 Found 406 hits with Last Name = 'bleasby' and Initial = 'k'
Found 406 hits with Last Name = 'bleasby' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382899

(CHEMBL2022787)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(C)c(Cl)c1 |r| Show InChI InChI=1S/C31H37ClF2N4O/c1-19-6-8-23(16-27(19)32)38-29(14-20(2)35-38)21-10-12-36(13-11-21)30(39)26-18-37(31(3,4)5)17-25(26)24-9-7-22(33)15-28(24)34/h6-9,14-16,21,25-26H,10-13,17-18H2,1-5H3/t25-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382904
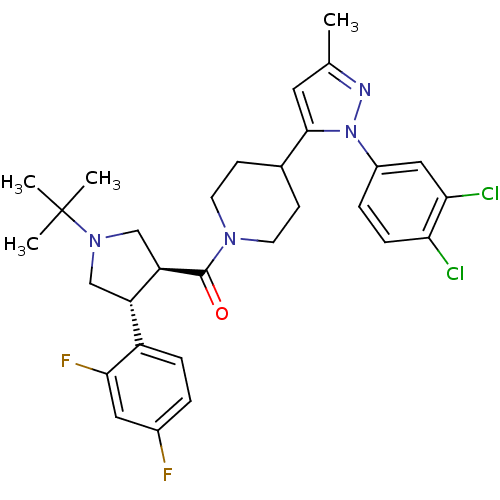
(CHEMBL2022793)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-28(38(35-18)21-6-8-25(31)26(32)15-21)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-5-20(33)14-27(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50383421
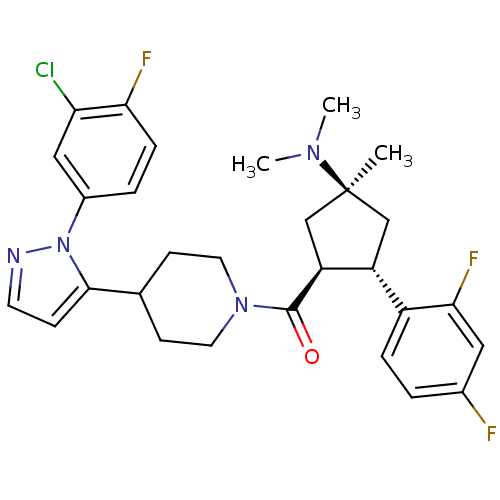
(CHEMBL2031595)Show SMILES CN(C)[C@]1(C)C[C@@H]([C@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1ccnn1-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C29H32ClF3N4O/c1-29(35(2)3)16-22(21-6-4-19(31)14-26(21)33)23(17-29)28(38)36-12-9-18(10-13-36)27-8-11-34-37(27)20-5-7-25(32)24(30)15-20/h4-8,11,14-15,18,22-23H,9-10,12-13,16-17H2,1-3H3/t22-,23+,29+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382913
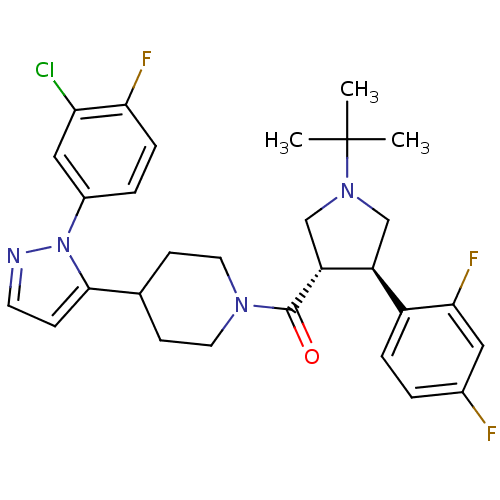
(CHEMBL2023210)Show SMILES CC(C)(C)N1C[C@H]([C@@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1ccnn1-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C29H32ClF3N4O/c1-29(2,3)36-16-22(21-6-4-19(31)14-26(21)33)23(17-36)28(38)35-12-9-18(10-13-35)27-8-11-34-37(27)20-5-7-25(32)24(30)15-20/h4-8,11,14-15,18,22-23H,9-10,12-13,16-17H2,1-3H3/t22-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50382913

