 Found 239 hits with Last Name = 'bernier' and Initial = 's'
Found 239 hits with Last Name = 'bernier' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate--tRNA ligase
(Escherichia coli) | BDBM18122
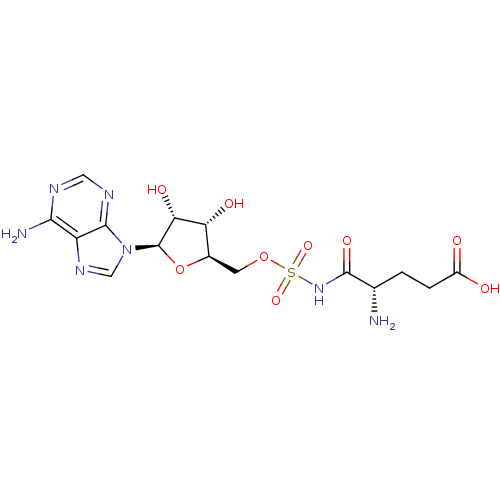
((4S)-4-amino-5-[({[(2R,3S,4R,5R)-5-(6-amino-9H-pur...)Show SMILES N[C@@H](CCC(O)=O)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C15H21N7O9S/c16-6(1-2-8(23)24)14(27)21-32(28,29)30-3-7-10(25)11(26)15(31-7)22-5-20-9-12(17)18-4-19-13(9)22/h4-7,10-11,15,25-26H,1-3,16H2,(H,21,27)(H,23,24)(H2,17,18,19)/t6-,7+,10+,11+,15+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.80 | -50.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
CREFSIP
| Assay Description
The Km and Kappm for the amino acid substrate were first calculated from Lineweaver-Burk plots. The Ki values were calculated from the Kappm vs [I] p... |
J Enzyme Inhib Med Chem 20: 61-7 (2005)
Article DOI: 10.1080/14756360400002007
BindingDB Entry DOI: 10.7270/Q2X0659C |
More data for this
Ligand-Target Pair | |
Aspartate--tRNA ligase
(Escherichia coli) | BDBM18127
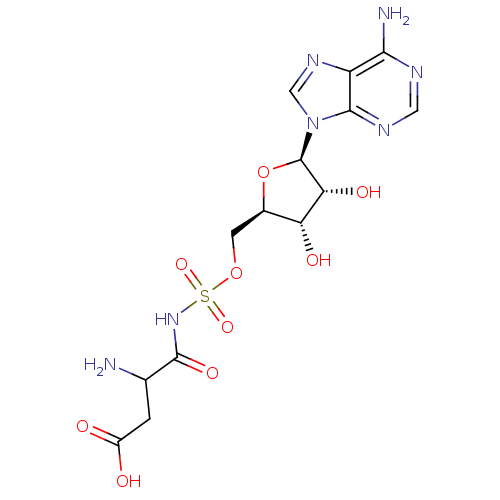
(3-amino-4-[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-...)Show SMILES NC(CC(O)=O)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C14H19N7O9S/c15-5(1-7(22)23)13(26)20-31(27,28)29-2-6-9(24)10(25)14(30-6)21-4-19-8-11(16)17-3-18-12(8)21/h3-6,9-10,14,24-25H,1-2,15H2,(H,20,26)(H,22,23)(H2,16,17,18)/t5?,6-,9-,10-,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
CREFSIP
| Assay Description
The Km and Kappm for the amino acid substrate were first calculated from Lineweaver-Burk plots. The Ki values were calculated from the Kappm vs [I] p... |
Bioorg Med Chem 13: 69-75 (2005)
Article DOI: 10.1016/j.bmc.2004.09.055
BindingDB Entry DOI: 10.7270/Q2S75DM3 |
More data for this
Ligand-Target Pair | |
Probable glutamate--tRNA ligase, mitochondrial
(Mus musculus (mouse)) | BDBM18122

((4S)-4-amino-5-[({[(2R,3S,4R,5R)-5-(6-amino-9H-pur...)Show SMILES N[C@@H](CCC(O)=O)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C15H21N7O9S/c16-6(1-2-8(23)24)14(27)21-32(28,29)30-3-7-10(25)11(26)15(31-7)22-5-20-9-12(17)18-4-19-13(9)22/h4-7,10-11,15,25-26H,1-3,16H2,(H,21,27)(H,23,24)(H2,17,18,19)/t6-,7+,10+,11+,15+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 70 | -41.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 30 |
CREFSIP
| Assay Description
The Km and Kappm for the amino acid substrate were first calculated from Lineweaver-Burk plots. The Ki values were calculated from the Kappm vs [I] p... |
J Enzyme Inhib Med Chem 20: 61-7 (2005)
Article DOI: 10.1080/14756360400002007
BindingDB Entry DOI: 10.7270/Q2X0659C |
More data for this
Ligand-Target Pair | |
Aspartate--tRNA ligase
(Escherichia coli) | BDBM18126
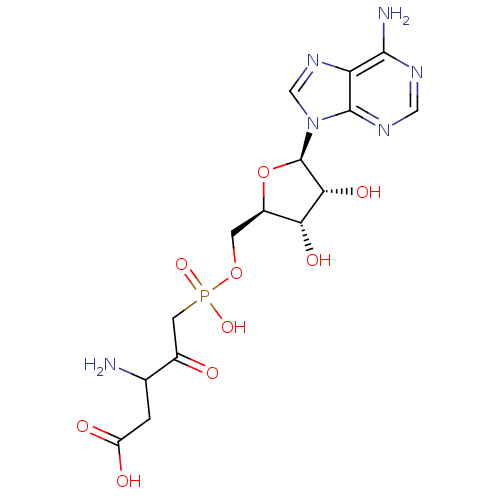
(3-amino-5-({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-y...)Show SMILES NC(CC(O)=O)C(=O)CP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C15H21N6O9P/c16-6(1-9(23)24)7(22)3-31(27,28)29-2-8-11(25)12(26)15(30-8)21-5-20-10-13(17)18-4-19-14(10)21/h4-6,8,11-12,15,25-26H,1-3,16H2,(H,23,24)(H,27,28)(H2,17,18,19)/t6?,8-,11-,12-,15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 123 | -41.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
CREFSIP
| Assay Description
The Km and Kappm for the amino acid substrate were first calculated from Lineweaver-Burk plots. The Ki values were calculated from the Kappm vs [I] p... |
Bioorg Med Chem 13: 69-75 (2005)
Article DOI: 10.1016/j.bmc.2004.09.055
BindingDB Entry DOI: 10.7270/Q2S75DM3 |
More data for this
Ligand-Target Pair | |
Glutamine--tRNA ligase
(Homo sapiens (Human)) | BDBM50366674

