 Found 605 hits with Last Name = 'chan' and Initial = 'th'
Found 605 hits with Last Name = 'chan' and Initial = 'th' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM25297
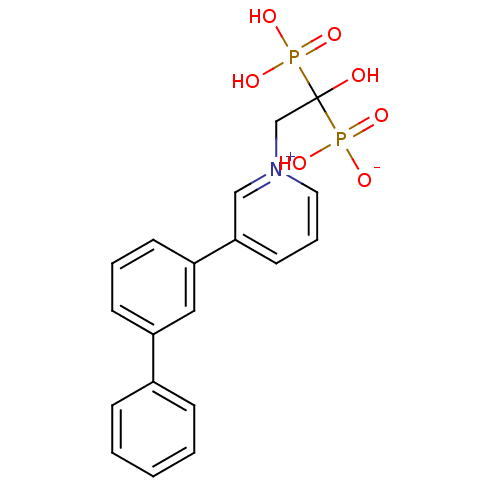
(1-(2-hydrogen phosphonato-2-hydroxy-2-phosphonoeth...)Show SMILES OC(C[n+]1cccc(c1)-c1cccc(c1)-c1ccccc1)(P(O)(O)=O)P(O)([O-])=O Show InChI InChI=1S/C19H19NO7P2/c21-19(28(22,23)24,29(25,26)27)14-20-11-5-10-18(13-20)17-9-4-8-16(12-17)15-6-2-1-3-7-15/h1-13,21H,14H2,(H3-,22,23,24,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25266
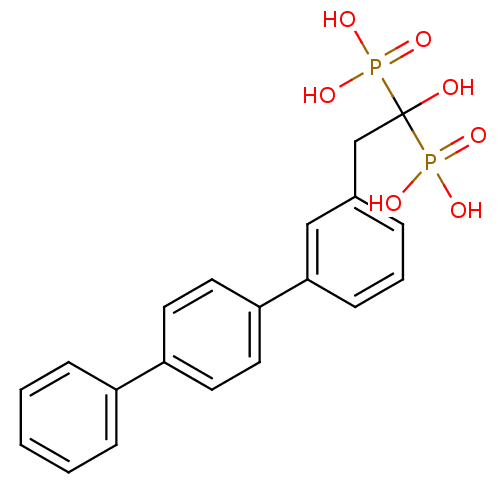
(BPH-628 | bisphosphonate, 21 | {1-hydroxy-2-[3-(4-...)Show SMILES OC(Cc1cccc(c1)-c1ccc(cc1)-c1ccccc1)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C20H20O7P2/c21-20(28(22,23)24,29(25,26)27)14-15-5-4-8-19(13-15)18-11-9-17(10-12-18)16-6-2-1-3-7-16/h1-13,21H,14H2,(H2,22,23,24)(H2,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to human GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM25279
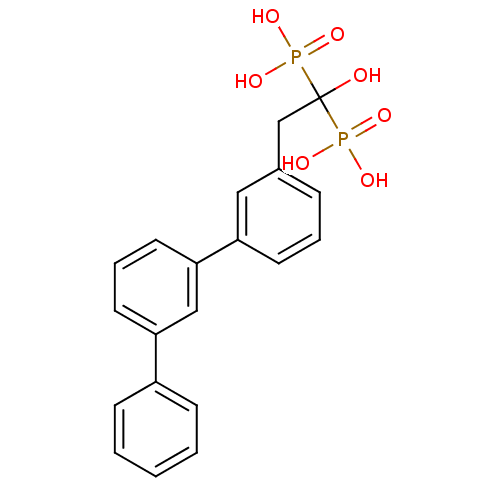
(BPH-608 | bisphosphonate, 31 | {1-hydroxy-2-[3-(3-...)Show SMILES OC(Cc1cccc(c1)-c1cccc(c1)-c1ccccc1)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C20H20O7P2/c21-20(28(22,23)24,29(25,26)27)14-15-6-4-9-17(12-15)19-11-5-10-18(13-19)16-7-2-1-3-8-16/h1-13,21H,14H2,(H2,22,23,24)(H2,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM25266

(BPH-628 | bisphosphonate, 21 | {1-hydroxy-2-[3-(4-...)Show SMILES OC(Cc1cccc(c1)-c1ccc(cc1)-c1ccccc1)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C20H20O7P2/c21-20(28(22,23)24,29(25,26)27)14-15-5-4-8-19(13-15)18-11-9-17(10-12-18)16-6-2-1-3-7-16/h1-13,21H,14H2,(H2,22,23,24)(H2,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM25289

(bisphosphonate, 38 | {1-hydroxy-2-[3-(3-phenylphen...)Show SMILES OC(COc1cccc(c1)-c1cccc(c1)-c1ccccc1)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C20H20O8P2/c21-20(29(22,23)24,30(25,26)27)14-28-19-11-5-10-18(13-19)17-9-4-8-16(12-17)15-6-2-1-3-7-15/h1-13,21H,14H2,(H2,22,23,24)(H2,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25279

(BPH-608 | bisphosphonate, 31 | {1-hydroxy-2-[3-(3-...)Show SMILES OC(Cc1cccc(c1)-c1cccc(c1)-c1ccccc1)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C20H20O7P2/c21-20(28(22,23)24,29(25,26)27)14-15-6-4-9-17(12-15)19-11-5-10-18(13-19)16-7-2-1-3-8-16/h1-13,21H,14H2,(H2,22,23,24)(H2,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to human GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25284
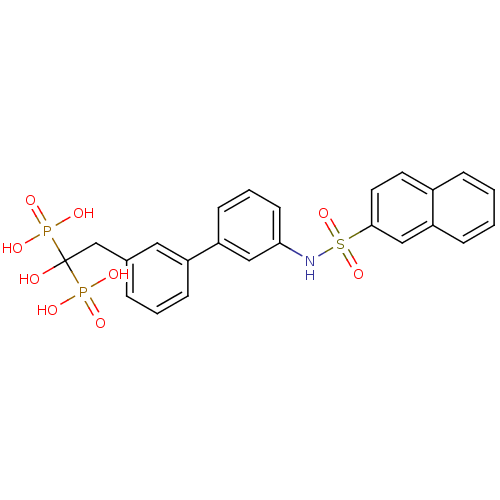
((1-hydroxy-2-{3-[3-(naphthalene-2-sulfonamido)phen...)Show SMILES OC(Cc1cccc(c1)-c1cccc(NS(=O)(=O)c2ccc3ccccc3c2)c1)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C24H23NO9P2S/c26-24(35(27,28)29,36(30,31)32)16-17-5-3-8-19(13-17)20-9-4-10-22(14-20)25-37(33,34)23-12-11-18-6-1-2-7-21(18)15-23/h1-15,25-26H,16H2,(H2,27,28,29)(H2,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to human GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM25284

