 Found 191 hits with Last Name = 'caulfield' and Initial = 'w'
Found 191 hits with Last Name = 'caulfield' and Initial = 'w' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416116

(CHEMBL1084315)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)N2CCCNCC2)c2ccccc12 Show InChI InChI=1S/C25H27N3O4S/c1-32-23-11-12-24(20-9-3-2-8-19(20)23)33(30,31)28-17-21(18-7-4-5-10-22(18)28)25(29)27-15-6-13-26-14-16-27/h2-5,7-12,21,26H,6,13-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416120
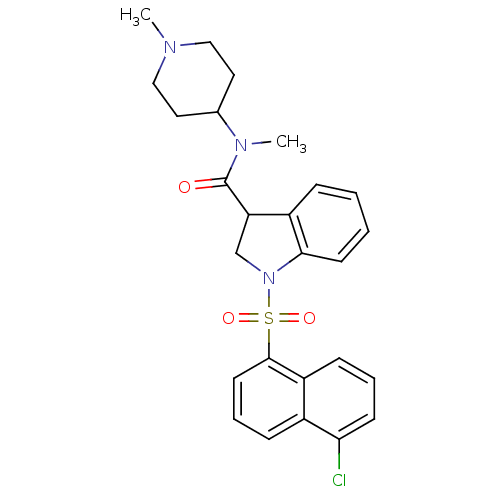
(CHEMBL1082484)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1cccc2c(Cl)cccc12 Show InChI InChI=1S/C26H28ClN3O3S/c1-28-15-13-18(14-16-28)29(2)26(31)22-17-30(24-11-4-3-7-20(22)24)34(32,33)25-12-6-8-19-21(25)9-5-10-23(19)27/h3-12,18,22H,13-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416108
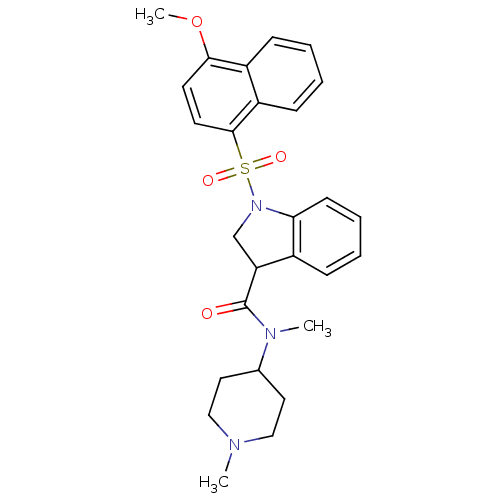
(CHEMBL1082818)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)N(C)C2CCN(C)CC2)c2ccccc12 Show InChI InChI=1S/C27H31N3O4S/c1-28-16-14-19(15-17-28)29(2)27(31)23-18-30(24-11-7-6-8-20(23)24)35(32,33)26-13-12-25(34-3)21-9-4-5-10-22(21)26/h4-13,19,23H,14-18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416114
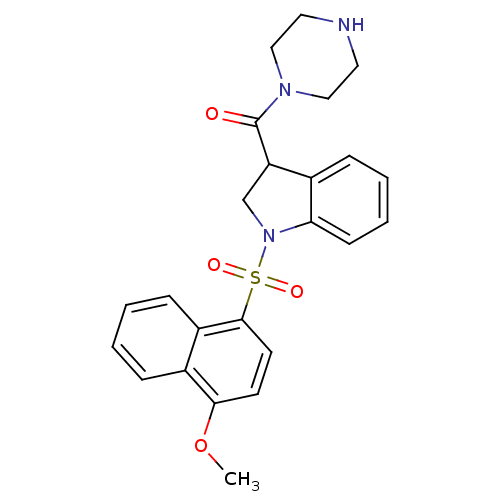
(CHEMBL1085608)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)N2CCNCC2)c2ccccc12 Show InChI InChI=1S/C24H25N3O4S/c1-31-22-10-11-23(19-8-3-2-7-18(19)22)32(29,30)27-16-20(17-6-4-5-9-21(17)27)24(28)26-14-12-25-13-15-26/h2-11,20,25H,12-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416117
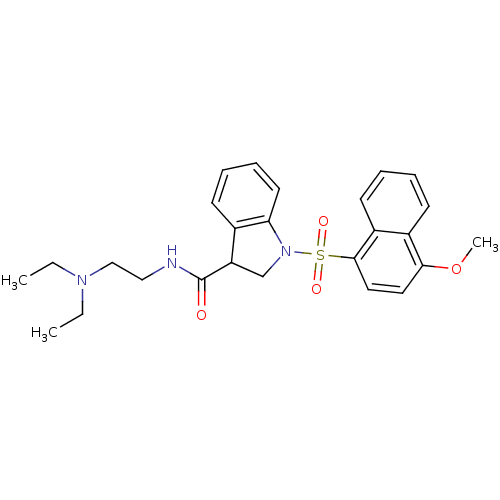
(CHEMBL1084316)Show SMILES CCN(CC)CCNC(=O)C1CN(c2ccccc12)S(=O)(=O)c1ccc(OC)c2ccccc12 Show InChI InChI=1S/C26H31N3O4S/c1-4-28(5-2)17-16-27-26(30)22-18-29(23-13-9-8-10-19(22)23)34(31,32)25-15-14-24(33-3)20-11-6-7-12-21(20)25/h6-15,22H,4-5,16-18H2,1-3H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416131
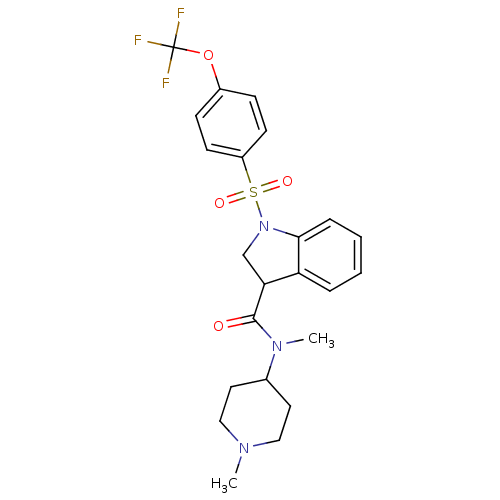
(CHEMBL1085965)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C23H26F3N3O4S/c1-27-13-11-16(12-14-27)28(2)22(30)20-15-29(21-6-4-3-5-19(20)21)34(31,32)18-9-7-17(8-10-18)33-23(24,25)26/h3-10,16,20H,11-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416132