(CHEMBL2023210)Show SMILES CC(C)(C)N1C[C@H]([C@@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1ccnn1-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C29H32ClF3N4O/c1-29(2,3)36-16-22(21-6-4-19(31)14-26(21)33)23(17-36)28(38)35-12-9-18(10-13-35)27-8-11-34-37(27)20-5-7-25(32)24(30)15-20/h4-8,11,14-15,18,22-23H,9-10,12-13,16-17H2,1-3H3/t22-,23+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50361786
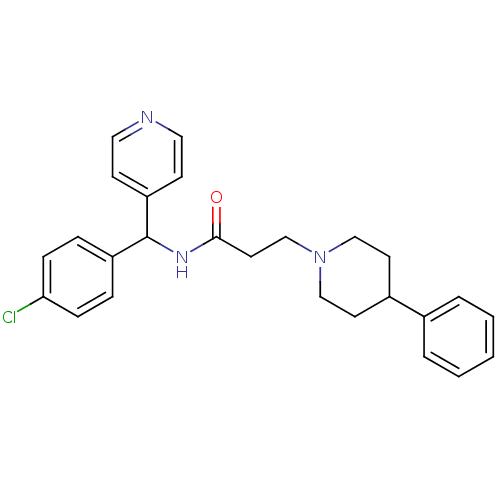
(CHEMBL1938522)Show SMILES Clc1ccc(cc1)C(NC(=O)CCN1CCC(CC1)c1ccccc1)c1ccncc1 Show InChI InChI=1S/C26H28ClN3O/c27-24-8-6-22(7-9-24)26(23-10-15-28-16-11-23)29-25(31)14-19-30-17-12-21(13-18-30)20-4-2-1-3-5-20/h1-11,15-16,21,26H,12-14,17-19H2,(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by continuous fluorometric assay |
Bioorg Med Chem Lett 22: 658-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.060
BindingDB Entry DOI: 10.7270/Q2B56K50 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382906

(CHEMBL2023202)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-27(38(35-18)28-14-20(31)5-8-25(28)32)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-6-21(33)15-26(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382906

(CHEMBL2023202)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-27(38(35-18)28-14-20(31)5-8-25(28)32)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-6-21(33)15-26(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50382906

(CHEMBL2023202)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-27(38(35-18)28-14-20(31)5-8-25(28)32)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-6-21(33)15-26(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50361774
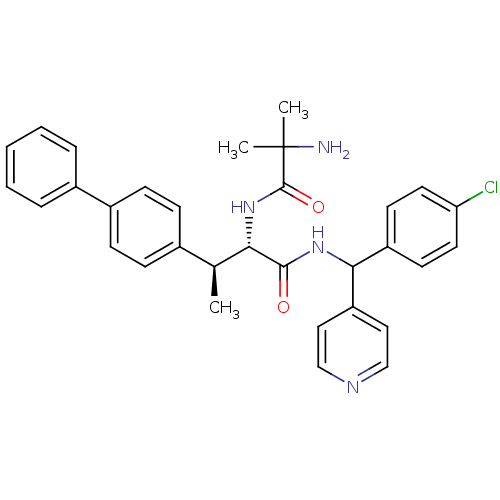
(CHEMBL1938510)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)N)C(=O)NC(c1ccncc1)c1ccc(Cl)cc1)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C32H33ClN4O2/c1-21(22-9-11-24(12-10-22)23-7-5-4-6-8-23)28(37-31(39)32(2,3)34)30(38)36-29(26-17-19-35-20-18-26)25-13-15-27(33)16-14-25/h4-21,28-29H,34H2,1-3H3,(H,36,38)(H,37,39)/t21-,28-,29?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mouse PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by continuous fluorometric assay |
Bioorg Med Chem Lett 22: 658-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.060
BindingDB Entry DOI: 10.7270/Q2B56K50 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50383427
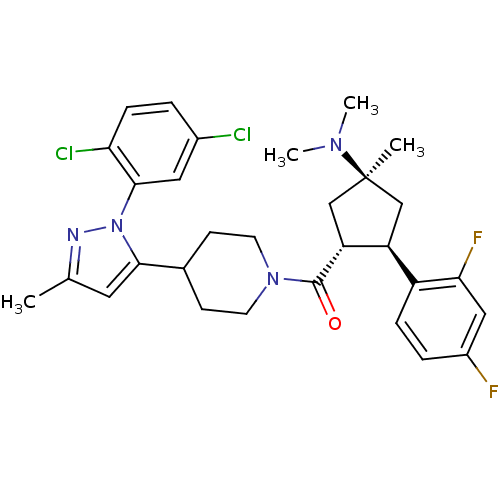
(CHEMBL2031588)Show SMILES CN(C)[C@]1(C)C[C@H]([C@@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1cc(C)nn1-c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-27(38(35-18)28-14-20(31)5-8-25(28)32)19-9-11-37(12-10-19)29(39)24-17-30(2,36(3)4)16-23(24)22-7-6-21(33)15-26(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+,30-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50383421

(CHEMBL2031595)Show SMILES CN(C)[C@]1(C)C[C@@H]([C@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1ccnn1-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C29H32ClF3N4O/c1-29(35(2)3)16-22(21-6-4-19(31)14-26(21)33)23(17-29)28(38)36-12-9-18(10-13-36)27-8-11-34-37(27)20-5-7-25(32)24(30)15-20/h4-8,11,14-15,18,22-23H,9-10,12-13,16-17H2,1-3H3/t22-,23+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50382906

(CHEMBL2023202)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-27(38(35-18)28-14-20(31)5-8-25(28)32)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-6-21(33)15-26(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382905

(CHEMBL2022794)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-28(38(35-18)27-8-5-20(31)14-25(27)32)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-6-21(33)15-26(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382912