(CHEMBL609187)Show SMILES NC(CCC(N)=O)COP([O-])(=O)OC[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C15H24N7O8P/c16-7(1-2-9(17)23)3-28-31(26,27)29-4-8-11(24)12(25)15(30-8)22-6-21-10-13(18)19-5-20-14(10)22/h5-8,11-12,15,24-25H,1-4,16H2,(H2,17,23)(H,26,27)(H2,18,19,20)/p-1/t7?,8-,11-,12-,15?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval
Curated by ChEMBL
| Assay Description
The compound was evaluated for inhibition of Glutaminyl-tRNA synthetase with respect to glutamine. |
Bioorg Med Chem Lett 10: 2441-4 (2001)
BindingDB Entry DOI: 10.7270/Q280533K |
More data for this
Ligand-Target Pair | |
Glutamine--tRNA ligase
(Homo sapiens (Human)) | BDBM50366674

(CHEMBL609187)Show SMILES NC(CCC(N)=O)COP([O-])(=O)OC[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C15H24N7O8P/c16-7(1-2-9(17)23)3-28-31(26,27)29-4-8-11(24)12(25)15(30-8)22-6-21-10-13(18)19-5-20-14(10)22/h5-8,11-12,15,24-25H,1-4,16H2,(H2,17,23)(H,26,27)(H2,18,19,20)/p-1/t7?,8-,11-,12-,15?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval
Curated by ChEMBL
| Assay Description
The compound was evaluated for inhibition of Glutaminyl-tRNA synthetase for Escherichia coli with respect to ATP. |
Bioorg Med Chem Lett 10: 2441-4 (2001)
BindingDB Entry DOI: 10.7270/Q280533K |
More data for this
Ligand-Target Pair | |
Glutamine--tRNA ligase
(Homo sapiens (Human)) | BDBM50366675
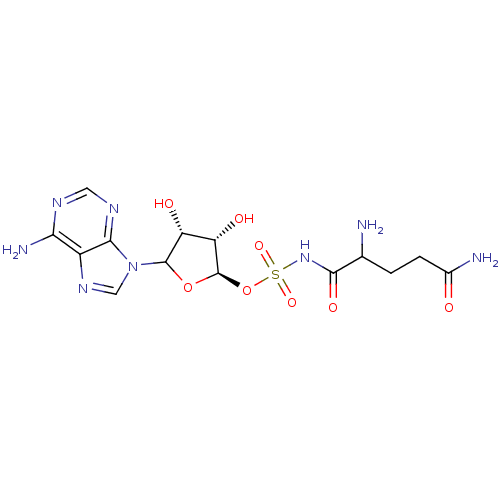
(CHEMBL609496)Show SMILES NC(CCC(N)=O)C(=O)NS(=O)(=O)O[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C14H20N8O8S/c15-5(1-2-6(16)23)12(26)21-31(27,28)30-14-9(25)8(24)13(29-14)22-4-20-7-10(17)18-3-19-11(7)22/h3-5,8-9,13-14,24-25H,1-2,15H2,(H2,16,23)(H,21,26)(H2,17,18,19)/t5?,8-,9+,13?,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval
Curated by ChEMBL
| Assay Description
The compound was evaluated for binding affinity to Glutaminyl-tRNA synthetase with respect to glutamine. |
Bioorg Med Chem Lett 10: 2441-4 (2001)
BindingDB Entry DOI: 10.7270/Q280533K |
More data for this
Ligand-Target Pair | |
Glutamine--tRNA ligase
(Homo sapiens (Human)) | BDBM50366673
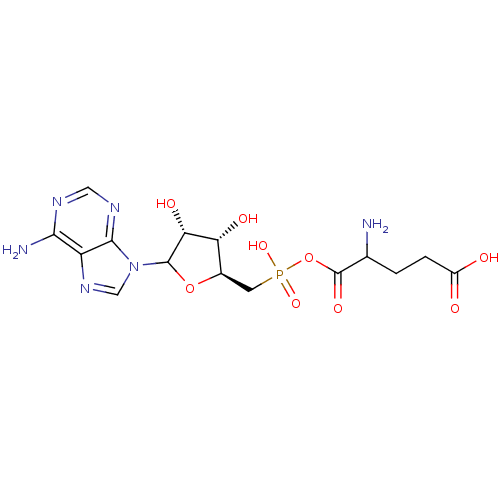
(CHEMBL608302)Show SMILES NC(CCC(O)=O)C(=O)OP(O)(=O)C[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C15H21N6O9P/c16-6(1-2-8(22)23)15(26)30-31(27,28)3-7-10(24)11(25)14(29-7)21-5-20-9-12(17)18-4-19-13(9)21/h4-7,10-11,14,24-25H,1-3,16H2,(H,22,23)(H,27,28)(H2,17,18,19)/t6?,7-,10-,11-,14?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval
Curated by ChEMBL
| Assay Description
The compound was evaluated for inhibition of Glutaminyl-tRNA synthetase with respect to glutamine. |
Bioorg Med Chem Lett 10: 2441-4 (2001)
BindingDB Entry DOI: 10.7270/Q280533K |
More data for this
Ligand-Target Pair | |
Glutamate--tRNA ligase
(Escherichia coli) | BDBM18123
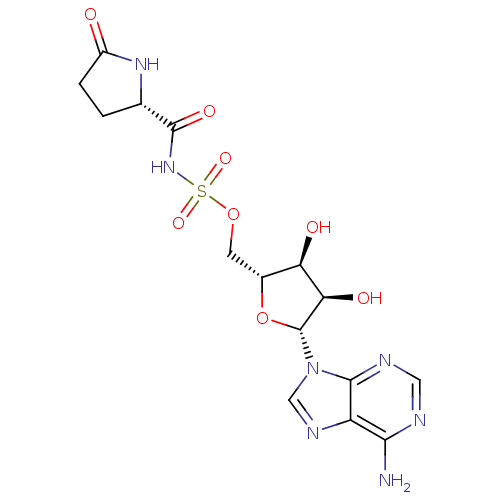
((5S)-5-{[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COS(=O)(=O)NC(=O)[C@@H]2CCC(=O)N2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H19N7O8S/c16-12-9-13(18-4-17-12)22(5-19-9)15-11(25)10(24)7(30-15)3-29-31(27,28)21-14(26)6-1-2-8(23)20-6/h4-7,10-11,15,24-25H,1-3H2,(H,20,23)(H,21,26)(H2,16,17,18)/t6-,7+,10+,11+,15+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+4 | -28.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
CREFSIP
| Assay Description
The Km and Kappm for the amino acid substrate were first calculated from Lineweaver-Burk plots. The Ki values were calculated from the Kappm vs [I] p... |
J Enzyme Inhib Med Chem 20: 61-7 (2005)
Article DOI: 10.1080/14756360400002007
BindingDB Entry DOI: 10.7270/Q2X0659C |
More data for this
Ligand-Target Pair | |
Glutamate--tRNA ligase
(Escherichia coli) | BDBM18118

