 Found 345 hits with Last Name = 'bryans' and Initial = 'j'
Found 345 hits with Last Name = 'bryans' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50256615
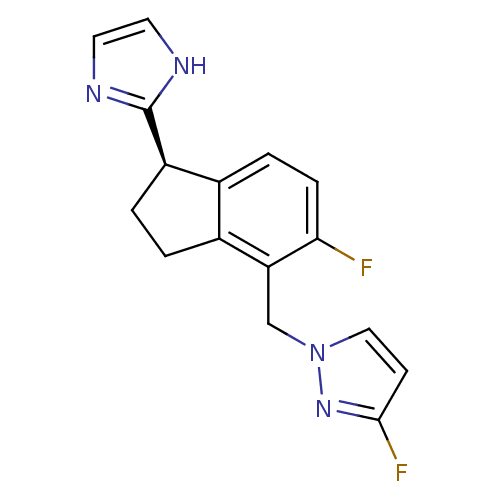
((R)-3-fluoro-1-((5-fluoro-1-(1H-imidazol-2-yl)-2,3...)Show SMILES Fc1ccn(Cc2c3CC[C@@H](c4ncc[nH]4)c3ccc2F)n1 |r| Show InChI InChI=1S/C16H14F2N4/c17-14-4-3-10-11(1-2-12(10)16-19-6-7-20-16)13(14)9-22-8-5-15(18)21-22/h3-8,12H,1-2,9H2,(H,19,20)/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to adrenergic alpha1A receptor (unknown origin) |
Bioorg Med Chem Lett 18: 6437-40 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.066
BindingDB Entry DOI: 10.7270/Q2C24W91 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50256563
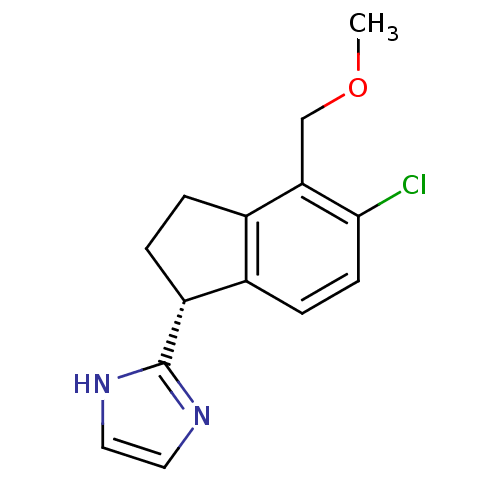
((R)-2-(5-chloro-4-(methoxymethyl)-2,3-dihydro-1H-i...)Show InChI InChI=1S/C14H15ClN2O/c1-18-8-12-10-2-3-11(14-16-6-7-17-14)9(10)4-5-13(12)15/h4-7,11H,2-3,8H2,1H3,(H,16,17)/t11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to adrenergic alpha1A receptor (unknown origin) |
Bioorg Med Chem Lett 18: 6437-40 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.066
BindingDB Entry DOI: 10.7270/Q2C24W91 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50354912
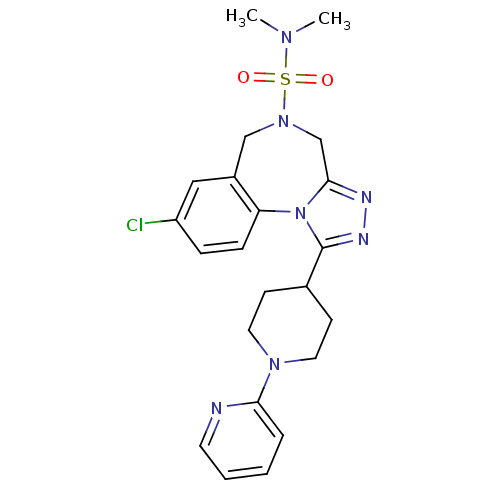
(CHEMBL1837039)Show SMILES CN(C)S(=O)(=O)N1Cc2nnc(C3CCN(CC3)c3ccccn3)n2-c2ccc(Cl)cc2C1 Show InChI InChI=1S/C22H26ClN7O2S/c1-27(2)33(31,32)29-14-17-13-18(23)6-7-19(17)30-21(15-29)25-26-22(30)16-8-11-28(12-9-16)20-5-3-4-10-24-20/h3-7,10,13,16H,8-9,11-12,14-15H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human V1A receptor expressed in CHO cells assessed as inhibition of AVP-induced intracellular calcium release after 30 seconds... |
Bioorg Med Chem Lett 21: 5684-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.038
BindingDB Entry DOI: 10.7270/Q2TB179T |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50354897
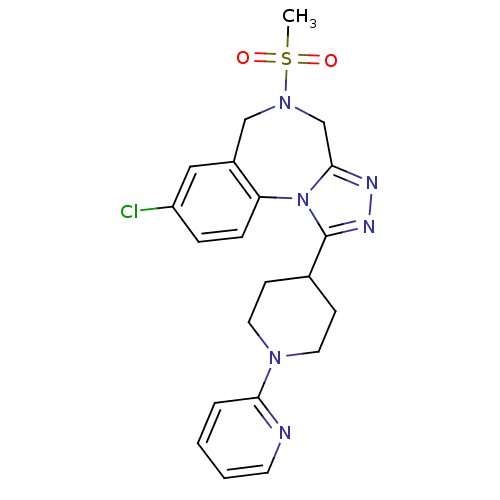
(CHEMBL1837041)Show SMILES CS(=O)(=O)N1Cc2nnc(C3CCN(CC3)c3ccccn3)n2-c2ccc(Cl)cc2C1 Show InChI InChI=1S/C21H23ClN6O2S/c1-31(29,30)27-13-16-12-17(22)5-6-18(16)28-20(14-27)24-25-21(28)15-7-10-26(11-8-15)19-4-2-3-9-23-19/h2-6,9,12,15H,7-8,10-11,13-14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human V1A receptor expressed in CHO cells assessed as inhibition of AVP-induced intracellular calcium release after 30 seconds... |
Bioorg Med Chem Lett 21: 5684-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.038
BindingDB Entry DOI: 10.7270/Q2TB179T |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50401913
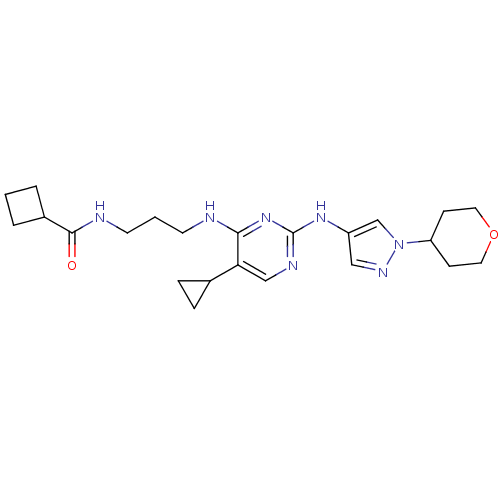
(CHEMBL2207214)Show SMILES O=C(NCCCNc1nc(Nc2cnn(c2)C2CCOCC2)ncc1C1CC1)C1CCC1 Show InChI InChI=1S/C23H33N7O2/c31-22(17-3-1-4-17)25-10-2-9-24-21-20(16-5-6-16)14-26-23(29-21)28-18-13-27-30(15-18)19-7-11-32-12-8-19/h13-17,19H,1-12H2,(H,25,31)(H2,24,26,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 by radiometry |
Bioorg Med Chem Lett 22: 7169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.063
BindingDB Entry DOI: 10.7270/Q2TT4S45 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50401928
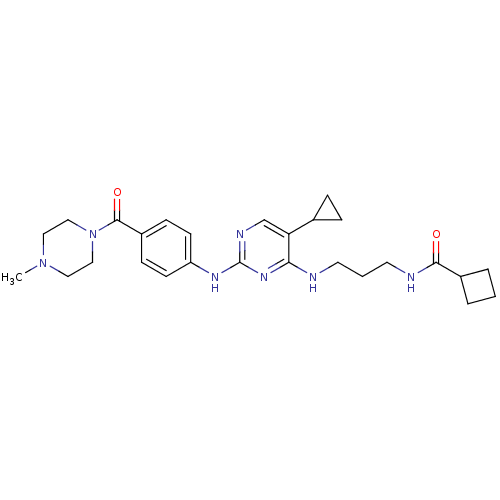
(CHEMBL2207195)Show SMILES CN1CCN(CC1)C(=O)c1ccc(Nc2ncc(C3CC3)c(NCCCNC(=O)C3CCC3)n2)cc1 Show InChI InChI=1S/C27H37N7O2/c1-33-14-16-34(17-15-33)26(36)21-8-10-22(11-9-21)31-27-30-18-23(19-6-7-19)24(32-27)28-12-3-13-29-25(35)20-4-2-5-20/h8-11,18-20H,2-7,12-17H2,1H3,(H,29,35)(H2,28,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 by radiometry |
Bioorg Med Chem Lett 22: 7169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.063
BindingDB Entry DOI: 10.7270/Q2TT4S45 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50401907

