 Found 509 hits with Last Name = 'cavallaro' and Initial = 'c'
Found 509 hits with Last Name = 'cavallaro' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440105
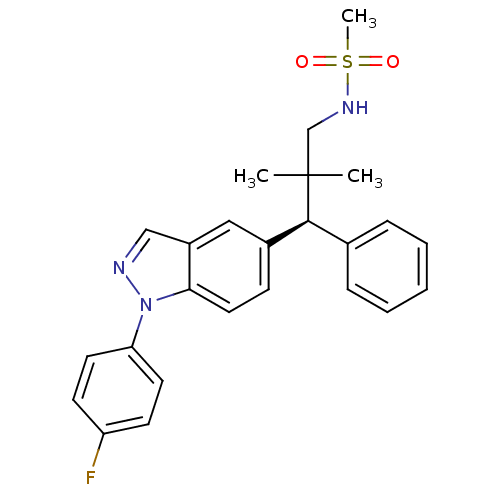
(CHEMBL2426114)Show SMILES CC(C)(CNS(C)(=O)=O)[C@@H](c1ccccc1)c1ccc2n(ncc2c1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C25H26FN3O2S/c1-25(2,17-28-32(3,30)31)24(18-7-5-4-6-8-18)19-9-14-23-20(15-19)16-27-29(23)22-12-10-21(26)11-13-22/h4-16,24,28H,17H2,1-3H3/t24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 23: 5448-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.085
BindingDB Entry DOI: 10.7270/Q22R3T2S |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440097
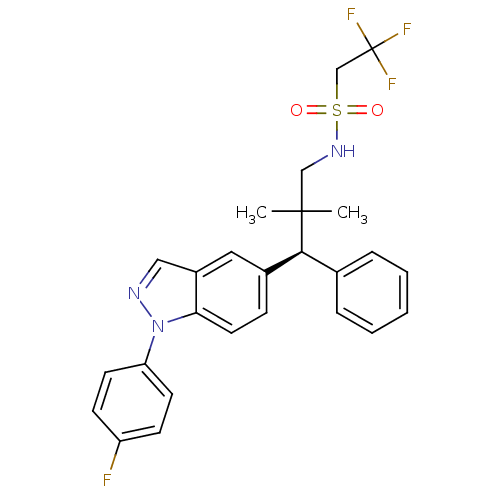
(CHEMBL2426118)Show SMILES CC(C)(CNS(=O)(=O)CC(F)(F)F)[C@@H](c1ccccc1)c1ccc2n(ncc2c1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H25F4N3O2S/c1-25(2,16-32-36(34,35)17-26(28,29)30)24(18-6-4-3-5-7-18)19-8-13-23-20(14-19)15-31-33(23)22-11-9-21(27)10-12-22/h3-15,24,32H,16-17H2,1-2H3/t24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 23: 5448-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.085
BindingDB Entry DOI: 10.7270/Q22R3T2S |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440104

(CHEMBL2426115)Show SMILES CCS(=O)(=O)NCC(C)(C)[C@@H](c1ccccc1)c1ccc2n(ncc2c1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H28FN3O2S/c1-4-33(31,32)29-18-26(2,3)25(19-8-6-5-7-9-19)20-10-15-24-21(16-20)17-28-30(24)23-13-11-22(27)12-14-23/h5-17,25,29H,4,18H2,1-3H3/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 23: 5448-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.085
BindingDB Entry DOI: 10.7270/Q22R3T2S |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440096
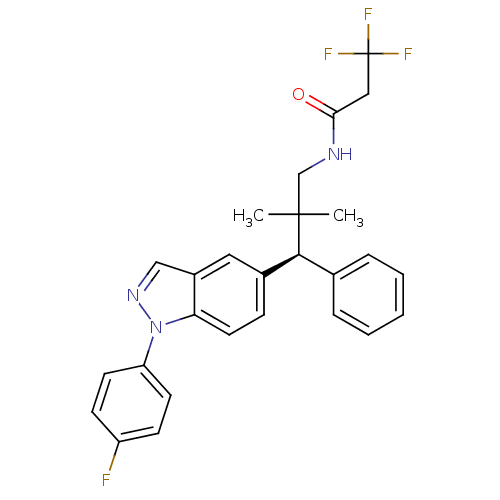
(CHEMBL2426124)Show SMILES CC(C)(CNC(=O)CC(F)(F)F)[C@@H](c1ccccc1)c1ccc2n(ncc2c1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C27H25F4N3O/c1-26(2,17-32-24(35)15-27(29,30)31)25(18-6-4-3-5-7-18)19-8-13-23-20(14-19)16-33-34(23)22-11-9-21(28)10-12-22/h3-14,16,25H,15,17H2,1-2H3,(H,32,35)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 23: 5448-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.085
BindingDB Entry DOI: 10.7270/Q22R3T2S |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440099

(CHEMBL2426121)Show SMILES CCS(=O)(=O)NCC(C)[C@@H](c1ccccc1)c1ccc2n(ncc2c1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C25H26FN3O2S/c1-3-32(30,31)28-16-18(2)25(19-7-5-4-6-8-19)20-9-14-24-21(15-20)17-27-29(24)23-12-10-22(26)11-13-23/h4-15,17-18,25,28H,3,16H2,1-2H3/t18?,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 23: 5448-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.085
BindingDB Entry DOI: 10.7270/Q22R3T2S |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440100

(CHEMBL2426120)Show SMILES CC(CNS(C)(=O)=O)[C@@H](c1ccccc1)c1ccc2n(ncc2c1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H24FN3O2S/c1-17(15-27-31(2,29)30)24(18-6-4-3-5-7-18)19-8-13-23-20(14-19)16-26-28(23)22-11-9-21(25)10-12-22/h3-14,16-17,24,27H,15H2,1-2H3/t17?,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 23: 5448-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.085
BindingDB Entry DOI: 10.7270/Q22R3T2S |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 23: 5448-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.085
BindingDB Entry DOI: 10.7270/Q22R3T2S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440102