((4S)-4-amino-6-({[(2R,3S,4R,5R)-5-(6-amino-9H-puri...)Show SMILES N[C@@H](CCC(O)=O)C(=O)CP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C16H23N6O9P/c17-7(1-2-10(24)25)8(23)4-32(28,29)30-3-9-12(26)13(27)16(31-9)22-6-21-11-14(18)19-5-20-15(11)22/h5-7,9,12-13,16,26-27H,1-4,17H2,(H,24,25)(H,28,29)(H2,18,19,20)/t7-,9+,12+,13+,16+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+4 | -28.2 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
CREFSIP
| Assay Description
The Km and Kappm for the amino acid substrate were first calculated from Lineweaver-Burk plots. The Ki values were calculated from the Kappm vs [I] p... |
Bioorg Med Chem 15: 295-304 (2007)
Article DOI: 10.1016/j.bmc.2006.09.056
BindingDB Entry DOI: 10.7270/Q21N7ZD3 |
More data for this
Ligand-Target Pair | |
Aspartate--tRNA ligase
(Escherichia coli) | BDBM18124
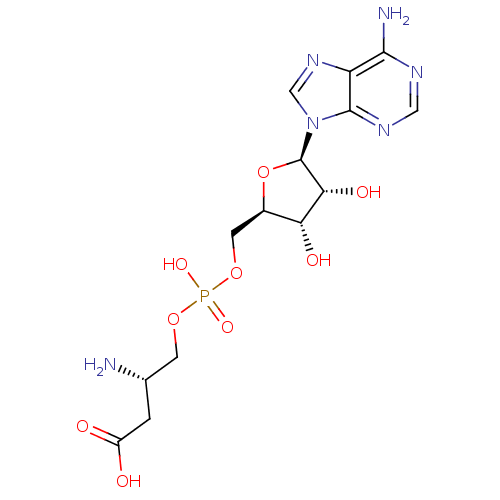
((3S)-3-amino-4-[({[(2R,3S,4R,5R)-5-(6-amino-9H-pur...)Show SMILES N[C@H](COP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)CC(O)=O |r| Show InChI InChI=1S/C14H21N6O9P/c15-6(1-8(21)22)2-27-30(25,26)28-3-7-10(23)11(24)14(29-7)20-5-19-9-12(16)17-4-18-13(9)20/h4-7,10-11,14,23-24H,1-3,15H2,(H,21,22)(H,25,26)(H2,16,17,18)/t6-,7+,10+,11+,14+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.50E+4 | -25.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
CREFSIP
| Assay Description
The Km and Kappm for the amino acid substrate were first calculated from Lineweaver-Burk plots. The Ki values were calculated from the Kappm vs [I] p... |
Bioorg Med Chem 13: 69-75 (2005)
Article DOI: 10.1016/j.bmc.2004.09.055
BindingDB Entry DOI: 10.7270/Q2S75DM3 |
More data for this
Ligand-Target Pair | |
Glutamine--tRNA ligase
(Escherichia coli) | BDBM18120

(Gln-KPA | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)...)Show SMILES N[C@@H](CCC(N)=O)C(=O)CP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C16H24N7O8P/c17-7(1-2-10(18)25)8(24)4-32(28,29)30-3-9-12(26)13(27)16(31-9)23-6-22-11-14(19)20-5-21-15(11)23/h5-7,9,12-13,16,26-27H,1-4,17H2,(H2,18,25)(H,28,29)(H2,19,20,21)/p-1/t7-,9+,12+,13+,16+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.50E+5 | -18.9 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
CREFSIP
| Assay Description
The Km and Kappm for the amino acid substrate were first calculated from Lineweaver-Burk plots. The Ki values were calculated from the Kappm vs [I] p... |
Bioorg Med Chem 15: 295-304 (2007)
Article DOI: 10.1016/j.bmc.2006.09.056
BindingDB Entry DOI: 10.7270/Q21N7ZD3 |
More data for this
Ligand-Target Pair | |
Glutamine--tRNA ligase
(Bos taurus (bovine)) | BDBM18118

((4S)-4-amino-6-({[(2R,3S,4R,5R)-5-(6-amino-9H-puri...)Show SMILES N[C@@H](CCC(O)=O)C(=O)CP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C16H23N6O9P/c17-7(1-2-10(24)25)8(23)4-32(28,29)30-3-9-12(26)13(27)16(31-9)22-6-21-11-14(18)19-5-20-15(11)22/h5-7,9,12-13,16,26-27H,1-4,17H2,(H,24,25)(H,28,29)(H2,18,19,20)/t7-,9+,12+,13+,16+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60E+6 | -15.3 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
CREFSIP
| Assay Description
The Km and Kappm for the amino acid substrate were first calculated from Lineweaver-Burk plots. The Ki values were calculated from the Kappm vs [I] p... |
Bioorg Med Chem 15: 295-304 (2007)
Article DOI: 10.1016/j.bmc.2006.09.056
BindingDB Entry DOI: 10.7270/Q21N7ZD3 |
More data for this
Ligand-Target Pair | |
Glutamine--tRNA ligase
(Escherichia coli) | BDBM18118

((4S)-4-amino-6-({[(2R,3S,4R,5R)-5-(6-amino-9H-puri...)Show SMILES N[C@@H](CCC(O)=O)C(=O)CP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C16H23N6O9P/c17-7(1-2-10(24)25)8(23)4-32(28,29)30-3-9-12(26)13(27)16(31-9)22-6-21-11-14(18)19-5-20-15(11)22/h5-7,9,12-13,16,26-27H,1-4,17H2,(H,24,25)(H,28,29)(H2,18,19,20)/t7-,9+,12+,13+,16+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+6 | -15.2 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
CREFSIP
| Assay Description
The Km and Kappm for the amino acid substrate were first calculated from Lineweaver-Burk plots. The Ki values were calculated from the Kappm vs [I] p... |
Bioorg Med Chem 15: 295-304 (2007)
Article DOI: 10.1016/j.bmc.2006.09.056
BindingDB Entry DOI: 10.7270/Q21N7ZD3 |
More data for this
Ligand-Target Pair | |
Glutamate--tRNA ligase
(Escherichia coli) | BDBM18120

