

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50067482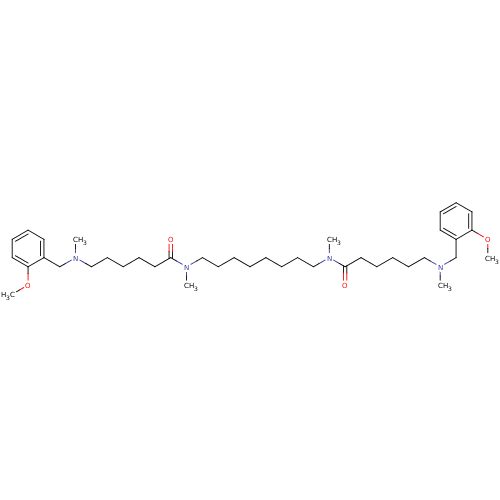 (6-[(2-Methoxy-benzyl)-methyl-amino]-hexanoic acid ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition constant determined against Acetylcholinesterase (AChE) receptor. | J Med Chem 41: 4186-9 (1998) Checked by Author Article DOI: 10.1021/jm9810452 BindingDB Entry DOI: 10.7270/Q2HH6KRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition constant determined against Acetylcholinesterase (AChE) receptor. | J Med Chem 41: 4186-9 (1998) Checked by Author Article DOI: 10.1021/jm9810452 BindingDB Entry DOI: 10.7270/Q2HH6KRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Binding affinity measured in CHO cells expressing human cloned Alpha-1D adrenergic receptor expressed as pKi | J Med Chem 46: 4895-903 (2003) Article DOI: 10.1021/jm030952q BindingDB Entry DOI: 10.7270/Q2ZK5KDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM82423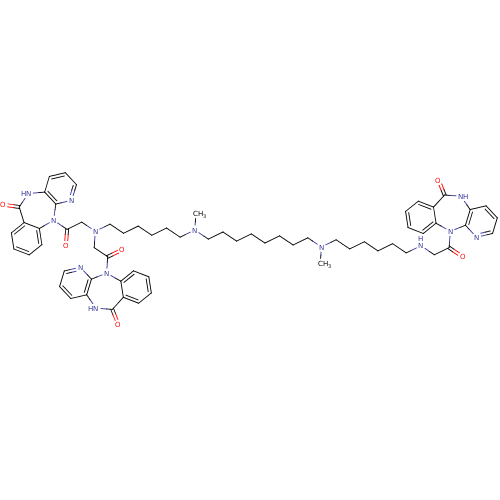 (CAS_132947 | NSC_132947 | TRIPITRAMINE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa Curated by PDSP Ki Database | Eur J Pharmacol 268: 459-62 (1994) Article DOI: 10.1016/0922-4106(94)90075-2 BindingDB Entry DOI: 10.7270/Q2348HWT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM82559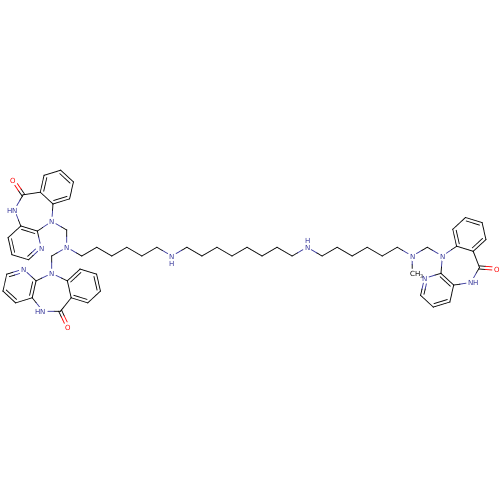 (methoctramine analog 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by PDSP Ki Database | J Med Chem 36: 3734-7 (1993) Article DOI: 10.1021/jm00075a032 BindingDB Entry DOI: 10.7270/Q2W957Q0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM82423 (CAS_132947 | NSC_132947 | TRIPITRAMINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to rat heart Muscarinic acetylcholine receptor M2 | J Med Chem 41: 4150-60 (1998) Checked by Author Article DOI: 10.1021/jm981038d BindingDB Entry DOI: 10.7270/Q2KH0PJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM82423 (CAS_132947 | NSC_132947 | TRIPITRAMINE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]N-methylscopolamine chloride from human cloned muscarinic M2 receptor expressed in CHOK1 cells | Bioorg Med Chem 16: 7311-20 (2008) Article DOI: 10.1016/j.bmc.2008.06.025 BindingDB Entry DOI: 10.7270/Q24F1S07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50110300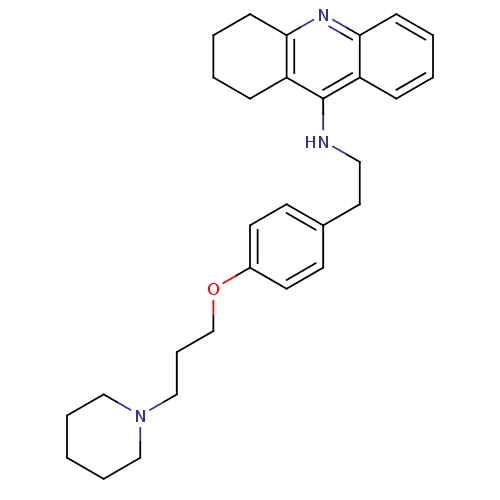 (CHEMBL15056 | N-(4-(3-(piperidin-1-yl)propoxy)phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Binding affinity to 5HT3 receptor | J Med Chem 51: 347-72 (2008) Article DOI: 10.1021/jm7009364 BindingDB Entry DOI: 10.7270/Q25B039W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50408535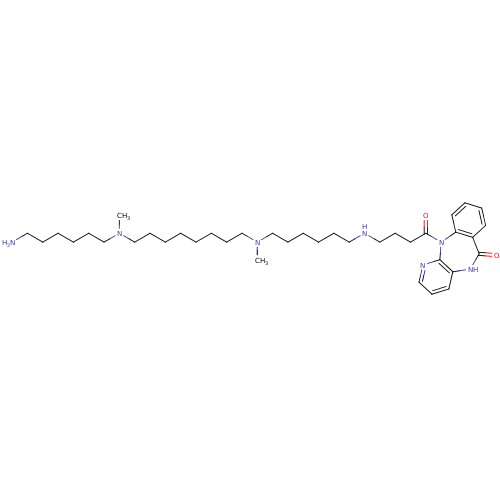 (CHEMBL131865) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to rat heart Muscarinic acetylcholine receptor M2 | J Med Chem 41: 4150-60 (1998) Checked by Author Article DOI: 10.1021/jm981038d BindingDB Entry DOI: 10.7270/Q2KH0PJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50198338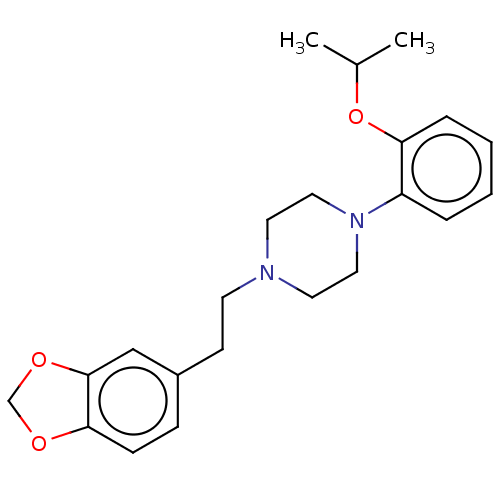 (CHEMBL3938038) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.389 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human alpha1A-adrenoceptor expressed in CHO cell membranes measured after 30 mins | Eur J Med Chem 122: 601-610 (2016) Article DOI: 10.1016/j.ejmech.2016.06.052 BindingDB Entry DOI: 10.7270/Q2JW8GW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.407 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Binding affinity measured in CHO cells expressing human cloned Alpha-1B adrenergic receptor expressed as pKi | J Med Chem 46: 4895-903 (2003) Article DOI: 10.1021/jm030952q BindingDB Entry DOI: 10.7270/Q2ZK5KDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM69602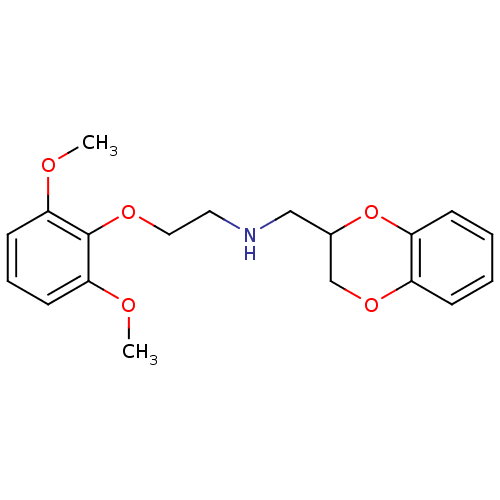 (2,3-dihydro-1,4-benzodioxin-3-ylmethyl-[2-(2,6-dim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.427 