(CHEMBL2426117)Show SMILES CC(C)(CNS(=O)(=O)C1CC1)[C@@H](c1ccccc1)c1ccc2n(ncc2c1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C27H28FN3O2S/c1-27(2,18-30-34(32,33)24-13-14-24)26(19-6-4-3-5-7-19)20-8-15-25-21(16-20)17-29-31(25)23-11-9-22(28)10-12-23/h3-12,15-17,24,26,30H,13-14,18H2,1-2H3/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 23: 5448-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.085
BindingDB Entry DOI: 10.7270/Q22R3T2S |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440101
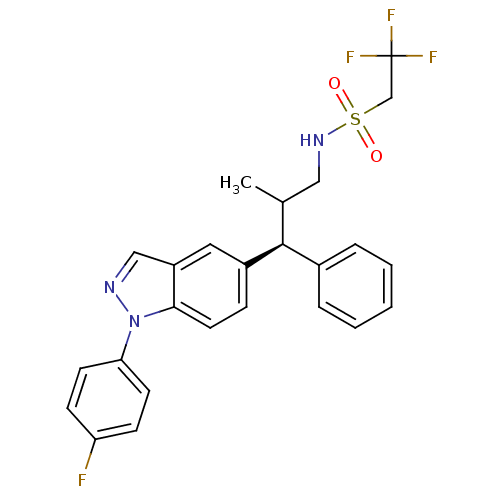
(CHEMBL2426119)Show SMILES CC(CNS(=O)(=O)CC(F)(F)F)[C@@H](c1ccccc1)c1ccc2n(ncc2c1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C25H23F4N3O2S/c1-17(14-31-35(33,34)16-25(27,28)29)24(18-5-3-2-4-6-18)19-7-12-23-20(13-19)15-30-32(23)22-10-8-21(26)9-11-22/h2-13,15,17,24,31H,14,16H2,1H3/t17?,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 23: 5448-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.085
BindingDB Entry DOI: 10.7270/Q22R3T2S |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440110

(CHEMBL2426128)Show SMILES CC(C)C[C@@H](c1ccc2n(ncc2c1)-c1ccc(F)cc1)C(C)(C)CNC(=O)CC(F)(F)F |r| Show InChI InChI=1S/C25H29F4N3O/c1-16(2)11-21(24(3,4)15-30-23(33)13-25(27,28)29)17-5-10-22-18(12-17)14-31-32(22)20-8-6-19(26)7-9-20/h5-10,12,14,16,21H,11,13,15H2,1-4H3,(H,30,33)/t21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 23: 5448-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.085
BindingDB Entry DOI: 10.7270/Q22R3T2S |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440107
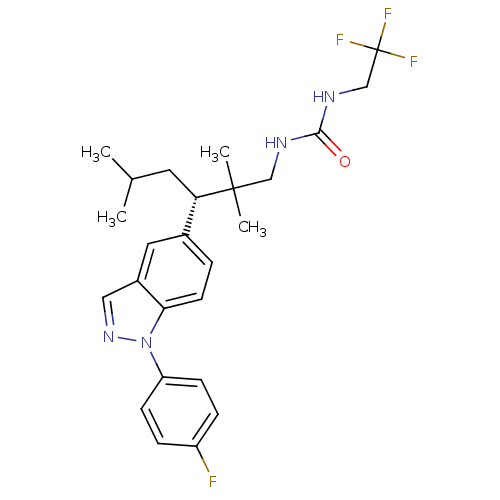
(CHEMBL2426111)Show SMILES CC(C)C[C@@H](c1ccc2n(ncc2c1)-c1ccc(F)cc1)C(C)(C)CNC(=O)NCC(F)(F)F |r| Show InChI InChI=1S/C25H30F4N4O/c1-16(2)11-21(24(3,4)14-30-23(34)31-15-25(27,28)29)17-5-10-22-18(12-17)13-32-33(22)20-8-6-19(26)7-9-20/h5-10,12-13,16,21H,11,14-15H2,1-4H3,(H2,30,31,34)/t21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 23: 5448-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.085
BindingDB Entry DOI: 10.7270/Q22R3T2S |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440103

(CHEMBL2426116)Show SMILES CCCS(=O)(=O)NCC(C)(C)[C@@H](c1ccccc1)c1ccc2n(ncc2c1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C27H30FN3O2S/c1-4-16-34(32,33)30-19-27(2,3)26(20-8-6-5-7-9-20)21-10-15-25-22(17-21)18-29-31(25)24-13-11-23(28)12-14-24/h5-15,17-18,26,30H,4,16,19H2,1-3H3/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 23: 5448-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.085
BindingDB Entry DOI: 10.7270/Q22R3T2S |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440114

(CHEMBL2426123)Show SMILES CC(C)(CNC(=O)C(F)(F)F)[C@@H](c1ccccc1)c1ccc2n(ncc2c1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H23F4N3O/c1-25(2,16-31-24(34)26(28,29)30)23(17-6-4-3-5-7-17)18-8-13-22-19(14-18)15-32-33(22)21-11-9-20(27)10-12-21/h3-15,23H,16H2,1-2H3,(H,31,34)/t23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 23: 5448-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.085
BindingDB Entry DOI: 10.7270/Q22R3T2S |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440112

(CHEMBL2426126)Show SMILES CC(C)(C)CC(=O)NCC(C)(C)[C@@H](c1ccccc1)c1ccc2n(ncc2c1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C30H34FN3O/c1-29(2,3)18-27(35)32-20-30(4,5)28(21-9-7-6-8-10-21)22-11-16-26-23(17-22)19-33-34(26)25-14-12-24(31)13-15-25/h6-17,19,28H,18,20H2,1-5H3,(H,32,35)/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 23: 5448-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.085
BindingDB Entry DOI: 10.7270/Q22R3T2S |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440113

(CHEMBL2426125)Show SMILES CC(C)(C)C(=O)NCC(C)(C)[C@@H](c1ccccc1)c1ccc2n(ncc2c1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C29H32FN3O/c1-28(2,3)27(34)31-19-29(4,5)26(20-9-7-6-8-10-20)21-11-16-25-22(17-21)18-32-33(25)24-14-12-23(30)13-15-24/h6-18,26H,19H2,1-5H3,(H,31,34)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 23: 5448-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.085
BindingDB Entry DOI: 10.7270/Q22R3T2S |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440109
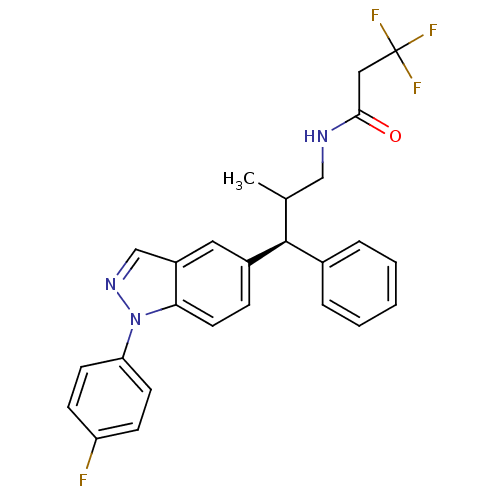
(CHEMBL2426109)Show SMILES CC(CNC(=O)CC(F)(F)F)[C@@H](c1ccccc1)c1ccc2n(ncc2c1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H23F4N3O/c1-17(15-31-24(34)14-26(28,29)30)25(18-5-3-2-4-6-18)19-7-12-23-20(13-19)16-32-33(23)22-10-8-21(27)9-11-22/h2-13,16-17,25H,14-15H2,1H3,(H,31,34)/t17?,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 23: 5448-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.085
BindingDB Entry DOI: 10.7270/Q22R3T2S |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440115