(Gln-KPA | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)...)Show SMILES N[C@@H](CCC(N)=O)C(=O)CP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C16H24N7O8P/c17-7(1-2-10(18)25)8(24)4-32(28,29)30-3-9-12(26)13(27)16(31-9)23-6-22-11-14(19)20-5-21-15(11)23/h5-7,9,12-13,16,26-27H,1-4,17H2,(H2,18,25)(H,28,29)(H2,19,20,21)/p-1/t7-,9+,12+,13+,16+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90E+6 | -15.1 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
CREFSIP
| Assay Description
The Km and Kappm for the amino acid substrate were first calculated from Lineweaver-Burk plots. The Ki values were calculated from the Kappm vs [I] p... |
Bioorg Med Chem 15: 295-304 (2007)
Article DOI: 10.1016/j.bmc.2006.09.056
BindingDB Entry DOI: 10.7270/Q21N7ZD3 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50277149
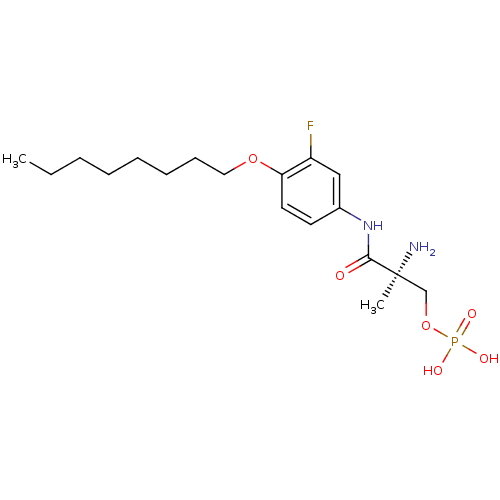
((S)-2-amino-3-(3-fluoro-4-(octyloxy)phenylamino)-2...)Show SMILES CCCCCCCCOc1ccc(NC(=O)[C@@](C)(N)COP(O)(O)=O)cc1F |r| Show InChI InChI=1S/C18H30FN2O6P/c1-3-4-5-6-7-8-11-26-16-10-9-14(12-15(16)19)21-17(22)18(2,20)13-27-28(23,24)25/h9-10,12H,3-8,11,13,20H2,1-2H3,(H,21,22)(H2,23,24,25)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50249294

((R)-2-amino-2-(4-(4-(5-phenylpentyloxy)phenyl)-1H-...)Show SMILES C[C@](N)(COP(O)(O)=O)c1nc(c[nH]1)-c1ccc(OCCCCCc2ccccc2)cc1 |r| Show InChI InChI=1S/C23H30N3O5P/c1-23(24,17-31-32(27,28)29)22-25-16-21(26-22)19-11-13-20(14-12-19)30-15-7-3-6-10-18-8-4-2-5-9-18/h2,4-5,8-9,11-14,16H,3,6-7,10,15,17,24H2,1H3,(H,25,26)(H2,27,28,29)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50249114
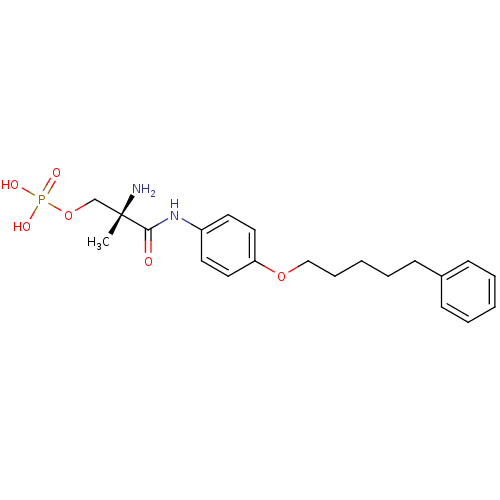
((S)-2-amino-2-methyl-3-oxo-3-(4-(5-phenylpentyloxy...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCCCCCc2ccccc2)cc1 |r| Show InChI InChI=1S/C21H29N2O6P/c1-21(22,16-29-30(25,26)27)20(24)23-18-11-13-19(14-12-18)28-15-7-3-6-10-17-8-4-2-5-9-17/h2,4-5,8-9,11-14H,3,6-7,10,15-16,22H2,1H3,(H,23,24)(H2,25,26,27)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50315562
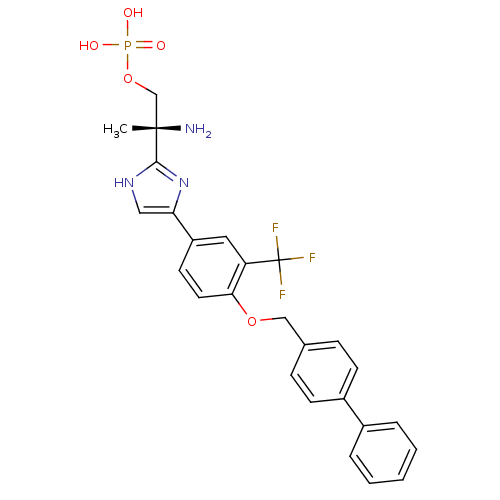
((R)-2-amino-2-(4-(4-(biphenyl-4-ylmethoxy)-3-(trif...)Show SMILES C[C@](N)(COP(O)(O)=O)c1nc(c[nH]1)-c1ccc(OCc2ccc(cc2)-c2ccccc2)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C26H25F3N3O5P/c1-25(30,16-37-38(33,34)35)24-31-14-22(32-24)20-11-12-23(21(13-20)26(27,28)29)36-15-17-7-9-19(10-8-17)18-5-3-2-4-6-18/h2-14H,15-16,30H2,1H3,(H,31,32)(H2,33,34,35)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 20: 2520-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.098
BindingDB Entry DOI: 10.7270/Q2XS5VJB |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50315559
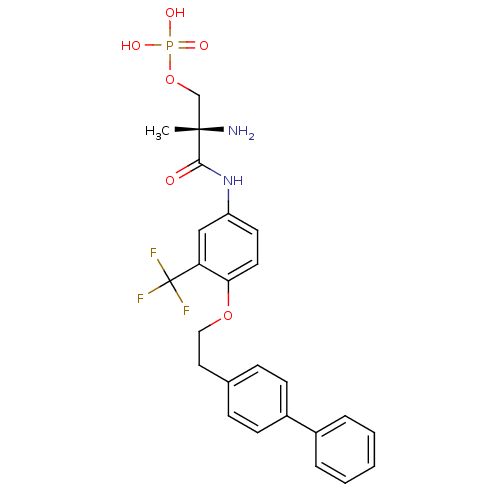
((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-(trif...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCCc2ccc(cc2)-c2ccccc2)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C25H26F3N2O6P/c1-24(29,16-36-37(32,33)34)23(31)30-20-11-12-22(21(15-20)25(26,27)28)35-14-13-17-7-9-19(10-8-17)18-5-3-2-4-6-18/h2-12,15H,13-14,16,29H2,1H3,(H,30,31)(H2,32,33,34)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 20: 2520-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.098
BindingDB Entry DOI: 10.7270/Q2XS5VJB |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50277186
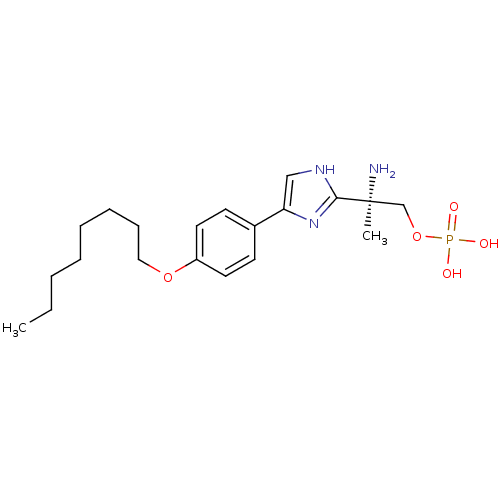
((R)-2-amino-2-(4-(4-(octyloxy)phenyl)-1H-imidazol-...)Show SMILES CCCCCCCCOc1ccc(cc1)-c1c[nH]c(n1)[C@@](C)(N)COP(O)(O)=O |r| Show InChI InChI=1S/C20H32N3O5P/c1-3-4-5-6-7-8-13-27-17-11-9-16(10-12-17)18-14-22-19(23-18)20(2,21)15-28-29(24,25)26/h9-12,14H,3-8,13,15,21H2,1-2H3,(H,22,23)(H2,24,25,26)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50277185
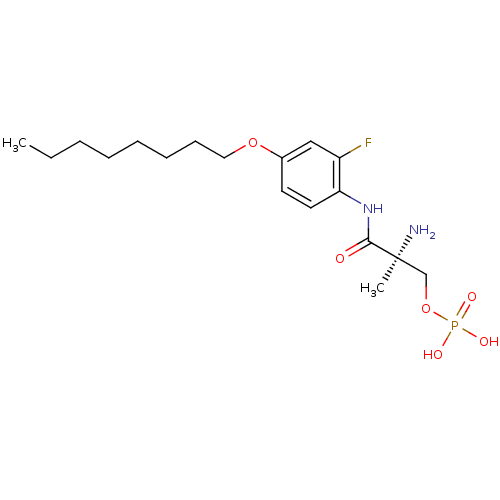
((S)-2-amino-3-(2-fluoro-4-(octyloxy)phenylamino)-2...)Show SMILES CCCCCCCCOc1ccc(NC(=O)[C@@](C)(N)COP(O)(O)=O)c(F)c1 |r| Show InChI InChI=1S/C18H30FN2O6P/c1-3-4-5-6-7-8-11-26-14-9-10-16(15(19)12-14)21-17(22)18(2,20)13-27-28(23,24)25/h9-10,12H,3-8,11,13,20H2,1-2H3,(H,21,22)(H2,23,24,25)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50277187
((R)-2-amino-2-(4-(3-fluoro-4-(octyloxy)phenyl)-1H-...)Show SMILES CCCCCCCCOc1ccc(cc1F)-c1c[nH]c(n1)[C@@](C)(N)COP(O)(O)=O |r| Show InChI InChI=1S/C20H31FN3O5P/c1-3-4-5-6-7-8-11-28-18-10-9-15(12-16(18)21)17-13-23-19(24-17)20(2,22)14-29-30(25,26)27/h9-10,12-13H,3-8,11,14,22H2,1-2H3,(H,23,24)(H2,25,26,27)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50249114

