

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50131251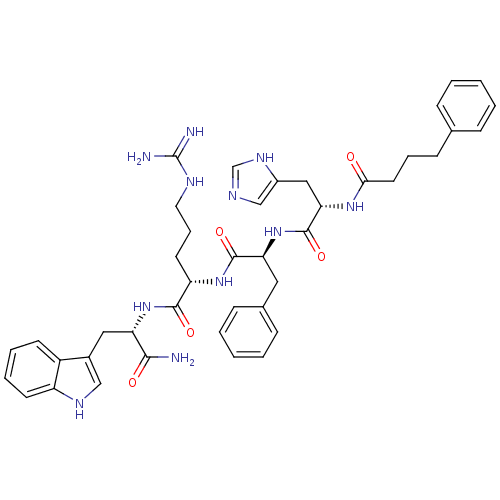 ((S)-5-Guanidino-2-{(S)-2-[(S)-3-(3H-imidazol-4-yl)...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati Curated by ChEMBL | Assay Description Binding affinity towards human Melanocortin 1 receptor (hMC1R) | Bioorg Med Chem Lett 13: 2647-50 (2003) BindingDB Entry DOI: 10.7270/Q2474BD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50189013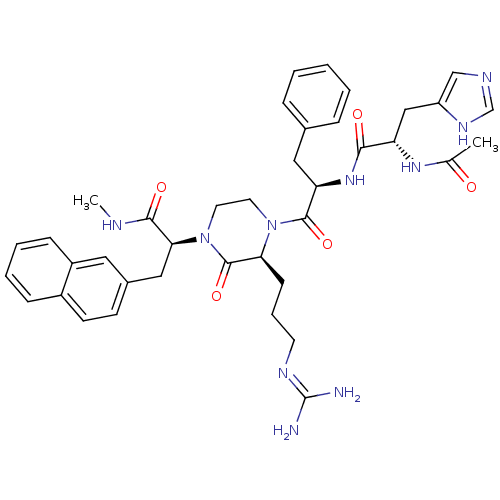 ((S)-2-[(S)-4-{(R)-2-[(S)-2-acetylamino-3-(3H-imida...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC1R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50189024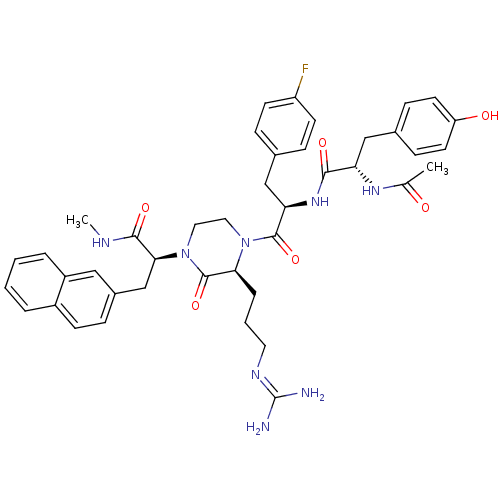 ((S)-2-[(S)-4-[(R)-2-[(S)-2-acetylamino-3-(4-hydrox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC4R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM82411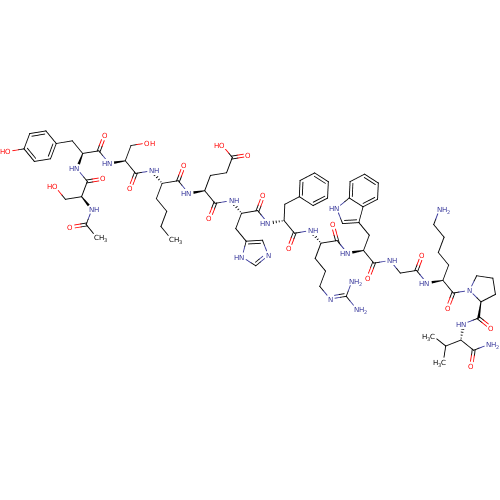 (CAS_75921-69-6 | NDP-MSH) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati Curated by ChEMBL | Assay Description Binding affinity towards human Melanocortin 1 receptor (hMC1R) | Bioorg Med Chem Lett 13: 2647-50 (2003) BindingDB Entry DOI: 10.7270/Q2474BD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129420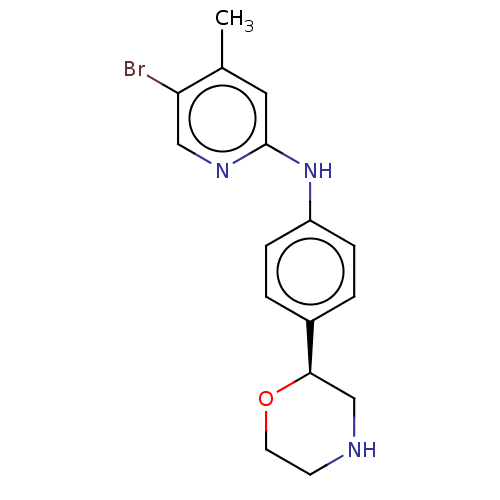 (US8802673, 62) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -55.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129366 (US8802673, 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -55.