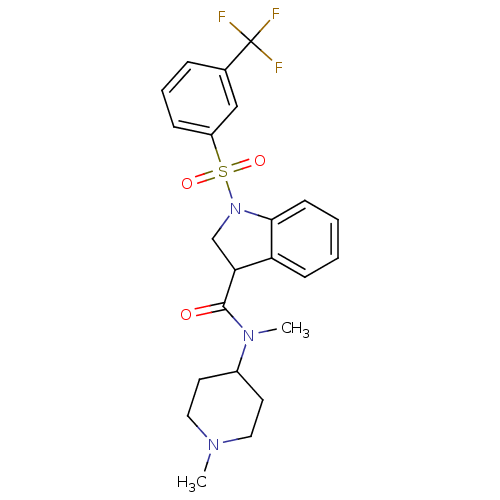
(CHEMBL1083297)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C23H26F3N3O3S/c1-27-12-10-17(11-13-27)28(2)22(30)20-15-29(21-9-4-3-8-19(20)21)33(31,32)18-7-5-6-16(14-18)23(24,25)26/h3-9,14,17,20H,10-13,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416122
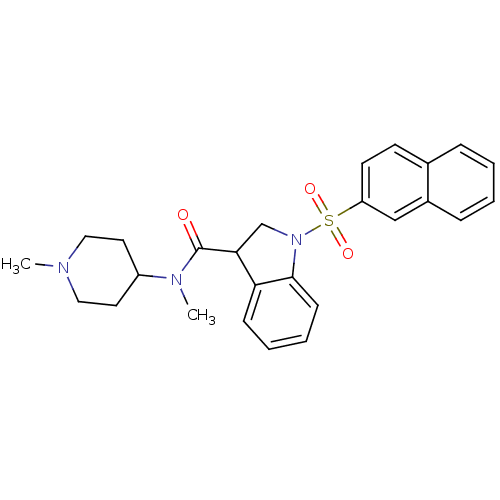
(CHEMBL1085351)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C26H29N3O3S/c1-27-15-13-21(14-16-27)28(2)26(30)24-18-29(25-10-6-5-9-23(24)25)33(31,32)22-12-11-19-7-3-4-8-20(19)17-22/h3-12,17,21,24H,13-16,18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416109
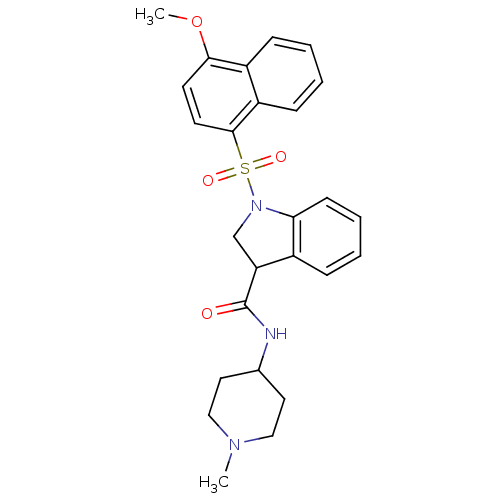
(CHEMBL1085835)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)NC2CCN(C)CC2)c2ccccc12 Show InChI InChI=1S/C26H29N3O4S/c1-28-15-13-18(14-16-28)27-26(30)22-17-29(23-10-6-5-7-19(22)23)34(31,32)25-12-11-24(33-2)20-8-3-4-9-21(20)25/h3-12,18,22H,13-17H2,1-2H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416130
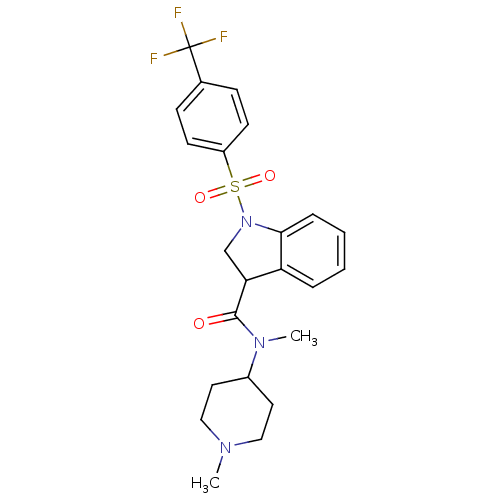
(CHEMBL1083909)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C23H26F3N3O3S/c1-27-13-11-17(12-14-27)28(2)22(30)20-15-29(21-6-4-3-5-19(20)21)33(31,32)18-9-7-16(8-10-18)23(24,25)26/h3-10,17,20H,11-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416113
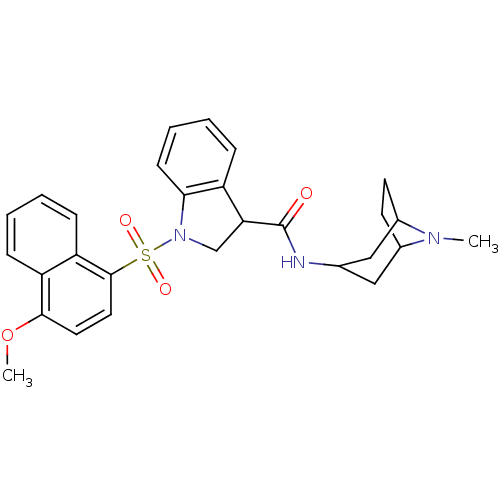
(CHEMBL1082494)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)NC2CC3CCC(C2)N3C)c2ccccc12 |TLB:20:21:24.25:28| Show InChI InChI=1S/C28H31N3O4S/c1-30-19-11-12-20(30)16-18(15-19)29-28(32)24-17-31(25-10-6-5-7-21(24)25)36(33,34)27-14-13-26(35-2)22-8-3-4-9-23(22)27/h3-10,13-14,18-20,24H,11-12,15-17H2,1-2H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416118
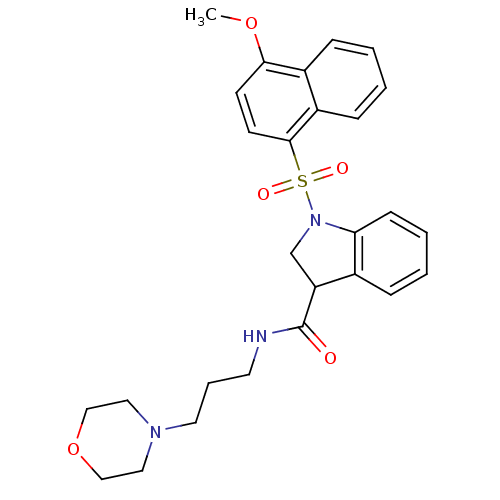
(CHEMBL1083122)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)NCCCN2CCOCC2)c2ccccc12 Show InChI InChI=1S/C27H31N3O5S/c1-34-25-11-12-26(22-9-3-2-8-21(22)25)36(32,33)30-19-23(20-7-4-5-10-24(20)30)27(31)28-13-6-14-29-15-17-35-18-16-29/h2-5,7-12,23H,6,13-19H2,1H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416123
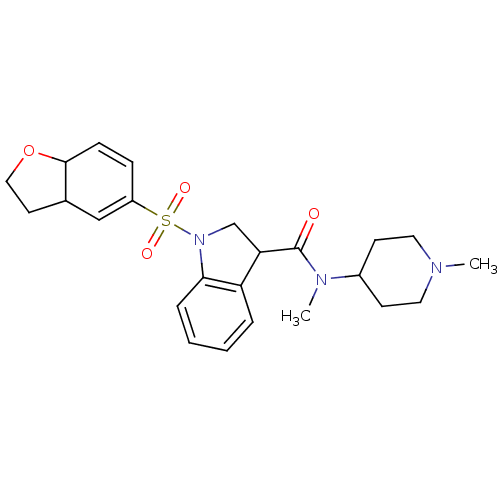