(CHEMBL2207181)Show InChI InChI=1S/C18H21BrFN5O/c19-15-11-23-18(24-14-7-2-6-13(20)10-14)25-16(15)21-8-3-9-22-17(26)12-4-1-5-12/h2,6-7,10-12H,1,3-5,8-9H2,(H,22,26)(H2,21,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 by radiometry |
Bioorg Med Chem Lett 22: 7169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.063
BindingDB Entry DOI: 10.7270/Q2TT4S45 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50401915
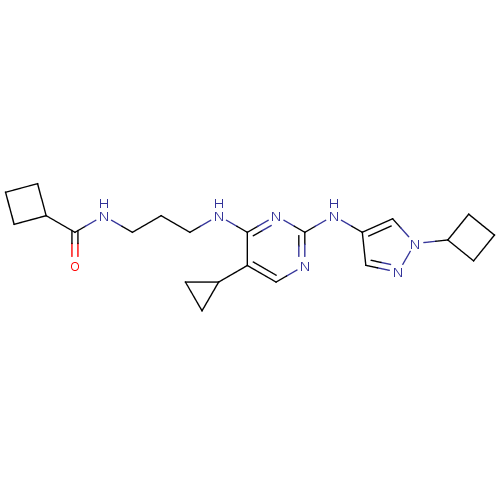
(CHEMBL2207212)Show SMILES O=C(NCCCNc1nc(Nc2cnn(c2)C2CCC2)ncc1C1CC1)C1CCC1 Show InChI InChI=1S/C22H31N7O/c30-21(16-4-1-5-16)24-11-3-10-23-20-19(15-8-9-15)13-25-22(28-20)27-17-12-26-29(14-17)18-6-2-7-18/h12-16,18H,1-11H2,(H,24,30)(H2,23,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 by radiometry |
Bioorg Med Chem Lett 22: 7169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.063
BindingDB Entry DOI: 10.7270/Q2TT4S45 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50401927
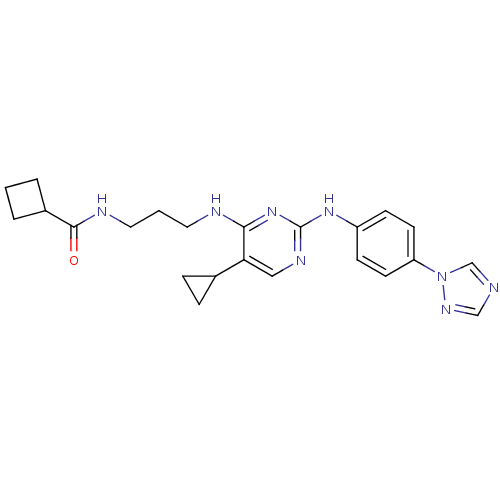
(CHEMBL2207199)Show SMILES O=C(NCCCNc1nc(Nc2ccc(cc2)-n2cncn2)ncc1C1CC1)C1CCC1 Show InChI InChI=1S/C23H28N8O/c32-22(17-3-1-4-17)26-12-2-11-25-21-20(16-5-6-16)13-27-23(30-21)29-18-7-9-19(10-8-18)31-15-24-14-28-31/h7-10,13-17H,1-6,11-12H2,(H,26,32)(H2,25,27,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 by radiometry |
Bioorg Med Chem Lett 22: 7169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.063
BindingDB Entry DOI: 10.7270/Q2TT4S45 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50401903

(CHEMBL2207194)Show SMILES O=C(NCCCNc1nc(Nc2cccc(Cn3cncn3)c2)ncc1C1CC1)C1CCC1 Show InChI InChI=1S/C24H30N8O/c33-23(19-5-2-6-19)27-11-3-10-26-22-21(18-8-9-18)13-28-24(31-22)30-20-7-1-4-17(12-20)14-32-16-25-15-29-32/h1,4,7,12-13,15-16,18-19H,2-3,5-6,8-11,14H2,(H,27,33)(H2,26,28,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 by radiometry |
Bioorg Med Chem Lett 22: 7169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.063
BindingDB Entry DOI: 10.7270/Q2TT4S45 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50354913
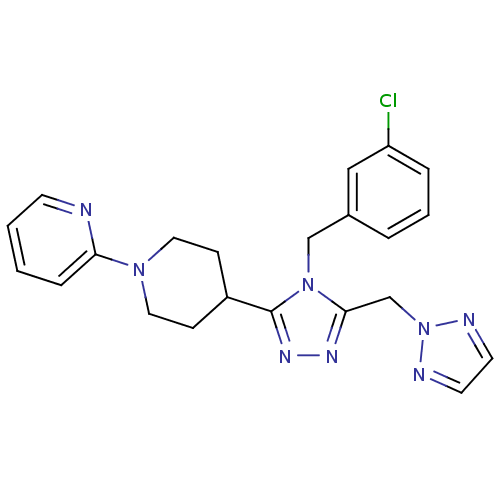
(CHEMBL1837028)Show SMILES Clc1cccc(Cn2c(Cn3nccn3)nnc2C2CCN(CC2)c2ccccn2)c1 Show InChI InChI=1S/C22H23ClN8/c23-19-5-3-4-17(14-19)15-30-21(16-31-25-10-11-26-31)27-28-22(30)18-7-12-29(13-8-18)20-6-1-2-9-24-20/h1-6,9-11,14,18H,7-8,12-13,15-16H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human V1A receptor expressed in CHO cells assessed as inhibition of AVP-induced intracellular calcium release after 30 seconds... |
Bioorg Med Chem Lett 21: 5684-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.038
BindingDB Entry DOI: 10.7270/Q2TB179T |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50354895
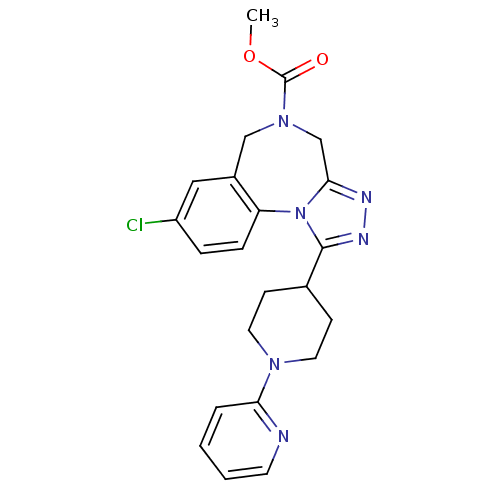
(CHEMBL1837038)Show SMILES COC(=O)N1Cc2nnc(C3CCN(CC3)c3ccccn3)n2-c2ccc(Cl)cc2C1 Show InChI InChI=1S/C22H23ClN6O2/c1-31-22(30)28-13-16-12-17(23)5-6-18(16)29-20(14-28)25-26-21(29)15-7-10-27(11-8-15)19-4-2-3-9-24-19/h2-6,9,12,15H,7-8,10-11,13-14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human V1A receptor expressed in CHO cells assessed as inhibition of AVP-induced intracellular calcium release after 30 seconds... |
Bioorg Med Chem Lett 21: 5684-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.038
BindingDB Entry DOI: 10.7270/Q2TB179T |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2 [G2019S]
(Homo sapiens (Human)) | BDBM482266
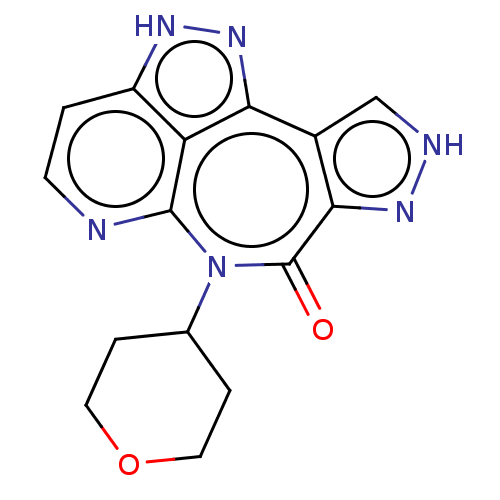
(US10918645, Example 65)Show SMILES O=c1n(C2CCOCC2)c2nccc3[nH]nc(c4c[nH]nc14)c23 Show InChI InChI=1S/C15H14N6O2/c22-15-13-9(7-17-19-13)12-11-10(18-20-12)1-4-16-14(11)21(15)8-2-5-23-6-3-8/h1,4,7-8H,2-3,5-6H2,(H,17,19)(H,18,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
LIFEARC; SUZHOU YABAO PHARMACEUTICAL R&D CO., LTD.