((1-hydroxy-2-{3-[3-(naphthalene-2-sulfonamido)phen...)Show SMILES OC(Cc1cccc(c1)-c1cccc(NS(=O)(=O)c2ccc3ccccc3c2)c1)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C24H23NO9P2S/c26-24(35(27,28)29,36(30,31)32)16-17-5-3-8-19(13-17)20-9-4-10-22(14-20)25-37(33,34)23-12-11-18-6-1-2-7-21(18)15-23/h1-15,25-26H,16H2,(H2,27,28,29)(H2,30,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25288

(BPH-629 | [1-hydroxy-2-(3-{8-oxatricyclo[7.4.0.0^{...)Show SMILES OC(Cc1cccc(c1)-c1cccc2c1oc1ccccc21)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C20H18O8P2/c21-20(29(22,23)24,30(25,26)27)12-13-5-3-6-14(11-13)15-8-4-9-17-16-7-1-2-10-18(16)28-19(15)17/h1-11,21H,12H2,(H2,22,23,24)(H2,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to human GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM25288

(BPH-629 | [1-hydroxy-2-(3-{8-oxatricyclo[7.4.0.0^{...)Show SMILES OC(Cc1cccc(c1)-c1cccc2c1oc1ccccc21)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C20H18O8P2/c21-20(29(22,23)24,30(25,26)27)12-13-5-3-6-14(11-13)15-8-4-9-17-16-7-1-2-10-18(16)28-19(15)17/h1-11,21H,12H2,(H2,22,23,24)(H2,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25289

(bisphosphonate, 38 | {1-hydroxy-2-[3-(3-phenylphen...)Show SMILES OC(COc1cccc(c1)-c1cccc(c1)-c1ccccc1)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C20H20O8P2/c21-20(29(22,23)24,30(25,26)27)14-28-19-11-5-10-18(13-19)17-9-4-8-16(12-17)15-6-2-1-3-7-15/h1-13,21H,14H2,(H2,22,23,24)(H2,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to human GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM25308
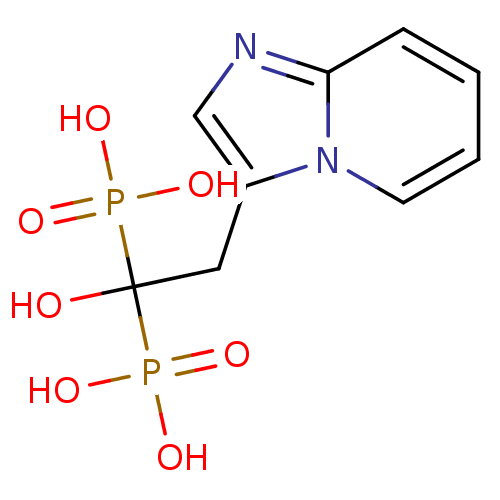
((1-hydroxy-2-{imidazo[1,2-a]pyridin-3-yl}-1-phosph...)Show InChI InChI=1S/C9H12N2O7P2/c12-9(19(13,14)15,20(16,17)18)5-7-6-10-8-3-1-2-4-11(7)8/h1-4,6,12H,5H2,(H2,13,14,15)(H2,16,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Multidrug resistance-associated protein 1
(Homo sapiens (Human)) | BDBM50295478

(1,16-Bis[40-((6-methyl)-4H-chromen-4-on-2-yl)pheny...)Show SMILES Cc1ccc2oc(cc(=O)c2c1)-c1ccc(OCCOCCOCCOCCOCCOc2ccc(cc2)-c2cc(=O)c3cc(C)ccc3o2)cc1 Show InChI InChI=1S/C42H42O10/c1-29-3-13-39-35(25-29)37(43)27-41(51-39)31-5-9-33(10-6-31)49-23-21-47-19-17-45-15-16-46-18-20-48-22-24-50-34-11-7-32(8-12-34)42-28-38(44)36-26-30(2)4-14-40(36)52-42/h3-14,25-28H,15-24H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong Polytechnic University
Curated by ChEMBL
| Assay Description
Inhibition of MRP1 in human 2008/MRP1 cells by dixon plot analysis |
J Med Chem 52: 5311-22 (2009)
Article DOI: 10.1021/jm900194w
BindingDB Entry DOI: 10.7270/Q25B02H5 |
More data for this
Ligand-Target Pair | |
Multidrug resistance-associated protein 1
(Homo sapiens (Human)) | BDBM50295478

(1,16-Bis[40-((6-methyl)-4H-chromen-4-on-2-yl)pheny...)Show SMILES Cc1ccc2oc(cc(=O)c2c1)-c1ccc(OCCOCCOCCOCCOCCOc2ccc(cc2)-c2cc(=O)c3cc(C)ccc3o2)cc1 Show InChI InChI=1S/C42H42O10/c1-29-3-13-39-35(25-29)37(43)27-41(51-39)31-5-9-33(10-6-31)49-23-21-47-19-17-45-15-16-46-18-20-48-22-24-50-34-11-7-32(8-12-34)42-28-38(44)36-26-30(2)4-14-40(36)52-42/h3-14,25-28H,15-24H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong Polytechnic University
Curated by ChEMBL
| Assay Description
Inhibition of MRP1 in human 2008/MRP1 cells by Lineweaver-Burke plot analysis |
J Med Chem 52: 5311-22 (2009)
Article DOI: 10.1021/jm900194w
BindingDB Entry DOI: 10.7270/Q25B02H5 |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25297

