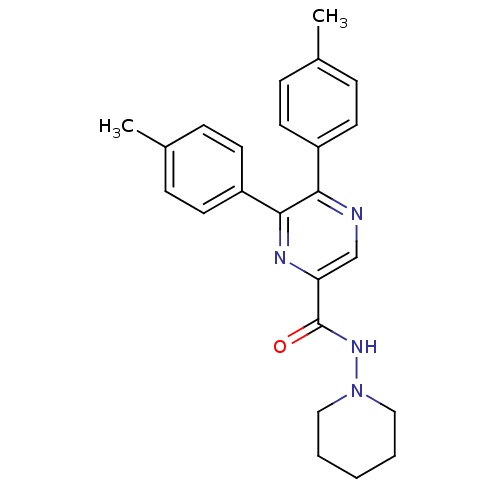 (5,6-di-p-tolyl-pyrazine-2-carboxylic acid piperidi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 30 |
AstraZeneca | Assay Description A GTP-gamma-S CB1 functional assay was used to assess the potency of the pyrazine derivatives. [35S]GTP-gamma-S binding assays were performed at 30 d... | Bioorg Med Chem 15: 4077-84 (2007) Article DOI: 10.1016/j.bmc.2007.03.075 BindingDB Entry DOI: 10.7270/Q2028PW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50539662 (CHEMBL4641579) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a stably expressed in HEK cell membrane measured after 60 mins by gamma counter method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM35877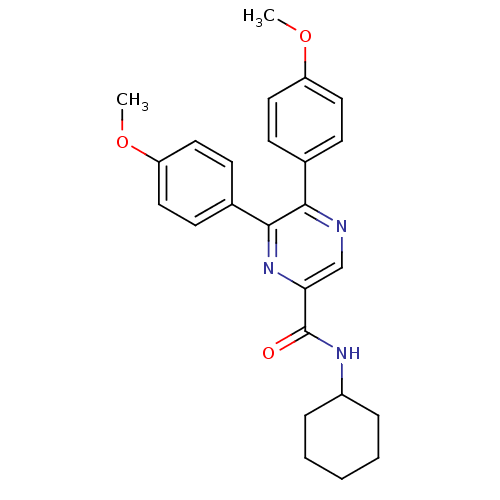 (5,6-bis-(4-methoxy-phenyl)-pyrazine-2-carboxylicac...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.4 | 30 |
AstraZeneca | Assay Description A GTP-gamma-S CB1 functional assay was used to assess the potency of the pyrazine derivatives. [35S]GTP-gamma-S binding assays were performed at 30 d... | Bioorg Med Chem 15: 4077-84 (2007) Article DOI: 10.1016/j.bmc.2007.03.075 BindingDB Entry DOI: 10.7270/Q2028PW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM35868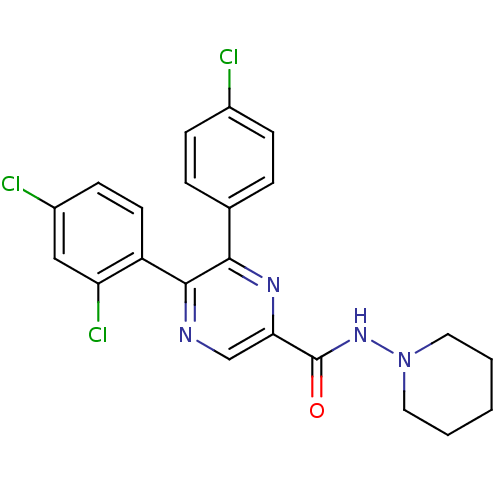 (5,6-diaryl-pyrazine-2-amide derivative, 2b | 6-(4-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | 30 |
AstraZeneca | Assay Description A GTP-gamma-S CB1 functional assay was used to assess the potency of the pyrazine derivatives. [35S]GTP-gamma-S binding assays were performed at 30 d... | Bioorg Med Chem 15: 4077-84 (2007) Article DOI: 10.1016/j.bmc.2007.03.075 BindingDB Entry DOI: 10.7270/Q2028PW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM35875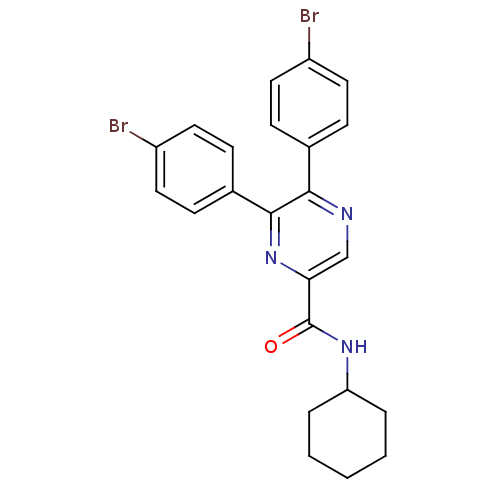 (5,6-bis-(4-bromo-phenyl)-pyrazine-2-carboxylicacid...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.4 | 30 |