US Patent
| Assay Description
TTBK1 (5-20 mU diluted in 50 mM Tris pH 7.5, 0.1 mM EGTA, 0.1% β-mercaptoethanol, 1 mg/ml BSA, 10 mM DTT) is assayed against RRKDLHDDEEDEAMSITA ... |
US Patent US10918645 (2021)
BindingDB Entry DOI: 10.7270/Q20Z76CB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50401914

(CHEMBL2207213)Show SMILES O=C(NCCCNc1nc(Nc2cnn(c2)C2CCCC2)ncc1C1CC1)C1CCC1 Show InChI InChI=1S/C23H33N7O/c31-22(17-5-3-6-17)25-12-4-11-24-21-20(16-9-10-16)14-26-23(29-21)28-18-13-27-30(15-18)19-7-1-2-8-19/h13-17,19H,1-12H2,(H,25,31)(H2,24,26,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 by radiometry |
Bioorg Med Chem Lett 22: 7169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.063
BindingDB Entry DOI: 10.7270/Q2TT4S45 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50401918
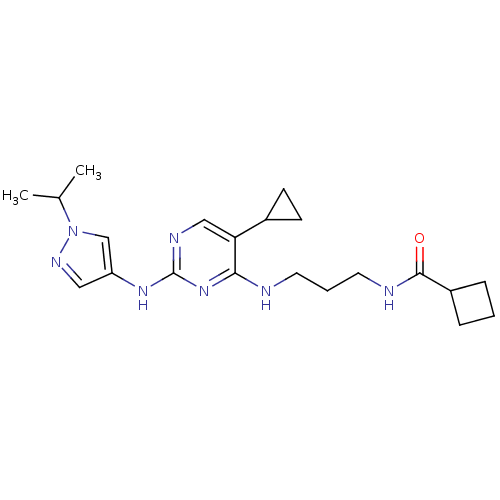
(CHEMBL2207209)Show SMILES CC(C)n1cc(Nc2ncc(C3CC3)c(NCCCNC(=O)C3CCC3)n2)cn1 Show InChI InChI=1S/C21H31N7O/c1-14(2)28-13-17(11-25-28)26-21-24-12-18(15-7-8-15)19(27-21)22-9-4-10-23-20(29)16-5-3-6-16/h11-16H,3-10H2,1-2H3,(H,23,29)(H2,22,24,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 by radiometry |
Bioorg Med Chem Lett 22: 7169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.063
BindingDB Entry DOI: 10.7270/Q2TT4S45 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50354896
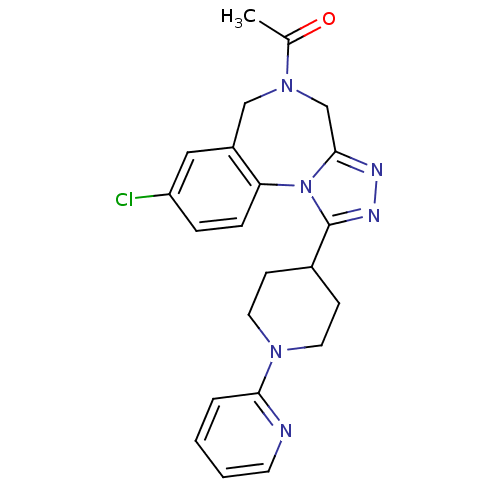
(CHEMBL1837040)Show SMILES CC(=O)N1Cc2nnc(C3CCN(CC3)c3ccccn3)n2-c2ccc(Cl)cc2C1 Show InChI InChI=1S/C22H23ClN6O/c1-15(30)28-13-17-12-18(23)5-6-19(17)29-21(14-28)25-26-22(29)16-7-10-27(11-8-16)20-4-2-3-9-24-20/h2-6,9,12,16H,7-8,10-11,13-14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human V1A receptor expressed in CHO cells assessed as inhibition of AVP-induced intracellular calcium release after 30 seconds... |
Bioorg Med Chem Lett 21: 5684-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.038
BindingDB Entry DOI: 10.7270/Q2TB179T |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50401910
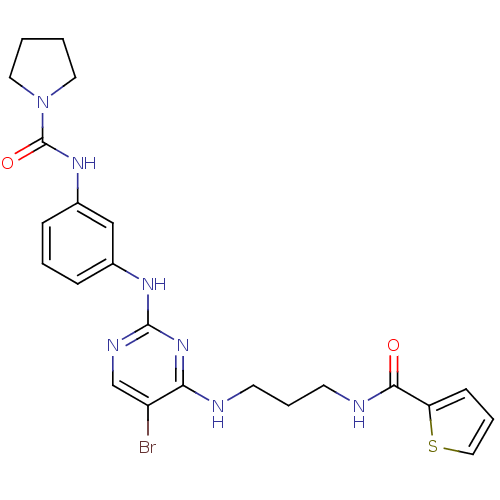
(CHEMBL2207217)Show SMILES Brc1cnc(Nc2cccc(NC(=O)N3CCCC3)c2)nc1NCCCNC(=O)c1cccs1 Show InChI InChI=1S/C23H26BrN7O2S/c24-18-15-27-22(30-20(18)25-9-5-10-26-21(32)19-8-4-13-34-19)28-16-6-3-7-17(14-16)29-23(33)31-11-1-2-12-31/h3-4,6-8,13-15H,1-2,5,9-12H2,(H,26,32)(H,29,33)(H2,25,27,28,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 by radiometry |
Bioorg Med Chem Lett 22: 7169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.063
BindingDB Entry DOI: 10.7270/Q2TT4S45 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50401902
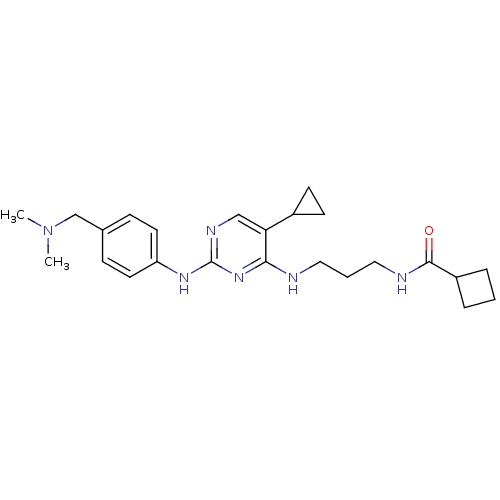
(CHEMBL2207196)Show SMILES CN(C)Cc1ccc(Nc2ncc(C3CC3)c(NCCCNC(=O)C3CCC3)n2)cc1 Show InChI InChI=1S/C24H34N6O/c1-30(2)16-17-7-11-20(12-8-17)28-24-27-15-21(18-9-10-18)22(29-24)25-13-4-14-26-23(31)19-5-3-6-19/h7-8,11-12,15,18-19H,3-6,9-10,13-14,16H2,1-2H3,(H,26,31)(H2,25,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 by radiometry |
Bioorg Med Chem Lett 22: 7169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.063
BindingDB Entry DOI: 10.7270/Q2TT4S45 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50401900
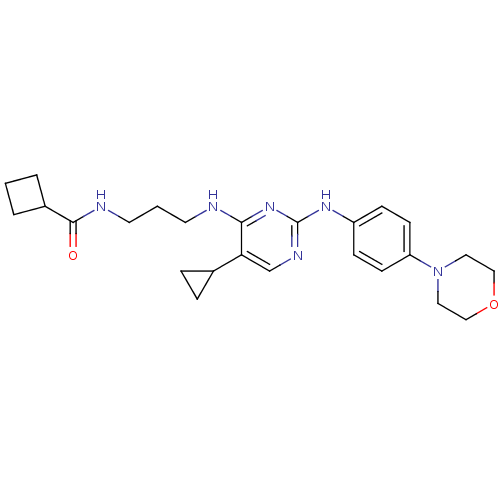
(CHEMBL2207198)Show SMILES O=C(NCCCNc1nc(Nc2ccc(cc2)N2CCOCC2)ncc1C1CC1)C1CCC1 Show InChI InChI=1S/C25H34N6O2/c32-24(19-3-1-4-19)27-12-2-11-26-23-22(18-5-6-18)17-28-25(30-23)29-20-7-9-21(10-8-20)31-13-15-33-16-14-31/h7-10,17-19H,1-6,11-16H2,(H,27,32)(H2,26,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 by radiometry |
Bioorg Med Chem Lett 22: 7169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.063
BindingDB Entry DOI: 10.7270/Q2TT4S45 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2 [G2019S]
(Homo sapiens (Human)) | BDBM482288

(8-ethyl-4-(tetrahydro-2H-pyran-4- yl)-5,11-dihydro...)Show SMILES CCc1cc2-c3n[nH]c4ccnc(N(Cc2cn1)C1CCOCC1)c34 Show InChI InChI=1S/C19H21N5O/c1-2-13-9-15-12(10-21-13)11-24(14-4-7-25-8-5-14)19-17-16(3-6-20-19)22-23-18(15)17/h3,6,9-10,14H,2,4-5,7-8,11H2,1H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
LIFEARC; SUZHOU YABAO PHARMACEUTICAL R&D CO., LTD.