(CHEMBL2426122)Show SMILES CC(=O)NCC(C)(C)[C@@H](c1ccccc1)c1ccc2n(ncc2c1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H26FN3O/c1-18(31)28-17-26(2,3)25(19-7-5-4-6-8-19)20-9-14-24-21(15-20)16-29-30(24)23-12-10-22(27)11-13-23/h4-16,25H,17H2,1-3H3,(H,28,31)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 23: 5448-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.085
BindingDB Entry DOI: 10.7270/Q22R3T2S |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440109

(CHEMBL2426109)Show SMILES CC(CNC(=O)CC(F)(F)F)[C@@H](c1ccccc1)c1ccc2n(ncc2c1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H23F4N3O/c1-17(15-31-24(34)14-26(28,29)30)25(18-5-3-2-4-6-18)19-7-12-23-20(13-19)16-32-33(23)22-10-8-21(27)9-11-22/h2-13,16-17,25H,14-15H2,1H3,(H,31,34)/t17?,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 23: 5448-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.085
BindingDB Entry DOI: 10.7270/Q22R3T2S |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440106

(CHEMBL2426113)Show SMILES CC(C)C[C@@H](c1ccc2n(ncc2c1)-c1ccc(F)cc1)C(C)(C)CNC(=O)NC(C)(C)C |r| Show InChI InChI=1S/C27H37FN4O/c1-18(2)14-23(27(6,7)17-29-25(33)31-26(3,4)5)19-8-13-24-20(15-19)16-30-32(24)22-11-9-21(28)10-12-22/h8-13,15-16,18,23H,14,17H2,1-7H3,(H2,29,31,33)/t23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 23: 5448-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.085
BindingDB Entry DOI: 10.7270/Q22R3T2S |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440098
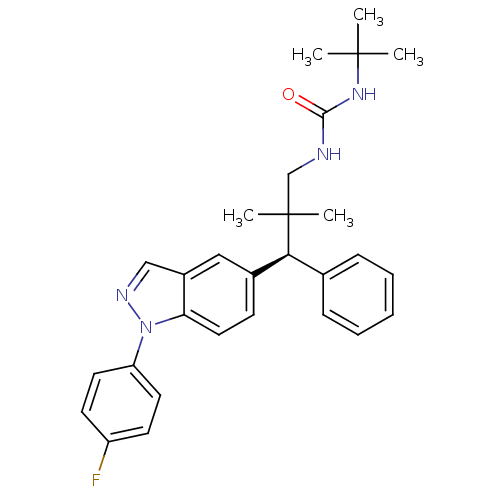
(CHEMBL2426112)Show SMILES CC(C)(C)NC(=O)NCC(C)(C)[C@@H](c1ccccc1)c1ccc2n(ncc2c1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C29H33FN4O/c1-28(2,3)33-27(35)31-19-29(4,5)26(20-9-7-6-8-10-20)21-11-16-25-22(17-21)18-32-34(25)24-14-12-23(30)13-15-24/h6-18,26H,19H2,1-5H3,(H2,31,33,35)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 23: 5448-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.085
BindingDB Entry DOI: 10.7270/Q22R3T2S |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440108

(CHEMBL2426110)Show SMILES CC(C)(CNC(=O)NCC(F)(F)F)[C@@H](c1ccccc1)c1ccc2n(ncc2c1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C27H26F4N4O/c1-26(2,16-32-25(36)33-17-27(29,30)31)24(18-6-4-3-5-7-18)19-8-13-23-20(14-19)15-34-35(23)22-11-9-21(28)10-12-22/h3-15,24H,16-17H2,1-2H3,(H2,32,33,36)/t24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 23: 5448-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.085
BindingDB Entry DOI: 10.7270/Q22R3T2S |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50071554

(3-Hydroxy-4-[(2S,3S)-3-methyl-2-(2-naphthalen-2-yl...)Show SMILES CCCCNC(=O)CC(O)C(Cc1ccccc1)NC(=O)[C@@H](NC(=O)Cc1ccc2ccccc2c1)[C@@H](C)CC Show InChI InChI=1S/C33H43N3O4/c1-4-6-18-34-30(38)22-29(37)28(20-24-12-8-7-9-13-24)35-33(40)32(23(3)5-2)36-31(39)21-25-16-17-26-14-10-11-15-27(26)19-25/h7-17,19,23,28-29,32,37H,4-6,18,20-22H2,1-3H3,(H,34,38)(H,35,40)(H,36,39)/t23-,28?,29?,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin D |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50071558
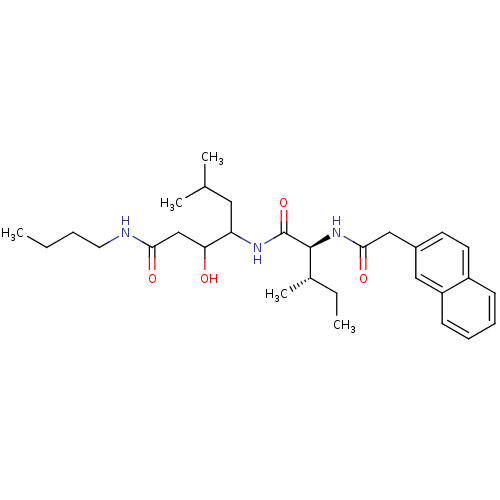
(3-Hydroxy-6-methyl-4-[(2S,3S)-3-methyl-2-(2-naphth...)Show SMILES CCCCNC(=O)CC(O)C(CC(C)C)NC(=O)[C@@H](NC(=O)Cc1ccc2ccccc2c1)[C@@H](C)CC Show InChI InChI=1S/C30H45N3O4/c1-6-8-15-31-27(35)19-26(34)25(16-20(3)4)32-30(37)29(21(5)7-2)33-28(36)18-22-13-14-23-11-9-10-12-24(23)17-22/h9-14,17,20-21,25-26,29,34H,6-8,15-16,18-19H2,1-5H3,(H,31,35)(H,32,37)(H,33,36)/t21-,25?,26?,29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against plasmepsin-2 from Plasmodium falciparum |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50071554

