

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50063292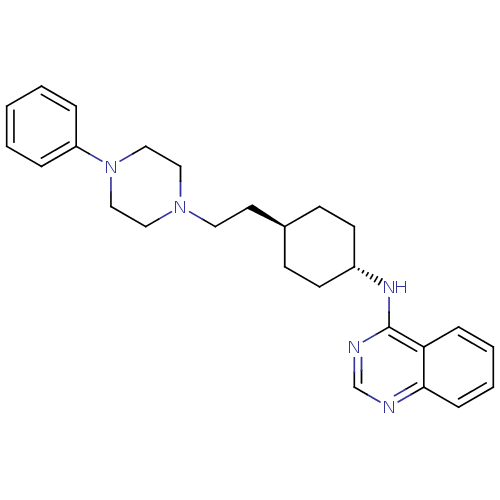 (CHEMBL349426 | {4-[2-(4-Phenyl-piperazin-1-yl)-eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity determined by measuring displacement of [3H]-spiperone from cloned Human Dopamine receptor D3 in CHO-K1 cells | J Med Chem 41: 760-71 (1998) Article DOI: 10.1021/jm9707378 BindingDB Entry DOI: 10.7270/Q20G3J97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50521464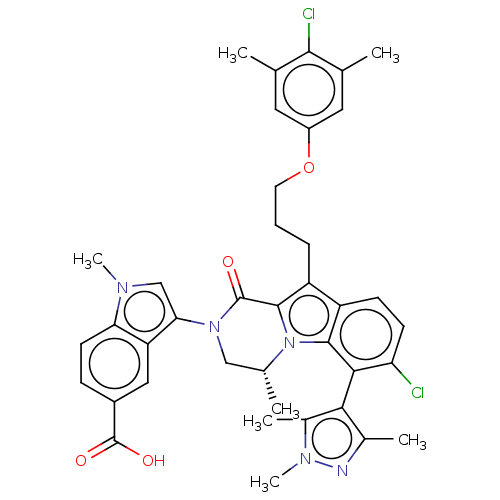 (CHEMBL4469869 | US11596639, Example 78) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of FITC-labeled Bak peptide binding to MBP-fused Mcl-1 (unknown origin) measured after 3 hrs by TR-FRET assay | J Med Chem 62: 3971-3988 (2019) Article DOI: 10.1021/acs.jmedchem.8b01991 BindingDB Entry DOI: 10.7270/Q2V69P04 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50521464 (CHEMBL4469869 | US11596639, Example 78) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of FITC-labeled Bak peptide binding to MBP-fused Mcl-1 (unknown origin) measured after 3 hrs by TR-FRET assay | J Med Chem 62: 3971-3988 (2019) Article DOI: 10.1021/acs.jmedchem.8b01991 BindingDB Entry DOI: 10.7270/Q2V69P04 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50521464 (CHEMBL4469869 | US11596639, Example 78) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of FITC-labeled Bak peptide binding to MBP-fused Mcl-1 (unknown origin) measured after 3 hrs by TR-FRET assay | J Med Chem 62: 3971-3988 (2019) Article DOI: 10.1021/acs.jmedchem.8b01991 BindingDB Entry DOI: 10.7270/Q2V69P04 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neurotensin