(CHEMBL1085352)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)C1=CC2CCOC2C=C1 |c:34,t:26| Show InChI InChI=1S/C24H31N3O4S/c1-25-12-9-18(10-13-25)26(2)24(28)21-16-27(22-6-4-3-5-20(21)22)32(29,30)19-7-8-23-17(15-19)11-14-31-23/h3-8,15,17-18,21,23H,9-14,16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416136
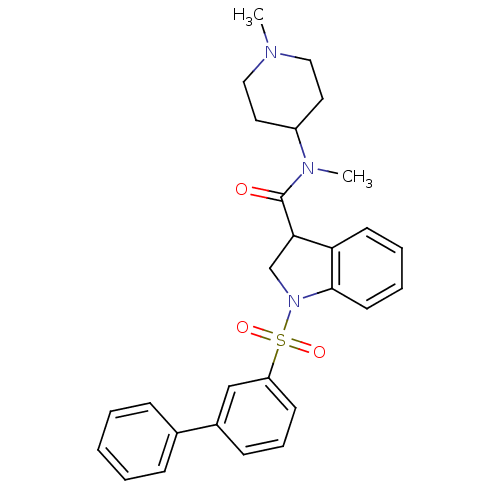
(CHEMBL1084483)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C28H31N3O3S/c1-29-17-15-23(16-18-29)30(2)28(32)26-20-31(27-14-7-6-13-25(26)27)35(33,34)24-12-8-11-22(19-24)21-9-4-3-5-10-21/h3-14,19,23,26H,15-18,20H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416121
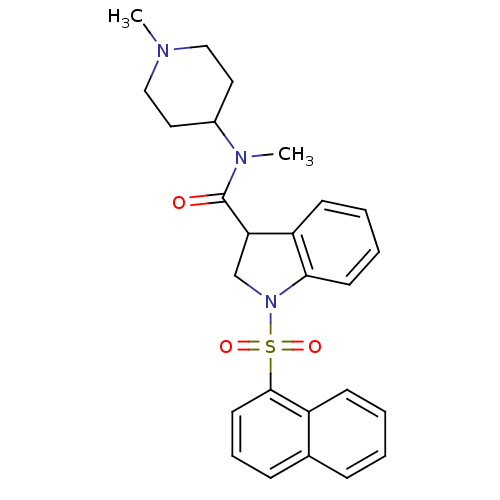
(CHEMBL1082485)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C26H29N3O3S/c1-27-16-14-20(15-17-27)28(2)26(30)23-18-29(24-12-6-5-11-22(23)24)33(31,32)25-13-7-9-19-8-3-4-10-21(19)25/h3-13,20,23H,14-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416124
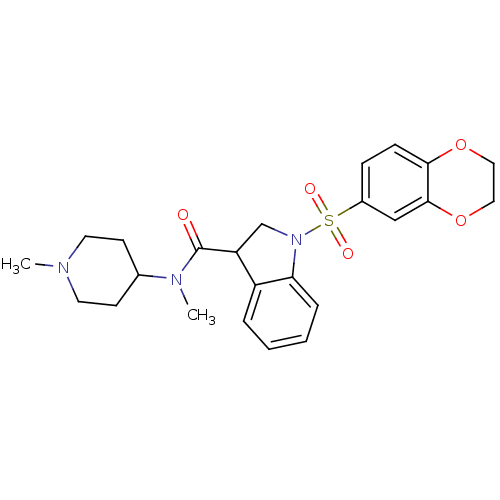
(CHEMBL1085353)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1ccc2OCCOc2c1 Show InChI InChI=1S/C24H29N3O5S/c1-25-11-9-17(10-12-25)26(2)24(28)20-16-27(21-6-4-3-5-19(20)21)33(29,30)18-7-8-22-23(15-18)32-14-13-31-22/h3-8,15,17,20H,9-14,16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416110

(CHEMBL1085836)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)NC2CCN(Cc3ccccc3)CC2)c2ccccc12 Show InChI InChI=1S/C32H33N3O4S/c1-39-30-15-16-31(27-13-6-5-12-26(27)30)40(37,38)35-22-28(25-11-7-8-14-29(25)35)32(36)33-24-17-19-34(20-18-24)21-23-9-3-2-4-10-23/h2-16,24,28H,17-22H2,1H3,(H,33,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416127
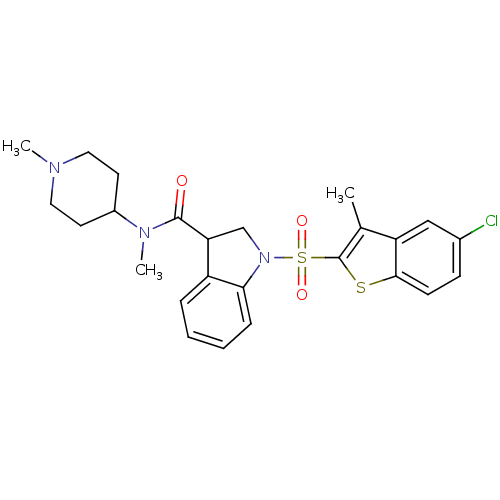
(CHEMBL1082765)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1sc2ccc(Cl)cc2c1C Show InChI InChI=1S/C25H28ClN3O3S2/c1-16-20-14-17(26)8-9-23(20)33-25(16)34(31,32)29-15-21(19-6-4-5-7-22(19)29)24(30)28(3)18-10-12-27(2)13-11-18/h4-9,14,18,21H,10-13,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 63.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416115
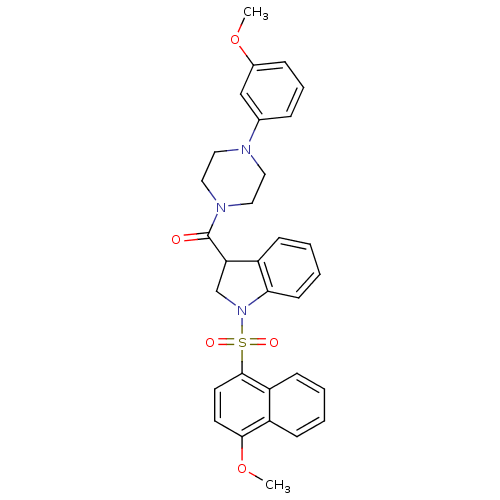