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129410 (US8802673, 52) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -55.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50189010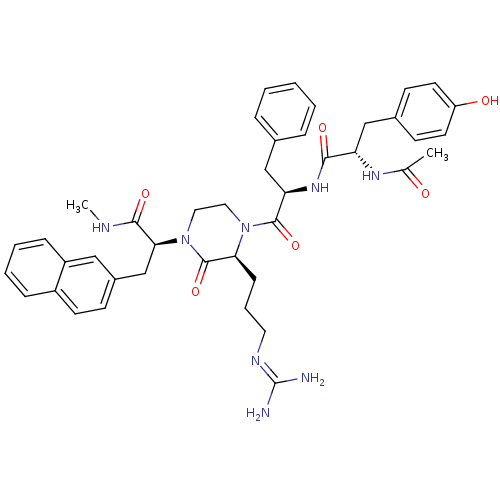 ((S)-2-[(S)-4-{(R)-2-[(S)-2-acetylamino-3-(4-hydrox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC4R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129522 (US8802673, 164) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129441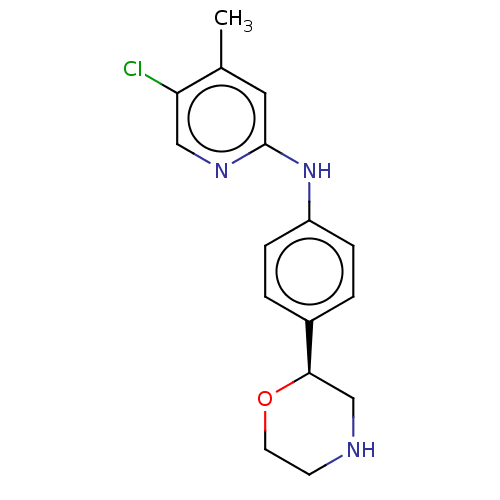 (US8802673, 83) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50191554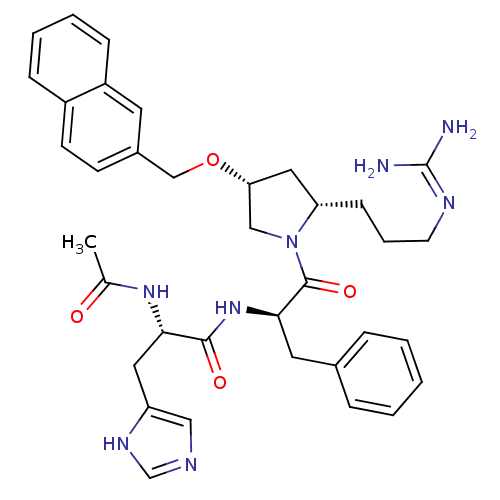 ((S)-2-acetamido-N-((R)-1-((2S,4R)-2-(3-guanidinopr...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of europium labeled NDP-alpha-MSH from human MC1R expressed in HEK293 cells | J Med Chem 49: 4745-61 (2006) Article DOI: 10.1021/jm060384p BindingDB Entry DOI: 10.7270/Q29W0F4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM82411 (CAS_75921-69-6 | NDP-MSH) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati Curated by ChEMBL | Assay Description Binding affinity towards human Melanocortin 3 receptor (hMC3R) | Bioorg Med Chem Lett 13: 2647-50 (2003) BindingDB Entry DOI: 10.7270/Q2474BD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129402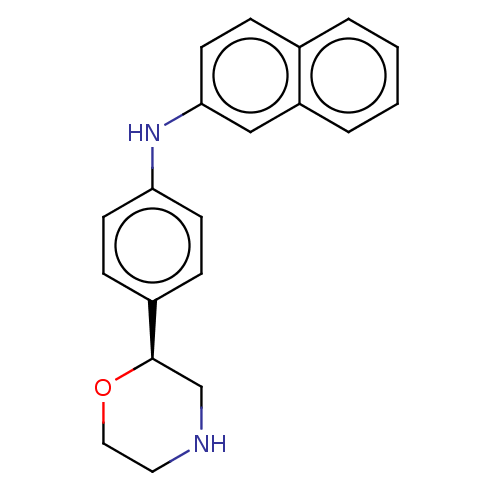 (US8802673, 44) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | -54.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50189013 ((S)-2-[(S)-4-{(R)-2-[(S)-2-acetylamino-3-(3H-imida...