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human cloned Alpha-1A adrenergic receptor | J Med Chem 42: 4214-24 (1999) Article DOI: 10.1021/jm991065j BindingDB Entry DOI: 10.7270/Q2TF012V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM69602 (2,3-dihydro-1,4-benzodioxin-3-ylmethyl-[2-(2,6-dim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.427 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Binding affinity measured in CHO cells expressing human cloned Alpha-1A adrenergic receptor expressed as pKi | J Med Chem 46: 4895-903 (2003) Article DOI: 10.1021/jm030952q BindingDB Entry DOI: 10.7270/Q2ZK5KDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50408530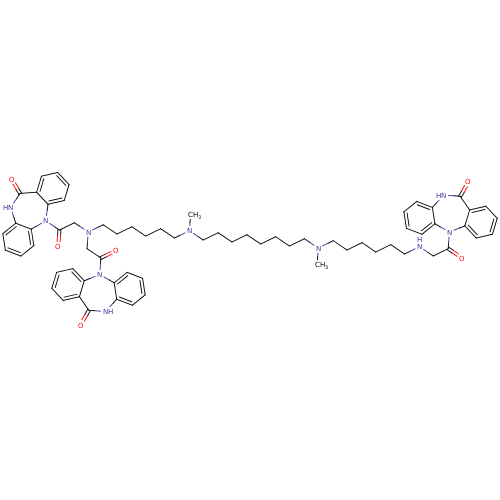 (CHEMBL1202003) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to rat heart Muscarinic acetylcholine receptor M2 | J Med Chem 41: 4150-60 (1998) Checked by Author Article DOI: 10.1021/jm981038d BindingDB Entry DOI: 10.7270/Q2KH0PJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50408535 (CHEMBL131865) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Binding affinity for rat cortex Muscarinic acetylcholine receptor M1 | J Med Chem 41: 4150-60 (1998) Checked by Author Article DOI: 10.1021/jm981038d BindingDB Entry DOI: 10.7270/Q2KH0PJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM69602 (2,3-dihydro-1,4-benzodioxin-3-ylmethyl-[2-(2,6-dim...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Binding affinity measured in CHO cells expressing human cloned Alpha-1D adrenergic receptor expressed as pKi | J Med Chem 46: 4895-903 (2003) Article DOI: 10.1021/jm030952q BindingDB Entry DOI: 10.7270/Q2ZK5KDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM69602 (2,3-dihydro-1,4-benzodioxin-3-ylmethyl-[2-(2,6-dim...