(1-(2-hydrogen phosphonato-2-hydroxy-2-phosphonoeth...)Show SMILES OC(C[n+]1cccc(c1)-c1cccc(c1)-c1ccccc1)(P(O)(O)=O)P(O)([O-])=O Show InChI InChI=1S/C19H19NO7P2/c21-19(28(22,23)24,29(25,26)27)14-20-11-5-10-18(13-20)17-9-4-8-16(12-17)15-6-2-1-3-7-15/h1-13,21H,14H2,(H3-,22,23,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to human GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50486029

(CHEMBL2172398)Show SMILES O=c1cc(oc2ccccc12)-c1ccc(OCCOCCN(CCOCCOc2ccc(cc2)-c2cc(=O)c3ccccc3o2)Cc2ccccc2)cc1 Show InChI InChI=1S/C45H41NO8/c47-40-30-44(53-42-12-6-4-10-38(40)42)34-14-18-36(19-15-34)51-28-26-49-24-22-46(32-33-8-2-1-3-9-33)23-25-50-27-29-52-37-20-16-35(17-21-37)45-31-41(48)39-11-5-7-13-43(39)54-45/h1-21,30-31H,22-29,32H2 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong Polytechnic University
Curated by ChEMBL
| Assay Description
Competitive inhibition of P-gp overexpressed in human MDA435/LCC6MDR cells assessed as accumulation of doxorubicin by Dixon plot analysis |
J Med Chem 55: 1999-2014 (2012)
Article DOI: 10.1021/jm201121b
BindingDB Entry DOI: 10.7270/Q2JW8HRN |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50486029

(CHEMBL2172398)Show SMILES O=c1cc(oc2ccccc12)-c1ccc(OCCOCCN(CCOCCOc2ccc(cc2)-c2cc(=O)c3ccccc3o2)Cc2ccccc2)cc1 Show InChI InChI=1S/C45H41NO8/c47-40-30-44(53-42-12-6-4-10-38(40)42)34-14-18-36(19-15-34)51-28-26-49-24-22-46(32-33-8-2-1-3-9-33)23-25-50-27-29-52-37-20-16-35(17-21-37)45-31-41(48)39-11-5-7-13-43(39)54-45/h1-21,30-31H,22-29,32H2 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong Polytechnic University
Curated by ChEMBL
| Assay Description
Competitive inhibition of P-gp overexpressed in human MDA435/LCC6MDR cells assessed as accumulation of doxorubicin by Lineweaver-Burk plot analysis |
J Med Chem 55: 1999-2014 (2012)
Article DOI: 10.1021/jm201121b
BindingDB Entry DOI: 10.7270/Q2JW8HRN |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM81939
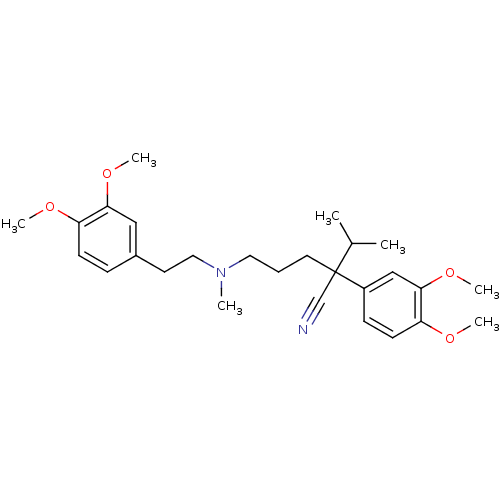
(CAS_52-53-9 | NSC_62969 | VERAPAMIL)Show SMILES COc1ccc(CCN(C)CCCC(C#N)(C(C)C)c2ccc(OC)c(OC)c2)cc1OC Show InChI InChI=1S/C27H38N2O4/c1-20(2)27(19-28,22-10-12-24(31-5)26(18-22)33-7)14-8-15-29(3)16-13-21-9-11-23(30-4)25(17-21)32-6/h9-12,17-18,20H,8,13-16H2,1-7H3 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong Polytechnic University
Curated by ChEMBL
| Assay Description
Competitive inhibition of P-gp overexpressed in human MDA435/LCC6MDR cells assessed as accumulation of doxorubicin by Dixon plot analysis |
J Med Chem 55: 1999-2014 (2012)
Article DOI: 10.1021/jm201121b
BindingDB Entry DOI: 10.7270/Q2JW8HRN |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM81939

(CAS_52-53-9 | NSC_62969 | VERAPAMIL)Show SMILES COc1ccc(CCN(C)CCCC(C#N)(C(C)C)c2ccc(OC)c(OC)c2)cc1OC Show InChI InChI=1S/C27H38N2O4/c1-20(2)27(19-28,22-10-12-24(31-5)26(18-22)33-7)14-8-15-29(3)16-13-21-9-11-23(30-4)25(17-21)32-6/h9-12,17-18,20H,8,13-16H2,1-7H3 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong Polytechnic University
Curated by ChEMBL
| Assay Description
Competitive inhibition of P-gp overexpressed in human MDA435/LCC6MDR cells assessed as accumulation of doxorubicin by Lineweaver-Burk plot analysis |
J Med Chem 55: 1999-2014 (2012)
Article DOI: 10.1021/jm201121b
BindingDB Entry DOI: 10.7270/Q2JW8HRN |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25308

((1-hydroxy-2-{imidazo[1,2-a]pyridin-3-yl}-1-phosph...)Show InChI InChI=1S/C9H12N2O7P2/c12-9(19(13,14)15,20(16,17)18)5-7-6-10-8-3-1-2-4-11(7)8/h1-4,6,12H,5H2,(H2,13,14,15)(H2,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to human GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Multidrug resistance-associated protein 1
(Homo sapiens (Human)) | BDBM15236