AstraZeneca | Assay Description A GTP-gamma-S CB1 functional assay was used to assess the potency of the pyrazine derivatives. [35S]GTP-gamma-S binding assays were performed at 30 d... | Bioorg Med Chem 15: 4077-84 (2007) Article DOI: 10.1016/j.bmc.2007.03.075 BindingDB Entry DOI: 10.7270/Q2028PW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM35876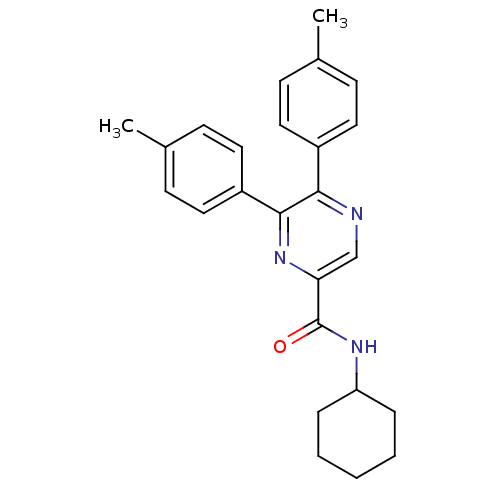 (5,6-di-p-tolyl-pyrazine-2-carboxylic acid cyclohex...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.4 | 30 |
AstraZeneca | Assay Description A GTP-gamma-S CB1 functional assay was used to assess the potency of the pyrazine derivatives. [35S]GTP-gamma-S binding assays were performed at 30 d... | Bioorg Med Chem 15: 4077-84 (2007) Article DOI: 10.1016/j.bmc.2007.03.075 BindingDB Entry DOI: 10.7270/Q2028PW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM35878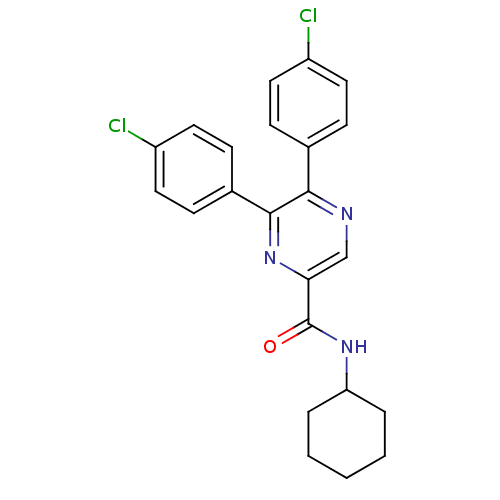 (5,6-bis(4-chlorophenyl)-N-cyclohexylpyrazine-2-car...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.4 | 30 |
AstraZeneca | Assay Description A GTP-gamma-S CB1 functional assay was used to assess the potency of the pyrazine derivatives. [35S]GTP-gamma-S binding assays were performed at 30 d... | Bioorg Med Chem 15: 4077-84 (2007) Article DOI: 10.1016/j.bmc.2007.03.075 BindingDB Entry DOI: 10.7270/Q2028PW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM35872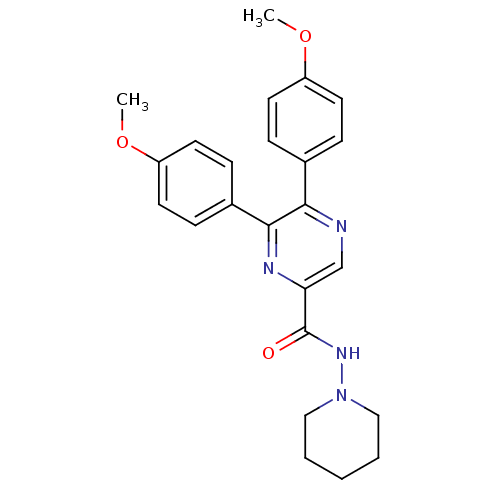 (5,6-bis(4-methoxyphenyl)-N-(piperidin-1-yl)pyrazin...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 7.4 | 30 |
AstraZeneca | Assay Description A GTP-gamma-S CB1 functional assay was used to assess the potency of the pyrazine derivatives. [35S]GTP-gamma-S binding assays were performed at 30 d... | Bioorg Med Chem 15: 4077-84 (2007) Article DOI: 10.1016/j.bmc.2007.03.075 BindingDB Entry DOI: 10.7270/Q2028PW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM35883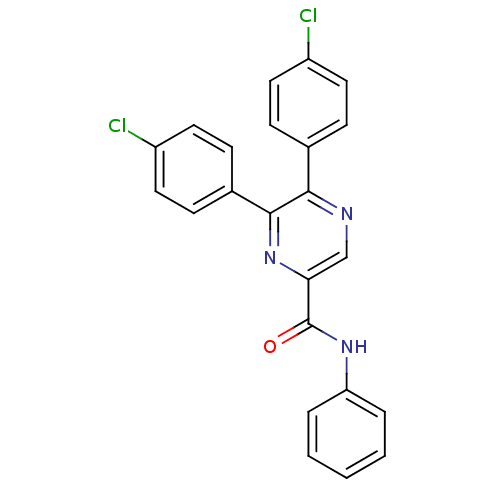 (5,6-bis-(4-chloro-phenyl)-pyrazine-2-carboxylicaci...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | 7.4 | 30 |