US Patent
| Assay Description
TTBK1 (5-20 mU diluted in 50 mM Tris pH 7.5, 0.1 mM EGTA, 0.1% β-mercaptoethanol, 1 mg/ml BSA, 10 mM DTT) is assayed against RRKDLHDDEEDEAMSITA ... |
US Patent US10918645 (2021)
BindingDB Entry DOI: 10.7270/Q20Z76CB |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50354894
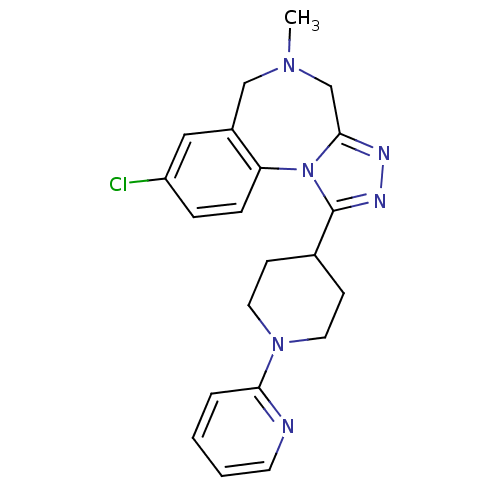
(CHEMBL1837037)Show SMILES CN1Cc2nnc(C3CCN(CC3)c3ccccn3)n2-c2ccc(Cl)cc2C1 Show InChI InChI=1S/C21H23ClN6/c1-26-13-16-12-17(22)5-6-18(16)28-20(14-26)24-25-21(28)15-7-10-27(11-8-15)19-4-2-3-9-23-19/h2-6,9,12,15H,7-8,10-11,13-14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human V1A receptor expressed in CHO cells assessed as inhibition of AVP-induced intracellular calcium release after 30 seconds... |
Bioorg Med Chem Lett 21: 5684-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.038
BindingDB Entry DOI: 10.7270/Q2TB179T |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50401922
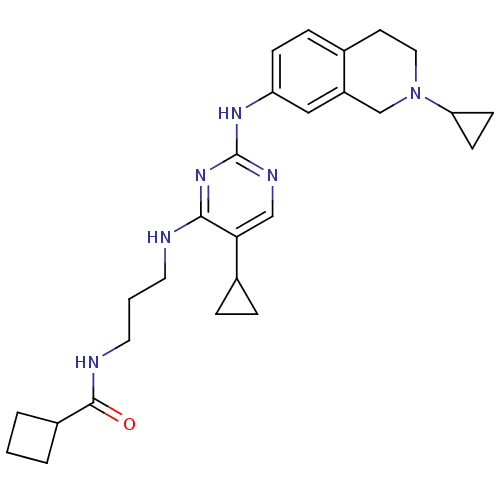
(CHEMBL2207204)Show SMILES O=C(NCCCNc1nc(Nc2ccc3CCN(Cc3c2)C2CC2)ncc1C1CC1)C1CCC1 Show InChI InChI=1S/C27H36N6O/c34-26(20-3-1-4-20)29-13-2-12-28-25-24(19-5-6-19)16-30-27(32-25)31-22-8-7-18-11-14-33(23-9-10-23)17-21(18)15-22/h7-8,15-16,19-20,23H,1-6,9-14,17H2,(H,29,34)(H2,28,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 by radiometry |
Bioorg Med Chem Lett 22: 7169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.063
BindingDB Entry DOI: 10.7270/Q2TT4S45 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50401920
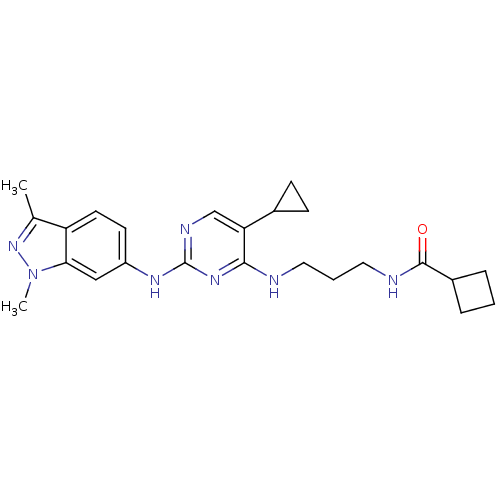
(CHEMBL2207206)Show SMILES Cc1nn(C)c2cc(Nc3ncc(C4CC4)c(NCCCNC(=O)C4CCC4)n3)ccc12 Show InChI InChI=1S/C24H31N7O/c1-15-19-10-9-18(13-21(19)31(2)30-15)28-24-27-14-20(16-7-8-16)22(29-24)25-11-4-12-26-23(32)17-5-3-6-17/h9-10,13-14,16-17H,3-8,11-12H2,1-2H3,(H,26,32)(H2,25,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 by radiometry |
Bioorg Med Chem Lett 22: 7169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.063
BindingDB Entry DOI: 10.7270/Q2TT4S45 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50401911
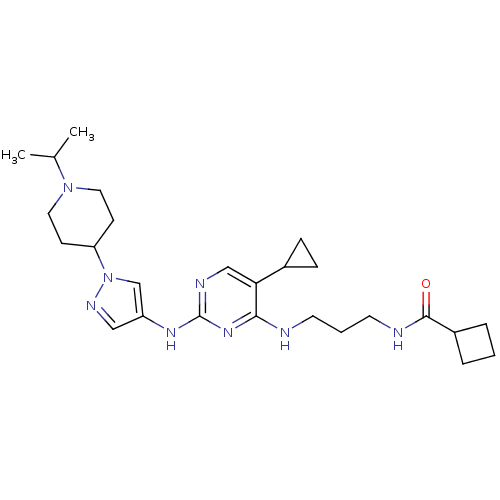
(CHEMBL2207216)Show SMILES CC(C)N1CCC(CC1)n1cc(Nc2ncc(C3CC3)c(NCCCNC(=O)C3CCC3)n2)cn1 Show InChI InChI=1S/C26H40N8O/c1-18(2)33-13-9-22(10-14-33)34-17-21(15-30-34)31-26-29-16-23(19-7-8-19)24(32-26)27-11-4-12-28-25(35)20-5-3-6-20/h15-20,22H,3-14H2,1-2H3,(H,28,35)(H2,27,29,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 by radiometry |
Bioorg Med Chem Lett 22: 7169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.063
BindingDB Entry DOI: 10.7270/Q2TT4S45 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2 [G2019S]
(Homo sapiens (Human)) | BDBM482268
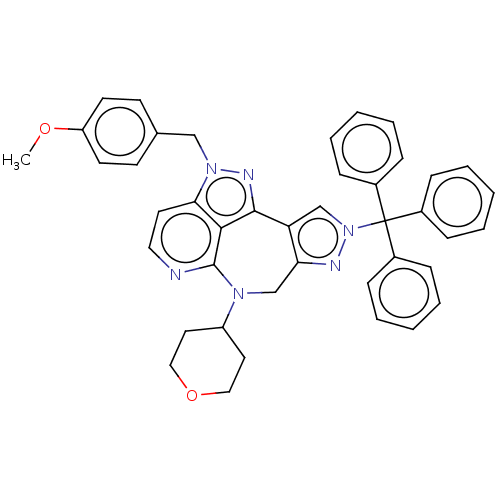
(US10918645, Example 66 | US10918645, Example 67)Show SMILES COc1ccc(Cn2nc3-c4cn(nc4CN(C4CCOCC4)c4nccc2c34)C(c2ccccc2)(c2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C42H38N6O2/c1-49-35-19-17-30(18-20-35)27-47-38-21-24-43-41-39(38)40(45-47)36-28-48(44-37(36)29-46(41)34-22-25-50-26-23-34)42(31-11-5-2-6-12-31,32-13-7-3-8-14-32)33-15-9-4-10-16-33/h2-21,24,28,34H,22-23,25-27,29H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
LIFEARC; SUZHOU YABAO PHARMACEUTICAL R&D CO., LTD.
