

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B2 bradykinin receptor (RAT) | BDBM50370083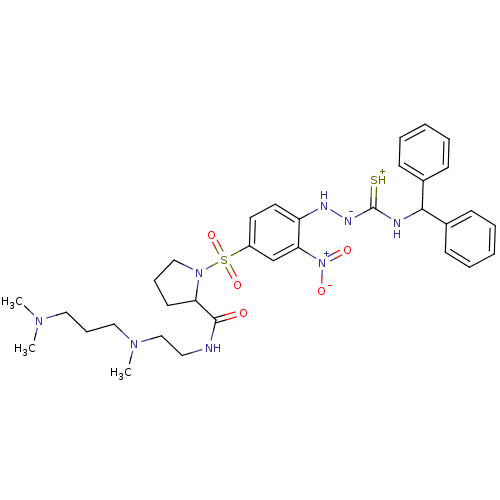 (CHEMBL1907651) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50355491 (CHEMBL1835870) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Inhibition of human recombinant FLT3 by radiometric assay | J Med Chem 55: 725-34 (2012) Article DOI: 10.1021/jm201198w BindingDB Entry DOI: 10.7270/Q2GQ6Z6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50370077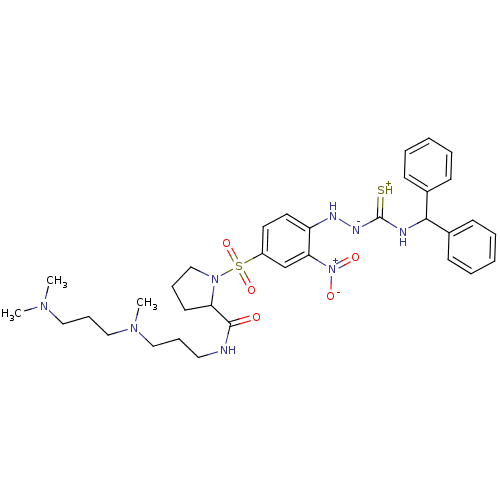 (CHEMBL1907652) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1C (Gallus gallus) | BDBM50472142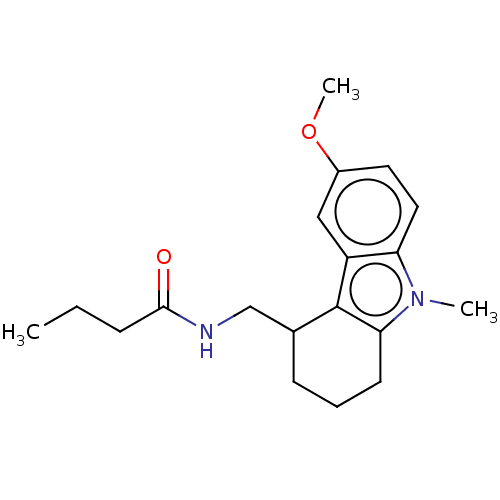 (CHEMBL142343) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.378 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Binding affinity towards melatonin receptor, determined in chicken brain membranes using [2-125I]melatonin | J Med Chem 41: 451-67 (1998) Article DOI: 10.1021/jm970246n BindingDB Entry DOI: 10.7270/Q2154KSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1C (Gallus gallus) | BDBM50472142 (CHEMBL142343) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.378 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Binding affinity towards melatonin receptor, determined in chicken brain membranes using [2-125I]melatonin | J Med Chem 41: 451-67 (1998) Article DOI: 10.1021/jm970246n BindingDB Entry DOI: 10.7270/Q2154KSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM9665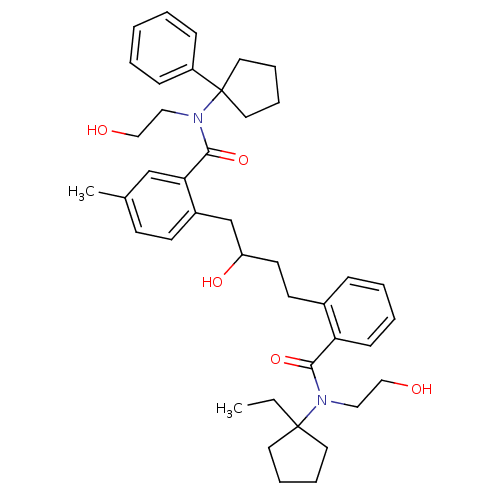 (2-(4-{2-[(1-ethylcyclopentyl)(2-hydroxyethyl)carba...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu-(p-NO2-Phe)-Glu-Ala-Nleu-Ser was use... | J Med Chem 39: 2795-811 (1996) Article DOI: 10.1021/jm960092w BindingDB Entry DOI: 10.7270/Q2028PRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50113263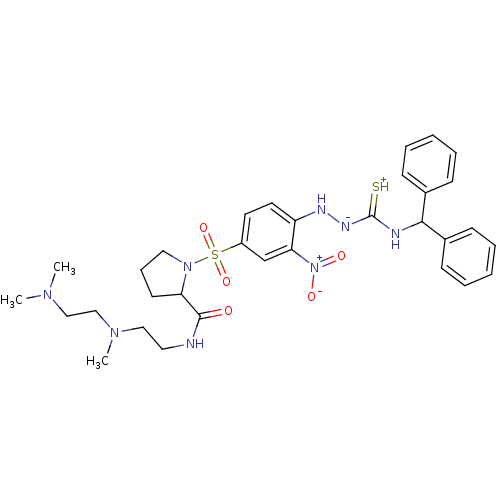 ((S)-1-[4-(4-benzhydrylthiosemicarbazido)-3-nitrobe...