((S)-2-amino-2-methyl-3-oxo-3-(4-(5-phenylpentyloxy...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCCCCCc2ccccc2)cc1 |r| Show InChI InChI=1S/C21H29N2O6P/c1-21(22,16-29-30(25,26)27)20(24)23-18-11-13-19(14-12-18)28-15-7-3-6-10-17-8-4-2-5-9-17/h2,4-5,8-9,11-14H,3,6-7,10,15-16,22H2,1H3,(H,23,24)(H2,25,26,27)/t21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P3 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50158348

((2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihy...)Show SMILES CCCCCCCCCCCCC\C=C\[C@@H](O)[C@@H](N)COP(O)(O)=O |r| Show InChI InChI=1S/C18H38NO5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(20)17(19)16-24-25(21,22)23/h14-15,17-18,20H,2-13,16,19H2,1H3,(H2,21,22,23)/b15-14+/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50158348

((2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihy...)Show SMILES CCCCCCCCCCCCC\C=C\[C@@H](O)[C@@H](N)COP(O)(O)=O |r| Show InChI InChI=1S/C18H38NO5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(20)17(19)16-24-25(21,22)23/h14-15,17-18,20H,2-13,16,19H2,1H3,(H2,21,22,23)/b15-14+/t17-,18+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P3 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50277148
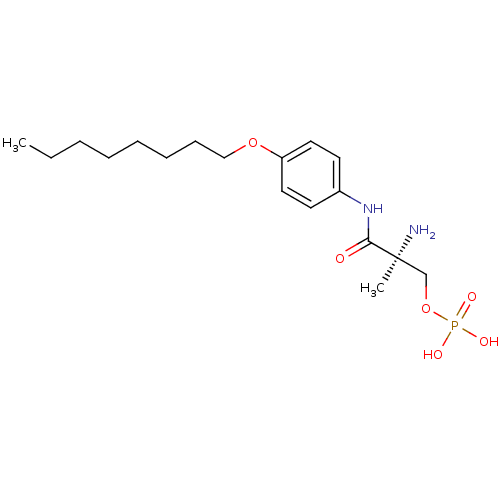
((S)-2-amino-2-methyl-3-(4-(octyloxy)phenylamino)-3...)Show SMILES CCCCCCCCOc1ccc(NC(=O)[C@@](C)(N)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C18H31N2O6P/c1-3-4-5-6-7-8-13-25-16-11-9-15(10-12-16)20-17(21)18(2,19)14-26-27(22,23)24/h9-12H,3-8,13-14,19H2,1-2H3,(H,20,21)(H2,22,23,24)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50249259
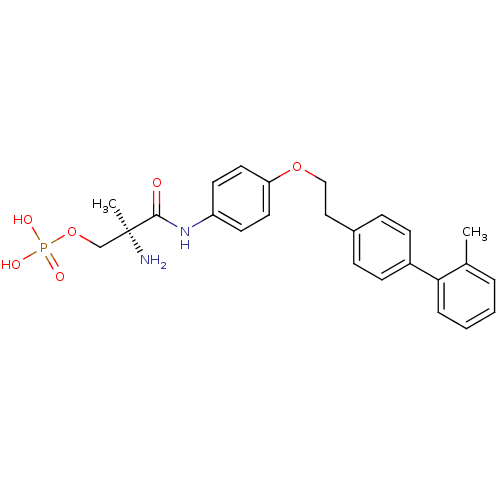
((S)-2-amino-2-methyl-3-(4-(2-(2'-methylbiphenyl-4-...)Show SMILES Cc1ccccc1-c1ccc(CCOc2ccc(NC(=O)[C@@](C)(N)COP(O)(O)=O)cc2)cc1 |r| Show InChI InChI=1S/C25H29N2O6P/c1-18-5-3-4-6-23(18)20-9-7-19(8-10-20)15-16-32-22-13-11-21(12-14-22)27-24(28)25(2,26)17-33-34(29,30)31/h3-14H,15-17,26H2,1-2H3,(H,27,28)(H2,29,30,31)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
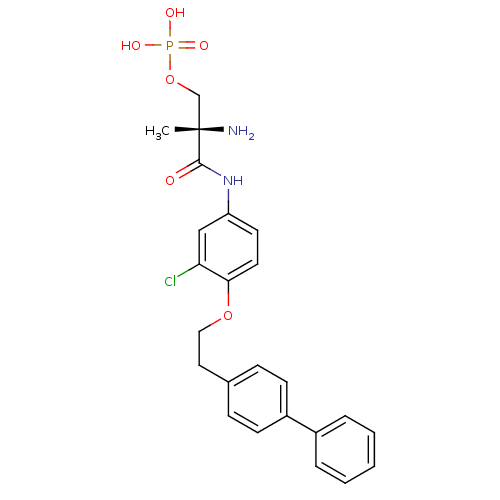
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50315556