(3-Hydroxy-4-[(2S,3S)-3-methyl-2-(2-naphthalen-2-yl...)Show SMILES CCCCNC(=O)CC(O)C(Cc1ccccc1)NC(=O)[C@@H](NC(=O)Cc1ccc2ccccc2c1)[C@@H](C)CC Show InChI InChI=1S/C33H43N3O4/c1-4-6-18-34-30(38)22-29(37)28(20-24-12-8-7-9-13-24)35-33(40)32(23(3)5-2)36-31(39)21-25-16-17-26-14-10-11-15-27(26)19-25/h7-17,19,23,28-29,32,37H,4-6,18,20-22H2,1-3H3,(H,34,38)(H,35,40)(H,36,39)/t23-,28?,29?,32-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasmepsin-2 from Plasmodium falciparum. |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50071546
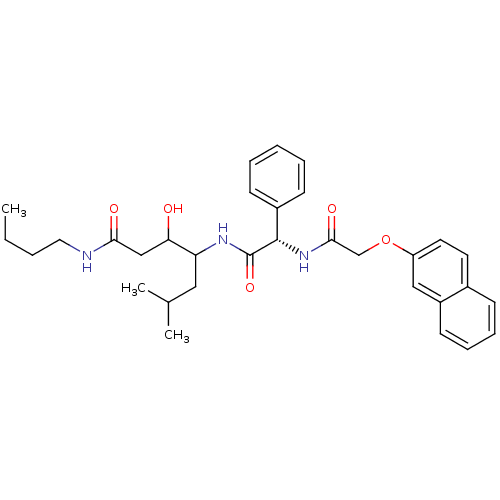
(3-Hydroxy-6-methyl-4-{(S)-2-[2-(naphthalen-2-yloxy...)Show SMILES CCCCNC(=O)CC(O)C(CC(C)C)NC(=O)[C@@H](NC(=O)COc1ccc2ccccc2c1)c1ccccc1 Show InChI InChI=1S/C32H41N3O5/c1-4-5-17-33-29(37)20-28(36)27(18-22(2)3)34-32(39)31(24-12-7-6-8-13-24)35-30(38)21-40-26-16-15-23-11-9-10-14-25(23)19-26/h6-16,19,22,27-28,31,36H,4-5,17-18,20-21H2,1-3H3,(H,33,37)(H,34,39)(H,35,38)/t27?,28?,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin D |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50071561
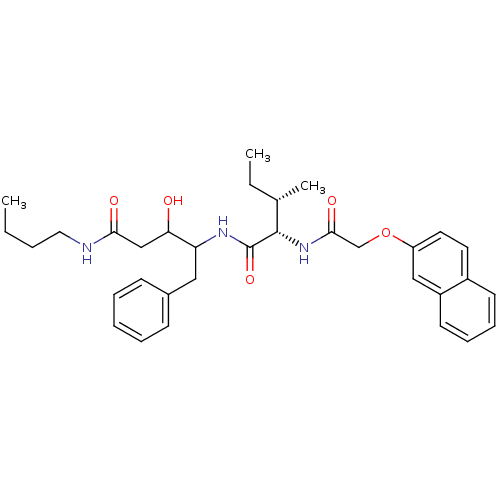
(3-Hydroxy-4-{(2S,3S)-3-methyl-2-[2-(naphthalen-2-y...)Show SMILES CCCCNC(=O)CC(O)C(Cc1ccccc1)NC(=O)[C@@H](NC(=O)COc1ccc2ccccc2c1)[C@@H](C)CC Show InChI InChI=1S/C33H43N3O5/c1-4-6-18-34-30(38)21-29(37)28(19-24-12-8-7-9-13-24)35-33(40)32(23(3)5-2)36-31(39)22-41-27-17-16-25-14-10-11-15-26(25)20-27/h7-17,20,23,28-29,32,37H,4-6,18-19,21-22H2,1-3H3,(H,34,38)(H,35,40)(H,36,39)/t23-,28?,29?,32-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasmepsin-2 from Plasmodium falciparum. |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50071555
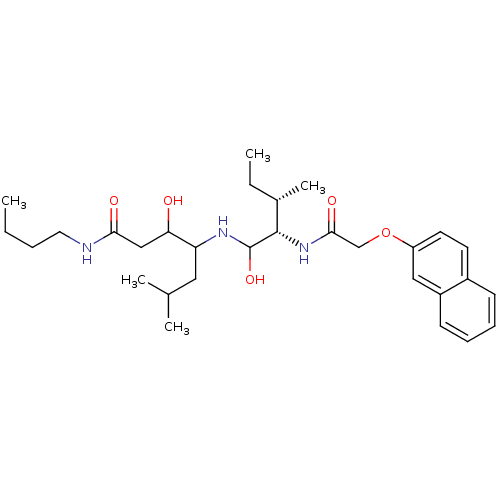
(3-Hydroxy-4-{(2S,3S)-1-hydroxy-3-methyl-2-[2-(naph...)Show SMILES CCCCNC(=O)CC(O)C(CC(C)C)NC(O)[C@@H](NC(=O)COc1ccc2ccccc2c1)[C@@H](C)CC Show InChI InChI=1S/C30H47N3O5/c1-6-8-15-31-27(35)18-26(34)25(16-20(3)4)32-30(37)29(21(5)7-2)33-28(36)19-38-24-14-13-22-11-9-10-12-23(22)17-24/h9-14,17,20-21,25-26,29-30,32,34,37H,6-8,15-16,18-19H2,1-5H3,(H,31,35)(H,33,36)/t21-,25?,26?,29-,30?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasmepsin-2 from Plasmodium falciparum. |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50071559
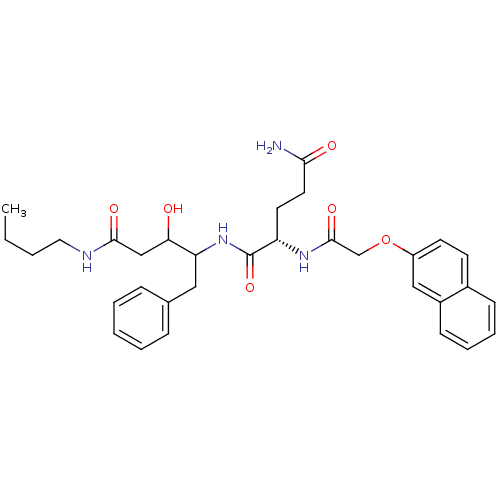
((S)-2-[2-(Naphthalen-2-yloxy)-acetylamino]-pentane...)Show SMILES CCCCNC(=O)CC(O)C(Cc1ccccc1)NC(=O)[C@H](CCC(N)=O)NC(=O)COc1ccc2ccccc2c1 Show InChI InChI=1S/C32H40N4O6/c1-2-3-17-34-30(39)20-28(37)27(18-22-9-5-4-6-10-22)36-32(41)26(15-16-29(33)38)35-31(40)21-42-25-14-13-23-11-7-8-12-24(23)19-25/h4-14,19,26-28,37H,2-3,15-18,20-21H2,1H3,(H2,33,38)(H,34,39)(H,35,40)(H,36,41)/t26-,27?,28?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin D |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50071562
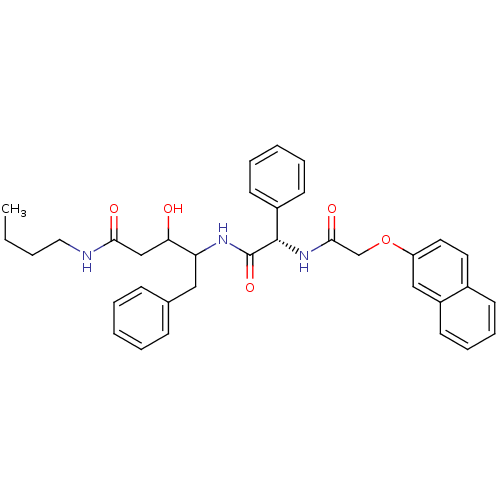
(3-Hydroxy-4-{(S)-2-[2-(naphthalen-2-yloxy)-acetyla...)Show SMILES CCCCNC(=O)CC(O)C(Cc1ccccc1)NC(=O)[C@@H](NC(=O)COc1ccc2ccccc2c1)c1ccccc1 Show InChI InChI=1S/C35H39N3O5/c1-2-3-20-36-32(40)23-31(39)30(21-25-12-6-4-7-13-25)37-35(42)34(27-15-8-5-9-16-27)38-33(41)24-43-29-19-18-26-14-10-11-17-28(26)22-29/h4-19,22,30-31,34,39H,2-3,20-21,23-24H2,1H3,(H,36,40)(H,37,42)(H,38,41)/t30?,31?,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin D |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50071556