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC4R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129440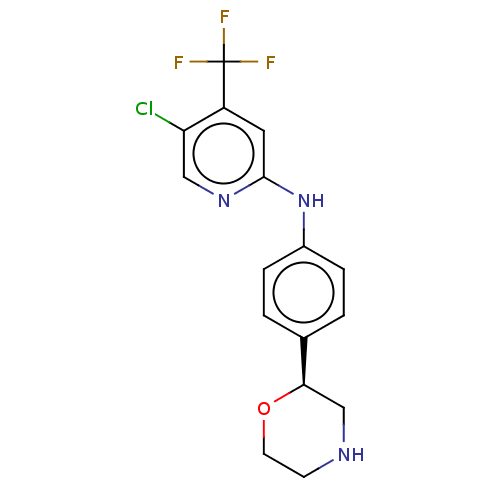 (US8802673, 82) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129404 (US8802673, 46) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129457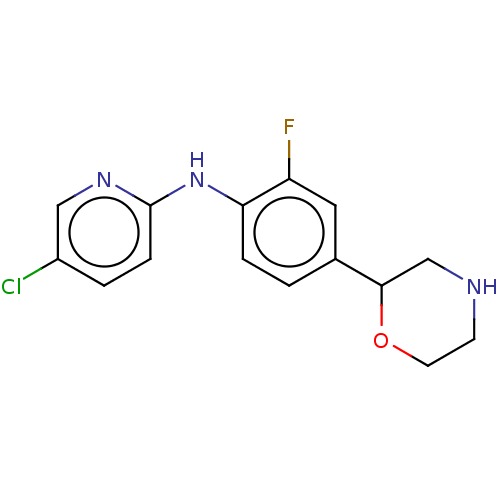 (US8802673, 99) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129529 (US8802673, 171) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129412 (US8802673, 54) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129505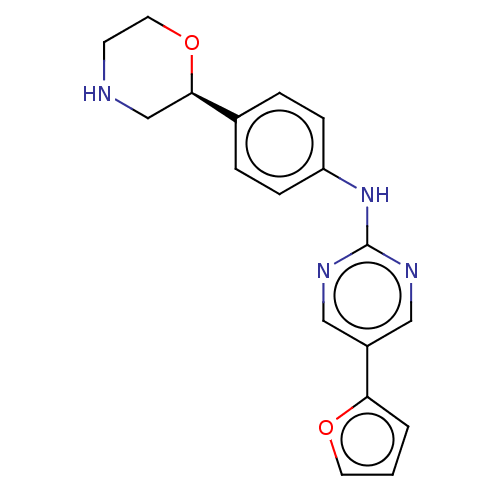 (US8802673, 147) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.900 | -53.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM50336205 ((N-(3-Ethoxy-phenyl)-4-pyrrolidin-1-yl-3-trifluoro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of mouse TAAR1 | Bioorg Med Chem Lett 21: 1227-31 (2011) Article DOI: 10.1016/j.bmcl.2010.12.075 BindingDB Entry DOI: 10.7270/Q25T3KRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM82411 (CAS_75921-69-6 | NDP-MSH) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati Curated by ChEMBL | Assay Description Binding affinity towards human melanocortin 4 receptor (hMC4R) | Bioorg Med Chem Lett 13: 2647-50 (2003) BindingDB Entry DOI: 10.7270/Q2474BD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50189010 ((S)-2-[(S)-4-{(R)-2-[(S)-2-acetylamino-3-(4-hydrox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC1R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50189010 ((S)-2-[(S)-4-{(R)-2-[(S)-2-acetylamino-3-(4-hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC3R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50189013 ((S)-2-[(S)-4-{(R)-2-[(S)-2-acetylamino-3-(3H-imida...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC3R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129549 (US8802673, 191) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -53.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 7b (Rattus norvegicus (Rat)) | BDBM129552 (US8802673, 194) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | -53.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing mouse TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat i... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50189025 ((S)-2-acetamido-N1-((R)-3-(4-fluorophenyl)-1-((S)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC4R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50189024 ((S)-2-[(S)-4-[(R)-2-[(S)-2-acetylamino-3-(4-hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC3R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129507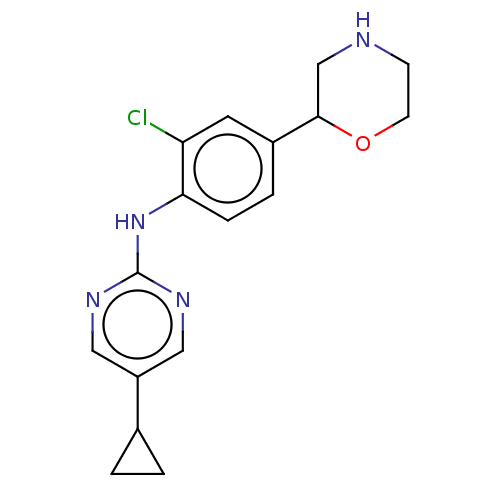 (US8802673, 149) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | -53.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129396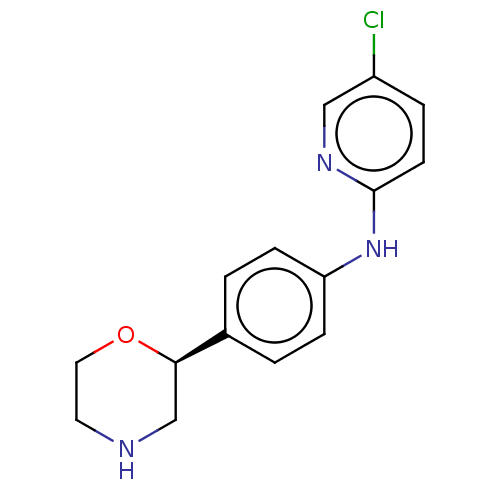 (US8802673, 38) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | -53.