((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-chlor...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCCc2ccc(cc2)-c2ccccc2)c(Cl)c1 |r| Show InChI InChI=1S/C24H26ClN2O6P/c1-24(26,16-33-34(29,30)31)23(28)27-20-11-12-22(21(25)15-20)32-14-13-17-7-9-19(10-8-17)18-5-3-2-4-6-18/h2-12,15H,13-14,16,26H2,1H3,(H,27,28)(H2,29,30,31)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 20: 2520-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.098
BindingDB Entry DOI: 10.7270/Q2XS5VJB |
More data for this
Ligand-Target Pair | |
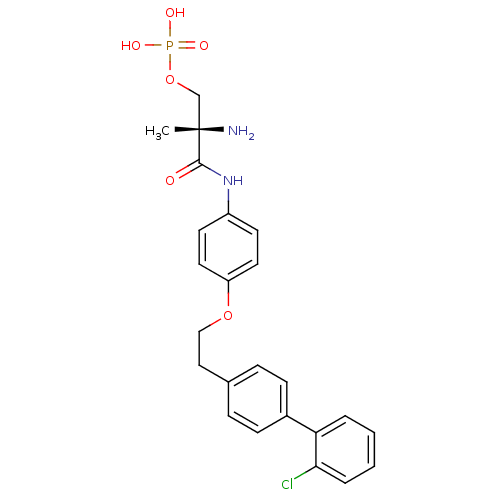
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50249260
((S)-2-amino-3-(4-(2-(2'-chlorobiphenyl-4-yl)ethoxy...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCCc2ccc(cc2)-c2ccccc2Cl)cc1 |r| Show InChI InChI=1S/C24H26ClN2O6P/c1-24(26,16-33-34(29,30)31)23(28)27-19-10-12-20(13-11-19)32-15-14-17-6-8-18(9-7-17)21-4-2-3-5-22(21)25/h2-13H,14-16,26H2,1H3,(H,27,28)(H2,29,30,31)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
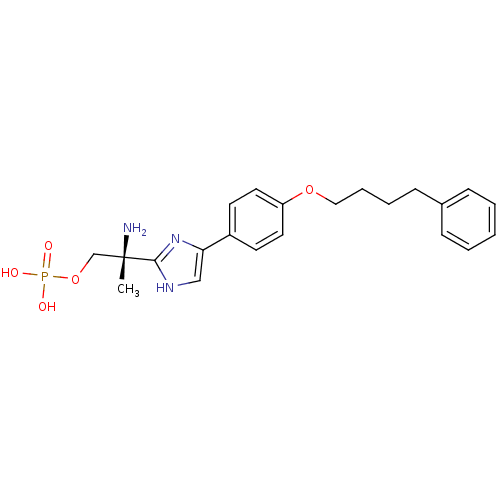
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50249293
((R)-2-amino-2-(4-(4-(4-phenylbutoxy)phenyl)-1H-imi...)Show SMILES C[C@](N)(COP(O)(O)=O)c1nc(c[nH]1)-c1ccc(OCCCCc2ccccc2)cc1 |r| Show InChI InChI=1S/C22H28N3O5P/c1-22(23,16-30-31(26,27)28)21-24-15-20(25-21)18-10-12-19(13-11-18)29-14-6-5-9-17-7-3-2-4-8-17/h2-4,7-8,10-13,15H,5-6,9,14,16,23H2,1H3,(H,24,25)(H2,26,27,28)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
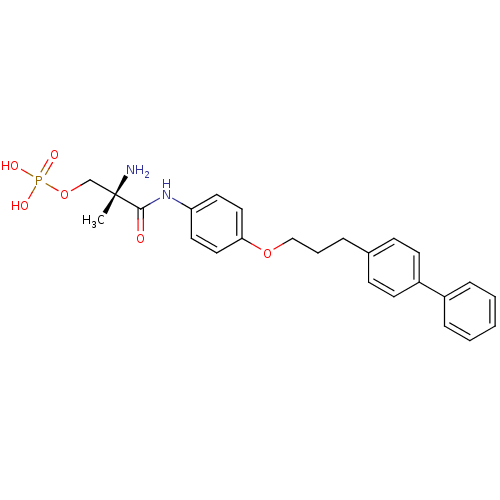
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50249263
((S)-2-amino-3-(4-(3-(biphenyl-4-yl)propoxy)phenyla...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCCCc2ccc(cc2)-c2ccccc2)cc1 |r| Show InChI InChI=1S/C25H29N2O6P/c1-25(26,18-33-34(29,30)31)24(28)27-22-13-15-23(16-14-22)32-17-5-6-19-9-11-21(12-10-19)20-7-3-2-4-8-20/h2-4,7-16H,5-6,17-18,26H2,1H3,(H,27,28)(H2,29,30,31)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50249266
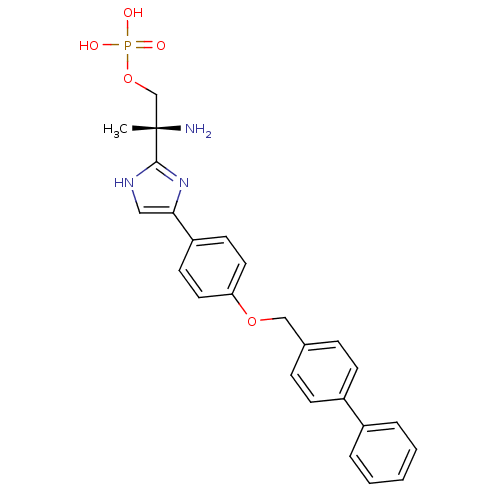
((R)-2-amino-2-(4-(4-(biphenyl-4-ylmethoxy)phenyl)-...)Show SMILES C[C@](N)(COP(O)(O)=O)c1nc(c[nH]1)-c1ccc(OCc2ccc(cc2)-c2ccccc2)cc1 |r| Show InChI InChI=1S/C25H26N3O5P/c1-25(26,17-33-34(29,30)31)24-27-15-23(28-24)21-11-13-22(14-12-21)32-16-18-7-9-20(10-8-18)19-5-3-2-4-6-19/h2-15H,16-17,26H2,1H3,(H,27,28)(H2,29,30,31)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.03 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50249266