(CHEMBL74943 | N-[(1S,2S)-1-(1-Benzyl-3-butylcarbam...)Show SMILES CCCCNC(=O)CC(O)C(Cc1ccccc1)NC(=O)[C@@H](NC(=O)c1ccc(CN2CCCC2)cc1)[C@@H](C)CC Show InChI InChI=1S/C33H48N4O4/c1-4-6-18-34-30(39)22-29(38)28(21-25-12-8-7-9-13-25)35-33(41)31(24(3)5-2)36-32(40)27-16-14-26(15-17-27)23-37-19-10-11-20-37/h7-9,12-17,24,28-29,31,38H,4-6,10-11,18-23H2,1-3H3,(H,34,39)(H,35,41)(H,36,40)/t24-,28?,29?,31-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasmepsin-2 from Plasmodium falciparum. |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50071550
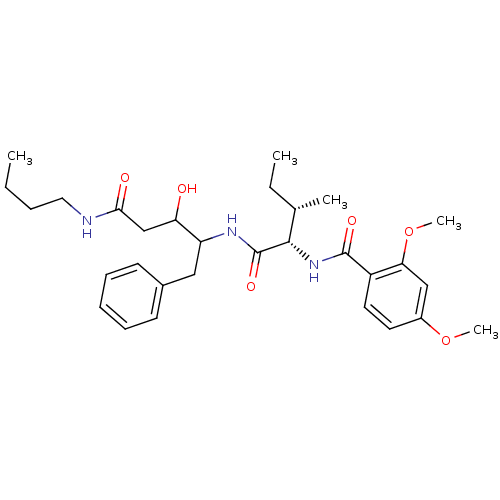
(CHEMBL74562 | N-[(1S,2S)-1-(1-Benzyl-3-butylcarbam...)Show SMILES CCCCNC(=O)CC(O)C(Cc1ccccc1)NC(=O)[C@@H](NC(=O)c1ccc(OC)cc1OC)[C@@H](C)CC Show InChI InChI=1S/C30H43N3O6/c1-6-8-16-31-27(35)19-25(34)24(17-21-12-10-9-11-13-21)32-30(37)28(20(3)7-2)33-29(36)23-15-14-22(38-4)18-26(23)39-5/h9-15,18,20,24-25,28,34H,6-8,16-17,19H2,1-5H3,(H,31,35)(H,32,37)(H,33,36)/t20-,24?,25?,28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasmepsin-2 from Plasmodium falciparum. |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50071558

(3-Hydroxy-6-methyl-4-[(2S,3S)-3-methyl-2-(2-naphth...)Show SMILES CCCCNC(=O)CC(O)C(CC(C)C)NC(=O)[C@@H](NC(=O)Cc1ccc2ccccc2c1)[C@@H](C)CC Show InChI InChI=1S/C30H45N3O4/c1-6-8-15-31-27(35)19-26(34)25(16-20(3)4)32-30(37)29(21(5)7-2)33-28(36)18-22-13-14-23-11-9-10-12-24(23)17-22/h9-14,17,20-21,25-26,29,34H,6-8,15-16,18-19H2,1-5H3,(H,31,35)(H,32,37)(H,33,36)/t21-,25?,26?,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin D |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50440097