receptor type 1 (MOUSE) | BDBM50286859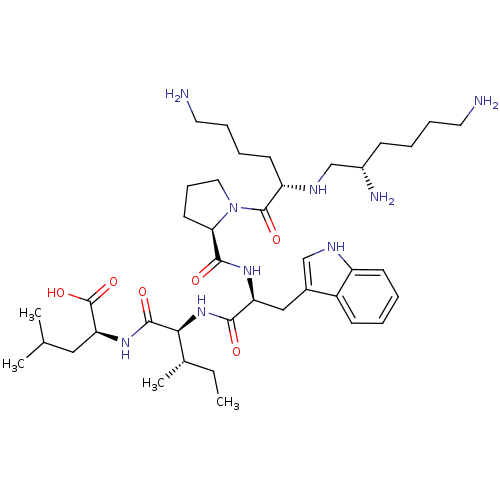 (2-{2-[(S)-2-({1-[(S)-6-Amino-2-((S)-(S)-2,6-diamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Neurotensin Receptor | Bioorg Med Chem Lett 5: 997-1002 (1995) Article DOI: 10.1016/0960-894X(95)00155-M BindingDB Entry DOI: 10.7270/Q2GQ6Z76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50031196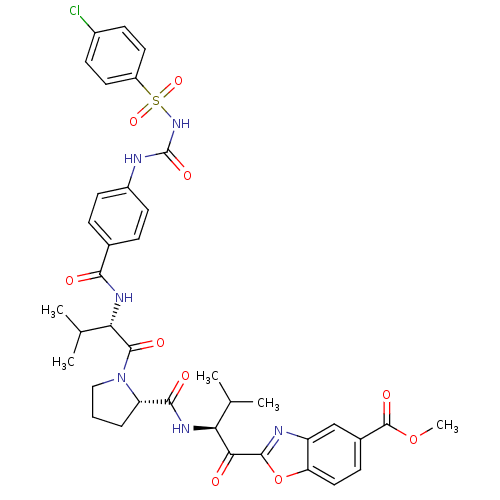 (2-[3-Methyl-2-({1-[3-methyl-2-(4-chlorophenyl-sulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 38: 3972-82 (1995) BindingDB Entry DOI: 10.7270/Q2T152NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (MOUSE) | BDBM50240339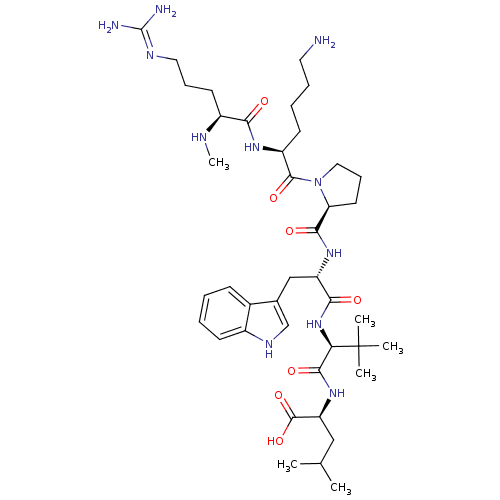 ((S)-2-((S)-2-((S)-2-((S)-1-((S)-6-amino-2-((S)-5-g...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for its binding affinity against Neurotensin Receptor after peripheral administration | Bioorg Med Chem Lett 5: 997-1002 (1995) Article DOI: 10.1016/0960-894X(95)00155-M BindingDB Entry DOI: 10.7270/Q2GQ6Z76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50521465 (CHEMBL4449014 | US11596639, Example 88) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of FITC-labeled Bak peptide binding to MBP-fused Mcl-1 (unknown origin) measured after 3 hrs by TR-FRET assay | J Med Chem 62: 3971-3988 (2019) Article DOI: 10.1021/acs.jmedchem.8b01991 BindingDB Entry DOI: 10.7270/Q2V69P04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22000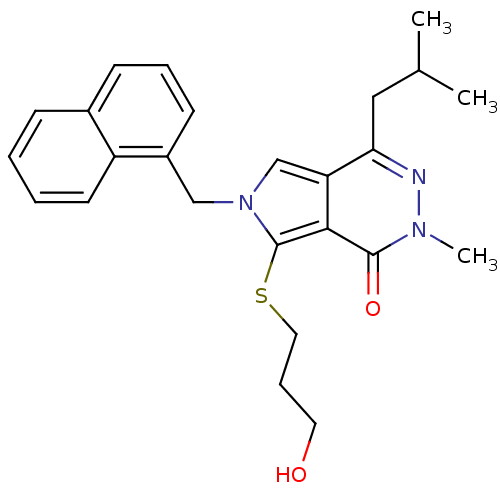 (7-[(3-hydroxypropyl)sulfanyl]-2-methyl-4-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | -56.