((R)-2-amino-2-(4-(4-(biphenyl-4-ylmethoxy)phenyl)-...)Show SMILES C[C@](N)(COP(O)(O)=O)c1nc(c[nH]1)-c1ccc(OCc2ccc(cc2)-c2ccccc2)cc1 |r| Show InChI InChI=1S/C25H26N3O5P/c1-25(26,17-33-34(29,30)31)24-27-15-23(28-24)21-11-13-22(14-12-21)32-16-18-7-9-20(10-8-18)19-5-3-2-4-6-19/h2-15H,16-17,26H2,1H3,(H,27,28)(H2,29,30,31)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.03 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 20: 2520-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.098
BindingDB Entry DOI: 10.7270/Q2XS5VJB |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50249240
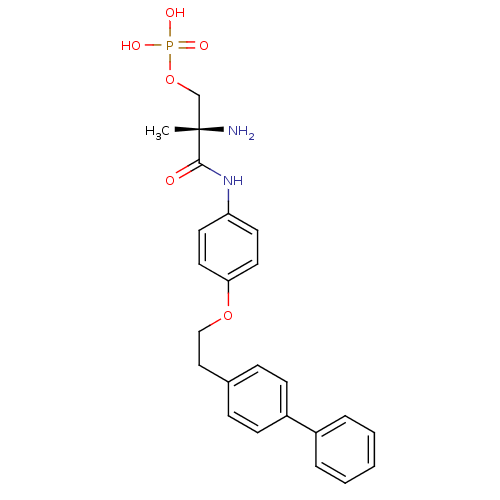
((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)phenylam...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCCc2ccc(cc2)-c2ccccc2)cc1 |r| Show InChI InChI=1S/C24H27N2O6P/c1-24(25,17-32-33(28,29)30)23(27)26-21-11-13-22(14-12-21)31-16-15-18-7-9-20(10-8-18)19-5-3-2-4-6-19/h2-14H,15-17,25H2,1H3,(H,26,27)(H2,28,29,30)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.05 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50249240

((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)phenylam...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCCc2ccc(cc2)-c2ccccc2)cc1 |r| Show InChI InChI=1S/C24H27N2O6P/c1-24(25,17-32-33(28,29)30)23(27)26-21-11-13-22(14-12-21)31-16-15-18-7-9-20(10-8-18)19-5-3-2-4-6-19/h2-14H,15-17,25H2,1H3,(H,26,27)(H2,28,29,30)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.05 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 20: 2520-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.098
BindingDB Entry DOI: 10.7270/Q2XS5VJB |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
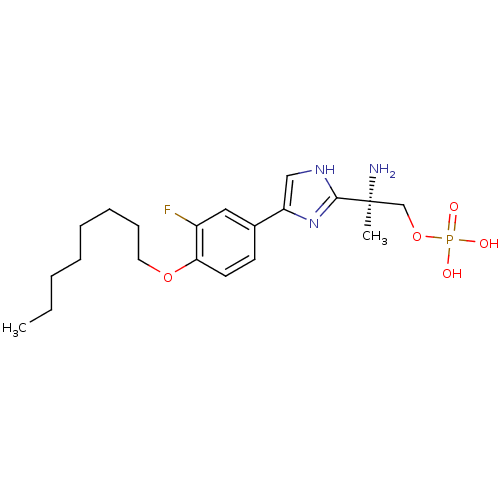
(Homo sapiens (Human)) | BDBM50277187

((R)-2-amino-2-(4-(3-fluoro-4-(octyloxy)phenyl)-1H-...)Show SMILES CCCCCCCCOc1ccc(cc1F)-c1c[nH]c(n1)[C@@](C)(N)COP(O)(O)=O |r| Show InChI InChI=1S/C20H31FN3O5P/c1-3-4-5-6-7-8-11-28-18-10-9-15(12-16(18)21)17-13-23-19(24-17)20(2,22)14-29-30(25,26)27/h9-10,12-13H,3-8,11,14,22H2,1-2H3,(H,23,24)(H2,25,26,27)/t20-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P5 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 4
(Homo sapiens (Human)) | BDBM50277186

((R)-2-amino-2-(4-(4-(octyloxy)phenyl)-1H-imidazol-...)Show SMILES CCCCCCCCOc1ccc(cc1)-c1c[nH]c(n1)[C@@](C)(N)COP(O)(O)=O |r| Show InChI InChI=1S/C20H32N3O5P/c1-3-4-5-6-7-8-13-27-17-11-9-16(10-12-17)18-14-22-19(23-18)20(2,21)15-28-29(24,25)26/h9-12,14H,3-8,13,15,21H2,1-2H3,(H,22,23)(H2,24,25,26)/t20-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P4 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50158348

((2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihy...)Show SMILES CCCCCCCCCCCCC\C=C\[C@@H](O)[C@@H](N)COP(O)(O)=O |r| Show InChI InChI=1S/C18H38NO5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(20)17(19)16-24-25(21,22)23/h14-15,17-18,20H,2-13,16,19H2,1H3,(H2,21,22,23)/b15-14+/t17-,18+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P5 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50249153

((S)-2-amino-3-(4-(4-cyclohexylbutoxy)phenylamino)-...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCCCCC2CCCCC2)cc1 |r| Show InChI InChI=1S/C20H33N2O6P/c1-20(21,15-28-29(24,25)26)19(23)22-17-10-12-18(13-11-17)27-14-6-5-9-16-7-3-2-4-8-16/h10-13,16H,2-9,14-15,21H2,1H3,(H,22,23)(H2,24,25,26)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 4
(Homo sapiens (Human)) | BDBM50249294