AstraZeneca | Assay Description A GTP-gamma-S CB1 functional assay was used to assess the potency of the pyrazine derivatives. [35S]GTP-gamma-S binding assays were performed at 30 d... | Bioorg Med Chem 15: 4077-84 (2007) Article DOI: 10.1016/j.bmc.2007.03.075 BindingDB Entry DOI: 10.7270/Q2028PW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM35867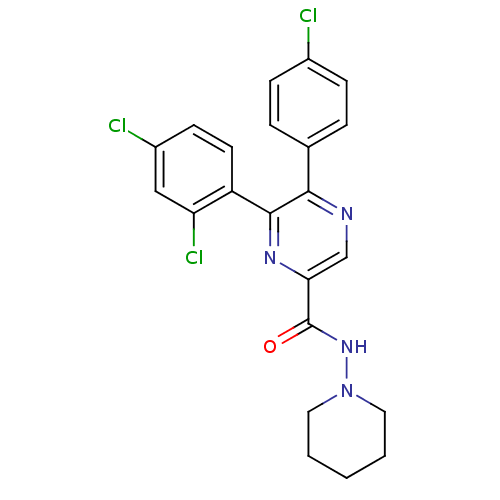 (5,6-diaryl-pyrazine-2-amide derivative, 2a | 5-(4-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | 7.4 | 30 |
AstraZeneca | Assay Description A GTP-gamma-S CB1 functional assay was used to assess the potency of the pyrazine derivatives. [35S]GTP-gamma-S binding assays were performed at 30 d... | Bioorg Med Chem 15: 4077-84 (2007) Article DOI: 10.1016/j.bmc.2007.03.075 BindingDB Entry DOI: 10.7270/Q2028PW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM35882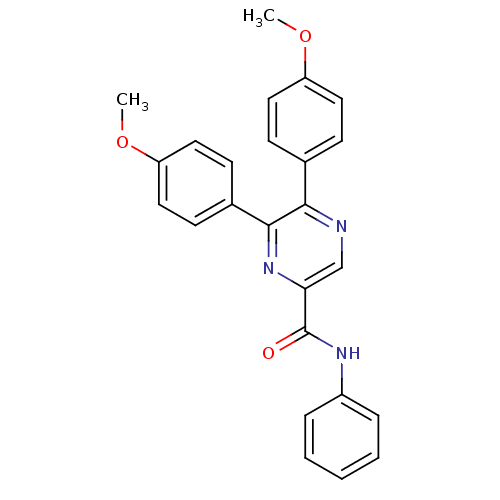 (5,6-bis(4-methoxyphenyl)-N-phenylpyrazine-2-carbox...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 119 | n/a | n/a | n/a | n/a | 7.4 | 30 |
AstraZeneca | Assay Description A GTP-gamma-S CB1 functional assay was used to assess the potency of the pyrazine derivatives. [35S]GTP-gamma-S binding assays were performed at 30 d... | Bioorg Med Chem 15: 4077-84 (2007) Article DOI: 10.1016/j.bmc.2007.03.075 BindingDB Entry DOI: 10.7270/Q2028PW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM35874 (5,6-bis(2-chlorophenyl)-N-(piperidin-1-yl)pyrazine...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 174 | n/a | n/a | n/a | n/a | 7.4 | 30 |
AstraZeneca | Assay Description A GTP-gamma-S CB1 functional assay was used to assess the potency of the pyrazine derivatives. [35S]GTP-gamma-S binding assays were performed at 30 d... | Bioorg Med Chem 15: 4077-84 (2007) Article DOI: 10.1016/j.bmc.2007.03.075 BindingDB Entry DOI: 10.7270/Q2028PW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM35881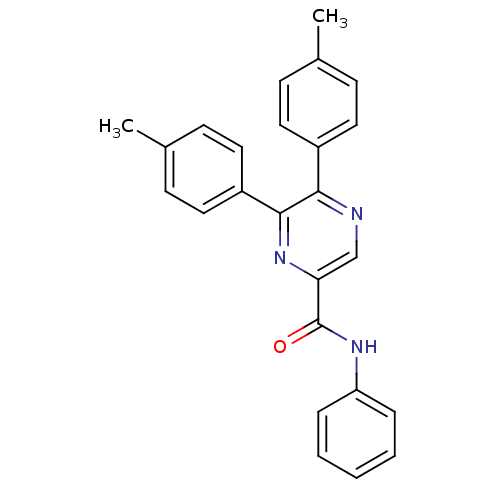 (5,6-di-p-tolyl-pyrazine-2-carboxylic acid phenylam...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 326 | n/a | n/a | n/a | n/a | 7.4 | 30 |