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human cloned Alpha-1D adrenergic receptor | J Med Chem 42: 4214-24 (1999) Article DOI: 10.1021/jm991065j BindingDB Entry DOI: 10.7270/Q2TF012V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50408527 (CHEMBL1202004) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to rat heart Muscarinic acetylcholine receptor M2 | J Med Chem 41: 4150-60 (1998) Checked by Author Article DOI: 10.1021/jm981038d BindingDB Entry DOI: 10.7270/Q2KH0PJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.589 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Binding affinity measured in CHO cells expressing human cloned Alpha-1A adrenergic receptor expressed as pKi | J Med Chem 46: 4895-903 (2003) Article DOI: 10.1021/jm030952q BindingDB Entry DOI: 10.7270/Q2ZK5KDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50200566 (CHEMBL3902626) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.603 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human alpha1D-adrenoceptor expressed in CHO cell membranes measured after 30 mins | Eur J Med Chem 122: 601-610 (2016) Article DOI: 10.1016/j.ejmech.2016.06.052 BindingDB Entry DOI: 10.7270/Q2JW8GW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 in Guinea pig brain membranes using [3H]DAMGO | J Med Chem 39: 1816-22 (1996) Article DOI: 10.1021/jm950807f BindingDB Entry DOI: 10.7270/Q20865Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50472355 (CHEMBL135974) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.832 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]8-hydroxy-2-(di-n-propylamino)tetralin from human 5-hydroxytryptamine 1A receptor | J Med Chem 42: 4214-24 (1999) Article DOI: 10.1021/jm991065j BindingDB Entry DOI: 10.7270/Q2TF012V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50408525 (CHEMBL1202000) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of [3H]NMS binding to rat heart Muscarinic acetylcholine receptor M2 | J Med Chem 41: 4150-60 (1998) Checked by Author Article DOI: 10.1021/jm981038d BindingDB Entry DOI: 10.7270/Q2KH0PJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50026917 (8-(2-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl)-8-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.933 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]8-hydroxy-2-(di-n-propylamino)tetralin from human 5-hydroxytryptamine 1A receptor | J Med Chem 42: 4214-24 (1999) Article DOI: 10.1021/jm991065j BindingDB Entry DOI: 10.7270/Q2TF012V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50408530 (CHEMBL1202003) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Binding affinity for rat cortex Muscarinic acetylcholine receptor M1 | J Med Chem 41: 4150-60 (1998) Checked by Author Article DOI: 10.1021/jm981038d BindingDB Entry DOI: 10.7270/Q2KH0PJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50049563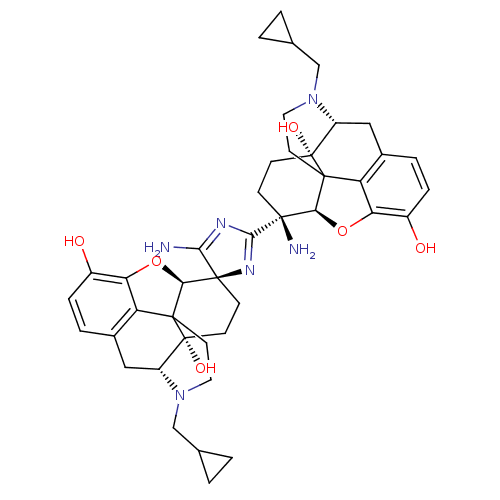 (5-amino-2,4(Bis-4-cyclopropylmethyl-10,17-dihydrox...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 in Guinea pig brain membranes | J Med Chem 39: 1816-22 (1996) Article DOI: 10.1021/jm950807f BindingDB Entry DOI: 10.7270/Q20865Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515472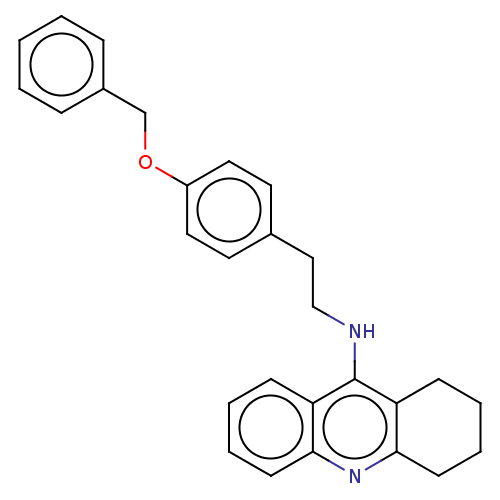 (CHEMBL4469822) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50200441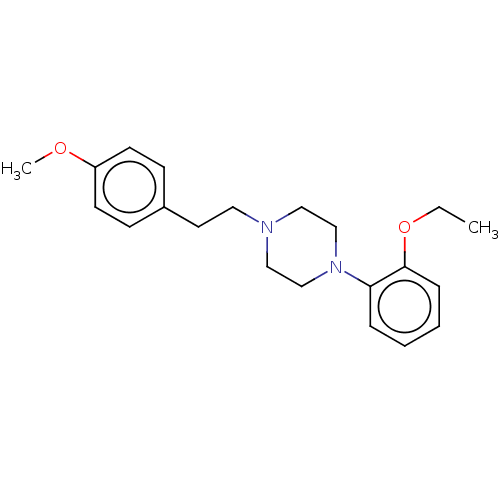 (CHEMBL3930616) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human alpha1A-adrenoceptor expressed in CHO cell membranes measured after 30 mins | Eur J Med Chem 122: 601-610 (2016) Article DOI: 10.1016/j.ejmech.2016.06.052 BindingDB Entry DOI: 10.7270/Q2JW8GW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50201790 (CHEMBL3917716) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human alpha1A-adrenoceptor expressed in CHO cell membranes measured after 30 mins | Eur J Med Chem 122: 601-610 (2016) Article