(CHEMBL2023209 | US8569299, 4)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C30H34ClF3N4O/c1-18-13-28(38(35-18)21-6-8-26(33)25(31)15-21)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-5-20(32)14-27(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382910
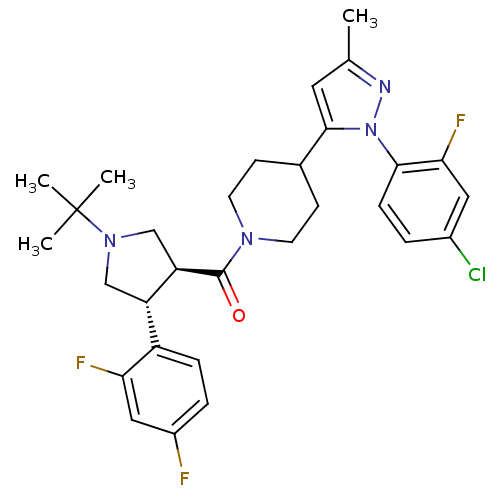
(CHEMBL2023207)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(Cl)cc1F |r| Show InChI InChI=1S/C30H34ClF3N4O/c1-18-13-28(38(35-18)27-8-5-20(31)14-26(27)34)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-6-21(32)15-25(22)33/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50383425
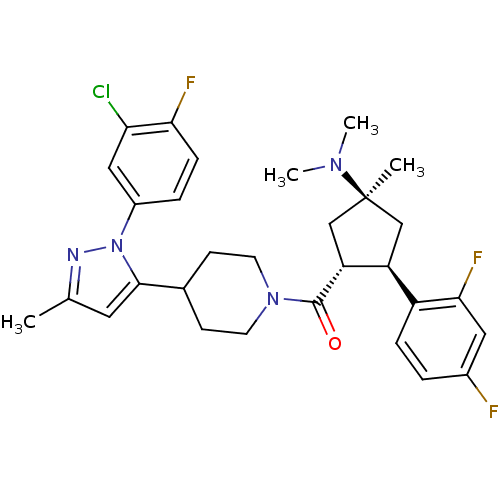
(CHEMBL2031590)Show SMILES CN(C)[C@]1(C)C[C@H]([C@@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1cc(C)nn1-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C30H34ClF3N4O/c1-18-13-28(38(35-18)21-6-8-26(33)25(31)15-21)19-9-11-37(12-10-19)29(39)24-17-30(2,36(3)4)16-23(24)22-7-5-20(32)14-27(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+,30-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50361774

(CHEMBL1938510)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)N)C(=O)NC(c1ccncc1)c1ccc(Cl)cc1)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C32H33ClN4O2/c1-21(22-9-11-24(12-10-22)23-7-5-4-6-8-23)28(37-31(39)32(2,3)34)30(38)36-29(26-17-19-35-20-18-26)25-13-15-27(33)16-14-25/h4-21,28-29H,34H2,1-3H3,(H,36,38)(H,37,39)/t21-,28-,29?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by continuous fluorometric assay |
Bioorg Med Chem Lett 22: 658-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.060
BindingDB Entry DOI: 10.7270/Q2B56K50 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382898
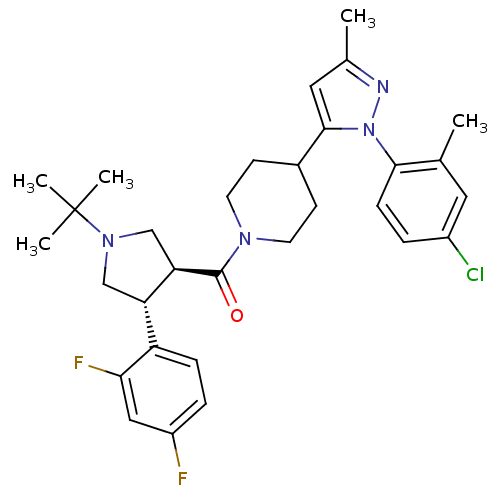
(CHEMBL2022786)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(Cl)cc1C |r| Show InChI InChI=1S/C31H37ClF2N4O/c1-19-14-22(32)6-9-28(19)38-29(15-20(2)35-38)21-10-12-36(13-11-21)30(39)26-18-37(31(3,4)5)17-25(26)24-8-7-23(33)16-27(24)34/h6-9,14-16,21,25-26H,10-13,17-18H2,1-5H3/t25-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50382912