((R)-2-amino-2-(4-(4-(5-phenylpentyloxy)phenyl)-1H-...)Show SMILES C[C@](N)(COP(O)(O)=O)c1nc(c[nH]1)-c1ccc(OCCCCCc2ccccc2)cc1 |r| Show InChI InChI=1S/C23H30N3O5P/c1-23(24,17-31-32(27,28)29)22-25-16-21(26-22)19-11-13-20(14-12-19)30-15-7-3-6-10-18-8-4-2-5-9-18/h2,4-5,8-9,11-14,16H,3,6-7,10,15,17,24H2,1H3,(H,25,26)(H2,27,28,29)/t23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.23 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P4 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50315557
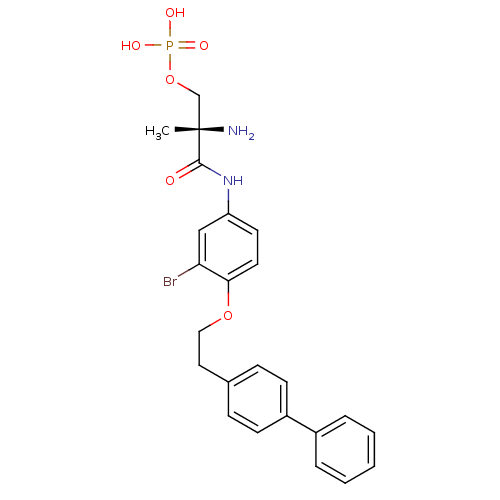
((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-bromo...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCCc2ccc(cc2)-c2ccccc2)c(Br)c1 |r| Show InChI InChI=1S/C24H26BrN2O6P/c1-24(26,16-33-34(29,30)31)23(28)27-20-11-12-22(21(25)15-20)32-14-13-17-7-9-19(10-8-17)18-5-3-2-4-6-18/h2-12,15H,13-14,16,26H2,1H3,(H,27,28)(H2,29,30,31)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 20: 2520-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.098
BindingDB Entry DOI: 10.7270/Q2XS5VJB |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50277186

((R)-2-amino-2-(4-(4-(octyloxy)phenyl)-1H-imidazol-...)Show SMILES CCCCCCCCOc1ccc(cc1)-c1c[nH]c(n1)[C@@](C)(N)COP(O)(O)=O |r| Show InChI InChI=1S/C20H32N3O5P/c1-3-4-5-6-7-8-13-27-17-11-9-16(10-12-17)18-14-22-19(23-18)20(2,21)15-28-29(24,25)26/h9-12,14H,3-8,13,15,21H2,1-2H3,(H,22,23)(H2,24,25,26)/t20-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P5 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 4
(Homo sapiens (Human)) | BDBM50277185

((S)-2-amino-3-(2-fluoro-4-(octyloxy)phenylamino)-2...)Show SMILES CCCCCCCCOc1ccc(NC(=O)[C@@](C)(N)COP(O)(O)=O)c(F)c1 |r| Show InChI InChI=1S/C18H30FN2O6P/c1-3-4-5-6-7-8-11-26-14-9-10-16(15(19)12-14)21-17(22)18(2,20)13-27-28(23,24)25/h9-10,12H,3-8,11,13,20H2,1-2H3,(H,21,22)(H2,23,24,25)/t18-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P4 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 4
(Homo sapiens (Human)) | BDBM50277187

((R)-2-amino-2-(4-(3-fluoro-4-(octyloxy)phenyl)-1H-...)Show SMILES CCCCCCCCOc1ccc(cc1F)-c1c[nH]c(n1)[C@@](C)(N)COP(O)(O)=O |r| Show InChI InChI=1S/C20H31FN3O5P/c1-3-4-5-6-7-8-11-28-18-10-9-15(12-16(18)21)17-13-23-19(24-17)20(2,22)14-29-30(25,26)27/h9-10,12-13H,3-8,11,14,22H2,1-2H3,(H,23,24)(H2,25,26,27)/t20-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P4 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50249113
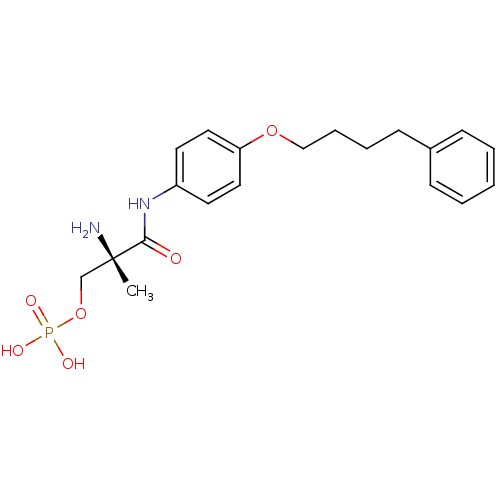
((S)-2-amino-2-methyl-3-oxo-3-(4-(4-phenylbutoxy)ph...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCCCCc2ccccc2)cc1 |r| Show InChI InChI=1S/C20H27N2O6P/c1-20(21,15-28-29(24,25)26)19(23)22-17-10-12-18(13-11-17)27-14-6-5-9-16-7-3-2-4-8-16/h2-4,7-8,10-13H,5-6,9,14-15,21H2,1H3,(H,22,23)(H2,24,25,26)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 4
(Homo sapiens (Human)) | BDBM50158348

((2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihy...)Show SMILES CCCCCCCCCCCCC\C=C\[C@@H](O)[C@@H](N)COP(O)(O)=O |r| Show InChI InChI=1S/C18H38NO5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(20)17(19)16-24-25(21,22)23/h14-15,17-18,20H,2-13,16,19H2,1H3,(H2,21,22,23)/b15-14+/t17-,18+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P4 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50249154
((S)-2-amino-3-(4-(biphenyl-4-ylmethoxy)phenylamino...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCc2ccc(cc2)-c2ccccc2)cc1 |r| Show InChI InChI=1S/C23H25N2O6P/c1-23(24,16-31-32(27,28)29)22(26)25-20-11-13-21(14-12-20)30-15-17-7-9-19(10-8-17)18-5-3-2-4-6-18/h2-14H,15-16,24H2,1H3,(H,25,26)(H2,27,28,29)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50249114

((S)-2-amino-2-methyl-3-oxo-3-(4-(5-phenylpentyloxy...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCCCCCc2ccccc2)cc1 |r| Show InChI InChI=1S/C21H29N2O6P/c1-21(22,16-29-30(25,26)27)20(24)23-18-11-13-19(14-12-18)28-15-7-3-6-10-17-8-4-2-5-9-17/h2,4-5,8-9,11-14H,3,6-7,10,15-16,22H2,1H3,(H,23,24)(H2,25,26,27)/t21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P5 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50277185

((S)-2-amino-3-(2-fluoro-4-(octyloxy)phenylamino)-2...)Show SMILES CCCCCCCCOc1ccc(NC(=O)[C@@](C)(N)COP(O)(O)=O)c(F)c1 |r| Show InChI InChI=1S/C18H30FN2O6P/c1-3-4-5-6-7-8-11-26-14-9-10-16(15(19)12-14)21-17(22)18(2,20)13-27-28(23,24)25/h9-10,12H,3-8,11,13,20H2,1-2H3,(H,21,22)(H2,23,24,25)/t18-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P5 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data