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129471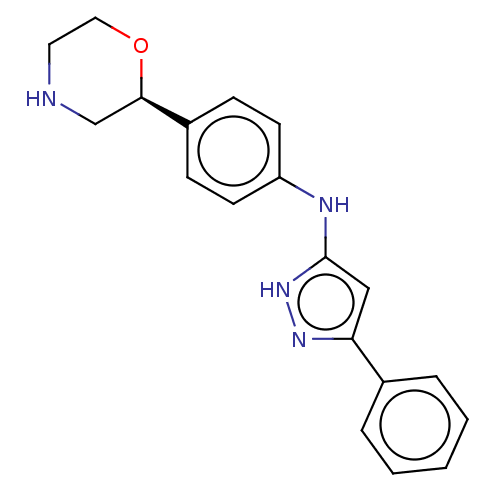 (US8802673, 113) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.10 | -53.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 7b (Rattus norvegicus (Rat)) | BDBM129529 (US8802673, 171) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | -53.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing mouse TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat i... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129363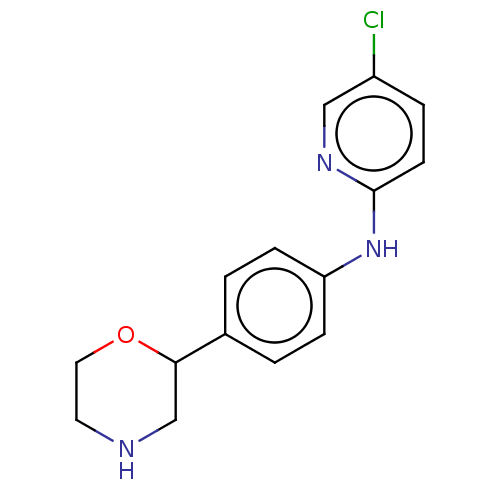 (US8802673, 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | -53.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 7b (Rattus norvegicus (Rat)) | BDBM129569 (US8802673, 211) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | -53.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing mouse TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat i... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129411 (US8802673, 53) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.20 | -53.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 7b (Rattus norvegicus (Rat)) | BDBM129528 (US8802673, 170) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.20 | -53.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing mouse TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat i... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129405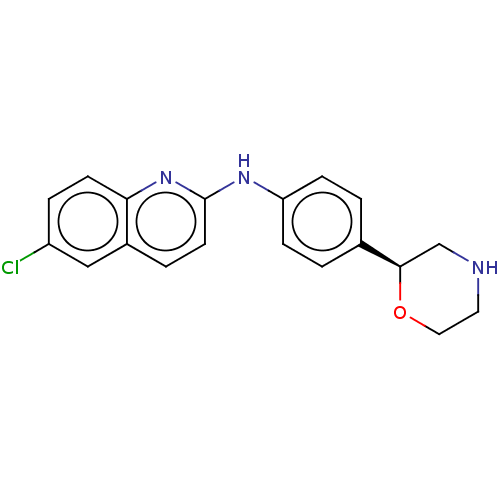 (US8802673, 47) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.20 | -53.