(CHEMBL1084614)Show SMILES COc1cccc(c1)N1CCN(CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1ccc(OC)c2ccccc12 Show InChI InChI=1S/C31H31N3O5S/c1-38-23-9-7-8-22(20-23)32-16-18-33(19-17-32)31(35)27-21-34(28-13-6-5-10-24(27)28)40(36,37)30-15-14-29(39-2)25-11-3-4-12-26(25)30/h3-15,20,27H,16-19,21H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416138
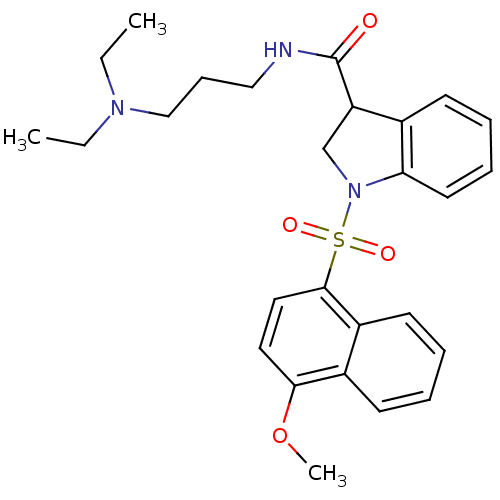
(CHEMBL1084317)Show SMILES CCN(CC)CCCNC(=O)C1CN(c2ccccc12)S(=O)(=O)c1ccc(OC)c2ccccc12 Show InChI InChI=1S/C27H33N3O4S/c1-4-29(5-2)18-10-17-28-27(31)23-19-30(24-14-9-8-11-20(23)24)35(32,33)26-16-15-25(34-3)21-12-6-7-13-22(21)26/h6-9,11-16,23H,4-5,10,17-19H2,1-3H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416112
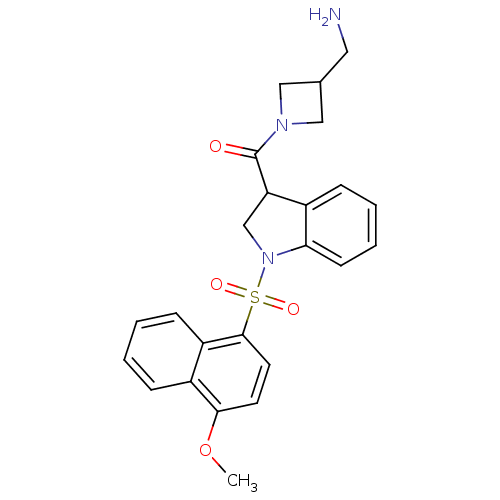
(CHEMBL1085362)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)N2CC(CN)C2)c2ccccc12 Show InChI InChI=1S/C24H25N3O4S/c1-31-22-10-11-23(19-8-3-2-7-18(19)22)32(29,30)27-15-20(17-6-4-5-9-21(17)27)24(28)26-13-16(12-25)14-26/h2-11,16,20H,12-15,25H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416119

(CHEMBL1082483)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)NCCCn2ccnc2)c2ccccc12 Show InChI InChI=1S/C26H26N4O4S/c1-34-24-11-12-25(21-9-3-2-8-20(21)24)35(32,33)30-17-22(19-7-4-5-10-23(19)30)26(31)28-13-6-15-29-16-14-27-18-29/h2-5,7-12,14,16,18,22H,6,13,15,17H2,1H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416134
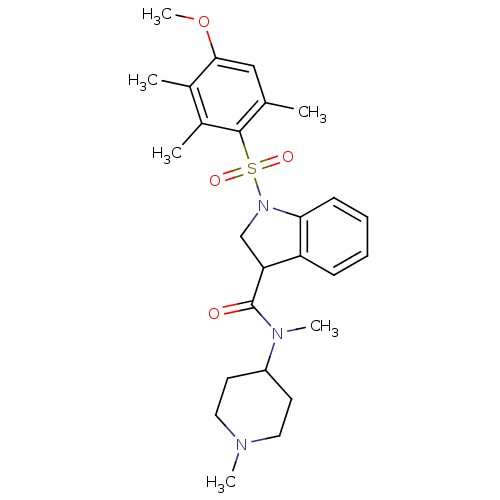
(CHEMBL1084214)Show SMILES COc1cc(C)c(c(C)c1C)S(=O)(=O)N1CC(C(=O)N(C)C2CCN(C)CC2)c2ccccc12 Show InChI InChI=1S/C26H35N3O4S/c1-17-15-24(33-6)18(2)19(3)25(17)34(31,32)29-16-22(21-9-7-8-10-23(21)29)26(30)28(5)20-11-13-27(4)14-12-20/h7-10,15,20,22H,11-14,16H2,1-6H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416133

(CHEMBL1083298)Show SMILES COc1ccc(c(C)c1C)S(=O)(=O)N1CC(C(=O)N(C)C2CCN(C)CC2)c2ccccc12 Show InChI InChI=1S/C25H33N3O4S/c1-17-18(2)24(11-10-23(17)32-5)33(30,31)28-16-21(20-8-6-7-9-22(20)28)25(29)27(4)19-12-14-26(3)15-13-19/h6-11,19,21H,12-16H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328879
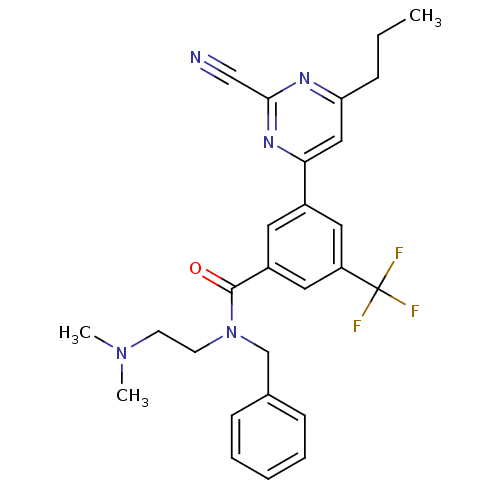
(CHEMBL1234898 | N-benzyl-3-(2-cyano-6-propylpyrimi...)Show SMILES CCCc1cc(nc(n1)C#N)-c1cc(cc(c1)C(F)(F)F)C(=O)N(CCN(C)C)Cc1ccccc1 Show InChI InChI=1S/C27H28F3N5O/c1-4-8-23-16-24(33-25(17-31)32-23)20-13-21(15-22(14-20)27(28,29)30)26(36)35(12-11-34(2)3)18-19-9-6-5-7-10-19/h5-7,9-10,13-16H,4,8,11-12,18H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416135