US Patent
| Assay Description
TTBK1 (5-20 mU diluted in 50 mM Tris pH 7.5, 0.1 mM EGTA, 0.1% β-mercaptoethanol, 1 mg/ml BSA, 10 mM DTT) is assayed against RRKDLHDDEEDEAMSITA ... |
US Patent US10918645 (2021)
BindingDB Entry DOI: 10.7270/Q20Z76CB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50401917
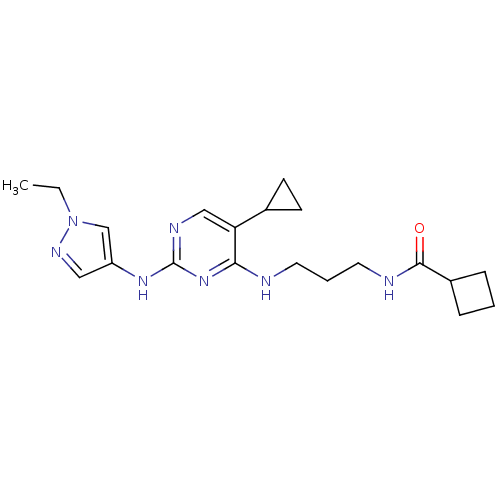
(CHEMBL2207210)Show SMILES CCn1cc(Nc2ncc(C3CC3)c(NCCCNC(=O)C3CCC3)n2)cn1 Show InChI InChI=1S/C20H29N7O/c1-2-27-13-16(11-24-27)25-20-23-12-17(14-7-8-14)18(26-20)21-9-4-10-22-19(28)15-5-3-6-15/h11-15H,2-10H2,1H3,(H,22,28)(H2,21,23,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 by radiometry |
Bioorg Med Chem Lett 22: 7169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.063
BindingDB Entry DOI: 10.7270/Q2TT4S45 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50401899

(CHEMBL2207208)Show SMILES Cn1cc(Nc2ncc(C3CC3)c(NCCCNC(=O)C3CCC3)n2)cn1 Show InChI InChI=1S/C19H27N7O/c1-26-12-15(10-23-26)24-19-22-11-16(13-6-7-13)17(25-19)20-8-3-9-21-18(27)14-4-2-5-14/h10-14H,2-9H2,1H3,(H,21,27)(H2,20,22,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 by radiometry |
Bioorg Med Chem Lett 22: 7169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.063
BindingDB Entry DOI: 10.7270/Q2TT4S45 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50401906
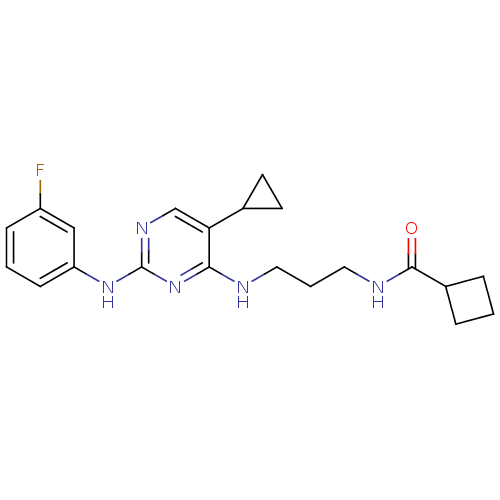
(CHEMBL2207187)Show SMILES Fc1cccc(Nc2ncc(C3CC3)c(NCCCNC(=O)C3CCC3)n2)c1 Show InChI InChI=1S/C21H26FN5O/c22-16-6-2-7-17(12-16)26-21-25-13-18(14-8-9-14)19(27-21)23-10-3-11-24-20(28)15-4-1-5-15/h2,6-7,12-15H,1,3-5,8-11H2,(H,24,28)(H2,23,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 by radiometry |
Bioorg Med Chem Lett 22: 7169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.063
BindingDB Entry DOI: 10.7270/Q2TT4S45 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50401908
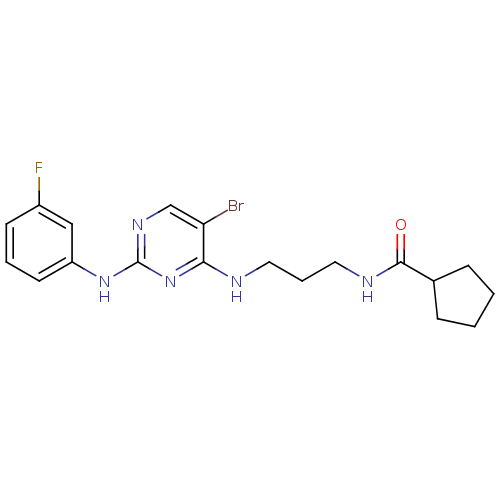
(CHEMBL2207180)Show SMILES Fc1cccc(Nc2ncc(Br)c(NCCCNC(=O)C3CCCC3)n2)c1 Show InChI InChI=1S/C19H23BrFN5O/c20-16-12-24-19(25-15-8-3-7-14(21)11-15)26-17(16)22-9-4-10-23-18(27)13-5-1-2-6-13/h3,7-8,11-13H,1-2,4-6,9-10H2,(H,23,27)(H2,22,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 by radiometry |
Bioorg Med Chem Lett 22: 7169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.063
BindingDB Entry DOI: 10.7270/Q2TT4S45 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50401901
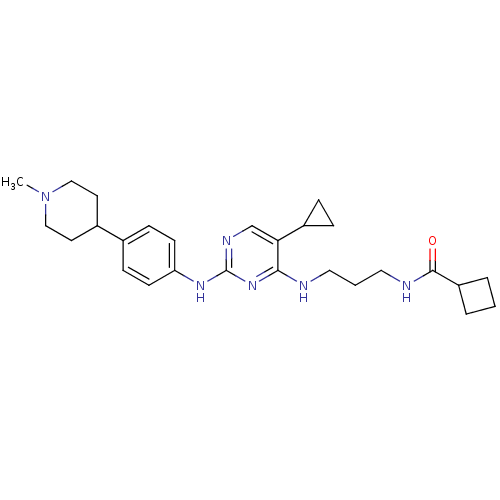
(CHEMBL2207197)Show SMILES CN1CCC(CC1)c1ccc(Nc2ncc(C3CC3)c(NCCCNC(=O)C3CCC3)n2)cc1 Show InChI InChI=1S/C27H38N6O/c1-33-16-12-20(13-17-33)19-8-10-23(11-9-19)31-27-30-18-24(21-6-7-21)25(32-27)28-14-3-15-29-26(34)22-4-2-5-22/h8-11,18,20-22H,2-7,12-17H2,1H3,(H,29,34)(H2,28,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 by radiometry |
Bioorg Med Chem Lett 22: 7169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.063
BindingDB Entry DOI: 10.7270/Q2TT4S45 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50401924
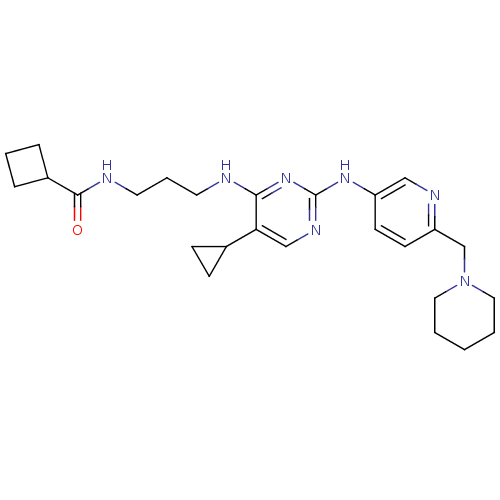
(CHEMBL2207202)Show SMILES O=C(NCCCNc1nc(Nc2ccc(CN3CCCCC3)nc2)ncc1C1CC1)C1CCC1 Show InChI InChI=1S/C26H37N7O/c34-25(20-6-4-7-20)28-13-5-12-27-24-23(19-8-9-19)17-30-26(32-24)31-21-10-11-22(29-16-21)18-33-14-2-1-3-15-33/h10-11,16-17,19-20H,1-9,12-15,18H2,(H,28,34)(H2,27,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 by radiometry |