DOI: 10.1016/j.ejmech.2016.06.052 BindingDB Entry DOI: 10.7270/Q2JW8GW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515454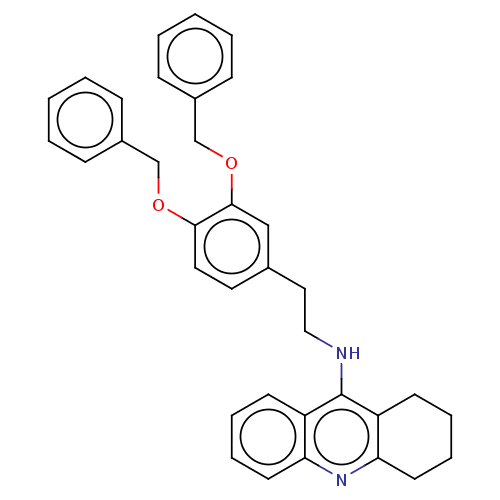 (CHEMBL4550977) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456708 (CHEMBL4204315) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456708 (CHEMBL4204315) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE assessed as enzyme-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver-Burk plot... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50049560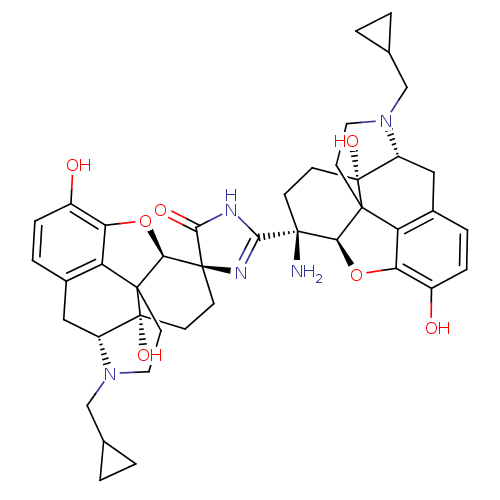 ((4S,5'R,13'R,17'S)-2-[(5R,13R,14R,17S)-14-amino-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity against Delta Opioid receptor in Guinea pig brain membranes | J Med Chem 39: 1816-22 (1996) Article DOI: 10.1021/jm950807f BindingDB Entry DOI: 10.7270/Q20865Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50393116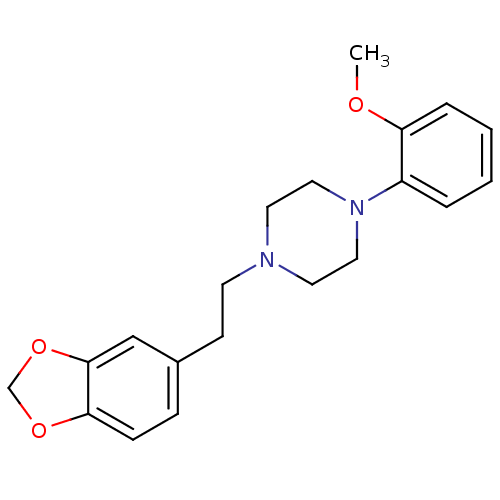 (CHEMBL2153423) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human alpha1D-adrenoceptor expressed in CHO cell membranes measured after 30 mins | Eur J Med Chem 122: 601-610 (2016) Article DOI: 10.1016/j.ejmech.2016.06.052 BindingDB Entry DOI: 10.7270/Q2JW8GW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50200441 (CHEMBL3930616) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human alpha1D-adrenoceptor expressed in CHO cell membranes measured after 30 mins | Eur J Med Chem 122: 601-610 (2016) Article DOI: 10.1016/j.ejmech.2016.06.052 BindingDB Entry DOI: 10.7270/Q2JW8GW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50201789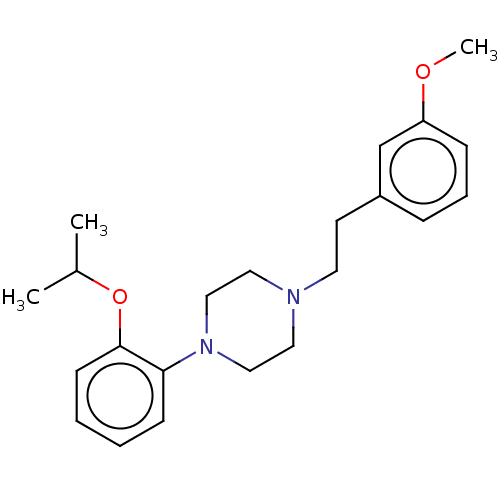 (CHEMBL3946843) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human alpha1D-adrenoceptor expressed in CHO cell membranes measured after 30 mins | Eur J Med Chem 122: 601-610 (2016) Article DOI: 10.1016/j.ejmech.2016.06.052 BindingDB Entry DOI: 