(3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...)Show SMILES Oc1cc(O)c2c(c1)oc(-c1cc(O)c(O)c(O)c1)c(O)c2=O Show InChI InChI=1S/C15H10O8/c16-6-3-7(17)11-10(4-6)23-15(14(22)13(11)21)5-1-8(18)12(20)9(19)2-5/h1-4,16-20,22H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong Polytechnic University
Curated by ChEMBL
| Assay Description
Inhibition of MRP1 transfected in human HeLa cells assessed as inhibition of [3H]LTC4 transport by rapid filtration assay |
J Med Chem 52: 5311-22 (2009)
Article DOI: 10.1021/jm900194w
BindingDB Entry DOI: 10.7270/Q25B02H5 |
More data for this
Ligand-Target Pair | |
Multidrug resistance-associated protein 1
(Homo sapiens (Human)) | BDBM23419
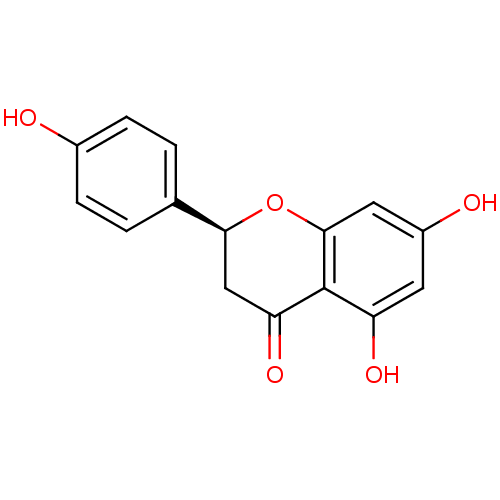
((2S)-5,7-dihydroxy-2-(4-hydroxyphenyl)-3,4-dihydro...)Show SMILES Oc1ccc(cc1)[C@@H]1CC(=O)c2c(O)cc(O)cc2O1 |r| Show InChI InChI=1S/C15H12O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-6,13,16-18H,7H2/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong Polytechnic University
Curated by ChEMBL
| Assay Description
Inhibition of MRP1 transfected in human HeLa cells assessed as inhibition of [3H]LTC4 transport by rapid filtration assay |
J Med Chem 52: 5311-22 (2009)
Article DOI: 10.1021/jm900194w
BindingDB Entry DOI: 10.7270/Q25B02H5 |
More data for this
Ligand-Target Pair | |
Multidrug resistance-associated protein 1
(Homo sapiens (Human)) | BDBM7458

(5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...)Show InChI InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong Polytechnic University
Curated by ChEMBL
| Assay Description
Inhibition of MRP1 transfected in human HeLa cells assessed as inhibition of [3H]LTC4 transport by rapid filtration assay |
J Med Chem 52: 5311-22 (2009)
Article DOI: 10.1021/jm900194w
BindingDB Entry DOI: 10.7270/Q25B02H5 |
More data for this
Ligand-Target Pair | |
Multidrug resistance-associated protein 1
(Homo sapiens (Human)) | BDBM7462

(3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...)Show InChI InChI=1S/C15H10O6/c16-8-3-1-7(2-4-8)15-14(20)13(19)12-10(18)5-9(17)6-11(12)21-15/h1-6,16-18,20H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong Polytechnic University
Curated by ChEMBL
| Assay Description
Inhibition of MRP1 transfected in human HeLa cells assessed as inhibition of [3H]LTC4 transport by rapid filtration assay |
J Med Chem 52: 5311-22 (2009)
Article DOI: 10.1021/jm900194w
BindingDB Entry DOI: 10.7270/Q25B02H5 |
More data for this
Ligand-Target Pair | |
Multidrug resistance-associated protein 1
(Homo sapiens (Human)) | BDBM7460

(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...)Show InChI InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong Polytechnic University
Curated by ChEMBL
| Assay Description
Inhibition of MRP1 transfected in human HeLa cells assessed as inhibition of [3H]LTC4 transport by rapid filtration assay |
J Med Chem 52: 5311-22 (2009)
Article DOI: 10.1021/jm900194w
BindingDB Entry DOI: 10.7270/Q25B02H5 |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to human GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Multidrug resistance-associated protein 1
(Homo sapiens (Human)) | BDBM50033767
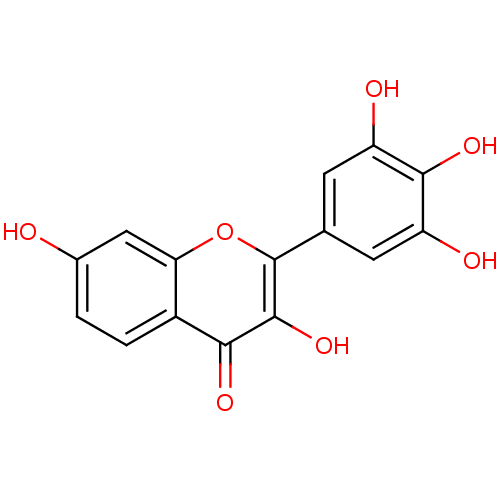
(3,3',4',5',7-pentahydroxy flavone | 3,7,3',4',5'-P...)Show InChI InChI=1S/C15H10O7/c16-7-1-2-8-11(5-7)22-15(14(21)12(8)19)6-3-9(17)13(20)10(18)4-6/h1-5,16-18,20-21H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong Polytechnic University
Curated by ChEMBL
| Assay Description
Inhibition of MRP1 transfected in MDCK2 cells assessed as inhibition of calcein transport |
J Med Chem 52: 5311-22 (2009)
Article DOI: 10.1021/jm900194w
BindingDB Entry DOI: 10.7270/Q25B02H5 |
More data for this
Ligand-Target Pair | |
Multidrug resistance-associated protein 1
(Homo sapiens (Human)) | BDBM81939