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (MOUSE) | BDBM50286864 ((S)-2-{(S)-2-[(S)-2-({(R)-1-[5-Guanidino-2-(3-guan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Neurotensin Receptor | Bioorg Med Chem Lett 5: 997-1002 (1995) Article DOI: 10.1016/0960-894X(95)00155-M BindingDB Entry DOI: 10.7270/Q2GQ6Z76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50031185 (2-{(S)-2-[((S)-1-{(S)-2-[4-(4-Chloro-benzenesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 38: 3972-82 (1995) BindingDB Entry DOI: 10.7270/Q2T152NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50063279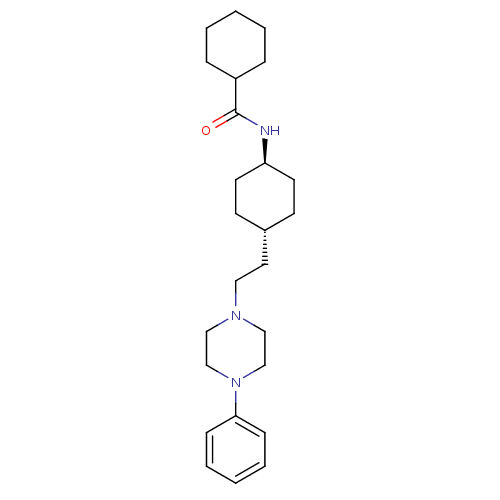 (CHEMBL309623 | Cyclohexanecarboxylic acid {4-[2-(4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity determined by measuring displacement of [3H]-spiperone from cloned Human Dopamine receptor D3 in CHO-K1 cells | J Med Chem 41: 760-71 (1998) Article DOI: 10.1021/jm9707378 BindingDB Entry DOI: 10.7270/Q20G3J97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (MOUSE) | BDBM50366427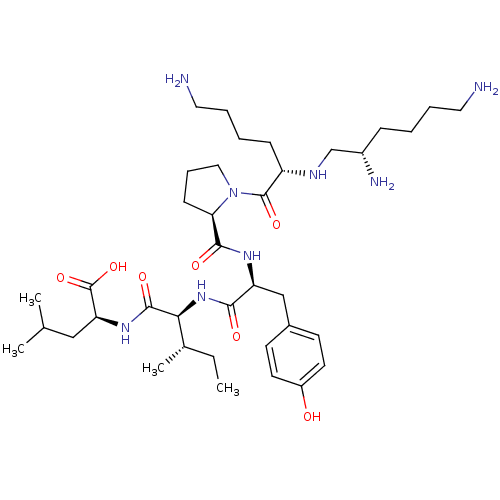 (CHEMBL1793865) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Neurotensin Receptor | Bioorg Med Chem Lett 5: 997-1002 (1995) Article DOI: 10.1016/0960-894X(95)00155-M BindingDB Entry DOI: 10.7270/Q2GQ6Z76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50031203 (2-{(S)-2-[((S)-1-{(S)-2-[4-(4-Chloro-benzenesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 38: 3972-82 (1995) BindingDB Entry DOI: 10.7270/Q2T152NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50031187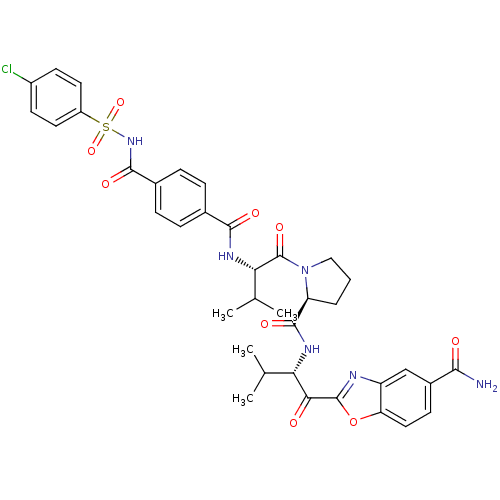 (2-{(S)-2-[((S)-1-{(S)-2-[4-(4-Chloro-benzenesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 38: 