10.7270/Q2JW8GW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50201791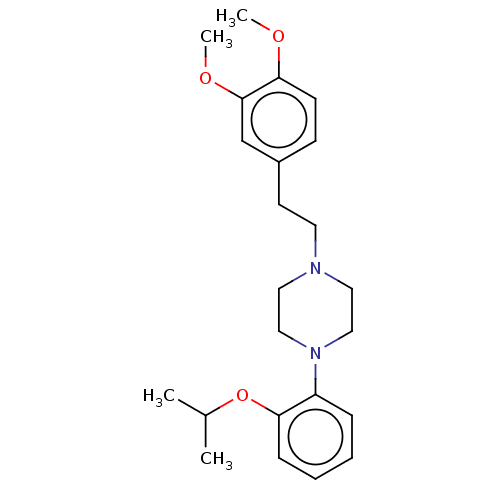 (CHEMBL3920110) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human alpha1D-adrenoceptor expressed in CHO cell membranes measured after 30 mins | Eur J Med Chem 122: 601-610 (2016) Article DOI: 10.1016/j.ejmech.2016.06.052 BindingDB Entry DOI: 10.7270/Q2JW8GW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (RAT) | BDBM50408535 (CHEMBL131865) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of NG 108-15 binding to Muscarinic acetylcholine receptor M4 | J Med Chem 41: 4150-60 (1998) Checked by Author Article DOI: 10.1021/jm981038d BindingDB Entry DOI: 10.7270/Q2KH0PJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50026917 (8-(2-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl)-8-a...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human cloned Alpha-1D adrenergic receptor | J Med Chem 42: 4214-24 (1999) Article DOI: 10.1021/jm991065j BindingDB Entry DOI: 10.7270/Q2TF012V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50158582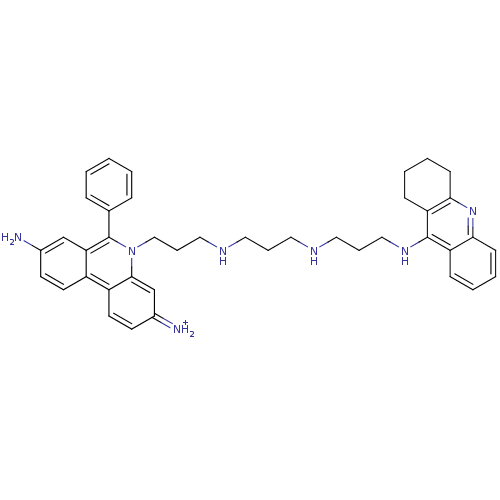 (3,8-Diamino-6-phenyl-5-(3-{3-[3-(1,2,3,4-tetrahydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Competitive inhibition constant for Acetylcholinesterase | J Med Chem 48: 24-7 (2005) Article DOI: 10.1021/jm049156q BindingDB Entry DOI: 10.7270/Q2P84CPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50201790 (CHEMBL3917716) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human alpha1D-adrenoceptor expressed in CHO cell membranes measured after 30 mins | Eur J Med Chem 122: 601-610 (2016) Article DOI: 10.1016/j.ejmech.2016.06.052 BindingDB Entry DOI: 10.7270/Q2JW8GW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515453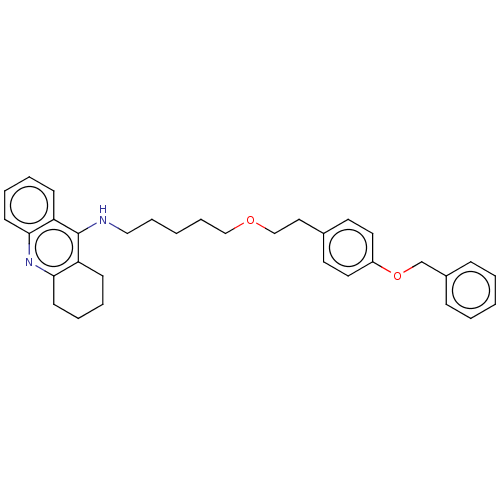 (CHEMBL4588525) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM82423 (CAS_132947 | NSC_132947 | TRIPITRAMINE) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa Curated by PDSP Ki Database | Eur J Pharmacol 268: 459-62 (1994) Article DOI: 10.1016/0922-4106(94)90075-2 BindingDB Entry DOI: 10.7270/Q2348HWT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM120262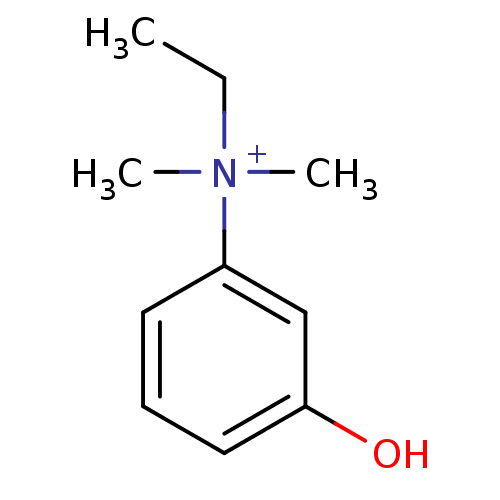 (EDROPHONIUM BROMIDE | EDROPHONIUM CHLORIDE | Edrop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition constant determined against Acetylcholinesterase (AChE) receptor. | J Med Chem 41: 4186-9 (1998) Checked by Author Article DOI: 10.1021/jm9810452 BindingDB Entry DOI: 10.7270/Q2HH6KRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515473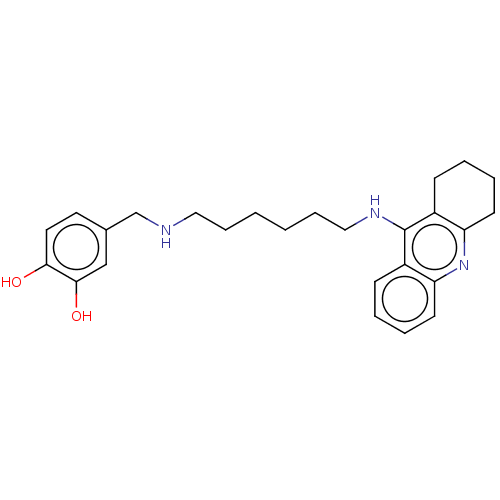 (CHEMBL4572757) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50198338 (CHEMBL3938038) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human alpha1D-adrenoceptor expressed in CHO cell membranes measured after 30 mins | Eur J Med Chem 122: 601-610 (2016) Article DOI: 10.1016/j.ejmech.2016.06.052 BindingDB Entry DOI: 10.7270/Q2JW8GW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515465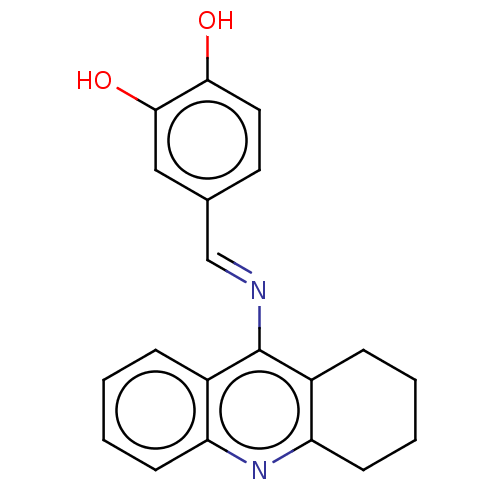 (CHEMBL4536715) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515470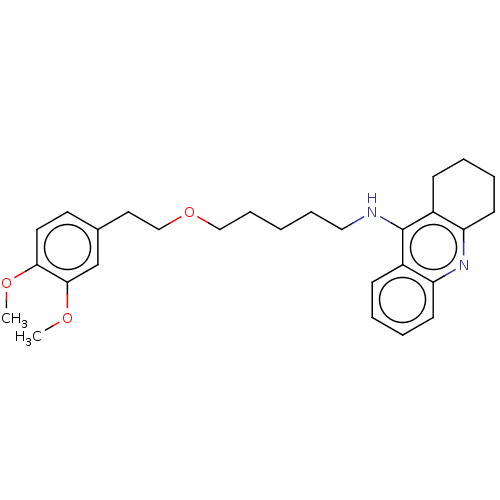 (CHEMBL4555120) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515453 (CHEMBL4588525) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-inhibitor complex using butyrylthiocholine iodide as substrate measured... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50201791 (CHEMBL3920110) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human alpha1A-adrenoceptor expressed in CHO cell membranes measured after 30 mins | Eur J Med Chem 122: 601-610 (2016) Article DOI: 10.1016/j.ejmech.2016.06.052 BindingDB Entry DOI: 10.7270/Q2JW8GW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1426 total ) | Next | Last >> |