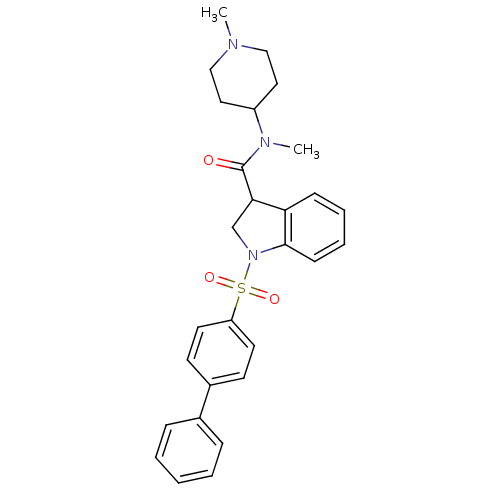
(CHEMBL1084482)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C28H31N3O3S/c1-29-18-16-23(17-19-29)30(2)28(32)26-20-31(27-11-7-6-10-25(26)27)35(33,34)24-14-12-22(13-15-24)21-8-4-3-5-9-21/h3-15,23,26H,16-20H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416126
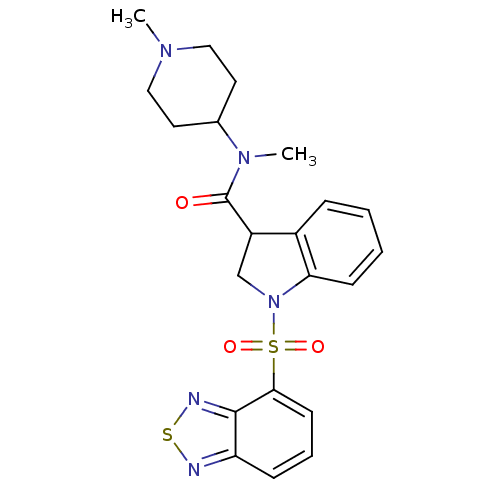
(CHEMBL1082764)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1cccc2nsnc12 Show InChI InChI=1S/C22H25N5O3S2/c1-25-12-10-15(11-13-25)26(2)22(28)17-14-27(19-8-4-3-6-16(17)19)32(29,30)20-9-5-7-18-21(20)24-31-23-18/h3-9,15,17H,10-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416111
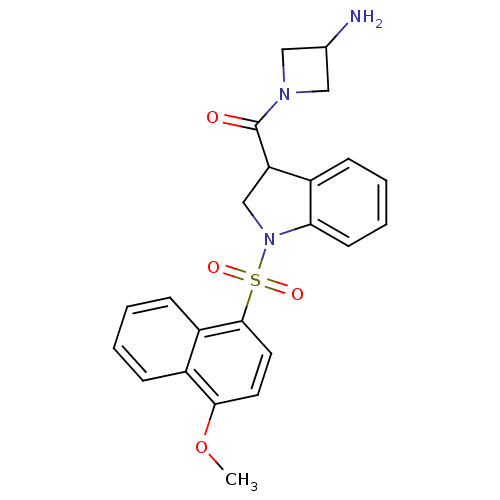
(CHEMBL1085361)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)N2CC(N)C2)c2ccccc12 Show InChI InChI=1S/C23H23N3O4S/c1-30-21-10-11-22(18-8-3-2-7-17(18)21)31(28,29)26-14-19(16-6-4-5-9-20(16)26)23(27)25-12-15(24)13-25/h2-11,15,19H,12-14,24H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50313476

(4-(3-(piperidin-1-yl)propyl)-6-(3-(trifluoromethyl...)Show SMILES FC(F)(F)c1cccc(c1)-c1cc(CCCN2CCCCC2)nc(n1)C#N Show InChI InChI=1S/C20H21F3N4/c21-20(22,23)16-7-4-6-15(12-16)18-13-17(25-19(14-24)26-18)8-5-11-27-9-2-1-3-10-27/h4,6-7,12-13H,1-3,5,8-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416125
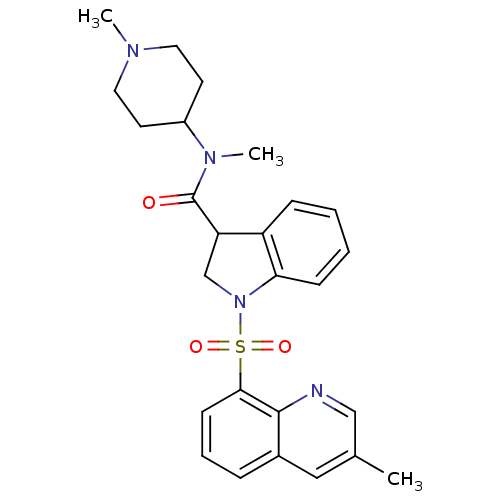
(CHEMBL1085354)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1cccc2cc(C)cnc12 Show InChI InChI=1S/C26H30N4O3S/c1-18-15-19-7-6-10-24(25(19)27-16-18)34(32,33)30-17-22(21-8-4-5-9-23(21)30)26(31)29(3)20-11-13-28(2)14-12-20/h4-10,15-16,20,22H,11-14,17H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416128
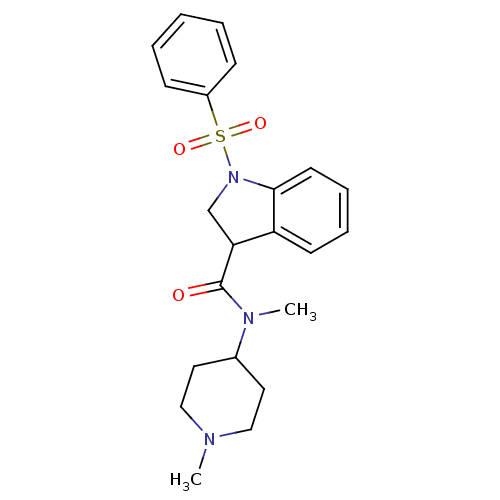
(CHEMBL1082767)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C22H27N3O3S/c1-23-14-12-17(13-15-23)24(2)22(26)20-16-25(21-11-7-6-10-19(20)21)29(27,28)18-8-4-3-5-9-18/h3-11,17,20H,12-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416129
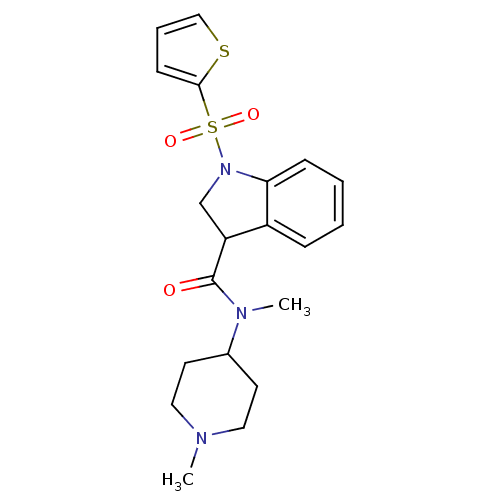