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1C (Gallus gallus) | BDBM9019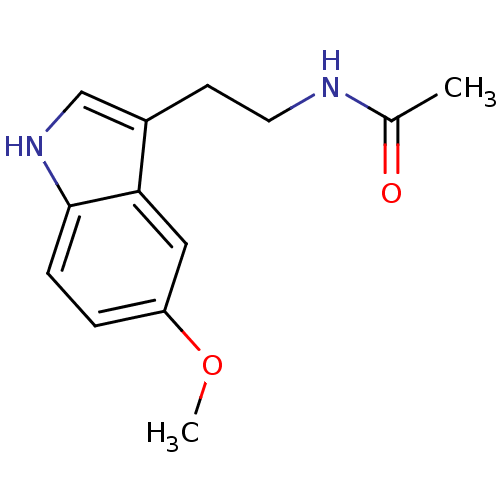 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Binding affinity towards melatonin receptor, determined in chicken brain membranes using [2-125I]melatonin | J Med Chem 41: 451-67 (1998) Article DOI: 10.1021/jm970246n BindingDB Entry DOI: 10.7270/Q2154KSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1C (Gallus gallus) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Binding affinity towards melatonin receptor, determined in chicken brain membranes using [2-125I]melatonin | J Med Chem 41: 451-67 (1998) Article DOI: 10.1021/jm970246n BindingDB Entry DOI: 10.7270/Q2154KSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50113263 ((S)-1-[4-(4-benzhydrylthiosemicarbazido)-3-nitrobe...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50471066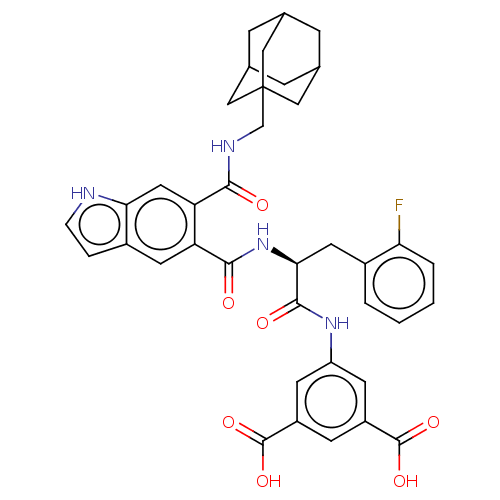 (CHEMBL415936) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.741 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes | J Med Chem 39: 1806-15 (1996) Article DOI: 10.1021/jm9508907 BindingDB Entry DOI: 10.7270/Q2571FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50409527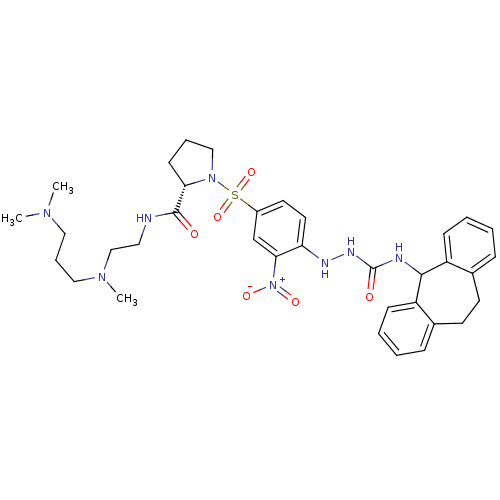 (CHEMBL2112283) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.900 | -53.7 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu-(p-NO2-Phe)-Glu-Ala-Nleu-Ser was use... | J Med Chem 39: 2795-811 (1996) Article DOI: 10.1021/jm960092w BindingDB Entry DOI: 10.7270/Q2028PRG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.900 | -53.7 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu- (p-NO2-Phe)-Glu-Ala-Nleu-Ser (Bache... | Proc Natl Acad Sci U S A 92: 3298-302 (1995) Article DOI: 10.1073/pnas.92.8.3298 BindingDB Entry DOI: 10.7270/Q2F769RX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melatonin receptor type 1C (Gallus gallus) | BDBM50471024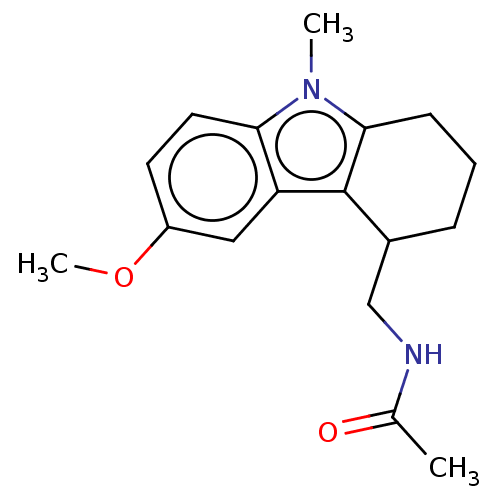 (CHEMBL53824) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Binding affinity towards melatonin receptor, determined in chicken brain membranes using [2-125I]melatonin | J Med Chem 41: 451-67 (1998) Article DOI: 10.1021/jm970246n BindingDB Entry DOI: 10.7270/Q2154KSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1C (Gallus gallus) | BDBM50471024 (CHEMBL53824) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Binding affinity towards melatonin receptor, determined in chicken brain membranes using [2-125I]melatonin | J Med Chem 41: 451-67 (1998) Article DOI: 10.1021/jm970246n BindingDB Entry DOI: 10.7270/Q2154KSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1C (Gallus gallus) | BDBM50471024 (CHEMBL53824) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Binding affinity towards melatonin receptor, determined in chicken brain membranes using [2-125I]melatonin | J Med Chem 41: 451-67 (1998) Article DOI: 10.1021/jm970246n BindingDB Entry DOI: 10.7270/Q2154KSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1C (Gallus gallus) | BDBM50471024 (CHEMBL53824) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Binding affinity towards melatonin receptor, determined in chicken brain membranes using [2-125I]melatonin | J Med Chem 41: 451-67 (1998) Article DOI: 10.1021/jm970246n BindingDB Entry DOI: 10.7270/Q2154KSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50355479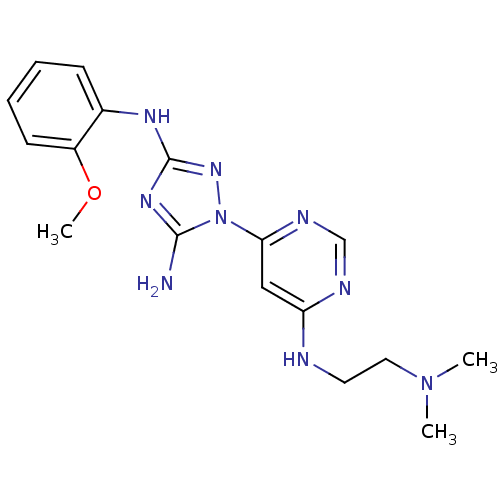 (CHEMBL1835746) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [33P]ATP from human recombinant FLT3 domain after 20 mins by scintillation counting | J Med Chem 54: 7184-92 (2011) Article DOI: 10.1021/jm200712h BindingDB Entry DOI: 10.7270/Q2DZ08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50213845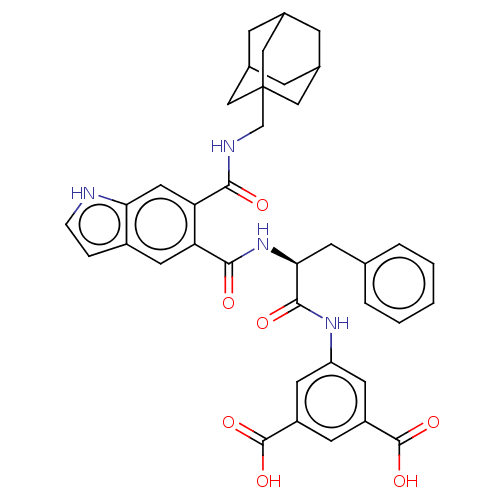 (CHEMBL14557) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes | J Med Chem 39: 1806-15 (1996) Article DOI: 10.1021/jm9508907 BindingDB