(CAS_52-53-9 | NSC_62969 | VERAPAMIL)Show SMILES COc1ccc(CCN(C)CCCC(C#N)(C(C)C)c2ccc(OC)c(OC)c2)cc1OC Show InChI InChI=1S/C27H38N2O4/c1-20(2)27(19-28,22-10-12-24(31-5)26(18-22)33-7)14-8-15-29(3)16-13-21-9-11-23(30-4)25(17-21)32-6/h9-12,17-18,20H,8,13-16H2,1-7H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong Polytechnic University
Curated by ChEMBL
| Assay Description
Inhibition of MRP1 in human 2008/MRP1 cells by Lineweaver-Burke plot analysis |
J Med Chem 52: 5311-22 (2009)
Article DOI: 10.1021/jm900194w
BindingDB Entry DOI: 10.7270/Q25B02H5 |
More data for this
Ligand-Target Pair | |
Multidrug resistance-associated protein 1
(Homo sapiens (Human)) | BDBM81939

(CAS_52-53-9 | NSC_62969 | VERAPAMIL)Show SMILES COc1ccc(CCN(C)CCCC(C#N)(C(C)C)c2ccc(OC)c(OC)c2)cc1OC Show InChI InChI=1S/C27H38N2O4/c1-20(2)27(19-28,22-10-12-24(31-5)26(18-22)33-7)14-8-15-29(3)16-13-21-9-11-23(30-4)25(17-21)32-6/h9-12,17-18,20H,8,13-16H2,1-7H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong Polytechnic University
Curated by ChEMBL
| Assay Description
Inhibition of MRP1 in human 2008/MRP1 cells by dixon plot analysis |
J Med Chem 52: 5311-22 (2009)
Article DOI: 10.1021/jm900194w
BindingDB Entry DOI: 10.7270/Q25B02H5 |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase type 2
(Klebsiella pneumoniae) | BDBM50548231
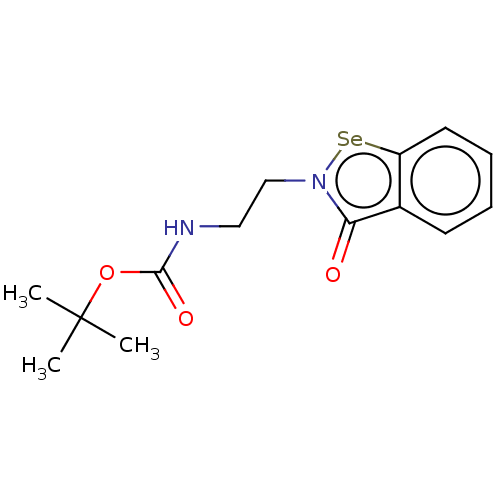
(CHEMBL4753599)Show SMILES [#6]C([#6])([#6])[#8]-[#6](=O)-[#7]-[#6]-[#6]-n1[se;v2]c2ccccc2c1=O | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type N-terminal His6-tagged Klebsiella pneumoniae NDM-1 expressed in Escherichia coli BL21 assessed as inhibition constant using n... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.06.007
BindingDB Entry DOI: 10.7270/Q2SB49CC |
More data for this
Ligand-Target Pair | |
Multidrug resistance-associated protein 1
(Homo sapiens (Human)) | BDBM50001285
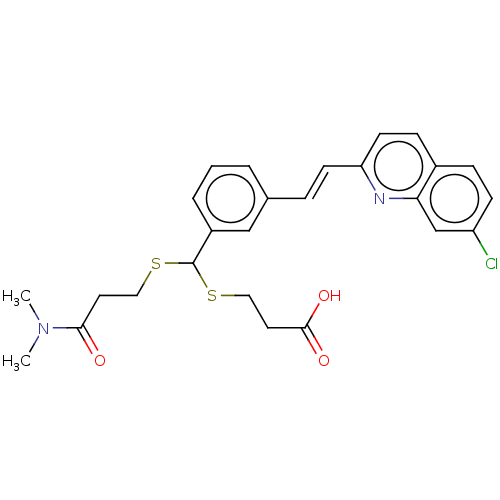
((E)-3-((3-(2-(7-chloroquinolin-2-yl)vinyl)phenyl)(...)Show SMILES CN(C)C(=O)CCSC(SCCC(O)=O)c1cccc(\C=C\c2ccc3ccc(Cl)cc3n2)c1 Show InChI InChI=1S/C26H27ClN2O3S2/c1-29(2)24(30)12-14-33-26(34-15-13-25(31)32)20-5-3-4-18(16-20)6-10-22-11-8-19-7-9-21(27)17-23(19)28-22/h3-11,16-17,26H,12-15H2,1-2H3,(H,31,32)/b10-6+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong Polytechnic University
Curated by ChEMBL
| Assay Description
Inhibition of MRP1 in human 2008/MRP1 cells by Lineweaver-Burke plot analysis |
J Med Chem 52: 5311-22 (2009)
Article DOI: 10.1021/jm900194w
BindingDB Entry DOI: 10.7270/Q25B02H5 |
More data for this
Ligand-Target Pair | |
Multidrug resistance-associated protein 1
(Homo sapiens (Human)) | BDBM50001285

((E)-3-((3-(2-(7-chloroquinolin-2-yl)vinyl)phenyl)(...)Show SMILES CN(C)C(=O)CCSC(SCCC(O)=O)c1cccc(\C=C\c2ccc3ccc(Cl)cc3n2)c1 Show InChI InChI=1S/C26H27ClN2O3S2/c1-29(2)24(30)12-14-33-26(34-15-13-25(31)32)20-5-3-4-18(16-20)6-10-22-11-8-19-7-9-21(27)17-23(19)28-22/h3-11,16-17,26H,12-15H2,1-2H3,(H,31,32)/b10-6+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong Polytechnic University
Curated by ChEMBL
| Assay Description
Inhibition of MRP1 in human 2008/MRP1 cells by dixon plot analysis |
J Med Chem 52: 5311-22 (2009)
Article DOI: 10.1021/jm900194w
BindingDB Entry DOI: 10.7270/Q25B02H5 |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50505459