(CHEMBL1082766)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1cccs1 Show InChI InChI=1S/C20H25N3O3S2/c1-21-11-9-15(10-12-21)22(2)20(24)17-14-23(18-7-4-3-6-16(17)18)28(25,26)19-8-5-13-27-19/h3-8,13,15,17H,9-12,14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328887
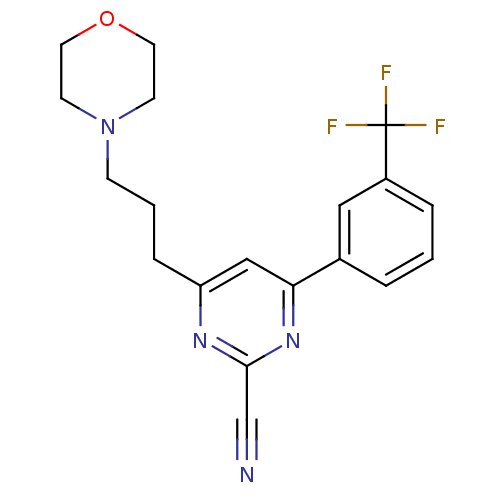
(4-(3-morpholinopropyl)-6-(3-(trifluoromethyl)pheny...)Show SMILES FC(F)(F)c1cccc(c1)-c1cc(CCCN2CCOCC2)nc(n1)C#N Show InChI InChI=1S/C19H19F3N4O/c20-19(21,22)15-4-1-3-14(11-15)17-12-16(24-18(13-23)25-17)5-2-6-26-7-9-27-10-8-26/h1,3-4,11-12H,2,5-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50313477

(4-(3-(pentan-3-ylamino)propyl)-6-(3-(trifluorometh...)Show SMILES CCC(CC)NCCCc1cc(nc(n1)C#N)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C20H23F3N4/c1-3-16(4-2)25-10-6-9-17-12-18(27-19(13-24)26-17)14-7-5-8-15(11-14)20(21,22)23/h5,7-8,11-12,16,25H,3-4,6,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328880
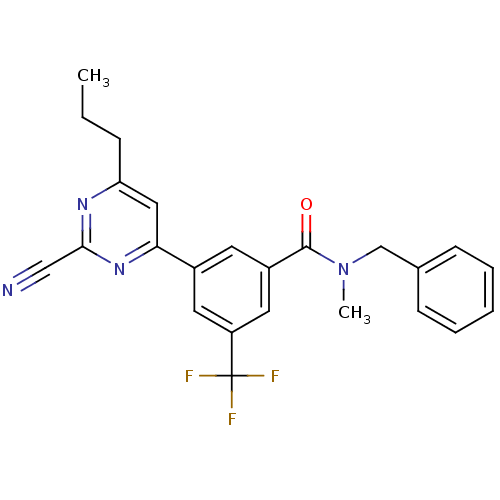
(CHEMBL1271174 | N-benzyl-3-(2-cyano-6-propylpyrimi...)Show SMILES CCCc1cc(nc(n1)C#N)-c1cc(cc(c1)C(F)(F)F)C(=O)N(C)Cc1ccccc1 Show InChI InChI=1S/C24H21F3N4O/c1-3-7-20-13-21(30-22(14-28)29-20)17-10-18(12-19(11-17)24(25,26)27)23(32)31(2)15-16-8-5-4-6-9-16/h4-6,8-13H,3,7,15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328890
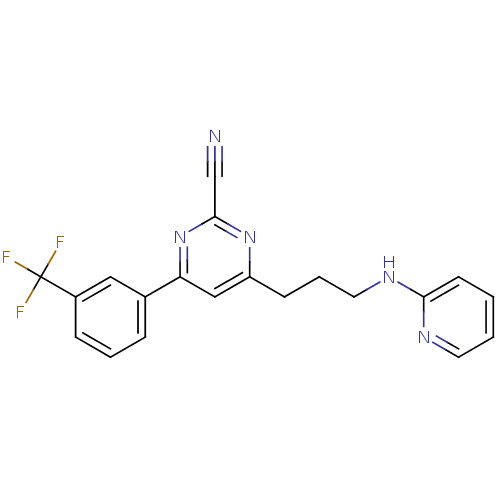
(4-(3-(pyridin-2-ylamino)propyl)-6-(3-(trifluoromet...)Show SMILES FC(F)(F)c1cccc(c1)-c1cc(CCCNc2ccccn2)nc(n1)C#N Show InChI InChI=1S/C20H16F3N5/c21-20(22,23)15-6-3-5-14(11-15)17-12-16(27-19(13-24)28-17)7-4-10-26-18-8-1-2-9-25-18/h1-3,5-6,8-9,11-12H,4,7,10H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328885

(4-(3-(2,2,2-trifluoroethylamino)propyl)-6-(3-(trif...)Show SMILES FC(F)(F)CNCCCc1cc(nc(n1)C#N)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C17H14F6N4/c18-16(19,20)10-25-6-2-5-13-8-14(27-15(9-24)26-13)11-3-1-4-12(7-11)17(21,22)23/h1,3-4,7-8,25H,2,5-6,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328888
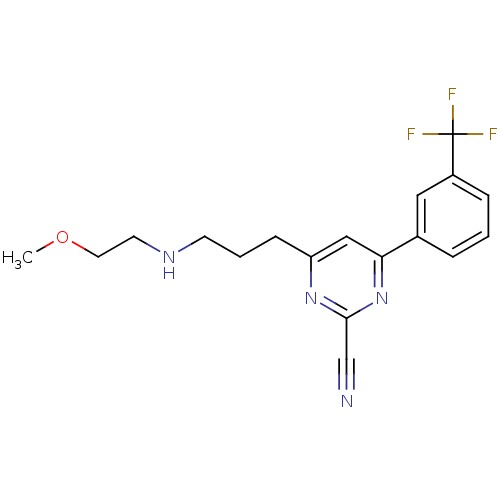
(4-(3-(2-methoxyethylamino)propyl)-6-(3-(trifluorom...)Show InChI InChI=1S/C18H19F3N4O/c1-26-9-8-23-7-3-6-15-11-16(25-17(12-22)24-15)13-4-2-5-14(10-13)18(19,20)21/h2,4-5,10-11,23H,3,6-9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