3972-82 (1995) BindingDB Entry DOI: 10.7270/Q2T152NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50031198 (1-(4-(((S)-1-((S)-2-(((S)-1-(benzo[d]oxazol-2-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 38: 3972-82 (1995) BindingDB Entry DOI: 10.7270/Q2T152NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM429076 (US10533010, Example I-174 | US11208415, Example I-...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of FITC-labeled Bak peptide binding to MBP-fused Mcl-1 (unknown origin) measured after 3 hrs by TR-FRET assay | J Med Chem 62: 3971-3988 (2019) Article DOI: 10.1021/acs.jmedchem.8b01991 BindingDB Entry DOI: 10.7270/Q2V69P04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50521464 (CHEMBL4469869 | US11596639, Example 78) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of FITC-labeled Bak peptide binding to MBP-fused Mcl-1 (unknown origin) measured after 3 hrs in presence of 1% fetal bovine serum by TR-FR... | J Med Chem 62: 3971-3988 (2019) Article DOI: 10.1021/acs.jmedchem.8b01991 BindingDB Entry DOI: 10.7270/Q2V69P04 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50521464 (CHEMBL4469869 | US11596639, Example 78) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of FITC-labeled Bak peptide binding to MBP-fused Mcl-1 (unknown origin) measured after 3 hrs in presence of 1% fetal bovine serum by TR-FR... | J Med Chem 62: 3971-3988 (2019) Article DOI: 10.1021/acs.jmedchem.8b01991 BindingDB Entry DOI: 10.7270/Q2V69P04 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50521464 (CHEMBL4469869 | US11596639, Example 78) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of FITC-labeled