AstraZeneca | Assay Description A GTP-gamma-S CB1 functional assay was used to assess the potency of the pyrazine derivatives. [35S]GTP-gamma-S binding assays were performed at 30 d... | Bioorg Med Chem 15: 4077-84 (2007) Article DOI: 10.1016/j.bmc.2007.03.075 BindingDB Entry DOI: 10.7270/Q2028PW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50539689 (CHEMBL4642043) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a stably expressed in HEK cell membrane measured after 60 mins by gamma counter method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM35884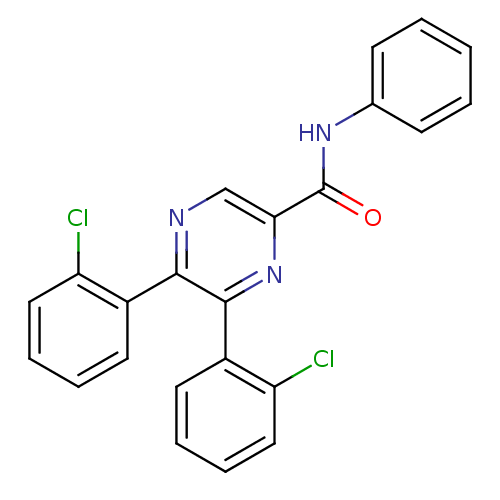 (5,6-bis-(2-chloro-phenyl)-pyrazine-2-carboxylicaci...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 763 | n/a | n/a | n/a | n/a | 7.4 | 30 |
AstraZeneca | Assay Description A GTP-gamma-S CB1 functional assay was used to assess the potency of the pyrazine derivatives. [35S]GTP-gamma-S binding assays were performed at 30 d... | Bioorg Med Chem 15: 4077-84 (2007) Article DOI: 10.1016/j.bmc.2007.03.075 BindingDB Entry DOI: 10.7270/Q2028PW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM35869 (5,6-diaryl-pyrazine-2-amide derivative, 5a | 5,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 766 | n/a | n/a | n/a | n/a | 7.4 | 30 |
AstraZeneca | Assay Description A GTP-gamma-S CB1 functional assay was used to assess the potency of the pyrazine derivatives. [35S]GTP-gamma-S binding assays were performed at 30 d... | Bioorg Med Chem 15: 4077-84 (2007) Article DOI: 10.1016/j.bmc.2007.03.075 BindingDB Entry DOI: 10.7270/Q2028PW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50539663 (CHEMBL4632411) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a stably expressed in HEK cell membrane measured after 60 mins by gamma counter method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50539685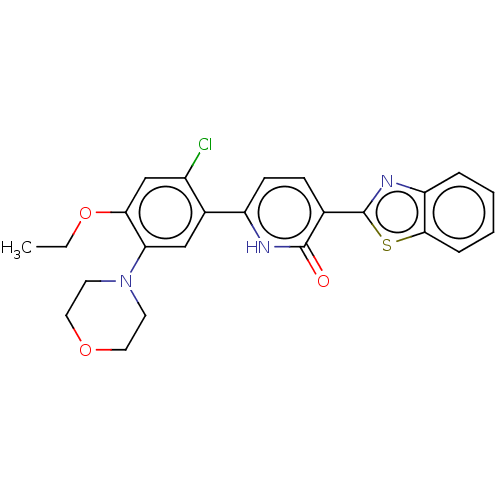 (CHEMBL4649089) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a stably expressed in HEK cell membrane measured after 60 mins by gamma counter method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM35879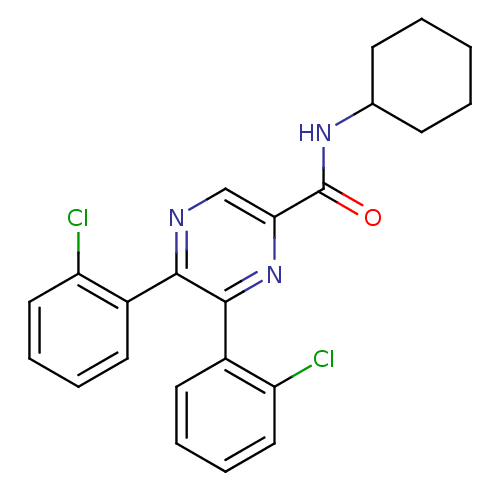 (5,6-bis-(2-chloro-phenyl)-pyrazine-2-carboxylicaci...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
AstraZeneca | Assay Description A GTP-gamma-S CB1 functional assay was used to assess the potency of the pyrazine derivatives. [35S]GTP-gamma-S binding assays were performed at 30 d... | Bioorg Med Chem 15: 4077-84 (2007) Article DOI: 10.1016/j.bmc.2007.03.075 BindingDB Entry DOI: 10.7270/Q2028PW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50539687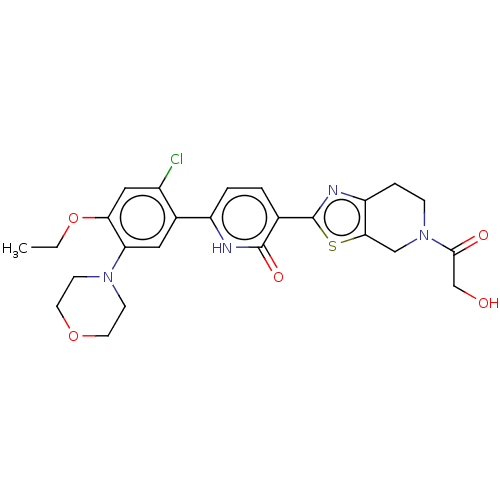 (CHEMBL4635089) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a stably expressed in HEK cell membrane measured after 60 mins by gamma counter method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM35880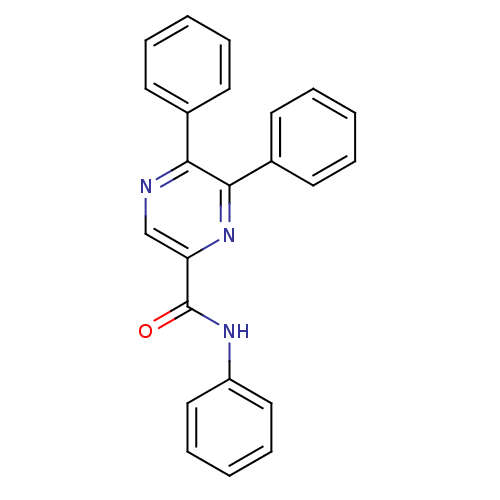 (5,6-diaryl-pyrazine-2-amide derivative, 7a | 5,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.79E+3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
AstraZeneca | Assay Description A GTP-gamma-S CB1 functional assay was used to assess the potency of the pyrazine derivatives. [35S]GTP-gamma-S binding assays were performed at 30 d... | Bioorg Med Chem 15: 4077-84 (2007) Article DOI: 10.1016/j.bmc.2007.03.075 BindingDB Entry DOI: 10.7270/Q2028PW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50539683 (CHEMBL4635119) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >4.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a stably expressed in HEK cell membrane measured after 60 mins by gamma counter method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50539686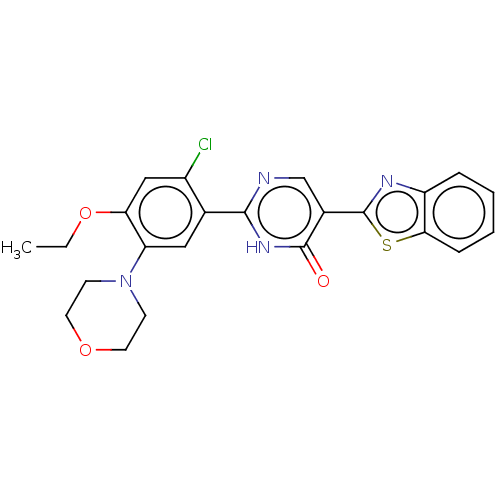 (CHEMBL4644587) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a stably expressed in HEK cell membrane measured after 60 