(CHEMBL2023209 | US8569299, 4)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C30H34ClF3N4O/c1-18-13-28(38(35-18)21-6-8-26(33)25(31)15-21)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-5-20(32)14-27(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50383420
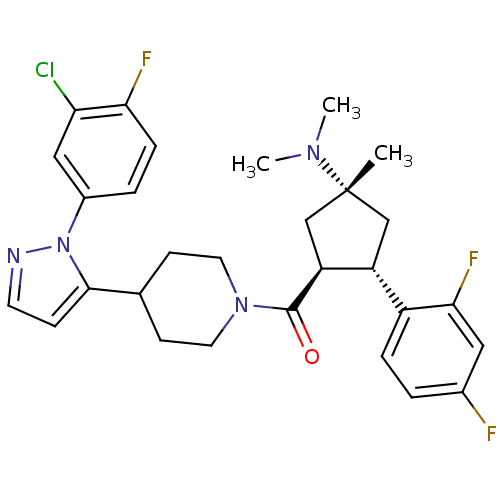
(CHEMBL2031596)Show SMILES CN(C)[C@@]1(C)C[C@@H]([C@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1ccnn1-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C29H32ClF3N4O/c1-29(35(2)3)16-22(21-6-4-19(31)14-26(21)33)23(17-29)28(38)36-12-9-18(10-13-36)27-8-11-34-37(27)20-5-7-25(32)24(30)15-20/h4-8,11,14-15,18,22-23H,9-10,12-13,16-17H2,1-3H3/t22-,23+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382886
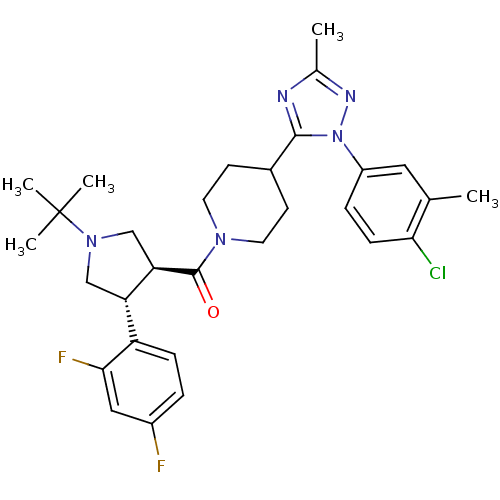
(CHEMBL2024198 | US8569299, 23)Show SMILES Cc1nc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(Cl)c(C)c1 |r| Show InChI InChI=1S/C30H36ClF2N5O/c1-18-14-22(7-9-26(18)31)38-28(34-19(2)35-38)20-10-12-36(13-11-20)29(39)25-17-37(30(3,4)5)16-24(25)23-8-6-21(32)15-27(23)33/h6-9,14-15,20,24-25H,10-13,16-17H2,1-5H3/t24-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382886

(CHEMBL2024198 | US8569299, 23)Show SMILES Cc1nc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(Cl)c(C)c1 |r| Show InChI InChI=1S/C30H36ClF2N5O/c1-18-14-22(7-9-26(18)31)38-28(34-19(2)35-38)20-10-12-36(13-11-20)29(39)25-17-37(30(3,4)5)16-24(25)23-8-6-21(32)15-27(23)33/h6-9,14-15,20,24-25H,10-13,16-17H2,1-5H3/t24-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50383425

(CHEMBL2031590)Show SMILES CN(C)[C@]1(C)C[C@H]([C@@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1cc(C)nn1-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C30H34ClF3N4O/c1-18-13-28(38(35-18)21-6-8-26(33)25(31)15-21)19-9-11-37(12-10-19)29(39)24-17-30(2,36(3)4)16-23(24)22-7-5-20(32)14-27(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50361766

(CHEMBL1938502)Show SMILES C[C@H](NC(=O)[C@@H](NC(=O)C(C)(C)N)[C@@H](C)c1ccc(cc1)-c1ccccc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C28H32ClN3O2/c1-18(20-10-12-23(13-11-20)22-8-6-5-7-9-22)25(32-27(34)28(3,4)30)26(33)31-19(2)21-14-16-24(29)17-15-21/h5-19,25H,30H2,1-4H3,(H,31,33)(H,32,34)/t18-,19-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by continuous fluorometric assay |
Bioorg Med Chem Lett 22: 658-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.060
BindingDB Entry DOI: 10.7270/Q2B56K50 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50383427