Bak peptide binding to MBP-fused Mcl-1 (unknown origin) measured after 3 hrs in presence of 1% fetal bovine serum by TR-FR... | J Med Chem 62: 3971-3988 (2019) Article DOI: 10.1021/acs.jmedchem.8b01991 BindingDB Entry DOI: 10.7270/Q2V69P04 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22001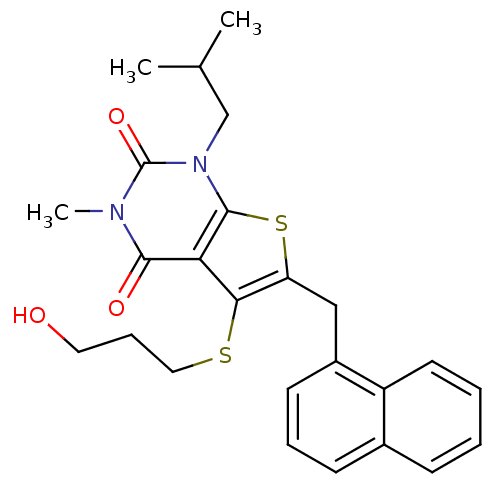 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 0.280 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22009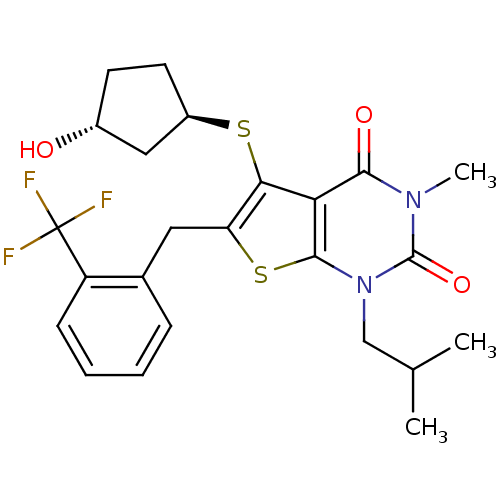 (5-{[(1R,3R)-3-hydroxycyclopentyl]sulfanyl}-3-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.290 | -53.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50031188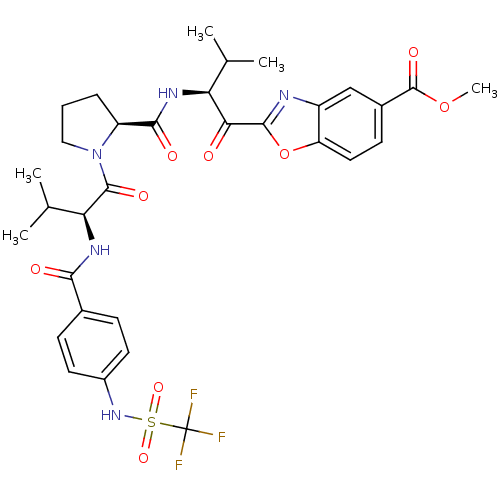 (2-[(S)-3-Methyl-2-({(S)-1-[(S)-3-methyl-2-(4-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 38: 3972-82 (1995) BindingDB Entry DOI: 10.7270/Q2T152NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM21986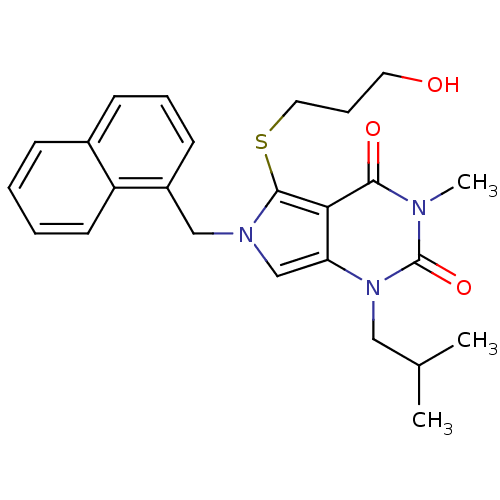 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.330 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50031209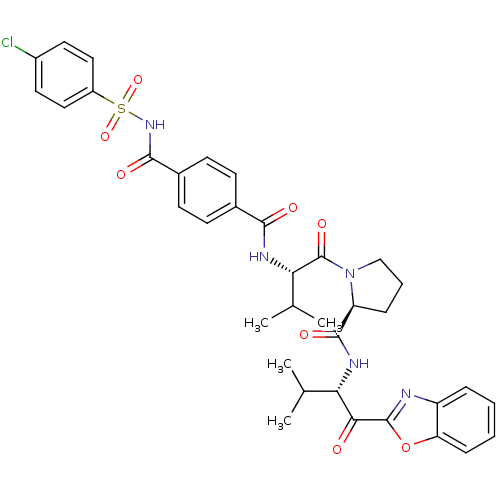 ((S)-1-{(S)-2-[4-(4-Chloro-benzenesulfonylaminocarb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 38: 3972-82 (1995) BindingDB Entry DOI: 10.7270/Q2T152NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50521467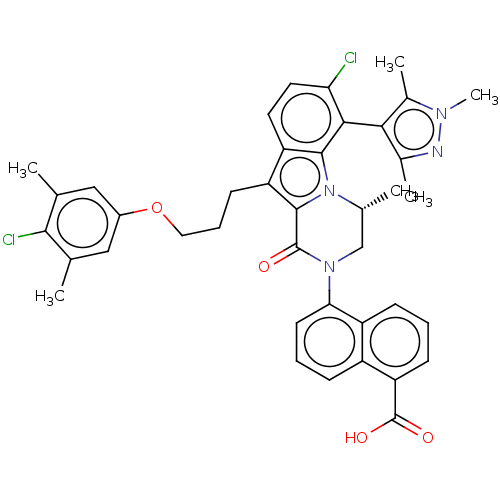 (CHEMBL4525487 | US11596639, Example 63) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of FITC-labeled Bak peptide binding to MBP-fused Mcl-1 (unknown origin) measured after 3 hrs by TR-FRET assay | J Med Chem 62: 3971-3988 (2019) Article DOI: 10.1021/acs.jmedchem.8b01991 