Bioorg Med Chem Lett 22: 7169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.063
BindingDB Entry DOI: 10.7270/Q2TT4S45 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50401912
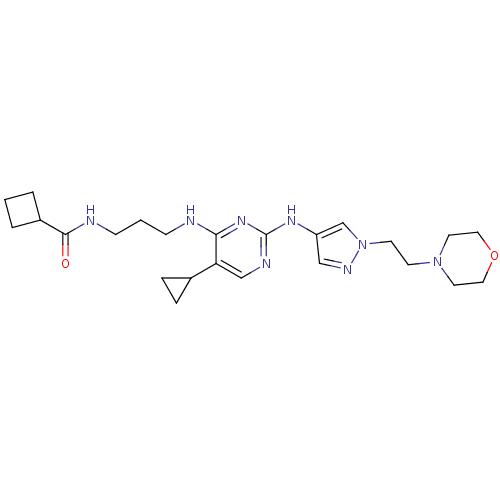
(CHEMBL2207215)Show SMILES O=C(NCCCNc1nc(Nc2cnn(CCN3CCOCC3)c2)ncc1C1CC1)C1CCC1 Show InChI InChI=1S/C24H36N8O2/c33-23(19-3-1-4-19)26-8-2-7-25-22-21(18-5-6-18)16-27-24(30-22)29-20-15-28-32(17-20)10-9-31-11-13-34-14-12-31/h15-19H,1-14H2,(H,26,33)(H2,25,27,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 by radiometry |
Bioorg Med Chem Lett 22: 7169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.063
BindingDB Entry DOI: 10.7270/Q2TT4S45 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50354914
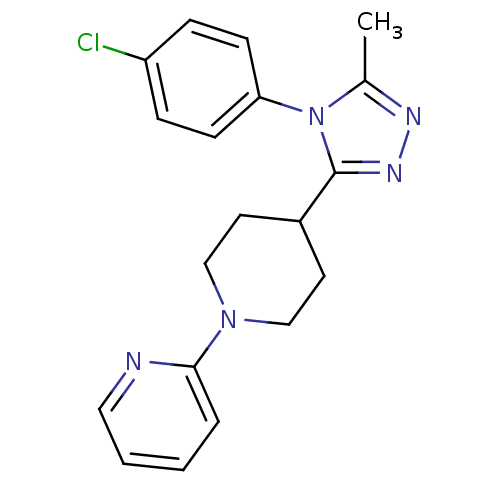
(CHEMBL1837017)Show InChI InChI=1S/C19H20ClN5/c1-14-22-23-19(25(14)17-7-5-16(20)6-8-17)15-9-12-24(13-10-15)18-4-2-3-11-21-18/h2-8,11,15H,9-10,12-13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human V1A receptor expressed in CHO cells assessed as inhibition of AVP-induced intracellular calcium release after 30 seconds... |
Bioorg Med Chem Lett 21: 5684-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.038
BindingDB Entry DOI: 10.7270/Q2TB179T |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50401909

(CHEMBL2207218)Show SMILES Fc1cccc(Nc2ncc(Br)c(NCCCNC(=O)c3cccs3)n2)c1 Show InChI InChI=1S/C18H17BrFN5OS/c19-14-11-23-18(24-13-5-1-4-12(20)10-13)25-16(14)21-7-3-8-22-17(26)15-6-2-9-27-15/h1-2,4-6,9-11H,3,7-8H2,(H,22,26)(H2,21,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 by radiometry |
Bioorg Med Chem Lett 22: 7169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.063
BindingDB Entry DOI: 10.7270/Q2TT4S45 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50401916
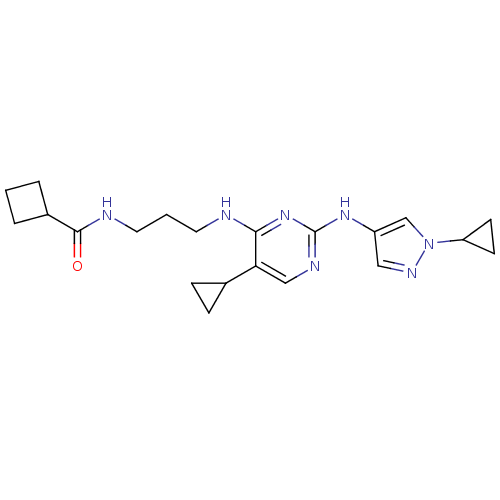
(CHEMBL2207211)Show SMILES O=C(NCCCNc1nc(Nc2cnn(c2)C2CC2)ncc1C1CC1)C1CCC1 Show InChI InChI=1S/C21H29N7O/c29-20(15-3-1-4-15)23-10-2-9-22-19-18(14-5-6-14)12-24-21(27-19)26-16-11-25-28(13-16)17-7-8-17/h11-15,17H,1-10H2,(H,23,29)(H2,22,24,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 by radiometry |
Bioorg Med Chem Lett 22: 7169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.063
BindingDB Entry DOI: 10.7270/Q2TT4S45 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50401905
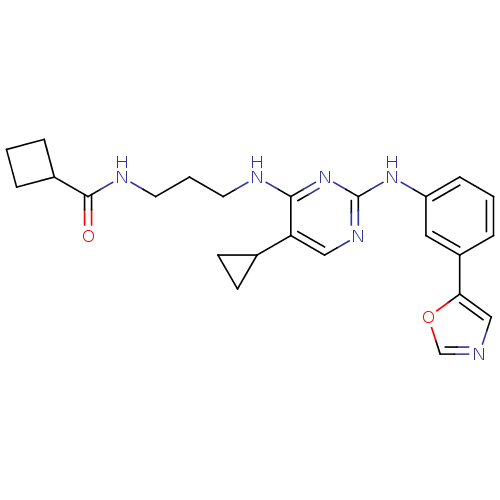
(CHEMBL2207192)Show SMILES O=C(NCCCNc1nc(Nc2cccc(c2)-c2cnco2)ncc1C1CC1)C1CCC1 Show InChI InChI=1S/C24H28N6O2/c31-23(17-4-1-5-17)27-11-3-10-26-22-20(16-8-9-16)13-28-24(30-22)29-19-7-2-6-18(12-19)21-14-25-15-32-21/h2,6-7,12-17H,1,3-5,8-11H2,(H,27,31)(H2,26,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 by radiometry |
Bioorg Med Chem Lett 22: 7169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.063
BindingDB Entry DOI: 10.7270/Q2TT4S45 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2 [G2019S]
(Homo sapiens (Human)) | BDBM482275
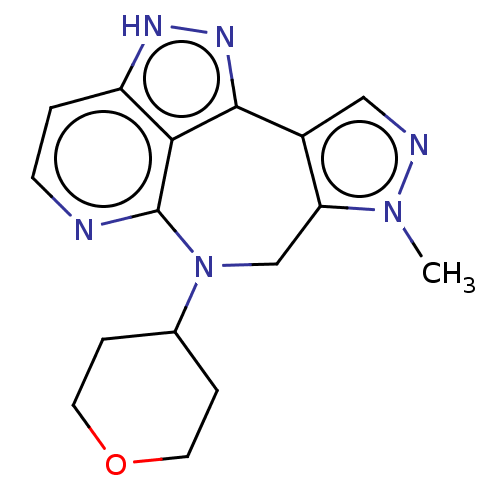
(1-methyl-9-(tetrahydro-2H-pyran- 4-yl)-1,5,9,10-te...)Show InChI InChI=1S/C16H18N6O/c1-21-13-9-22(10-3-6-23-7-4-10)16-14-12(2-5-17-16)19-20-15(14)11(13)8-18-21/h2,5,8,10H,3-4,6-7,9H2,1H3,(H,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
LIFEARC; SUZHOU YABAO PHARMACEUTICAL R&D CO., LTD.