(CHEMBL2426118)Show SMILES CC(C)(CNS(=O)(=O)CC(F)(F)F)[C@@H](c1ccccc1)c1ccc2n(ncc2c1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H25F4N3O2S/c1-25(2,16-32-36(34,35)17-26(28,29)30)24(18-6-4-3-5-7-18)19-8-13-23-20(14-19)15-31-33(23)22-11-9-21(27)10-12-22/h3-15,24,32H,16-17H2,1-2H3/t24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 406 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to progesterone receptor (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 23: 5448-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.085
BindingDB Entry DOI: 10.7270/Q22R3T2S |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50071552
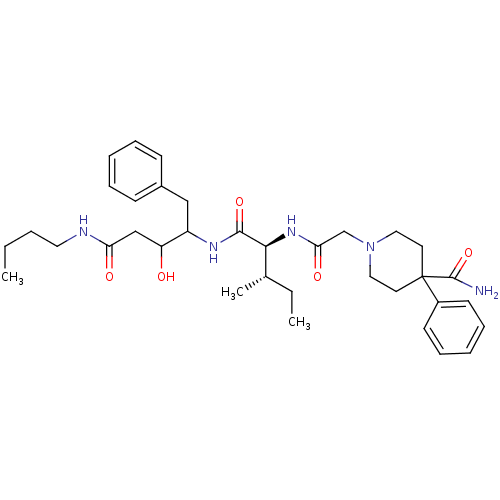
(1-{[(1S,2S)-1-(1-Benzyl-3-butylcarbamoyl-2-hydroxy...)Show SMILES CCCCNC(=O)CC(O)C(Cc1ccccc1)NC(=O)[C@@H](NC(=O)CN1CCC(CC1)(C(N)=O)c1ccccc1)[C@@H](C)CC Show InChI InChI=1S/C35H51N5O5/c1-4-6-19-37-30(42)23-29(41)28(22-26-13-9-7-10-14-26)38-33(44)32(25(3)5-2)39-31(43)24-40-20-17-35(18-21-40,34(36)45)27-15-11-8-12-16-27/h7-16,25,28-29,32,41H,4-6,17-24H2,1-3H3,(H2,36,45)(H,37,42)(H,38,44)(H,39,43)/t25-,28?,29?,32-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasmepsin-2 from Plasmodium falciparum. |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50146770
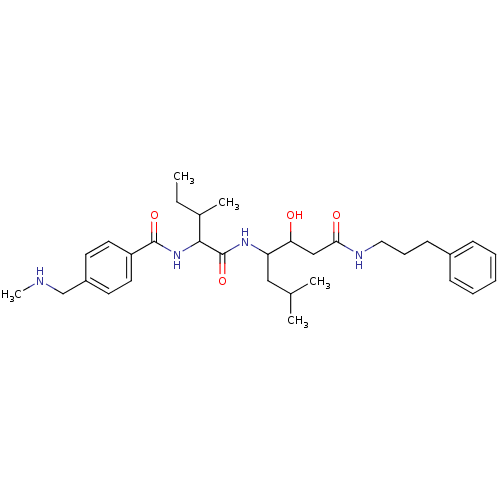
(CHEMBL94646 | N-(1-{1-[1-Hydroxy-2-(3-phenyl-propy...)Show SMILES CCC(C)C(NC(=O)c1ccc(CNC)cc1)C(=O)NC(CC(C)C)C(O)CC(=O)NCCCc1ccccc1 Show InChI InChI=1S/C32H48N4O4/c1-6-23(4)30(36-31(39)26-16-14-25(15-17-26)21-33-5)32(40)35-27(19-22(2)3)28(37)20-29(38)34-18-10-13-24-11-8-7-9-12-24/h7-9,11-12,14-17,22-23,27-28,30,33,37H,6,10,13,18-21H2,1-5H3,(H,34,38)(H,35,40)(H,36,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasmepsin-2 from Plasmodium falciparum. |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50071551
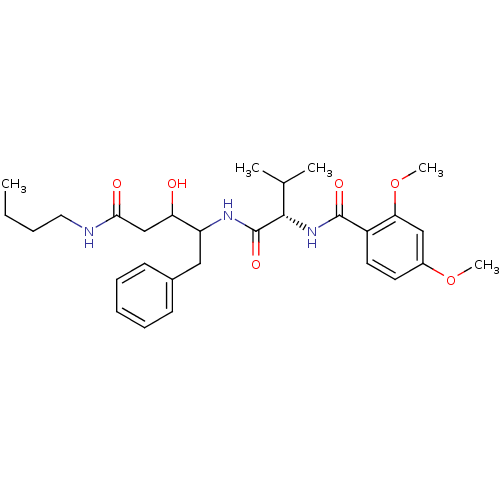
(CHEMBL427946 | N-[(S)-1-(1-Benzyl-3-butylcarbamoyl...)Show SMILES CCCCNC(=O)CC(O)C(Cc1ccccc1)NC(=O)[C@@H](NC(=O)c1ccc(OC)cc1OC)C(C)C Show InChI InChI=1S/C29H41N3O6/c1-6-7-15-30-26(34)18-24(33)23(16-20-11-9-8-10-12-20)31-29(36)27(19(2)3)32-28(35)22-14-13-21(37-4)17-25(22)38-5/h8-14,17,19,23-24,27,33H,6-7,15-16,18H2,1-5H3,(H,30,34)(H,31,36)(H,32,35)/t23?,24?,27-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasmepsin-2 from Plasmodium falciparum. |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50071562

(3-Hydroxy-4-{(S)-2-[2-(naphthalen-2-yloxy)-acetyla...)Show SMILES CCCCNC(=O)CC(O)C(Cc1ccccc1)NC(=O)[C@@H](NC(=O)COc1ccc2ccccc2c1)c1ccccc1 Show InChI InChI=1S/C35H39N3O5/c1-2-3-20-36-32(40)23-31(39)30(21-25-12-6-4-7-13-25)37-35(42)34(27-15-8-5-9-16-27)38-33(41)24-43-29-19-18-26-14-10-11-17-28(26)22-29/h4-19,22,30-31,34,39H,2-3,20-21,23-24H2,1H3,(H,36,40)(H,37,42)(H,38,41)/t30?,31?,34-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasmepsin-2 from Plasmodium falciparum. |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50071555