BindingDB Entry DOI: 10.7270/Q2V69P04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50031217 (CHEMBL262516 | N-((S)-1-{(S)-2-[(S)-1-(Benzooxazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 38: 3972-82 (1995) BindingDB Entry DOI: 10.7270/Q2T152NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50031197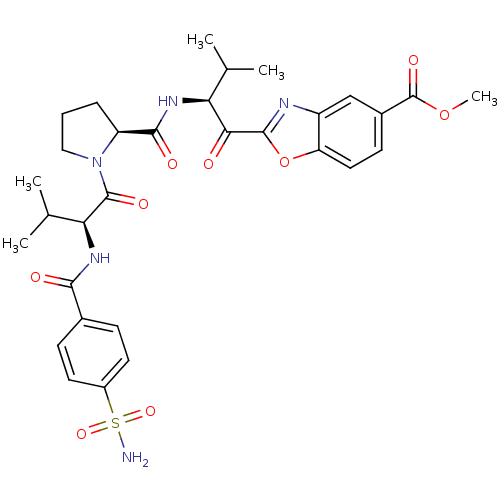 (2-[(S)-3-Methyl-2-({(S)-1-[(S)-3-methyl-2-(4-sulfa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 38: 3972-82 (1995) BindingDB Entry DOI: 10.7270/Q2T152NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22002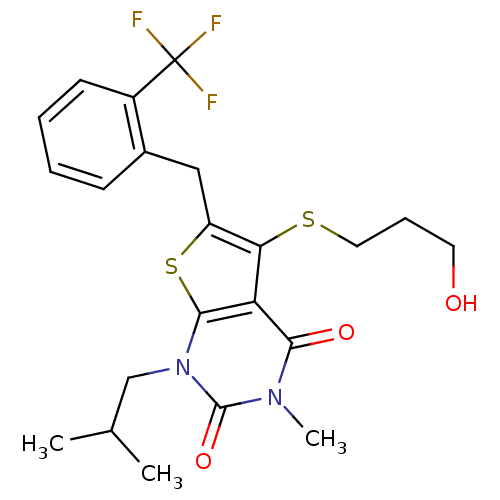 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.350 | -53.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50085072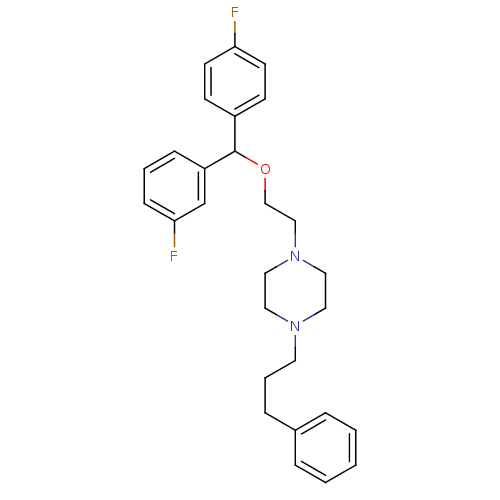 (1-{2-[(3-Fluoro-phenyl)-(4-fluoro-phenyl)-methoxy]...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Competitive binding versus [N-methyl-3H]-WIN 35,428 in murine kidney cells transfected with human dopamine transporter | J Med Chem 43: 639-48 (2000) BindingDB Entry DOI: 10.7270/Q27S7N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50031210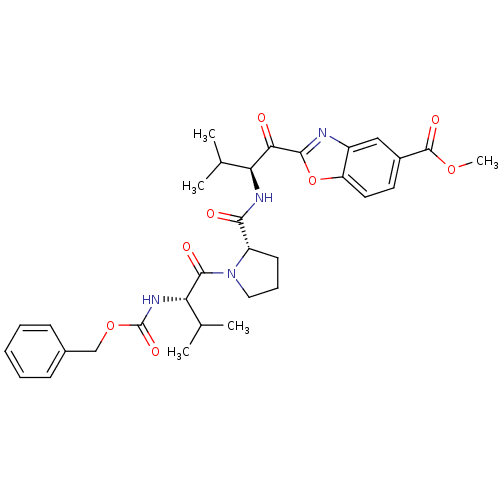 (2-((S)-2-{[(S)-1-((S)-2-Benzyloxycarbonylamino-3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 38: 3972-82 (1995) BindingDB Entry DOI: 10.7270/Q2T152NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50521465 (CHEMBL4449014 | US11596639, Example 88) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of FITC-labeled Bak peptide binding to MBP-fused Mcl-1 (unknown origin) measured after 3 hrs in presence of 1% fetal bovine serum by TR-FR... | J Med