Entry DOI: 10.7270/Q2571FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50471075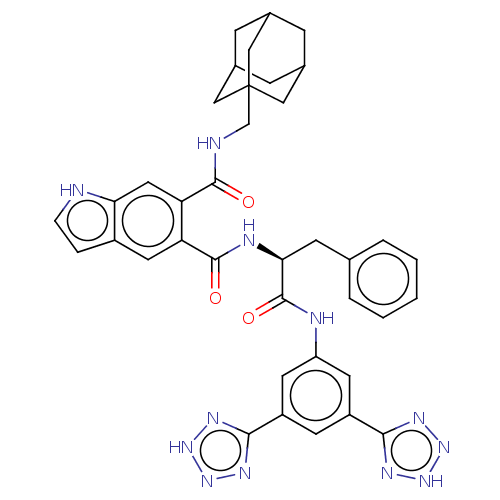 (CHEMBL299540) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes | J Med Chem 39: 1806-15 (1996) Article DOI: 10.1021/jm9508907 BindingDB Entry DOI: 10.7270/Q2571FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50471067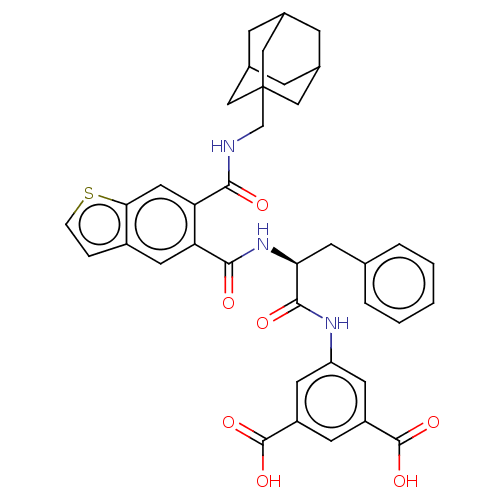 (CHEMBL298521) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes | J Med Chem 39: 1806-15 (1996) Article DOI: 10.1021/jm9508907 BindingDB Entry DOI: 10.7270/Q2571FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1C (Gallus gallus) | BDBM50472177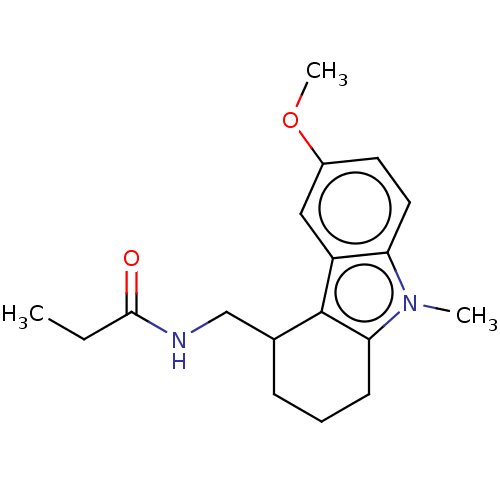 (CHEMBL127826) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Binding affinity towards melatonin receptor, determined in chicken brain membranes using [2-125I]melatonin | J Med Chem 41: 451-67 (1998) Article DOI: 10.1021/jm970246n BindingDB Entry DOI: 10.7270/Q2154KSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1C (Gallus gallus) | BDBM50472177 (CHEMBL127826) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Binding affinity towards melatonin receptor, determined in chicken brain membranes using [2-125I]melatonin | J Med Chem 41: 451-67 (1998) Article DOI: 10.1021/jm970246n BindingDB Entry DOI: 10.7270/Q2154KSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50409529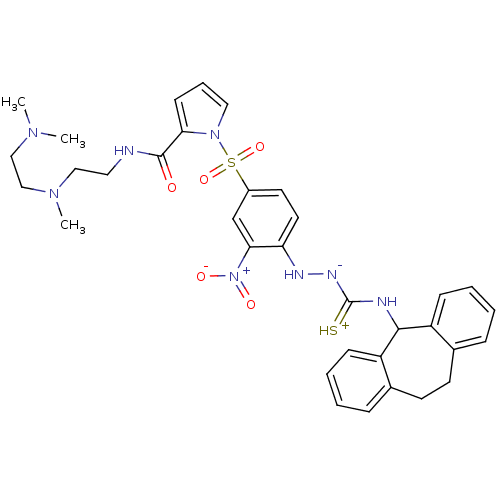 (CHEMBL2112221) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM3414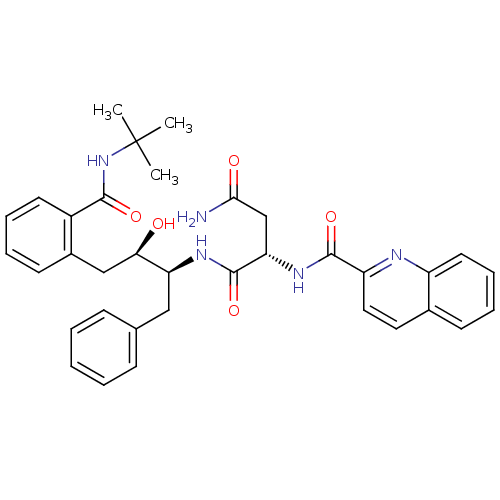 ((2S)-N-[(2S,3R)-4-[2-(tert-butylcarbamoyl)phenyl]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | -52.2 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu- (p-NO2-Phe)-Glu-Ala-Nleu-Ser (Bache... | Proc Natl Acad Sci U S A 92: 3298-302 (1995) Article DOI: 10.1073/pnas.92.8.3298 BindingDB Entry DOI: 10.7270/Q2F769RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50471065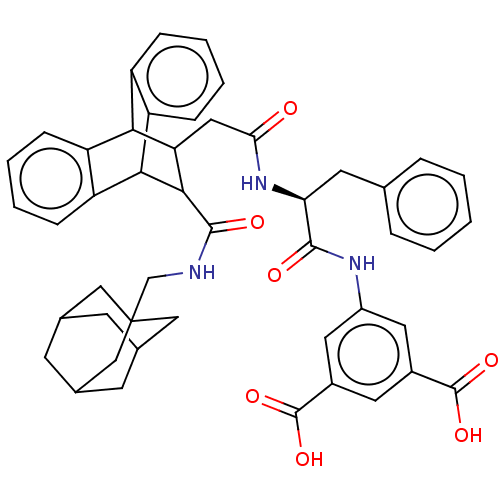 (CHEMBL296167) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes | J Med Chem 39: 1806-15 (1996) Article DOI: 10.1021/jm9508907 BindingDB Entry DOI: 10.7270/Q2571FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1C (Gallus gallus) | BDBM50472180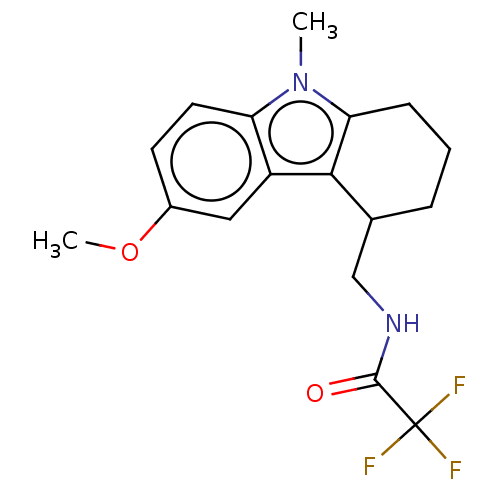 (CHEMBL359451) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Binding affinity towards melatonin receptor, determined in chicken brain membranes using [2-125I]melatonin | J Med Chem 41: 451-67 (1998) Article DOI: 10.1021/jm970246n BindingDB Entry DOI: 10.7270/Q2154KSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM9674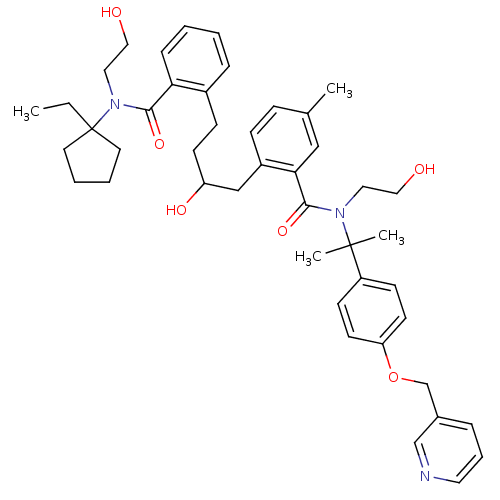 (2-(4-{2-[(1-ethylcyclopentyl)(2-hydroxyethyl)carba...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu-(p-NO2-Phe)-Glu-Ala-Nleu-Ser was use... | J Med Chem 39: 2795-811 (1996) Article DOI: 10.1021/jm960092w BindingDB Entry DOI: 10.7270/Q2028PRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1C (Gallus gallus) | BDBM50472180 (CHEMBL359451) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Binding affinity towards melatonin receptor, determined in chicken brain membranes using [2-125I]melatonin | J Med Chem 41: 451-67 (1998) Article DOI: 10.1021/jm970246n BindingDB Entry DOI: 10.7270/Q2154KSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50061306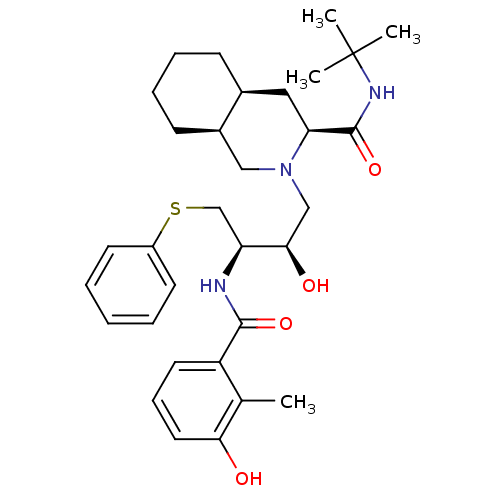 ((3S,4aS,8aS)-2-[(2R,3R)-2-Hydroxy-3-(3-hydroxy-2-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 40: 3979-85 (1998) Article DOI: 10.1021/jm9704098 BindingDB Entry DOI: 10.7270/Q2V69K71 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM3425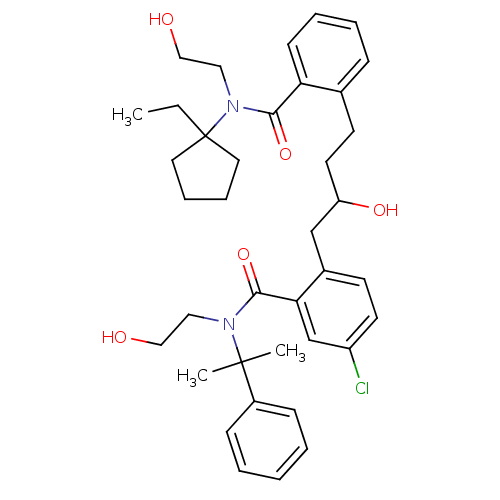 (5-chloro-2-(4-{2-[(1-ethylcyclopentyl)(2-hydroxyet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | -51.6 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu- (p-NO2-Phe)-Glu-Ala-Nleu-Ser (Bache... | Proc Natl Acad Sci U S A 92: 3298-302 (1995) Article