(3-Hydroxy-4-{(2S,3S)-1-hydroxy-3-methyl-2-[2-(naph...)Show SMILES CCCCNC(=O)CC(O)C(CC(C)C)NC(O)[C@@H](NC(=O)COc1ccc2ccccc2c1)[C@@H](C)CC Show InChI InChI=1S/C30H47N3O5/c1-6-8-15-31-27(35)18-26(34)25(16-20(3)4)32-30(37)29(21(5)7-2)33-28(36)19-38-24-14-13-22-11-9-10-12-23(22)17-24/h9-14,17,20-21,25-26,29-30,32,34,37H,6-8,15-16,18-19H2,1-5H3,(H,31,35)(H,33,36)/t21-,25?,26?,29-,30?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin D |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50071549
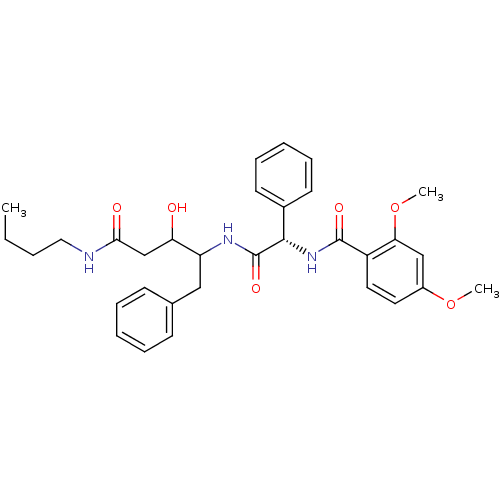
(CHEMBL309056 | N-[(S)-(1-Benzyl-3-butylcarbamoyl-2...)Show SMILES CCCCNC(=O)CC(O)C(Cc1ccccc1)NC(=O)[C@@H](NC(=O)c1ccc(OC)cc1OC)c1ccccc1 Show InChI InChI=1S/C32H39N3O6/c1-4-5-18-33-29(37)21-27(36)26(19-22-12-8-6-9-13-22)34-32(39)30(23-14-10-7-11-15-23)35-31(38)25-17-16-24(40-2)20-28(25)41-3/h6-17,20,26-27,30,36H,4-5,18-19,21H2,1-3H3,(H,33,37)(H,34,39)(H,35,38)/t26?,27?,30-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasmepsin-2 from Plasmodium falciparum. |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Plasmepsin II
(Plasmodium falciparum) | BDBM50071553
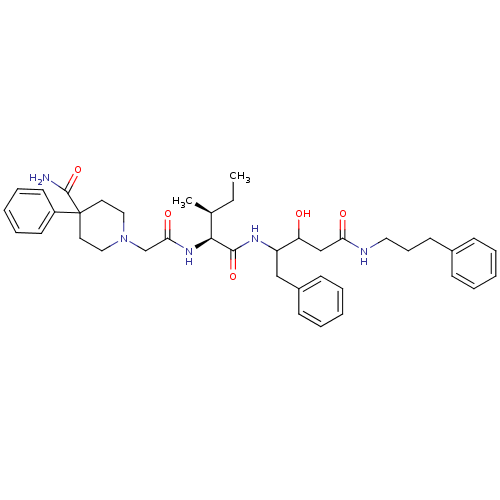
(1-({(1S,2S)-1-[1-Benzyl-2-hydroxy-3-(3-phenyl-prop...)Show SMILES CC[C@H](C)[C@H](NC(=O)CN1CCC(CC1)(C(N)=O)c1ccccc1)C(=O)NC(Cc1ccccc1)C(O)CC(=O)NCCCc1ccccc1 Show InChI InChI=1S/C40H53N5O5/c1-3-29(2)37(44-36(48)28-45-24-21-40(22-25-45,39(41)50)32-19-11-6-12-20-32)38(49)43-33(26-31-16-9-5-10-17-31)34(46)27-35(47)42-23-13-18-30-14-7-4-8-15-30/h4-12,14-17,19-20,29,33-34,37,46H,3,13,18,21-28H2,1-2H3,(H2,41,50)(H,42,47)(H,43,49)(H,44,48)/t29-,33?,34?,37-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasmepsin-2 from Plasmodium falciparum. |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50071547
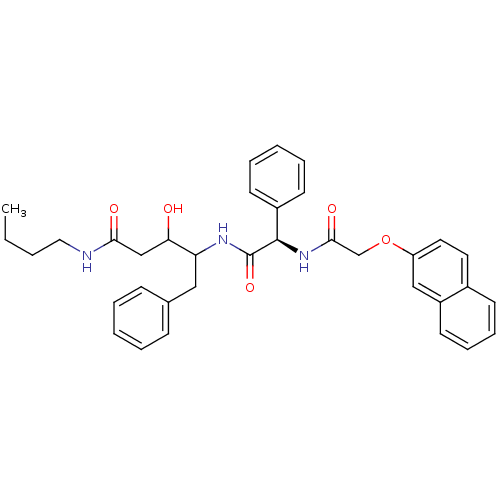
(3-Hydroxy-4-{(R)-2-[2-(naphthalen-2-yloxy)-acetyla...)Show SMILES CCCCNC(=O)CC(O)C(Cc1ccccc1)NC(=O)[C@H](NC(=O)COc1ccc2ccccc2c1)c1ccccc1 Show InChI InChI=1S/C35H39N3O5/c1-2-3-20-36-32(40)23-31(39)30(21-25-12-6-4-7-13-25)37-35(42)34(27-15-8-5-9-16-27)38-33(41)24-43-29-19-18-26-14-10-11-17-28(26)22-29/h4-19,22,30-31,34,39H,2-3,20-21,23-24H2,1H3,(H,36,40)(H,37,42)(H,38,41)/t30?,31?,34-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin D |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50071557
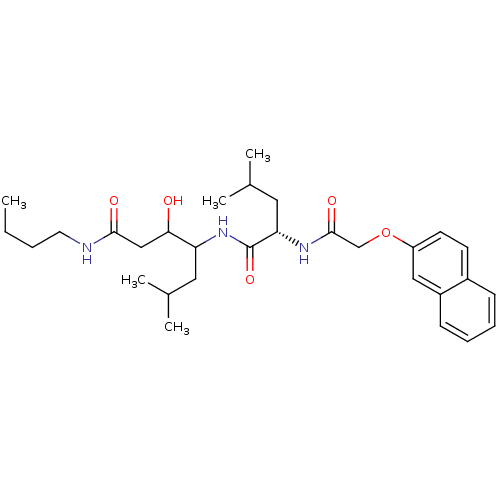
(3-Hydroxy-6-methyl-4-{(S)-4-methyl-2-[2-(naphthale...)Show SMILES CCCCNC(=O)CC(O)C(CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)COc1ccc2ccccc2c1 Show InChI InChI=1S/C30H45N3O5/c1-6-7-14-31-28(35)18-27(34)25(15-20(2)3)33-30(37)26(16-21(4)5)32-29(36)19-38-24-13-12-22-10-8-9-11-23(22)17-24/h8-13,17,20-21,25-27,34H,6-7,14-16,18-19H2,1-5H3,(H,31,35)(H,32,36)(H,33,37)/t25?,26-,27?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin D |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50071560
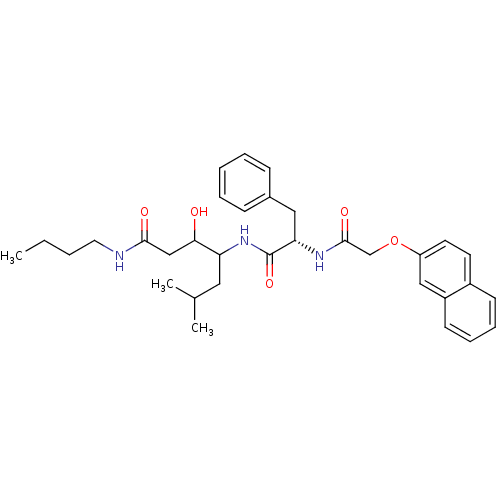
(3-Hydroxy-6-methyl-4-{(S)-2-[2-(naphthalen-2-yloxy...)Show SMILES CCCCNC(=O)CC(O)C(CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)COc1ccc2ccccc2c1 Show InChI InChI=1S/C33H43N3O5/c1-4-5-17-34-31(38)21-30(37)28(18-23(2)3)36-33(40)29(19-24-11-7-6-8-12-24)35-32(39)22-41-27-16-15-25-13-9-10-14-26(25)20-27/h6-16,20,23,28-30,37H,4-5,17-19,21-22H2,1-3H3,(H,34,38)(H,35,39)(H,36,40)/t28?,29-,30?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin D |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50146770