(CHEMBL2031588)Show SMILES CN(C)[C@]1(C)C[C@H]([C@@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1cc(C)nn1-c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-27(38(35-18)28-14-20(31)5-8-25(28)32)19-9-11-37(12-10-19)29(39)24-17-30(2,36(3)4)16-23(24)22-7-6-21(33)15-26(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50383426
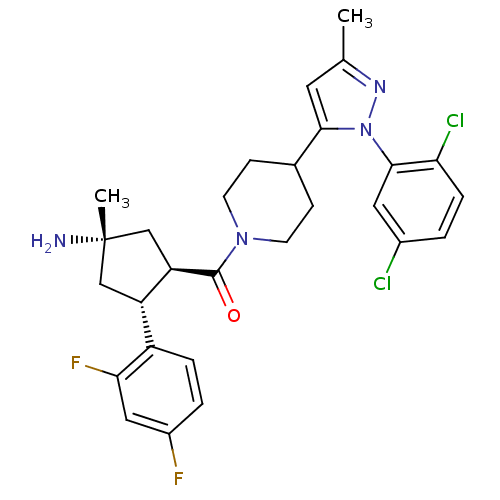
(CHEMBL2031589)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2C[C@@](C)(N)C[C@H]2c2ccc(F)cc2F)n(n1)-c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C28H30Cl2F2N4O/c1-16-11-25(36(34-16)26-12-18(29)3-6-23(26)30)17-7-9-35(10-8-17)27(37)22-15-28(2,33)14-21(22)20-5-4-19(31)13-24(20)32/h3-6,11-13,17,21-22H,7-10,14-15,33H2,1-2H3/t21-,22+,28-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406127
(CHEMBL5290844)Show SMILES Cc1cc(C)c(CCCP(O)(O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C22H28FO5P/c1-14-9-15(2)19(20(10-14)17-6-7-21(23)16(3)11-17)5-4-8-29(27,28)13-18(24)12-22(25)26/h6-7,9-11,27-29H,4-5,8,12-13H2,1-3H3,(H,25,26) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382897
(CHEMBL2022785)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(Cl)c(C)c1 |r| Show InChI InChI=1S/C31H37ClF2N4O/c1-19-14-23(7-9-27(19)32)38-29(15-20(2)35-38)21-10-12-36(13-11-21)30(39)26-18-37(31(3,4)5)17-25(26)24-8-6-22(33)16-28(24)34/h6-9,14-16,21,25-26H,10-13,17-18H2,1-5H3/t25-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
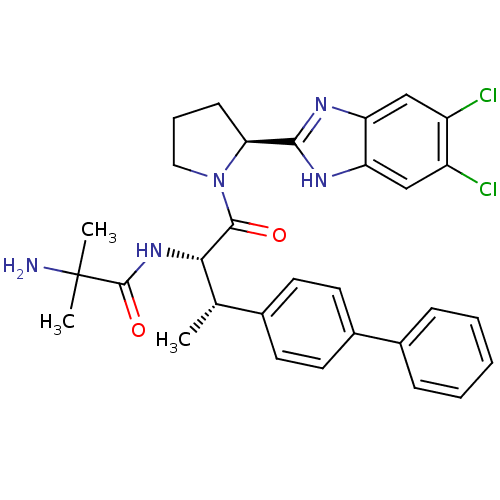
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328523
(2-Amino-N-{(2S,3S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5,...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)N)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C31H33Cl2N5O2/c1-18(19-11-13-21(14-12-19)20-8-5-4-6-9-20)27(37-30(40)31(2,3)34)29(39)38-15-7-10-26(38)28-35-24-16-22(32)23(33)17-25(24)36-28/h4-6,8-9,11-14,16-18,26-27H,7,10,15,34H2,1-3H3,(H,35,36)(H,37,40)/t18-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by continuous fluorometric assay |
Bioorg Med Chem Lett 22: 658-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.060
BindingDB Entry DOI: 10.7270/Q2B56K50 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50382905

(CHEMBL2022794)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-28(38(35-18)27-8-5-20(31)14-25(27)32)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-6-21(33)15-26(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50382904

(CHEMBL2022793)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-28(38(35-18)21-6-8-25(31)26(32)15-21)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-5-20(33)14-27(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382900
(CHEMBL2022789)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C30H35ClF2N4O/c1-19-15-28(37(34-19)23-8-5-21(31)6-9-23)20-11-13-35(14-12-20)29(38)26-18-36(30(2,3)4)17-25(26)24-10-7-22(32)16-27(24)33/h5-10,15-16,20,25-26H,11-14,17-18H2,1-4H3/t25-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Stearoyl-CoA desaturase
(Homo sapiens (Human)) | BDBM50362592
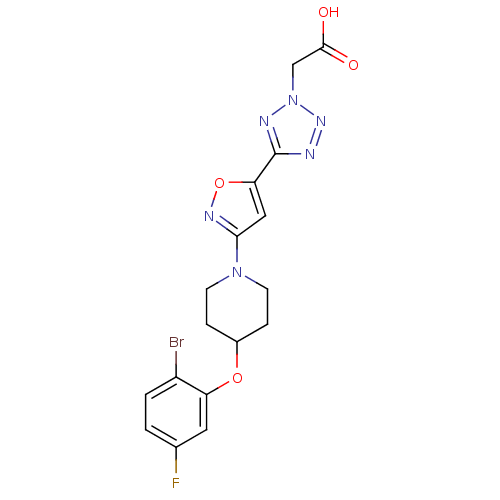
(CHEMBL1938870)Show SMILES OC(=O)Cn1nnc(n1)-c1cc(no1)N1CCC(CC1)Oc1cc(F)ccc1Br Show InChI InChI=1S/C17H16BrFN6O4/c18-12-2-1-10(19)7-13(12)28-11-3-5-24(6-4-11)15-8-14(29-22-15)17-20-23-25(21-17)9-16(26)27/h1-2,7-8,11H,3-6,9H2,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human SCD-1 |
J Med Chem 54: 5082-96 (2011)
Article DOI: 10.1021/jm200319u
BindingDB Entry DOI: 10.7270/Q2348MGP |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50383419
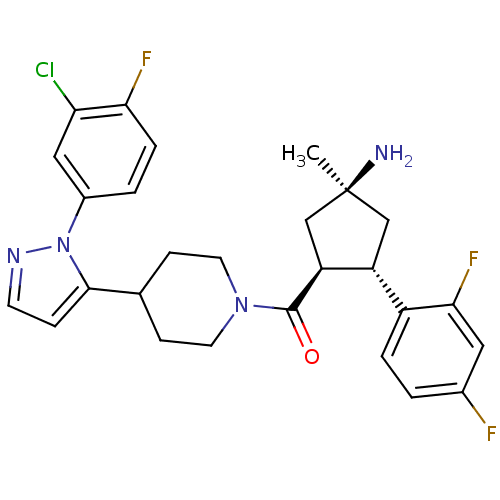
(CHEMBL2031597)Show SMILES C[C@@]1(N)C[C@@H]([C@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1ccnn1-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C27H28ClF3N4O/c1-27(32)14-20(19-4-2-17(29)12-24(19)31)21(15-27)26(36)34-10-7-16(8-11-34)25-6-9-33-35(25)18-3-5-23(30)22(28)13-18/h2-6,9,12-13,16,20-21H,7-8,10-11,14-15,32H2,1H3/t20-,21+,27+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
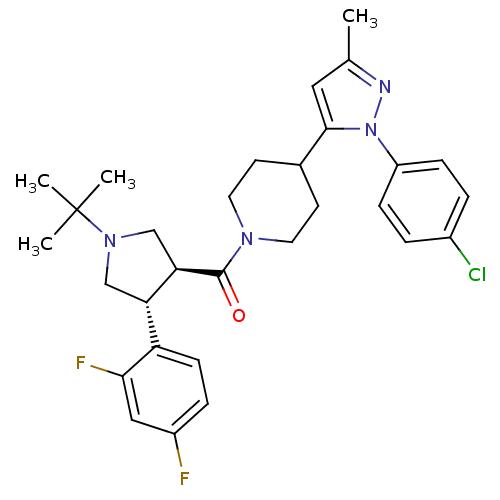
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382914