DOI: 10.1073/pnas.92.8.3298 BindingDB Entry DOI: 10.7270/Q2F769RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50044287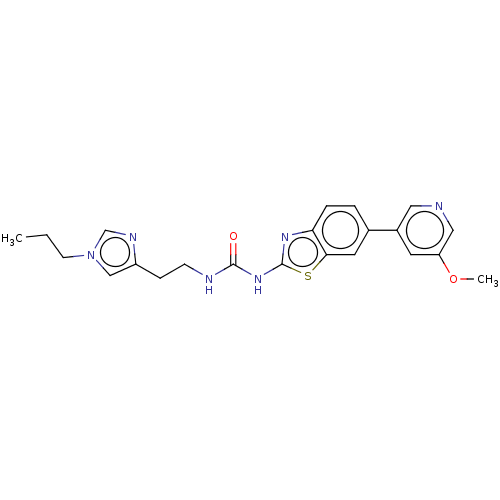 (CHEMBL3356900) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method | J Med Chem 61: 5245-5256 (2018) Article DOI: 10.1021/acs.jmedchem.8b00085 BindingDB Entry DOI: 10.7270/Q2V98BK8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM3414 ((2S)-N-[(2S,3R)-4-[2-(tert-butylcarbamoyl)phenyl]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | -51.6 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu-(p-NO2-Phe)-Glu-Ala-Nleu-Ser was use... | J Med Chem 39: 2795-811 (1996) Article DOI: 10.1021/jm960092w BindingDB Entry DOI: 10.7270/Q2028PRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50355491 (CHEMBL1835870) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [33P]ATP from human recombinant FLT3 domain after 20 mins by scintillation counting | J Med Chem 54: 7184-92 (2011) Article DOI: 10.1021/jm200712h BindingDB Entry DOI: 10.7270/Q2DZ08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM3425 (5-chloro-2-(4-{2-[(1-ethylcyclopentyl)(2-hydroxyet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu-(p-NO2-Phe)-Glu-Ala-Nleu-Ser was use... | J Med Chem 39: 2795-811 (1996) Article DOI: 10.1021/jm960092w BindingDB Entry DOI: 10.7270/Q2028PRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50355489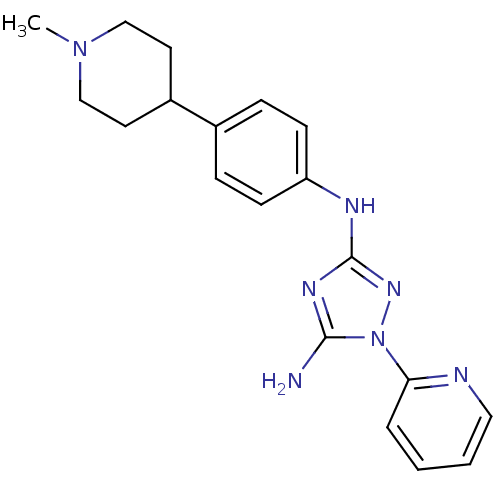 (CHEMBL1835867) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [33P]ATP from human recombinant FLT3 domain after 20 mins by scintillation counting | J Med Chem 54: 7184-92 (2011) Article DOI: 10.1021/jm200712h BindingDB Entry DOI: 10.7270/Q2DZ08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1C (Gallus gallus) | BDBM50472145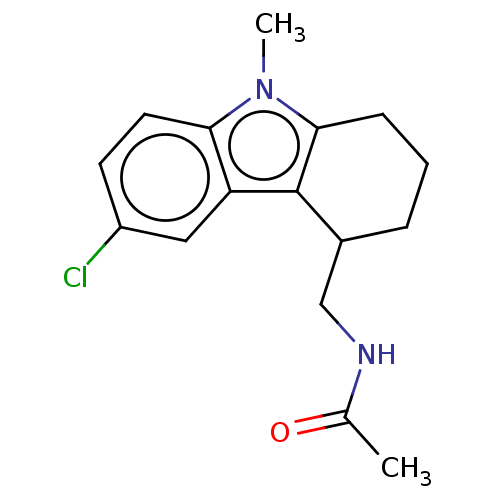 (CHEMBL141146) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Binding affinity towards melatonin receptor, determined in