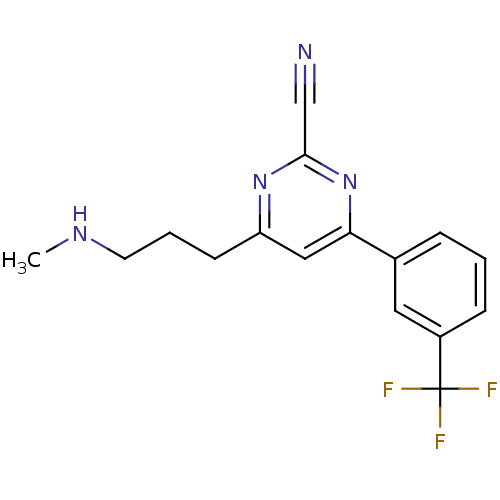
(Homo sapiens (Human)) | BDBM50328884
(4-(3-(methylamino)propyl)-6-(3-(trifluoromethyl)ph...)Show InChI InChI=1S/C16H15F3N4/c1-21-7-3-6-13-9-14(23-15(10-20)22-13)11-4-2-5-12(8-11)16(17,18)19/h2,4-5,8-9,21H,3,6-7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50313479

(4-cycloheptyl-6-(3-(piperidin-1-yl)propyl)pyrimidi...)Show InChI InChI=1S/C20H30N4/c21-16-20-22-18(11-8-14-24-12-6-3-7-13-24)15-19(23-20)17-9-4-1-2-5-10-17/h15,17H,1-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328878
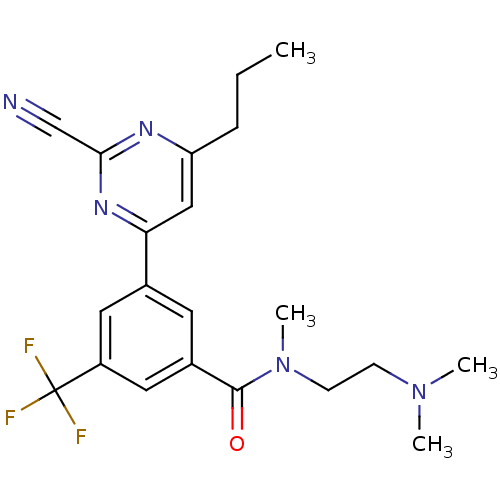
(3-(2-cyano-6-propylpyrimidin-4-yl)-N-(2-(dimethyla...)Show SMILES CCCc1cc(nc(n1)C#N)-c1cc(cc(c1)C(F)(F)F)C(=O)N(C)CCN(C)C Show InChI InChI=1S/C21H24F3N5O/c1-5-6-17-12-18(27-19(13-25)26-17)14-9-15(11-16(10-14)21(22,23)24)20(30)29(4)8-7-28(2)3/h9-12H,5-8H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328886
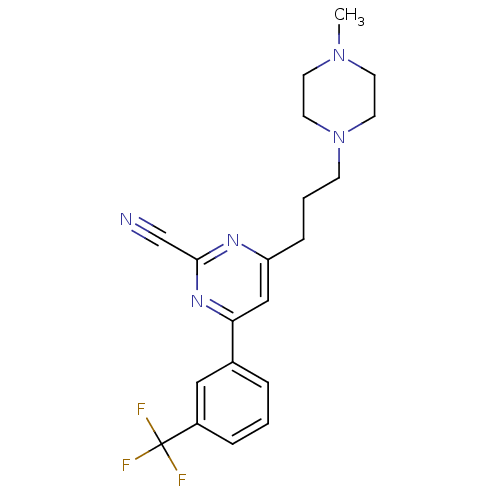
(4-(3-(4-methylpiperazin-1-yl)propyl)-6-(3-(trifluo...)Show SMILES CN1CCN(CCCc2cc(nc(n2)C#N)-c2cccc(c2)C(F)(F)F)CC1 Show InChI InChI=1S/C20H22F3N5/c1-27-8-10-28(11-9-27)7-3-6-17-13-18(26-19(14-24)25-17)15-4-2-5-16(12-15)20(21,22)23/h2,4-5,12-13H,3,6-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328889
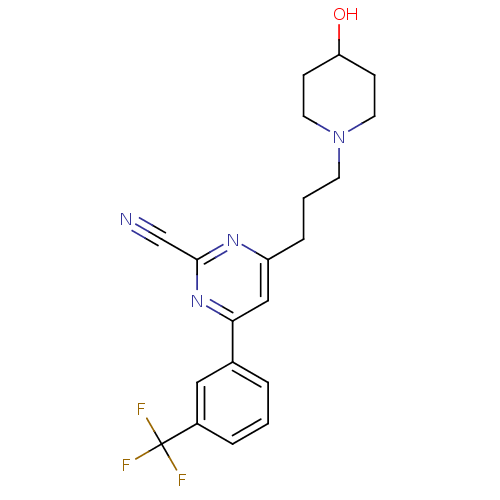
(4-(3-(4-hydroxypiperidin-1-yl)propyl)-6-(3-(triflu...)Show SMILES OC1CCN(CCCc2cc(nc(n2)C#N)-c2cccc(c2)C(F)(F)F)CC1 Show InChI InChI=1S/C20H21F3N4O/c21-20(22,23)15-4-1-3-14(11-15)18-12-16(25-19(13-24)26-18)5-2-8-27-9-6-17(28)7-10-27/h1,3-4,11-12,17,28H,2,5-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50416137
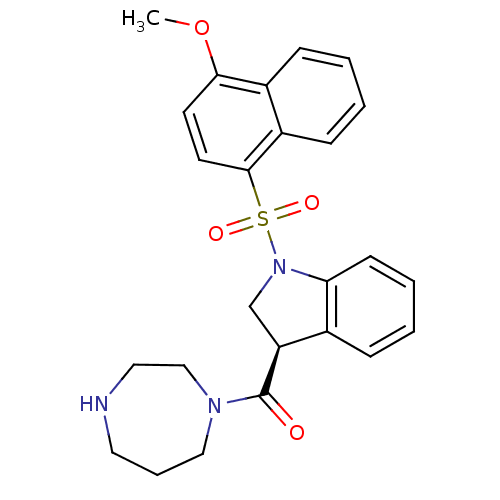
(CHEMBL1084215)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1C[C@H](C(=O)N2CCCNCC2)c2ccccc12 |r| Show InChI InChI=1S/C25H27N3O4S/c1-32-23-11-12-24(20-9-3-2-8-19(20)23)33(30,31)28-17-21(18-7-4-5-10-22(18)28)25(29)27-15-6-13-26-14-16-27/h2-5,7-12,21,26H,6,13-17H2,1H3/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetolide from human ERG channel in HEK293 cells |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328895