(CHEMBL2023211)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1cc(Cl)ccc1F |r| Show InChI InChI=1S/C30H34ClF3N4O/c1-18-13-27(38(35-18)28-14-20(31)5-8-25(28)33)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-6-21(32)15-26(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
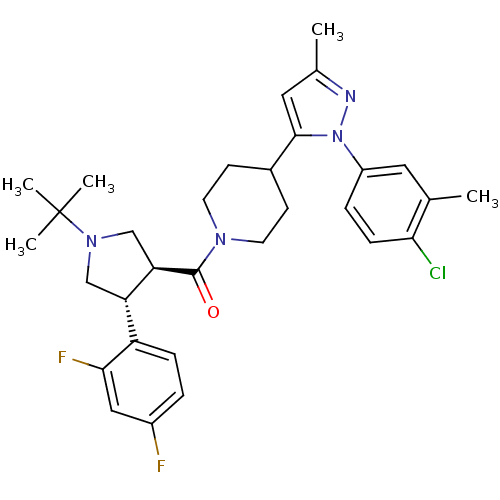
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50383435
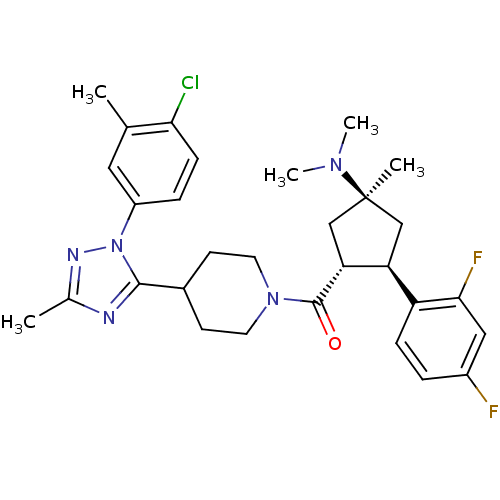
(CHEMBL2031580)Show SMILES CN(C)[C@]1(C)C[C@H]([C@@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1nc(C)nn1-c1ccc(Cl)c(C)c1 |r| Show InChI InChI=1S/C30H36ClF2N5O/c1-18-14-22(7-9-26(18)31)38-28(34-19(2)35-38)20-10-12-37(13-11-20)29(39)25-17-30(3,36(4)5)16-24(25)23-8-6-21(32)15-27(23)33/h6-9,14-15,20,24-25H,10-13,16-17H2,1-5H3/t24-,25+,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50382899