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50189023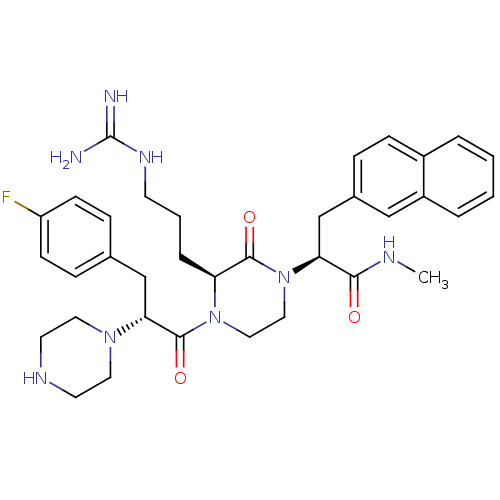 ((S)-2-((S)-4-((R)-3-(4-fluorophenyl)-2-(piperazin-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC4R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50191569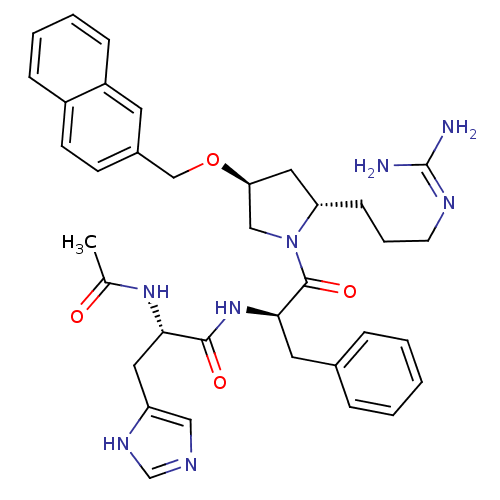 ((S)-2-Acetamido-N-((R)-1-((2S,4S)-2-(3-guanidinopr...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of europium labeled NDP-alpha-MSH from human MC1R expressed in HEK293 cells | J Med Chem 49: 4745-61 (2006) Article DOI: 10.1021/jm060384p BindingDB Entry DOI: 10.7270/Q29W0F4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50189014 ((S)-2-((S)-4-((R)-2-acetamido-3-(4-fluorophenyl)pr...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC4R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 7b (Rattus norvegicus (Rat)) | BDBM129507 (US8802673, 149) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.40 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing mouse TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat i... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129427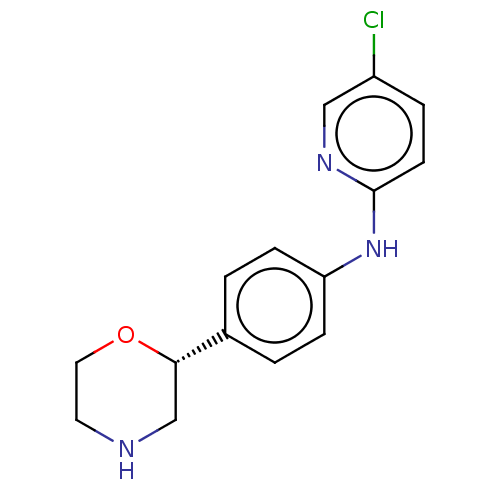 (US8802673, 69) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.40 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129443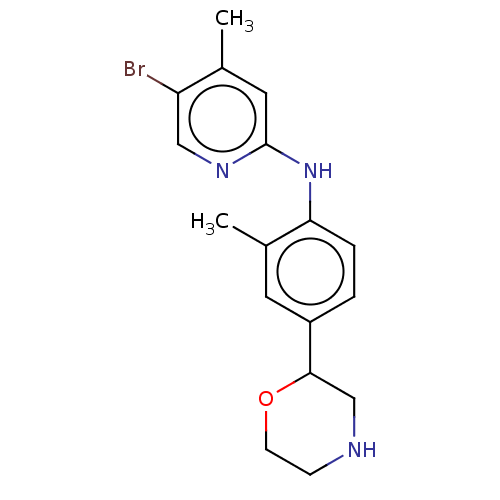 (US8802673, 85) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.40 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50253660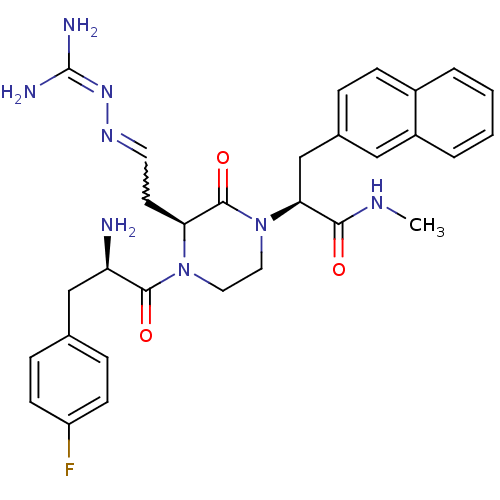 (2-{3-(2-Amino-ethyl-guanidino)-4-[2-amino-3-(4-flu...