(CHEMBL4589907)Show SMILES COC(=O)c1cccc(COc2c(oc3ccccc3c2=O)-c2ccc(OCCOCCn3cc(CN(Cc4cn(CCOCCOc5ccc(cc5)-c5oc6ccccc6c(=O)c5OCc5cccc(c5)C(=O)OC)nn4)Cc4ccccc4)nn3)cc2)c1 Show InChI InChI=1S/C69H63N7O14/c1-81-68(79)52-16-10-14-48(38-52)45-87-66-62(77)58-18-6-8-20-60(58)89-64(66)50-22-26-56(27-23-50)85-36-34-83-32-30-75-43-54(70-72-75)41-74(40-47-12-4-3-5-13-47)42-55-44-76(73-71-55)31-33-84-35-37-86-57-28-24-51(25-29-57)65-67(63(78)59-19-7-9-21-61(59)90-65)88-46-49-15-11-17-53(39-49)69(80)82-2/h3-29,38-39,43-44H,30-37,40-42,45-46H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong Polytechnic University
Curated by ChEMBL
| Assay Description
Inhibition of BCRP (unknown origin) transfected in human HEK293/R2 cells assessed as potentiation of topotecan-induced cytotoxicity measured as topot... |
J Med Chem 62: 8578-8608 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00963
BindingDB Entry DOI: 10.7270/Q28S4T5Q |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50505456
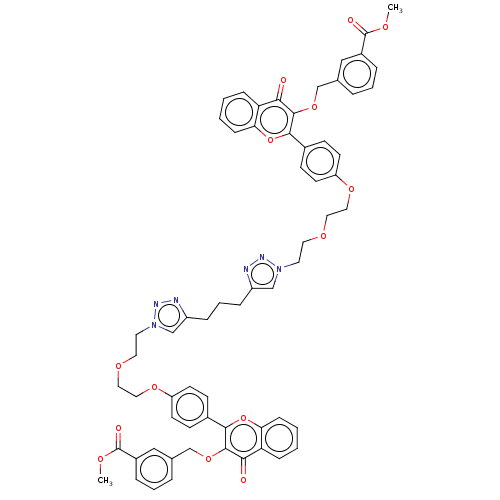
(CHEMBL4440681)Show SMILES COC(=O)c1cccc(COc2c(oc3ccccc3c2=O)-c2ccc(OCCOCCn3cc(CCCc4cn(CCOCCOc5ccc(cc5)-c5oc6ccccc6c(=O)c5OCc5cccc(c5)C(=O)OC)nn4)nn3)cc2)c1 Show InChI InChI=1S/C63H58N6O14/c1-74-62(72)46-12-7-10-42(36-46)40-80-60-56(70)52-16-3-5-18-54(52)82-58(60)44-20-24-50(25-21-44)78-34-32-76-30-28-68-38-48(64-66-68)14-9-15-49-39-69(67-65-49)29-31-77-33-35-79-51-26-22-45(23-27-51)59-61(57(71)53-17-4-6-19-55(53)83-59)81-41-43-11-8-13-47(37-43)63(73)75-2/h3-8,10-13,16-27,36-39H,9,14-15,28-35,40-41H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong Polytechnic University
Curated by ChEMBL
| Assay Description
Inhibition of BCRP (unknown origin) transfected in human HEK293/R2 cells assessed as potentiation of topotecan-induced cytotoxicity measured as topot... |
J Med Chem 62: 8578-8608 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00963
BindingDB Entry DOI: 10.7270/Q28S4T5Q |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50505460
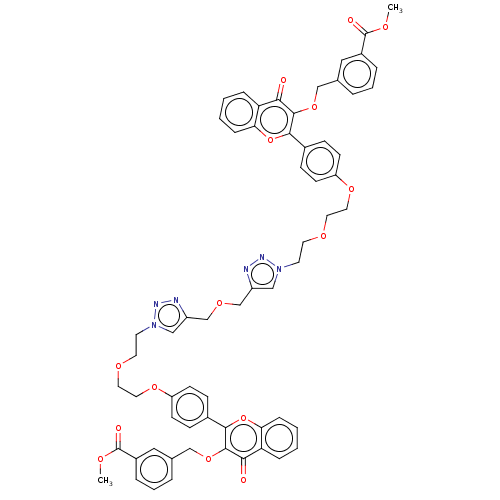
(CHEMBL4474920)Show SMILES COC(=O)c1cccc(COc2c(oc3ccccc3c2=O)-c2ccc(OCCOCCn3cc(COCc4cn(CCOCCOc5ccc(cc5)-c5oc6ccccc6c(=O)c5OCc5cccc(c5)C(=O)OC)nn4)nn3)cc2)c1 Show InChI InChI=1S/C62H56N6O15/c1-73-61(71)45-11-7-9-41(33-45)37-80-59-55(69)51-13-3-5-15-53(51)82-57(59)43-17-21-49(22-18-43)78-31-29-75-27-25-67-35-47(63-65-67)39-77-40-48-36-68(66-64-48)26-28-76-30-32-79-50-23-19-44(20-24-50)58-60(56(70)52-14-4-6-16-54(52)83-58)81-38-42-10-8-12-46(34-42)62(72)74-2/h3-24,33-36H,25-32,37-40H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong Polytechnic University
Curated by ChEMBL
| Assay Description
Inhibition of BCRP (unknown origin) transfected in human HEK293/R2 cells assessed as potentiation of topotecan-induced cytotoxicity measured as topot... |
J Med Chem 62: 8578-8608 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00963
BindingDB Entry DOI: 10.7270/Q28S4T5Q |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50305083
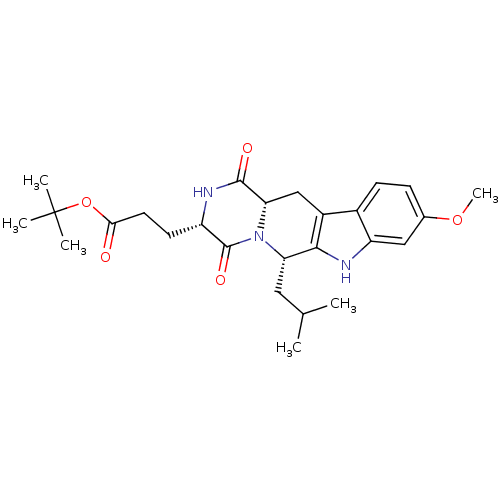
(3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...)Show SMILES COc1ccc2c3C[C@@H]4N([C@@H](CC(C)C)c3[nH]c2c1)C(=O)[C@H](CCC(=O)OC(C)(C)C)NC4=O |r| Show InChI InChI=1S/C26H35N3O5/c1-14(2)11-20-23-17(16-8-7-15(33-6)12-19(16)27-23)13-21-24(31)28-18(25(32)29(20)21)9-10-22(30)34-26(3,4)5/h7-8,12,14,18,20-21,27H,9-11,13H2,1-6H3,(H,28,31)/t18-,20-,21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong Polytechnic University
Curated by ChEMBL
| Assay Description
Inhibition of BCRP (unknown origin) transfected in human HEK293/R2 cells assessed as potentiation of topotecan-induced cytotoxicity measured as topot... |
J Med Chem 62: 8578-8608 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00963
BindingDB Entry DOI: 10.7270/Q28S4T5Q |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50505458
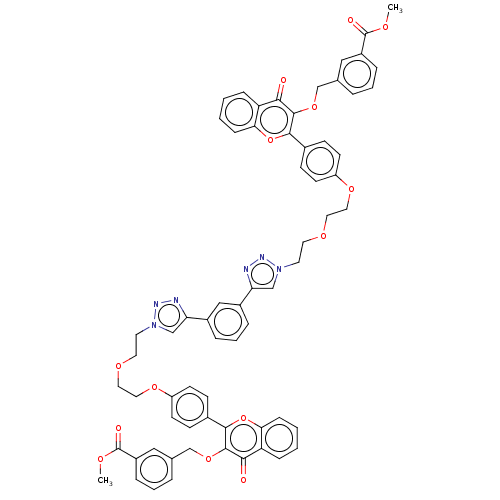
(CHEMBL4453052)Show SMILES COC(=O)c1cccc(COc2c(oc3ccccc3c2=O)-c2ccc(OCCOCCn3cc(nn3)-c3cccc(c3)-c3cn(CCOCCOc4ccc(cc4)-c4oc5ccccc5c(=O)c4OCc4cccc(c4)C(=O)OC)nn3)cc2)c1 Show InChI InChI=1S/C66H56N6O14/c1-77-65(75)49-14-7-10-43(36-49)41-83-63-59(73)53-16-3-5-18-57(53)85-61(63)45-20-24-51(25-21-45)81-34-32-79-30-28-71-39-55(67-69-71)47-12-9-13-48(38-47)56-40-72(70-68-56)29-31-80-33-35-82-52-26-22-46(23-27-52)62-64(60(74)54-17-4-6-19-58(54)86-62)84-42-44-11-8-15-50(37-44)66(76)78-2/h3-27,36-40H,28-35,41-42H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong Polytechnic University
Curated by ChEMBL
| Assay Description
Inhibition of BCRP (unknown origin) transfected in human HEK293/R2 cells assessed as potentiation of topotecan-induced cytotoxicity measured as topot... |
J Med Chem 62: 8578-8608 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00963
BindingDB Entry DOI: 10.7270/Q28S4T5Q |
More data for this
Ligand-Target Pair | |
Multidrug resistance-associated protein 1
(Homo sapiens (Human)) | BDBM67690
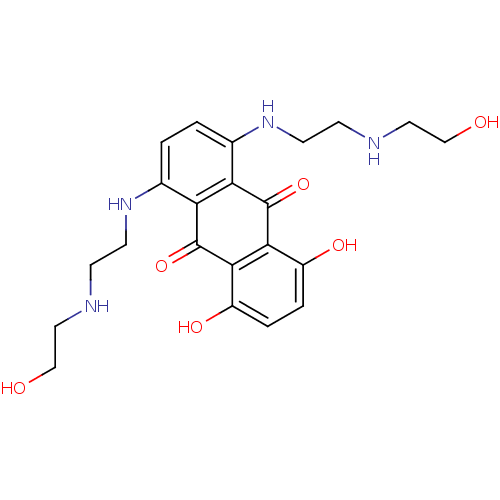
(1,4-bis[2-(2-hydroxyethylamino)ethylamino]-5,8-bis...)Show SMILES OCCNCCNc1ccc(NCCNCCO)c2C(=O)c3c(O)ccc(O)c3C(=O)c12 Show InChI InChI=1S/C22H28N4O6/c27-11-9-23-5-7-25-13-1-2-14(26-8-6-24-10-12-28)18-17(13)21(31)19-15(29)3-4-16(30)20(19)22(18)32/h1-4,23-30H,5-12H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong Polytechnic University
Curated by ChEMBL
| Assay Description
Inhibition of MRP1 in human 2008/MRP1 cells assessed as reduction in cell viability after 5 days by MTS/PMS assay |
J Med Chem 62: 8578-8608 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00963
BindingDB Entry DOI: 10.7270/Q28S4T5Q |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM25297