mins by gamma counter method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50539696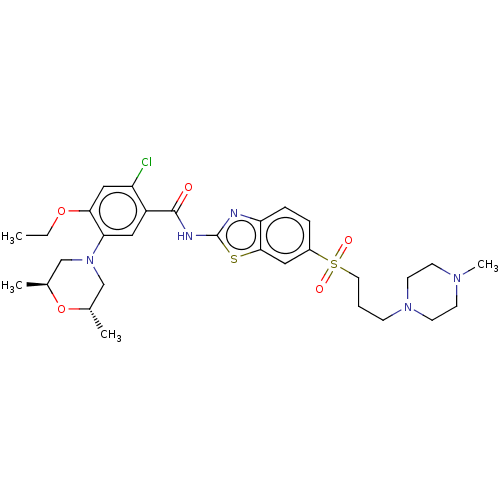 (CHEMBL4641503) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a stably expressed in HEK cell membrane measured after 60 mins by gamma counter method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50539690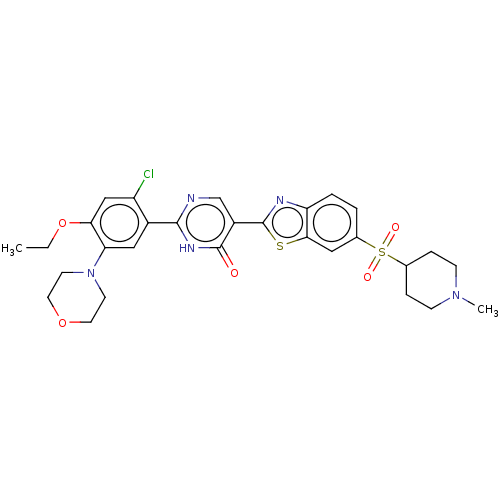 (CHEMBL4645314) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a stably expressed in HEK cell membrane measured after 60 mins by gamma counter method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50539695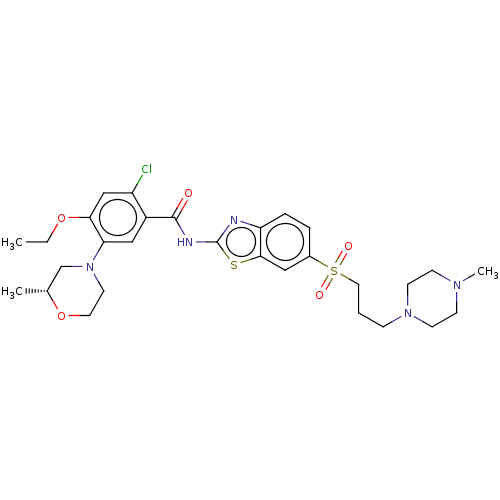 (CHEMBL4649741) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a stably expressed in HEK cell membrane measured after 60 mins by gamma counter method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50539697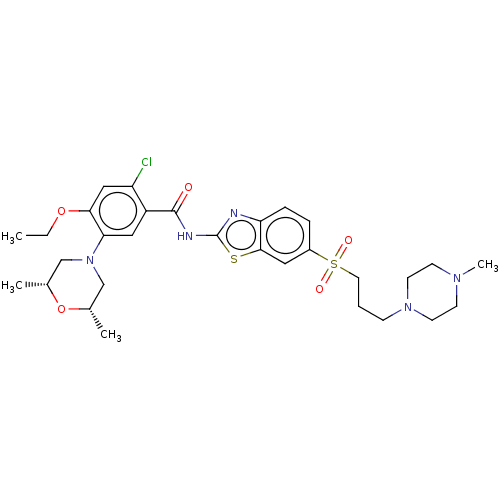 (CHEMBL4636832) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a stably expressed in HEK cell membrane measured after 60 mins by gamma counter method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50539693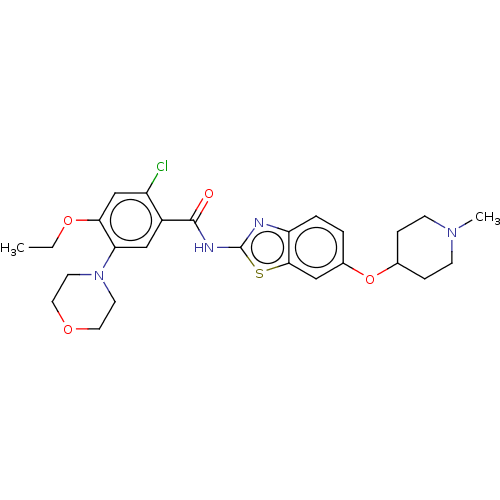 (CHEMBL4646005) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a stably expressed in HEK cell membrane measured after 60 mins by gamma counter method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50539691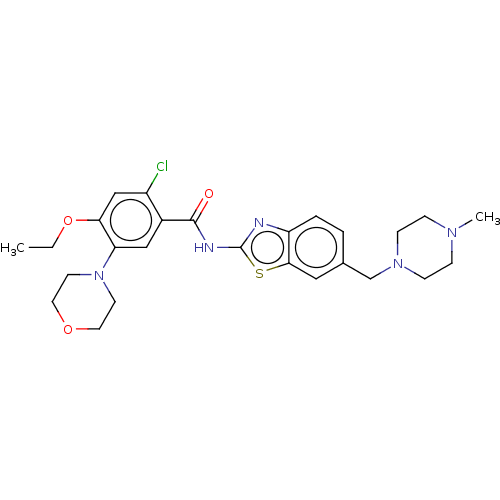 (CHEMBL4642179) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a stably expressed in HEK cell membrane measured after 60 mins by gamma counter method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50539692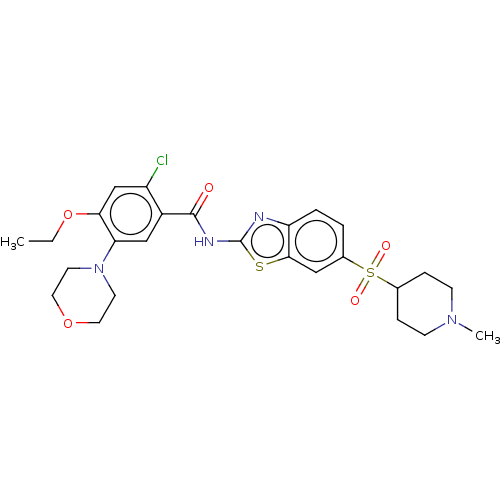 (CHEMBL4638676) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a stably expressed in HEK cell membrane measured after 60 mins by gamma counter method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50539694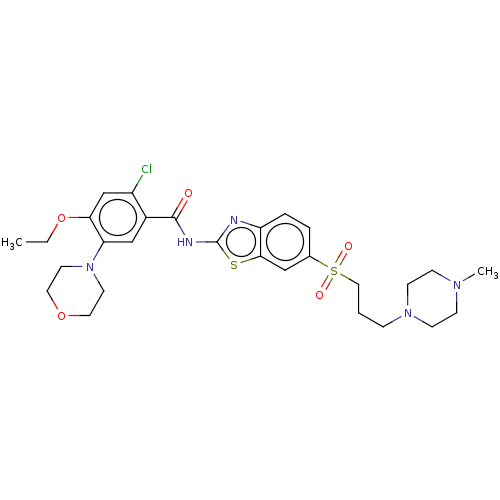 (CHEMBL4633635) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a