(CHEMBL2022787)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(C)c(Cl)c1 |r| Show InChI InChI=1S/C31H37ClF2N4O/c1-19-6-8-23(16-27(19)32)38-29(14-20(2)35-38)21-10-12-36(13-11-21)30(39)26-18-37(31(3,4)5)17-25(26)24-9-7-22(33)15-28(24)34/h6-9,14-16,21,25-26H,10-13,17-18H2,1-5H3/t25-,26+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
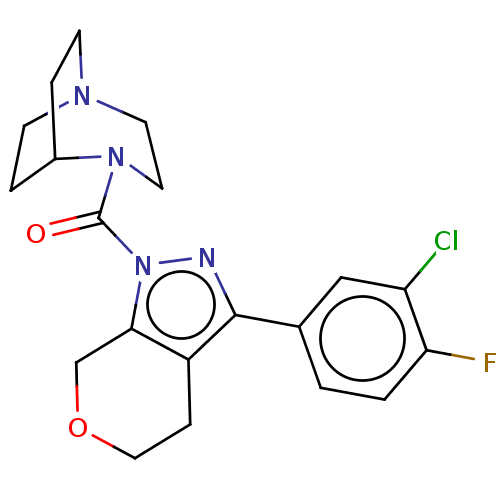
(Homo sapiens (Human)) | BDBM50338007

((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Cn1nc(cc1-c1ccncc1)C1CCN(CCC(=O)N2CCC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)CC1 |r| Show InChI InChI=1S/C28H31Cl2N7O/c1-35-26(19-4-9-31-10-5-19)17-22(34-35)18-6-12-36(13-7-18)14-8-27(38)37-11-2-3-25(37)28-32-23-15-20(29)21(30)16-24(23)33-28/h4-5,9-10,15-18,25H,2-3,6-8,11-14H2,1H3,(H,32,33)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate after 30 mins by fluorescence analysis |
Bioorg Med Chem Lett 22: 1727-30 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.098
BindingDB Entry DOI: 10.7270/Q2GT5NNH |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50361787
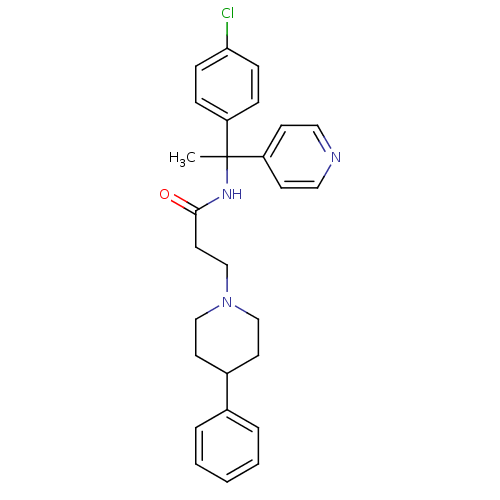
(CHEMBL1938523)Show SMILES CC(NC(=O)CCN1CCC(CC1)c1ccccc1)(c1ccncc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C27H30ClN3O/c1-27(24-11-16-29-17-12-24,23-7-9-25(28)10-8-23)30-26(32)15-20-31-18-13-22(14-19-31)21-5-3-2-4-6-21/h2-12,16-17,22H,13-15,18-20H2,1H3,(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by continuous fluorometric assay |
Bioorg Med Chem Lett 22: 658-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.060
BindingDB Entry DOI: 10.7270/Q2B56K50 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50361783
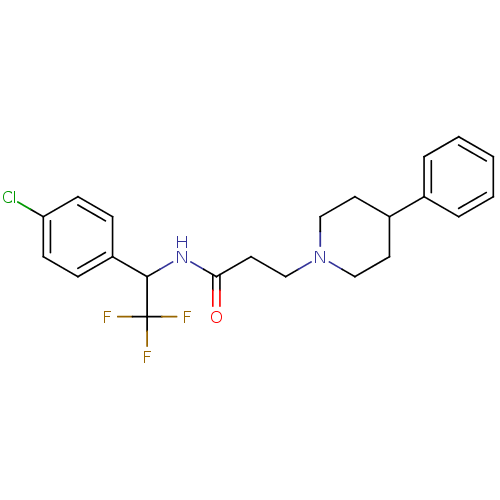
(CHEMBL1938519)Show SMILES FC(F)(F)C(NC(=O)CCN1CCC(CC1)c1ccccc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C22H24ClF3N2O/c23-19-8-6-18(7-9-19)21(22(24,25)26)27-20(29)12-15-28-13-10-17(11-14-28)16-4-2-1-3-5-16/h1-9,17,21H,10-15H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by continuous fluorometric assay |
Bioorg Med Chem Lett 22: 658-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.060
BindingDB Entry DOI: 10.7270/Q2B56K50 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50361783

(CHEMBL1938519)Show SMILES FC(F)(F)C(NC(=O)CCN1CCC(CC1)c1ccccc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C22H24ClF3N2O/c23-19-8-6-18(7-9-19)21(22(24,25)26)27-20(29)12-15-28-13-10-17(11-14-28)16-4-2-1-3-5-16/h1-9,17,21H,10-15H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by continuous fluorometric assay |
Bioorg Med Chem Lett 22: 658-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.060
BindingDB Entry DOI: 10.7270/Q2B56K50 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382911

(CHEMBL2023208)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(Cl)c(F)c1 |r| Show InChI InChI=1S/C30H34ClF3N4O/c1-18-13-28(38(35-18)21-6-8-25(31)27(34)15-21)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-5-20(32)14-26(22)33/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50383420