Chem 62: 3971-3988 (2019) Article DOI: 10.1021/acs.jmedchem.8b01991 BindingDB Entry DOI: 10.7270/Q2V69P04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (MOUSE) | BDBM50286868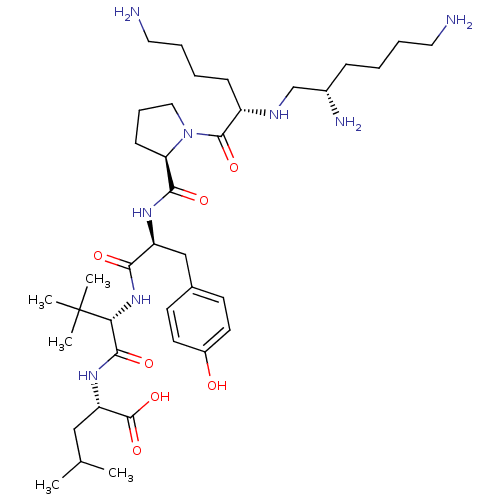 ((1S,2S,4S)-2-{2-[(S)-2-({1-[6-Amino-2-(2,6-diamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Neurotensin Receptor | Bioorg Med Chem Lett 5: 997-1002 (1995) Article DOI: 10.1016/0960-894X(95)00155-M BindingDB Entry DOI: 10.7270/Q2GQ6Z76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036037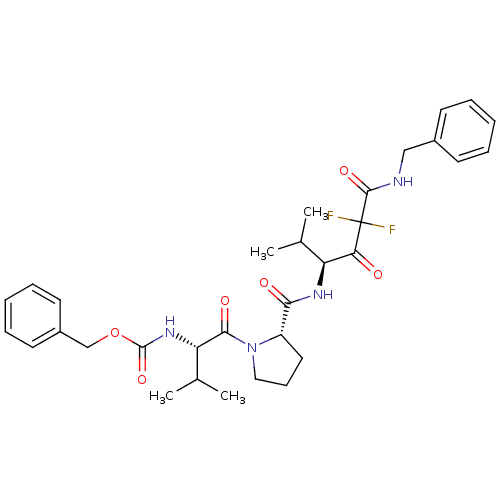 (CHEMBL165759 | {(S)-1-[(S)-2-((S)-3-Benzylcarbamoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22010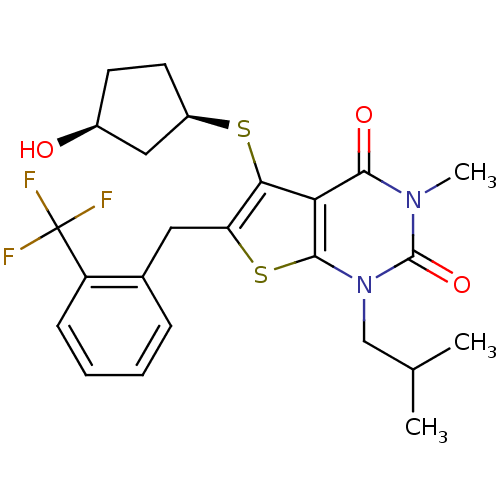 (5-{[(1R,3S)-3-hydroxycyclopentyl]sulfanyl}-3-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.420 | -53.0 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50521468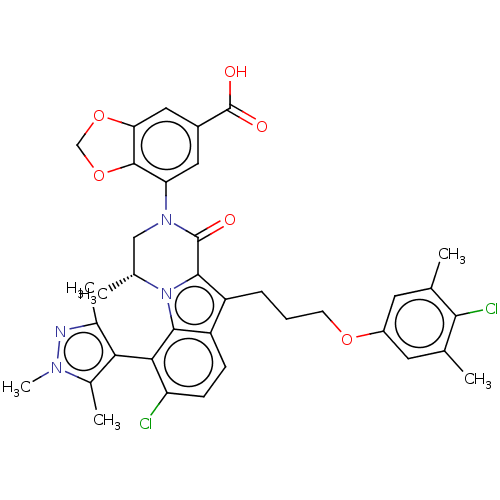 (CHEMBL4524392 | US11596639, Example 33) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of FITC-labeled Bak peptide binding to MBP-fused Mcl-1 (unknown origin) measured after 3 hrs by TR-FRET assay | J Med Chem 62: 3971-3988 (2019) Article DOI: 10.1021/acs.jmedchem.8b01991 BindingDB Entry DOI: 10.7270/Q2V69P04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin (GUINEA PIG) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 273: 1015-22 (1995) BindingDB Entry DOI: 10.7270/Q2QV3K1C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50031192 (CHEMBL339650 | [(S)-1-((S)-2-{(S)-1-[5-(tert-Butyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 38: 3972-82 (1995) BindingDB Entry DOI: 