stably expressed in HEK cell membrane measured after 60 mins by gamma counter method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50539684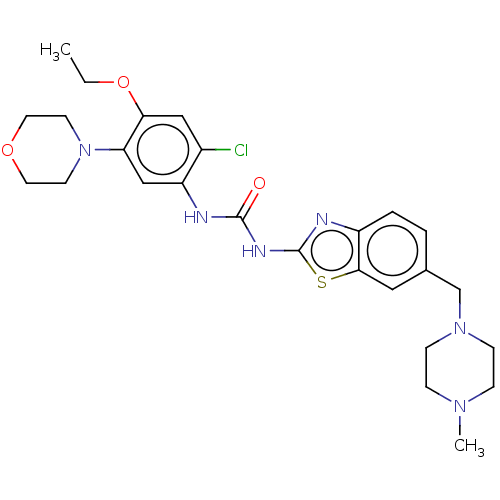 (CHEMBL4641120) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a stably expressed in HEK cell membrane measured after 60 mins by gamma counter method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50539682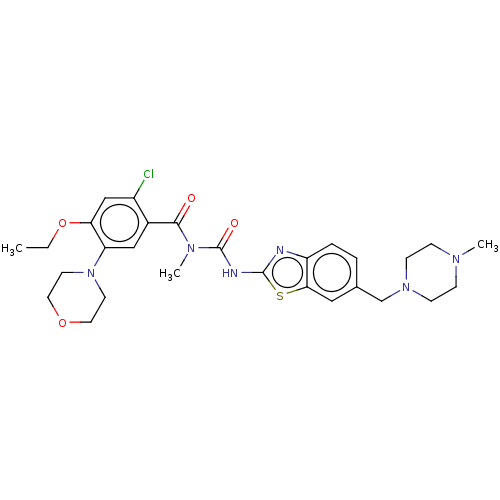 (CHEMBL4642413) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a stably expressed in HEK cell membrane measured after 60 mins by gamma counter method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50539698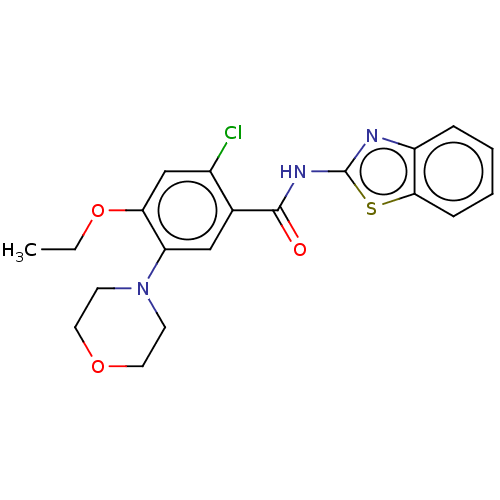 (CHEMBL4641449) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a stably expressed in HEK cell membrane measured after 60 mins by gamma counter method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50539688 (CHEMBL4638263) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a stably expressed in HEK cell membrane measured after 60 mins by gamma counter method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 1 (Homo sapiens (Human)) | BDBM50539662 (CHEMBL4641579) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Agonist activity at human GPR81 overexpressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 1 (Homo sapiens (Human)) | BDBM50539663 (CHEMBL4632411) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 74 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Agonist activity at human GPR81 overexpressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 1 (Homo sapiens (Human)) | BDBM50539664 (CHEMBL4647314) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >3.70E+3 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Agonist activity at human GPR81 overexpressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 1 (Homo sapiens (Human)) | BDBM50539665 (CHEMBL4637486) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Agonist activity