US Patent
| Assay Description
TTBK1 (5-20 mU diluted in 50 mM Tris pH 7.5, 0.1 mM EGTA, 0.1% β-mercaptoethanol, 1 mg/ml BSA, 10 mM DTT) is assayed against RRKDLHDDEEDEAMSITA ... |
US Patent US10918645 (2021)
BindingDB Entry DOI: 10.7270/Q20Z76CB |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2 [G2019S]
(Homo sapiens (Human)) | BDBM482286

(8-methyl-4-(tetrahydro-2H-pyran-4- yl)-5,11-dihydr...)Show InChI InChI=1S/C18H19N5O/c1-11-8-14-12(9-20-11)10-23(13-3-6-24-7-4-13)18-16-15(2-5-19-18)21-22-17(14)16/h2,5,8-9,13H,3-4,6-7,10H2,1H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
LIFEARC; SUZHOU YABAO PHARMACEUTICAL R&D CO., LTD.
US Patent
| Assay Description
TTBK1 (5-20 mU diluted in 50 mM Tris pH 7.5, 0.1 mM EGTA, 0.1% β-mercaptoethanol, 1 mg/ml BSA, 10 mM DTT) is assayed against RRKDLHDDEEDEAMSITA ... |
US Patent US10918645 (2021)
BindingDB Entry DOI: 10.7270/Q20Z76CB |
More data for this
Ligand-Target Pair | |
MAP/microtubule affinity-regulating kinase 3
(Homo sapiens (Human)) | BDBM50401900

(CHEMBL2207198)Show SMILES O=C(NCCCNc1nc(Nc2ccc(cc2)N2CCOCC2)ncc1C1CC1)C1CCC1 Show InChI InChI=1S/C25H34N6O2/c32-24(19-3-1-4-19)27-12-2-11-26-23-22(18-5-6-18)17-28-25(30-23)29-20-7-9-21(10-8-20)31-13-15-33-16-14-31/h7-10,17-19H,1-6,11-16H2,(H,27,32)(H2,26,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of MARK3 |
Bioorg Med Chem Lett 22: 7169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.063
BindingDB Entry DOI: 10.7270/Q2TT4S45 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50401926

(CHEMBL2207200)Show InChI InChI=1S/C20H26N6O/c27-19(15-4-1-5-15)23-11-3-10-22-18-17(14-7-8-14)13-24-20(26-18)25-16-6-2-9-21-12-16/h2,6,9,12-15H,1,3-5,7-8,10-11H2,(H,23,27)(H2,22,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 by radiometry |
Bioorg Med Chem Lett 22: 7169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.063
BindingDB Entry DOI: 10.7270/Q2TT4S45 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2 [G2019S]
(Homo sapiens (Human)) | BDBM482290
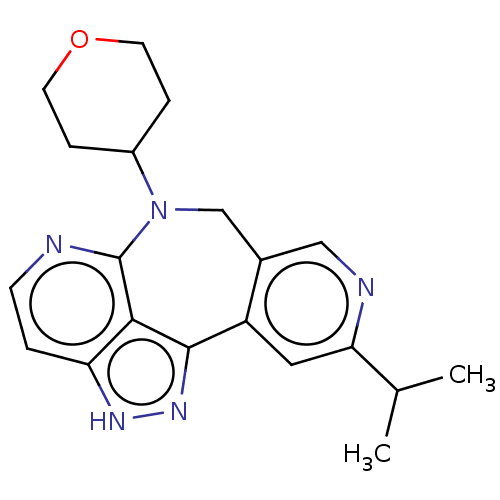
(8-isopropyl-4-(tetrahydro-2H-pyran- 4-yl)-5,11-dih...)Show SMILES CC(C)c1cc2-c3n[nH]c4ccnc(N(Cc2cn1)C1CCOCC1)c34 Show InChI InChI=1S/C20H23N5O/c1-12(2)17-9-15-13(10-22-17)11-25(14-4-7-26-8-5-14)20-18-16(3-6-21-20)23-24-19(15)18/h3,6,9-10,12,14H,4-5,7-8,11H2,1-2H3,(H,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
LIFEARC; SUZHOU YABAO PHARMACEUTICAL R&D CO., LTD.
US Patent
| Assay Description
TTBK1 (5-20 mU diluted in 50 mM Tris pH 7.5, 0.1 mM EGTA, 0.1% β-mercaptoethanol, 1 mg/ml BSA, 10 mM DTT) is assayed against RRKDLHDDEEDEAMSITA ... |
US Patent US10918645 (2021)
BindingDB Entry DOI: 10.7270/Q20Z76CB |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50114031
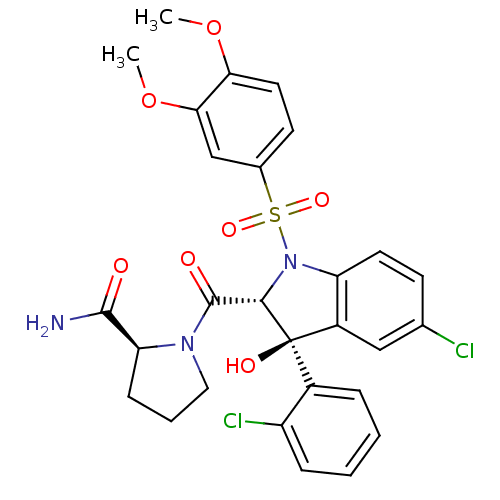
((2S)-1-[(2R,3S)-5-chloro-3-(2-chlorophenyl)-1-(3,4...)Show SMILES COc1ccc(cc1OC)S(=O)(=O)N1[C@@H](C(=O)N2CCC[C@H]2C(N)=O)[C@](O)(c2cc(Cl)ccc12)c1ccccc1Cl |r| Show InChI InChI=1S/C28H27Cl2N3O7S/c1-39-23-12-10-17(15-24(23)40-2)41(37,38)33-21-11-9-16(29)14-19(21)28(36,18-6-3-4-7-20(18)30)25(33)27(35)32-13-5-8-22(32)26(31)34/h3-4,6-7,9-12,14-15,22,25,36H,5,8,13H2,1-2H3,(H2,31,34)/t22-,25-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human V1A receptor expressed in CHO cells assessed as inhibition of AVP-induced intracellular calcium release after 30 seconds... |
Bioorg Med Chem Lett 21: 5684-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.038
BindingDB Entry DOI: 10.7270/Q2TB179T |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50401925
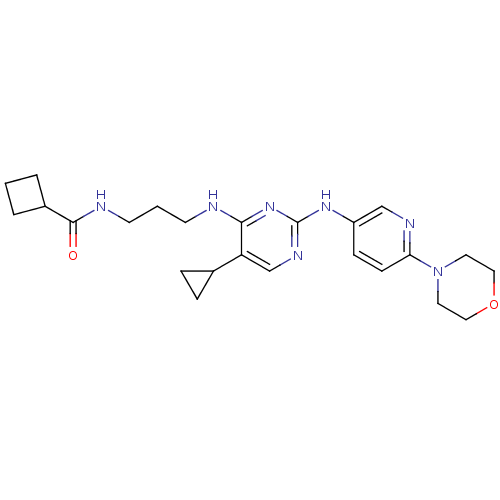
(CHEMBL2207201)Show SMILES O=C(NCCCNc1nc(Nc2ccc(nc2)N2CCOCC2)ncc1C1CC1)C1CCC1 Show InChI InChI=1S/C24H33N7O2/c32-23(18-3-1-4-18)26-10-2-9-25-22-20(17-5-6-17)16-28-24(30-22)29-19-7-8-21(27-15-19)31-11-13-33-14-12-31/h7-8,15-18H,1-6,9-14H2,(H,26,32)(H2,25,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 by radiometry |
Bioorg Med Chem Lett 22: 7169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.063
BindingDB Entry DOI: 10.7270/Q2TT4S45 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2 [G2019S]
(Homo sapiens (Human)) | BDBM482277
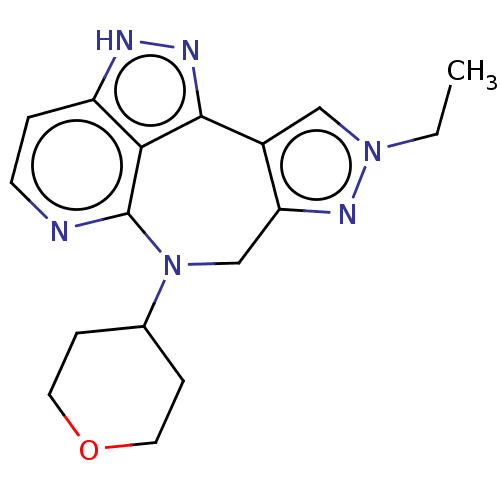
(2-ethyl-9-(tetrahydro-2H-pyran-4- yl)-2,5,9,10-tet...)Show SMILES CCn1cc-2c(CN(C3CCOCC3)c3nccc4[nH]nc-2c34)n1 Show InChI InChI=1S/C17H20N6O/c1-2-22-9-12-14(21-22)10-23(11-4-7-24-8-5-11)17-15-13(3-6-18-17)19-20-16(12)15/h3,6,9,11H,2,4-5,7-8,10H2,1H3,(H,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
LIFEARC; SUZHOU YABAO PHARMACEUTICAL R&D CO., LTD.