(CHEMBL94646 | N-(1-{1-[1-Hydroxy-2-(3-phenyl-propy...)Show SMILES CCC(C)C(NC(=O)c1ccc(CNC)cc1)C(=O)NC(CC(C)C)C(O)CC(=O)NCCCc1ccccc1 Show InChI InChI=1S/C32H48N4O4/c1-6-23(4)30(36-31(39)26-16-14-25(15-17-26)21-33-5)32(40)35-27(19-22(2)3)28(37)20-29(38)34-18-10-13-24-11-8-7-9-12-24/h7-9,11-12,14-17,22-23,27-28,30,33,37H,6,10,13,18-21H2,1-5H3,(H,34,38)(H,35,40)(H,36,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin D |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50071551

(CHEMBL427946 | N-[(S)-1-(1-Benzyl-3-butylcarbamoyl...)Show SMILES CCCCNC(=O)CC(O)C(Cc1ccccc1)NC(=O)[C@@H](NC(=O)c1ccc(OC)cc1OC)C(C)C Show InChI InChI=1S/C29H41N3O6/c1-6-7-15-30-26(34)18-24(33)23(16-20-11-9-8-10-12-20)31-29(36)27(19(2)3)32-28(35)22-14-13-21(37-4)17-25(22)38-5/h8-14,17,19,23-24,27,33H,6-7,15-16,18H2,1-5H3,(H,30,34)(H,31,36)(H,32,35)/t23?,24?,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin D |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50071550

(CHEMBL74562 | N-[(1S,2S)-1-(1-Benzyl-3-butylcarbam...)Show SMILES CCCCNC(=O)CC(O)C(Cc1ccccc1)NC(=O)[C@@H](NC(=O)c1ccc(OC)cc1OC)[C@@H](C)CC Show InChI InChI=1S/C30H43N3O6/c1-6-8-16-31-27(35)19-25(34)24(17-21-12-10-9-11-13-21)32-30(37)28(20(3)7-2)33-29(36)23-15-14-22(38-4)18-26(23)39-5/h9-15,18,20,24-25,28,34H,6-8,16-17,19H2,1-5H3,(H,31,35)(H,32,37)(H,33,36)/t20-,24?,25?,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin D |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50071553

(1-({(1S,2S)-1-[1-Benzyl-2-hydroxy-3-(3-phenyl-prop...)Show SMILES CC[C@H](C)[C@H](NC(=O)CN1CCC(CC1)(C(N)=O)c1ccccc1)C(=O)NC(Cc1ccccc1)C(O)CC(=O)NCCCc1ccccc1 Show InChI InChI=1S/C40H53N5O5/c1-3-29(2)37(44-36(48)28-45-24-21-40(22-25-45,39(41)50)32-19-11-6-12-20-32)38(49)43-33(26-31-16-9-5-10-17-31)34(46)27-35(47)42-23-13-18-30-14-7-4-8-15-30/h4-12,14-17,19-20,29,33-34,37,46H,3,13,18,21-28H2,1-2H3,(H2,41,50)(H,42,47)(H,43,49)(H,44,48)/t29-,33?,34?,37-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin D |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50440097

(CHEMBL2426118)Show SMILES CC(C)(CNS(=O)(=O)CC(F)(F)F)[C@@H](c1ccccc1)c1ccc2n(ncc2c1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H25F4N3O2S/c1-25(2,16-32-36(34,35)17-26(28,29)30)24(18-6-4-3-5-7-18)19-8-13-23-20(14-19)15-31-33(23)22-11-9-21(27)10-12-22/h3-15,24,32H,16-17H2,1-2H3/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to androgen receptor (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 23: 5448-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.085
BindingDB Entry DOI: 10.7270/Q22R3T2S |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50440098

(CHEMBL2426112)Show SMILES CC(C)(C)NC(=O)NCC(C)(C)[C@@H](c1ccccc1)c1ccc2n(ncc2c1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C29H33FN4O/c1-28(2,3)33-27(35)31-19-29(4,5)26(20-9-7-6-8-10-20)21-11-16-25-22(17-21)18-32-34(25)24-14-12-23(30)13-15-24/h6-18,26H,19H2,1-5H3,(H2,31,33,35)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to progesterone receptor (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 23: 5448-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.085
BindingDB Entry DOI: 10.7270/Q22R3T2S |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50440096

(CHEMBL2426124)Show SMILES CC(C)(CNC(=O)CC(F)(F)F)[C@@H](c1ccccc1)c1ccc2n(ncc2c1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C27H25F4N3O/c1-26(2,17-32-24(35)15-27(29,30)31)25(18-6-4-3-5-7-18)19-8-13-23-20(14-19)16-33-34(23)22-11-9-21(28)10-12-22/h3-14,16,25H,15,17H2,1-2H3,(H,32,35)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to androgen receptor (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 23: 5448-51 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.085
BindingDB Entry DOI: 10.7270/Q22R3T2S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data