chicken brain membranes using [2-125I]melatonin | J Med Chem 41: 451-67 (1998) Article DOI: 10.1021/jm970246n BindingDB Entry DOI: 10.7270/Q2154KSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1C (Gallus gallus) | BDBM50472145 (CHEMBL141146) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Binding affinity towards melatonin receptor, determined in chicken brain membranes using [2-125I]melatonin | J Med Chem 41: 451-67 (1998) Article DOI: 10.1021/jm970246n BindingDB Entry DOI: 10.7270/Q2154KSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50409528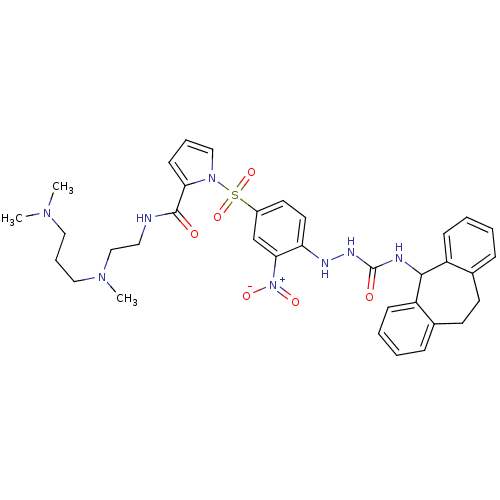 (CHEMBL2112220) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50355464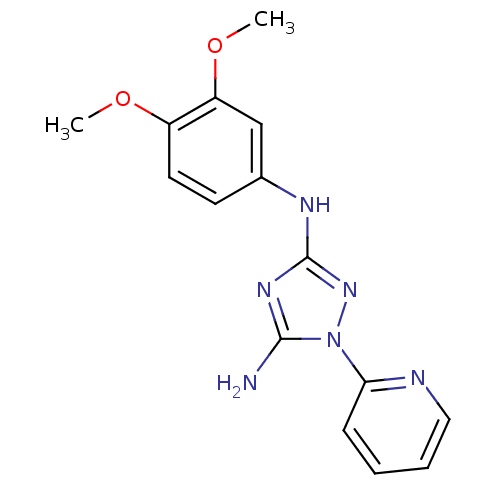 (CHEMBL1835740) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [33P]ATP from human recombinant FLT3 domain after 20 mins by scintillation counting | J Med Chem 54: 7184-92 (2011) Article DOI: 10.1021/jm200712h BindingDB Entry DOI: 10.7270/Q2DZ08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50061307 (AG-1254 | CHEMBL128696 | N-[(1R,2R)-3-(2-tert-Buty...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity HIV-1 protease enzyme | Bioorg Med Chem Lett 5: 727-732 (1995) Article DOI: 10.1016/0960-894X(95)00103-Z BindingDB Entry DOI: 10.7270/Q29S1R0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50549837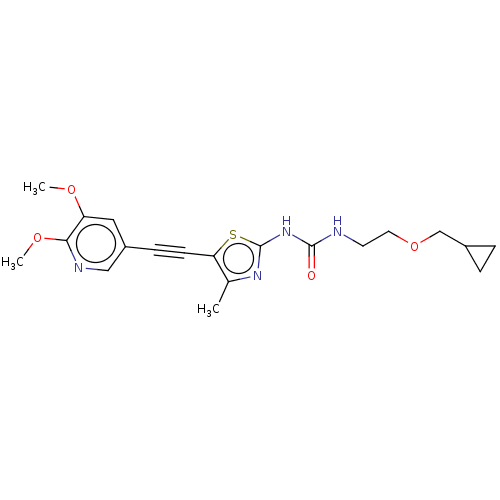 (CHEMBL4759283) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate measured after 15 mins in presence of [33P]-ATP by liquid scintillation counting met... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00573 BindingDB Entry DOI: 10.7270/Q2HD8081 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50549829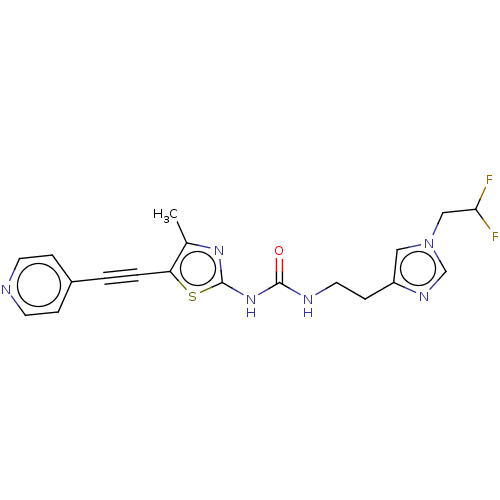 (CHEMBL4760520) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate measured after 15 mins in presence of [33P]-ATP by liquid scintillation counting met... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00573 BindingDB Entry DOI: 10.7270/Q2HD8081 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50549855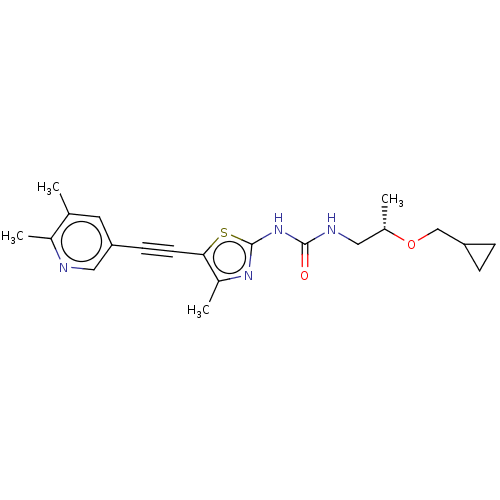 (CHEMBL4756664) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate measured after 15 mins in presence of [33P]-ATP by liquid scintillation counting met... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00573 BindingDB Entry DOI: 10.7270/Q2HD8081 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50061307 (AG-1254 | CHEMBL128696 | N-[(1R,2R)-3-(2-tert-Buty...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 40: 3979-85 (1998) Article DOI: 10.1021/jm9704098 BindingDB Entry DOI: 10.7270/Q2V69K71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50274571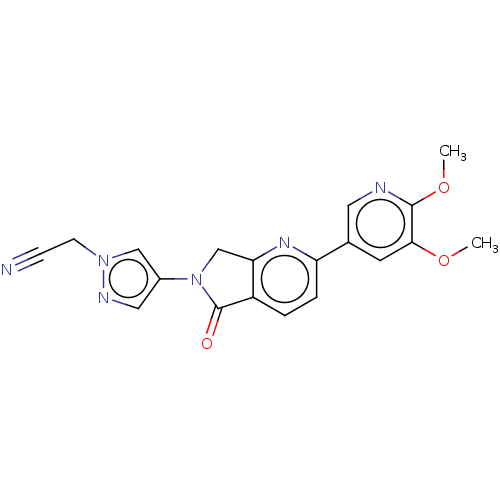 (CHEMBL4127784) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method | J Med Chem 61: 5245-5256 (2018) Article DOI: 10.1021/acs.jmedchem.8b00085 BindingDB Entry DOI: 10.7270/Q2V98BK8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50471076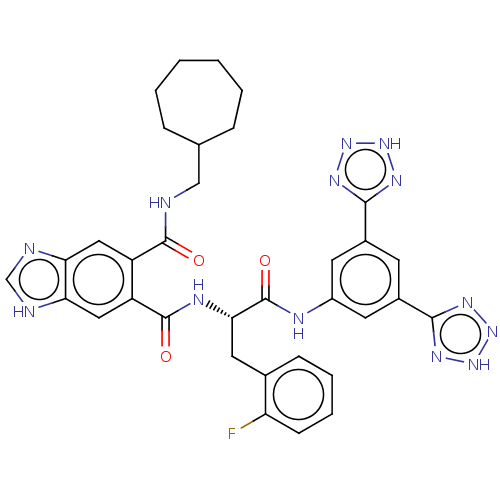 (CHEMBL299387) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes | J Med Chem 39: 1806-15 (1996) Article DOI: 10.1021/jm9508907 BindingDB Entry DOI: 10.7270/Q2571FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM9669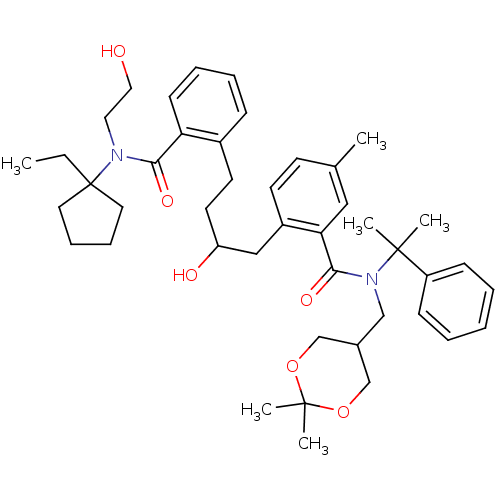 (N-(1-Ethylcyclopentyl)-2-[4-[2-[(2,2-dimethyl[1,3]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu-(p-NO2-Phe)-Glu-Ala-Nleu-Ser was use... | J Med Chem 39: 2795-811 (1996) Article DOI: 10.1021/jm960092w BindingDB Entry DOI: 10.7270/Q2028PRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50274538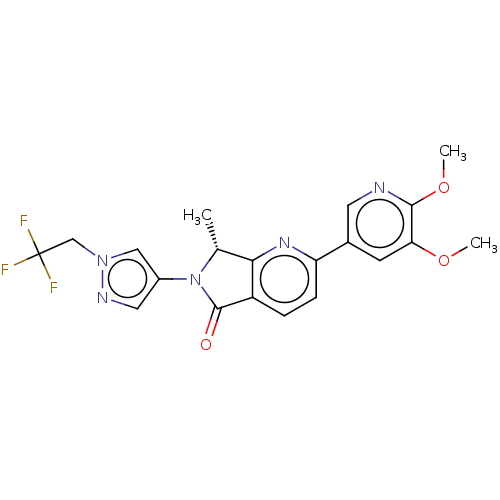 (CHEMBL4126773) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method | J Med Chem 61: 5245-5256 (2018) Article DOI: 10.1021/acs.jmedchem.8b00085 BindingDB Entry DOI: 10.7270/Q2V98BK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1342 total ) | Next | Last >> |