(1-(2-hydrogen phosphonato-2-hydroxy-2-phosphonoeth...)Show SMILES OC(C[n+]1cccc(c1)-c1cccc(c1)-c1ccccc1)(P(O)(O)=O)P(O)([O-])=O Show InChI InChI=1S/C19H19NO7P2/c21-19(28(22,23)24,29(25,26)27)14-20-11-5-10-18(13-20)17-9-4-8-16(12-17)15-6-2-1-3-7-15/h1-13,21H,14H2,(H3-,22,23,24,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM25279

(BPH-608 | bisphosphonate, 31 | {1-hydroxy-2-[3-(3-...)Show SMILES OC(Cc1cccc(c1)-c1cccc(c1)-c1ccccc1)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C20H20O7P2/c21-20(28(22,23)24,29(25,26)27)14-15-6-4-9-17(12-15)19-11-5-10-18(13-19)16-7-2-1-3-8-16/h1-13,21H,14H2,(H2,22,23,24)(H2,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM25266

(BPH-628 | bisphosphonate, 21 | {1-hydroxy-2-[3-(4-...)Show SMILES OC(Cc1cccc(c1)-c1ccc(cc1)-c1ccccc1)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C20H20O7P2/c21-20(28(22,23)24,29(25,26)27)14-15-5-4-8-19(13-15)18-11-9-17(10-12-18)16-6-2-1-3-7-16/h1-13,21H,14H2,(H2,22,23,24)(H2,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25256