(CHEMBL2031596)Show SMILES CN(C)[C@@]1(C)C[C@@H]([C@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1ccnn1-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C29H32ClF3N4O/c1-29(35(2)3)16-22(21-6-4-19(31)14-26(21)33)23(17-29)28(38)36-12-9-18(10-13-36)27-8-11-34-37(27)20-5-7-25(32)24(30)15-20/h4-8,11,14-15,18,22-23H,9-10,12-13,16-17H2,1-3H3/t22-,23+,29-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50361773

(CHEMBL1938509)Show SMILES C[C@H]([C@H](N)C(=O)NC(c1ccncc1)c1ccc(Cl)cc1)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C28H26ClN3O/c1-19(20-7-9-22(10-8-20)21-5-3-2-4-6-21)26(30)28(33)32-27(24-15-17-31-18-16-24)23-11-13-25(29)14-12-23/h2-19,26-27H,30H2,1H3,(H,32,33)/t19-,26-,27?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mouse PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by continuous fluorometric assay |
Bioorg Med Chem Lett 22: 658-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.060
BindingDB Entry DOI: 10.7270/Q2B56K50 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50382914

(CHEMBL2023211)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1cc(Cl)ccc1F |r| Show InChI InChI=1S/C30H34ClF3N4O/c1-18-13-27(38(35-18)28-14-20(31)5-8-25(28)33)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-6-21(32)15-26(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50361780
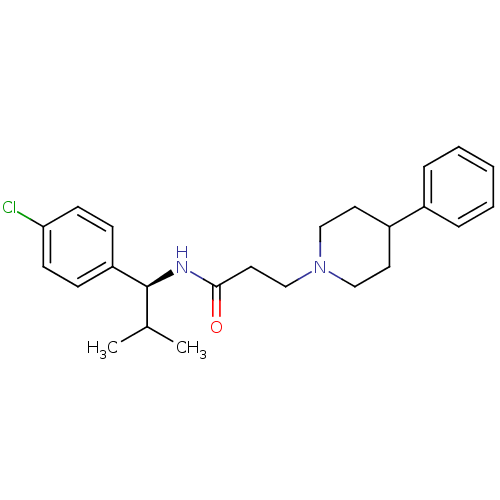
(CHEMBL1938516)Show SMILES CC(C)[C@H](NC(=O)CCN1CCC(CC1)c1ccccc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H31ClN2O/c1-18(2)24(21-8-10-22(25)11-9-21)26-23(28)14-17-27-15-12-20(13-16-27)19-6-4-3-5-7-19/h3-11,18,20,24H,12-17H2,1-2H3,(H,26,28)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by continuous fluorometric assay |
Bioorg Med Chem Lett 22: 658-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.060
BindingDB Entry DOI: 10.7270/Q2B56K50 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50383433
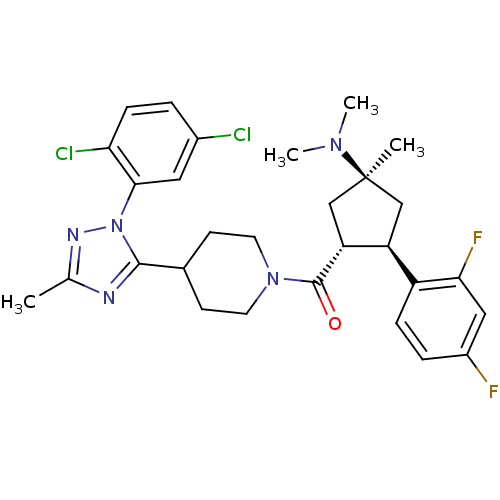
(CHEMBL2031582)Show SMILES CN(C)[C@]1(C)C[C@H]([C@@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1nc(C)nn1-c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C29H33Cl2F2N5O/c1-17-34-27(38(35-17)26-13-19(30)5-8-24(26)31)18-9-11-37(12-10-18)28(39)23-16-29(2,36(3)4)15-22(23)21-7-6-20(32)14-25(21)33/h5-8,13-14,18,22-23H,9-12,15-16H2,1-4H3/t22-,23+,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406134

(CHEMBL5275403)Show SMILES Cc1cc(C)c(C=CP(O)(=O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 |w:7.7| Show InChI InChI=1S/C21H22FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-10H,11-12H2,1-3H3,(H,24,25)(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50361772

(CHEMBL1938508)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)N)C(=O)NC(c1ccc(Cl)cc1)C(F)(F)F)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C28H29ClF3N3O2/c1-17(18-9-11-20(12-10-18)19-7-5-4-6-8-19)23(34-26(37)27(2,3)33)25(36)35-24(28(30,31)32)21-13-15-22(29)16-14-21/h4-17,23-24H,33H2,1-3H3,(H,34,37)(H,35,36)/t17-,23-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by continuous fluorometric assay |
Bioorg Med Chem Lett 22: 658-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.060
BindingDB Entry DOI: 10.7270/Q2B56K50 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data