at human GPR81 overexpressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 1 (Homo sapiens (Human)) | BDBM50539666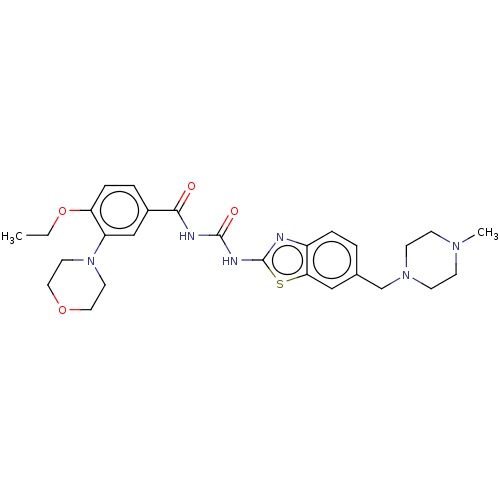 (CHEMBL4641372) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 740 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Agonist activity at human GPR81 overexpressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 1 (Homo sapiens (Human)) | BDBM50539667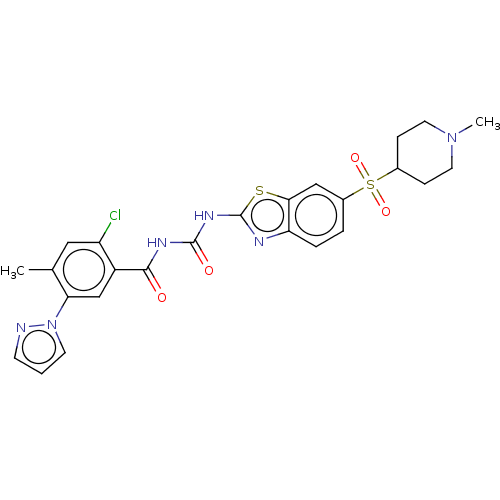 (CHEMBL4646399) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Agonist activity at human GPR81 overexpressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 1 (Homo sapiens (Human)) | BDBM50539668 (CHEMBL4637505) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Agonist activity at human GPR81 overexpressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 1 (Homo sapiens (Human)) | BDBM50047422 (CHEMBL3319403) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Agonist activity at human GPR81 overexpressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 1 (Homo sapiens (Human)) | BDBM50539669 (CHEMBL4649373) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Agonist activity at human GPR81 overexpressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 1 (Homo sapiens (Human)) | BDBM50539670 (CHEMBL4632482) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Agonist activity at human GPR81 overexpressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 1 (Homo sapiens (Human)) | BDBM50539671 (CHEMBL4641219) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Agonist activity at human GPR81 overexpressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 1 (Homo sapiens (Human)) | BDBM50539672 (CHEMBL4640189) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Agonist activity at human GPR81 overexpressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126953 BindingDB Entry DOI: 10.7270/Q2125X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 93 total ) | Next | Last >> |