US Patent
| Assay Description
TTBK1 (5-20 mU diluted in 50 mM Tris pH 7.5, 0.1 mM EGTA, 0.1% β-mercaptoethanol, 1 mg/ml BSA, 10 mM DTT) is assayed against RRKDLHDDEEDEAMSITA ... |
US Patent US10918645 (2021)
BindingDB Entry DOI: 10.7270/Q20Z76CB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50401921
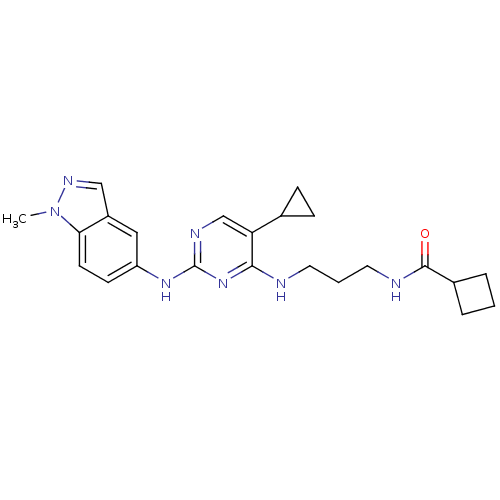
(CHEMBL2207205)Show SMILES Cn1ncc2cc(Nc3ncc(C4CC4)c(NCCCNC(=O)C4CCC4)n3)ccc12 Show InChI InChI=1S/C23H29N7O/c1-30-20-9-8-18(12-17(20)13-27-30)28-23-26-14-19(15-6-7-15)21(29-23)24-10-3-11-25-22(31)16-4-2-5-16/h8-9,12-16H,2-7,10-11H2,1H3,(H,25,31)(H2,24,26,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 by radiometry |
Bioorg Med Chem Lett 22: 7169-73 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.063
BindingDB Entry DOI: 10.7270/Q2TT4S45 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2 [G2019S]
(Homo sapiens (Human)) | BDBM482283
(1-isopropyl-9-(tetrahydro-2H- pyran-4-yl)-1,5,9,10...)Show SMILES CC(C)n1ncc-2c1CN(C1CCOCC1)c1nccc3[nH]nc-2c13 Show InChI InChI=1S/C18H22N6O/c1-11(2)24-15-10-23(12-4-7-25-8-5-12)18-16-14(3-6-19-18)21-22-17(16)13(15)9-20-24/h3,6,9,11-12H,4-5,7-8,10H2,1-2H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
LIFEARC; SUZHOU YABAO PHARMACEUTICAL R&D CO., LTD.
US Patent
| Assay Description
TTBK1 (5-20 mU diluted in 50 mM Tris pH 7.5, 0.1 mM EGTA, 0.1% β-mercaptoethanol, 1 mg/ml BSA, 10 mM DTT) is assayed against RRKDLHDDEEDEAMSITA ... |
US Patent US10918645 (2021)
BindingDB Entry DOI: 10.7270/Q20Z76CB |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50354916
(CHEMBL1837031)Show SMILES Clc1ccc-2c(COCc3nnc(C4CCN(CC4)c4ccccn4)n-23)c1 Show InChI InChI=1S/C20H20ClN5O/c21-16-4-5-17-15(11-16)12-27-13-19-23-24-20(26(17)19)14-6-9-25(10-7-14)18-3-1-2-8-22-18/h1-5,8,11,14H,6-7,9-10,12-13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human V1A receptor expressed in CHO cells assessed as inhibition of AVP-induced intracellular calcium release after 30 seconds... |
Bioorg Med Chem Lett 21: 5684-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.038
BindingDB Entry DOI: 10.7270/Q2TB179T |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50354915
(CHEMBL1837022)Show InChI InChI=1S/C23H29N5/c1-2-3-11-22-25-26-23(28(22)18-19-9-5-4-6-10-19)20-13-16-27(17-14-20)21-12-7-8-15-24-21/h4-10,12,15,20H,2-3,11,13-14,16-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human V1A receptor expressed in CHO cells assessed as inhibition of AVP-induced intracellular calcium release after 30 seconds... |
Bioorg Med Chem Lett 21: 5684-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.038
BindingDB Entry DOI: 10.7270/Q2TB179T |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50354917
(CHEMBL1837027)Show SMILES C(c1nnc(C2CCN(CC2)c2ccccn2)n1Cc1ccccc1)n1nccn1 Show InChI InChI=1S/C22H24N8/c1-2-6-18(7-3-1)16-29-21(17-30-24-12-13-25-30)26-27-22(29)19-9-14-28(15-10-19)20-8-4-5-11-23-20/h1-8,11-13,19H,9-10,14-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human V1A receptor expressed in CHO cells assessed as inhibition of AVP-induced intracellular calcium release after 30 seconds... |
Bioorg Med Chem Lett 21: 5684-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.038
BindingDB Entry DOI: 10.7270/Q2TB179T |
More data for this
Ligand-Target Pair | |
Voltage-dependent calcium channel alpha-2 delta subunit
(Sus scrofa) | BDBM50305900
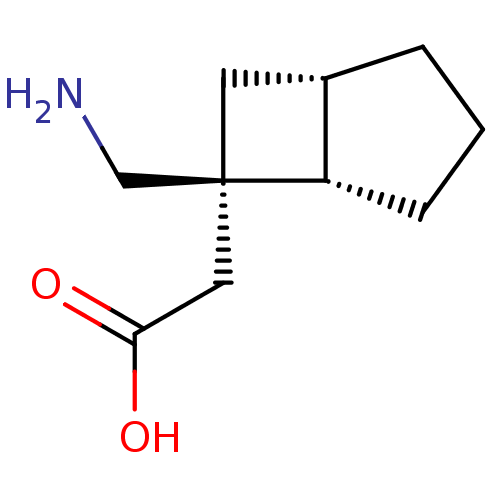
((+)-2-((1S,5S,6R)-6-(aminomethyl)bicyclo[3.2.0]hep...)Show InChI InChI=1S/C10H17NO2/c11-6-10(5-9(12)13)4-7-2-1-3-8(7)10/h7-8H,1-6,11H2,(H,12,13)/t7-,8-,10-/m0/s1 | PDB
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]gabapentin from calcium channel alpha2delta in pig cerebral cortex membrane after 30 mins |
Bioorg Med Chem Lett 20: 461-4 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.118
BindingDB Entry DOI: 10.7270/Q25H7GCQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data