(bisphosphonate, 9 | hydrogen [2-(dodecyldimethylph...)Show SMILES CCCCCCCCCCCC[P+](C)(C)CC(P(O)(O)=O)P(O)([O-])=O Show InChI InChI=1S/C16H37O6P3/c1-4-5-6-7-8-9-10-11-12-13-14-23(2,3)15-16(24(17,18)19)25(20,21)22/h16H,4-15H2,1-3H3,(H3-,17,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University
| Assay Description
The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... |
J Med Chem 51: 5594-607 (2008)
Article DOI: 10.1021/jm800325y
BindingDB Entry DOI: 10.7270/Q2028PVT |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM25289

(bisphosphonate, 38 | {1-hydroxy-2-[3-(3-phenylphen...)Show SMILES OC(COc1cccc(c1)-c1cccc(c1)-c1ccccc1)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C20H20O8P2/c21-20(29(22,23)24,30(25,26)27)14-28-19-11-5-10-18(13-19)17-9-4-8-16(12-17)15-6-2-1-3-7-15/h1-13,21H,14H2,(H2,22,23,24)(H2,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM25284

((1-hydroxy-2-{3-[3-(naphthalene-2-sulfonamido)phen...)Show SMILES OC(Cc1cccc(c1)-c1cccc(NS(=O)(=O)c2ccc3ccccc3c2)c1)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C24H23NO9P2S/c26-24(35(27,28)29,36(30,31)32)16-17-5-3-8-19(13-17)20-9-4-10-22(14-20)25-37(33,34)23-12-11-18-6-1-2-7-21(18)15-23/h1-15,25-26H,16H2,(H2,27,28,29)(H2,30,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25259

(3-(decyloxy)-1-(2-hydrogen phosphonato-2-phosphono...)Show SMILES CCCCCCCCCCOc1ccc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C17H31NO7P2/c1-2-3-4-5-6-7-8-9-13-25-16-11-10-12-18(14-16)15-17(26(19,20)21)27(22,23)24/h10-12,14,17H,2-9,13,15H2,1H3,(H3-,19,20,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University
| Assay Description
The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... |
J Med Chem 51: 5594-607 (2008)
Article DOI: 10.1021/jm800325y
BindingDB Entry DOI: 10.7270/Q2028PVT |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM25288

(BPH-629 | [1-hydroxy-2-(3-{8-oxatricyclo[7.4.0.0^{...)Show SMILES OC(Cc1cccc(c1)-c1cccc2c1oc1ccccc21)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C20H18O8P2/c21-20(29(22,23)24,30(25,26)27)12-13-5-3-6-14(11-13)15-8-4-9-17-16-7-1-2-10-18(16)28-19(15)17/h1-11,21H,12H2,(H2,22,23,24)(H2,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25258
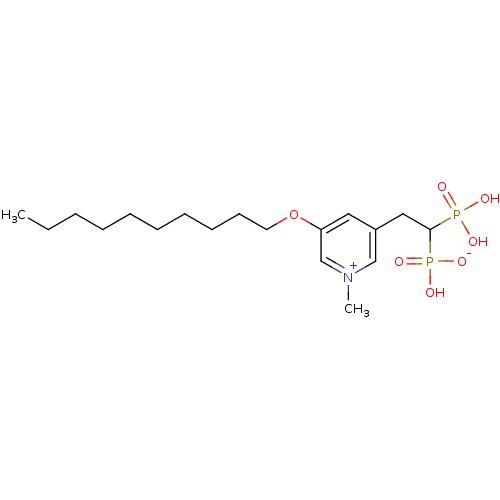
(3-(decyloxy)-5-(2-hydrogen phosphonato-2-phosphono...)Show SMILES CCCCCCCCCCOc1cc(CC(P(O)(O)=O)P(O)([O-])=O)c[n+](C)c1 Show InChI InChI=1S/C18H33NO7P2/c1-3-4-5-6-7-8-9-10-11-26-17-12-16(14-19(2)15-17)13-18(27(20,21)22)28(23,24)25/h12,14-15,18H,3-11,13H2,1-2H3,(H3-,20,21,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University
| Assay Description
The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... |
J Med Chem 51: 5594-607 (2008)
Article DOI: 10.1021/jm800325y
BindingDB Entry DOI: 10.7270/Q2028PVT |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM25308

((1-hydroxy-2-{imidazo[1,2-a]pyridin-3-yl}-1-phosph...)Show InChI InChI=1S/C9H12N2O7P2/c12-9(19(13,14)15,20(16,17)18)5-7-6-10-8-3-1-2-4-11(7)8/h1-4,6,12H,5H2,(H2,13,14,15)(H2,16,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25260
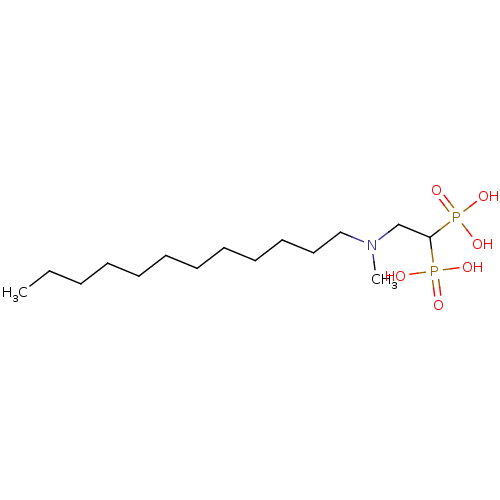
(bisphosphonate, 16 | {2-[dodecyl(methyl)amino]-1-p...)Show InChI InChI=1S/C15H35NO6P2/c1-3-4-5-6-7-8-9-10-11-12-13-16(2)14-15(23(17,18)19)24(20,21)22/h15H,3-14H2,1-2H3,(H2,17,18,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University
| Assay Description
The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... |
J Med Chem 51: 5594-607 (2008)
Article DOI: 10.1021/jm800325y
BindingDB Entry DOI: 10.7270/Q2028PVT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data