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of europium-labeled NDP-alpha-MSH from human MC4R expressed in HEK293 cells | J Med Chem 51: 6055-66 (2008) Article DOI: 10.1021/jm800525p BindingDB Entry DOI: 10.7270/Q2DV1JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129442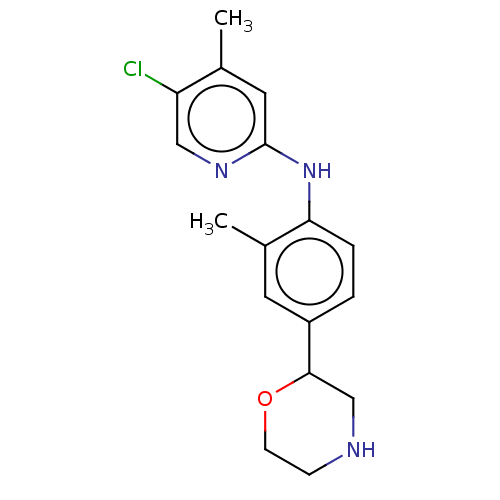 (US8802673, 84) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.5 | -52.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129369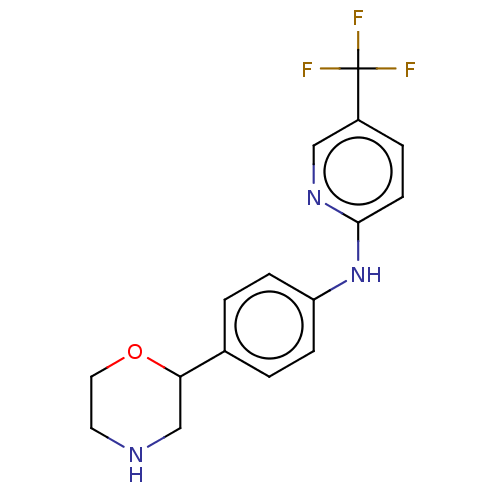 (US8802673, 11) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.5 | -52.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129368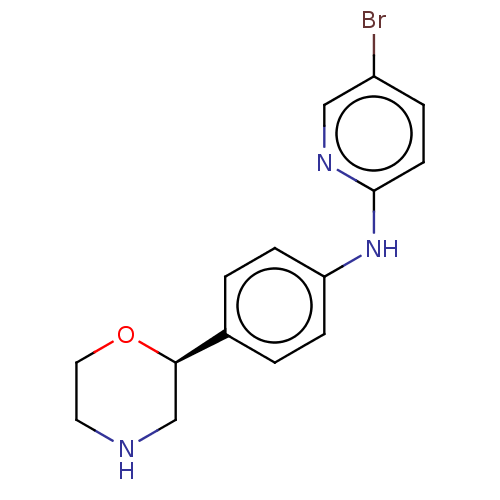 (US8802673, 10) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.5 | -52.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129546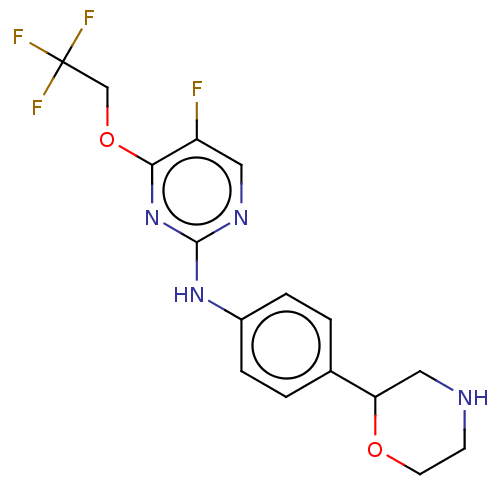 (US8802673, 188) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.60 | -52.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50236369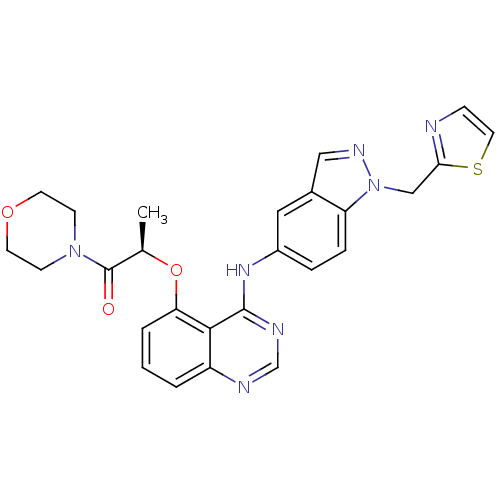 ((R)-1-morpholino-2-(4-(1-(thiazol-2-ylmethyl)-1H-i...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches Curated by ChEMBL | Assay Description Inhibition of EGFR | Bioorg Med Chem Lett 18: 1799-803 (2008) Article DOI: 10.1016/j.bmcl.2008.02.035 BindingDB Entry DOI: 10.7270/Q23T9H0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2690 total ) | Next | Last >> |