(1-(3-(2-cyano-6-(3-(trifluoromethyl)phenyl)pyrimid...)Show SMILES NC(=O)C1(CC1)NCCCc1cc(nc(n1)C#N)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C19H18F3N5O/c20-19(21,22)13-4-1-3-12(9-13)15-10-14(26-16(11-23)27-15)5-2-8-25-18(6-7-18)17(24)28/h1,3-4,9-10,25H,2,5-8H2,(H2,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328896
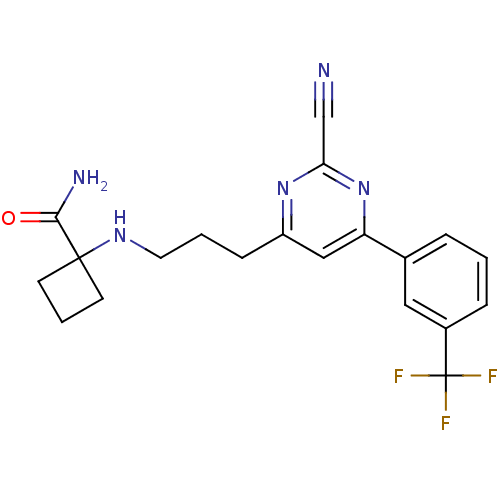
(1-(3-(2-cyano-6-(3-(trifluoromethyl)phenyl)pyrimid...)Show SMILES NC(=O)C1(CCC1)NCCCc1cc(nc(n1)C#N)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C20H20F3N5O/c21-20(22,23)14-5-1-4-13(10-14)16-11-15(27-17(12-24)28-16)6-2-9-26-19(18(25)29)7-3-8-19/h1,4-5,10-11,26H,2-3,6-9H2,(H2,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328894
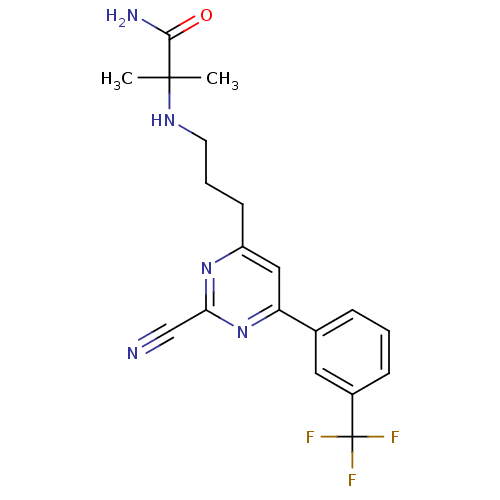
(2-(3-(2-cyano-6-(3-(trifluoromethyl)phenyl)pyrimid...)Show SMILES CC(C)(NCCCc1cc(nc(n1)C#N)-c1cccc(c1)C(F)(F)F)C(N)=O Show InChI InChI=1S/C19H20F3N5O/c1-18(2,17(24)28)25-8-4-7-14-10-15(27-16(11-23)26-14)12-5-3-6-13(9-12)19(20,21)22/h3,5-6,9-10,25H,4,7-8H2,1-2H3,(H2,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by patch clamp assay |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328894

(2-(3-(2-cyano-6-(3-(trifluoromethyl)phenyl)pyrimid...)Show SMILES CC(C)(NCCCc1cc(nc(n1)C#N)-c1cccc(c1)C(F)(F)F)C(N)=O Show InChI InChI=1S/C19H20F3N5O/c1-18(2,17(24)28)25-8-4-7-14-10-15(27-16(11-23)26-14)12-5-3-6-13(9-12)19(20,21)22/h3,5-6,9-10,25H,4,7-8H2,1-2H3,(H2,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328883

(3-(2-cyano-6-(3-(trifluoromethyl)phenyl)pyrimidin-...)Show SMILES CCC(CC)NC(=O)CCc1cc(nc(n1)C#N)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C20H21F3N4O/c1-3-15(4-2)26-19(28)9-8-16-11-17(27-18(12-24)25-16)13-6-5-7-14(10-13)20(21,22)23/h5-7,10-11,15H,3-4,8-9H2,1-2H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328893
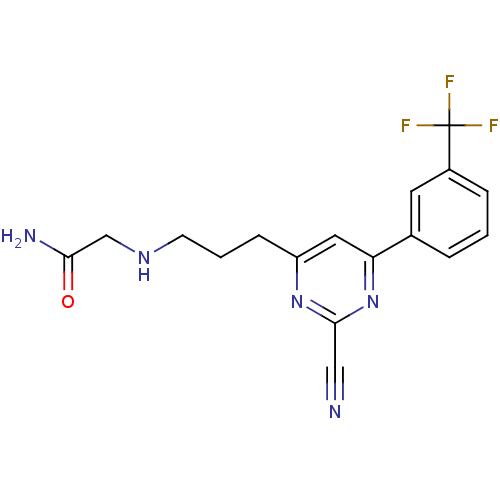
(2-(3-(2-cyano-6-(3-(trifluoromethyl)phenyl)pyrimid...)Show SMILES NC(=O)CNCCCc1cc(nc(n1)C#N)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C17H16F3N5O/c18-17(19,20)12-4-1-3-11(7-12)14-8-13(24-16(9-21)25-14)5-2-6-23-10-15(22)26/h1,3-4,7-8,23H,2,5-6,10H2,(H2,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data