10.7270/Q2T152NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin (GUINEA PIG) | BDBM82403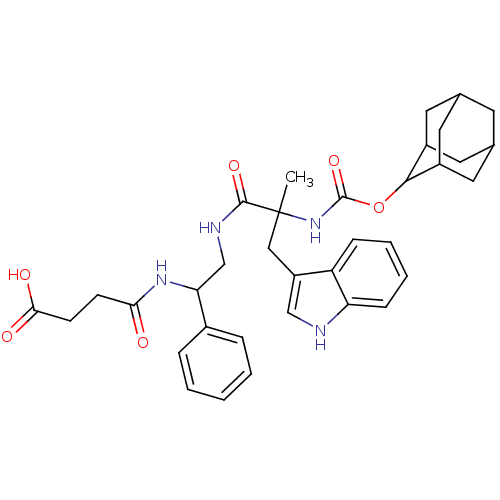 (CAS_108186 | CI-988 | NSC_108186) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 273: 1015-22 (1995) BindingDB Entry DOI: 10.7270/Q2QV3K1C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (GUINEA PIG) | BDBM50005463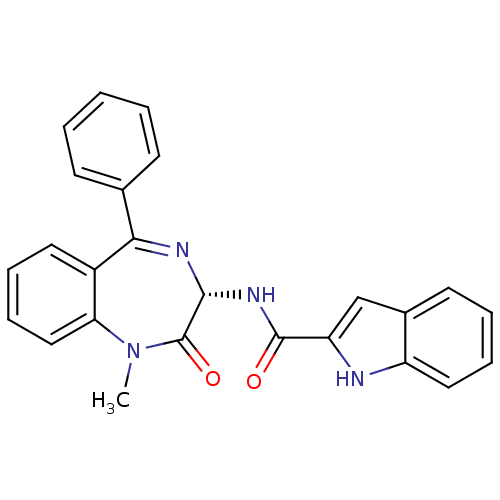 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 273: 1015-22 (1995) BindingDB Entry DOI: 10.7270/Q2QV3K1C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin (GUINEA PIG) | BDBM82404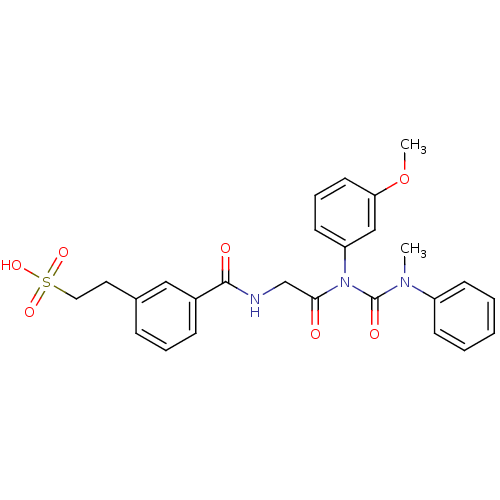 (Glycinamide,N-[[[3-(2-sulfoethyl)phenyl]amino]carb...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 273: 1015-22 (1995) BindingDB Entry DOI: 10.7270/Q2QV3K1C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50085071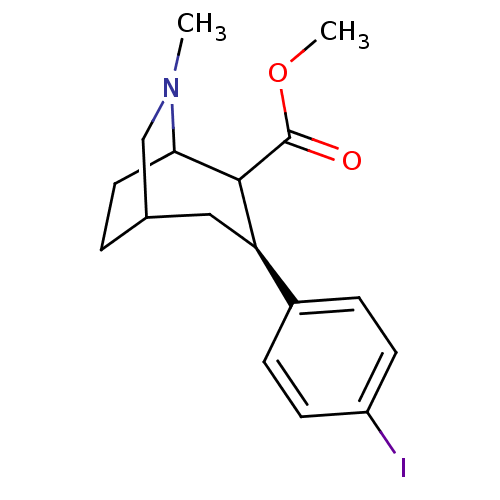 (3-(4-Iodo-phenyl)-6-methyl-6-aza-bicyclo[3.2.2]non...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description In vitro affinity determined using [3H]-WIN- 35428 in murine kidney cells transfected with human dopamine transporter (DAT) | J Med Chem 43: 639-48 (2000) BindingDB Entry DOI: 10.7270/Q27S7N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50031201 ((S)-1-{(S)-2-[4-(4-Chloro-benzenesulfonylaminocarb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 38: 3972-82 (1995) BindingDB Entry DOI: 10.7270/Q2T152NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50031194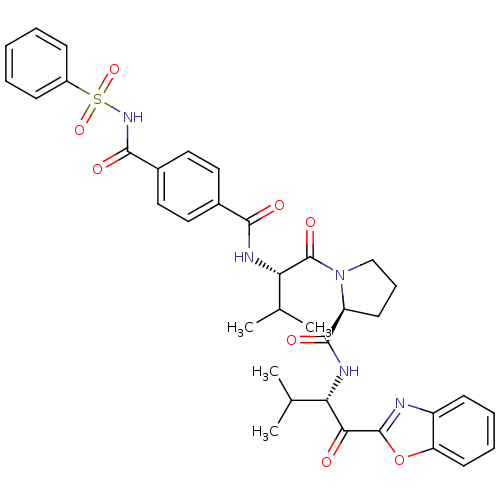 ((S)-1-[(S)-2-(4-Benzenesulfonylaminocarbonyl-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 38: 3972-82 (1995) BindingDB Entry DOI: 10.7270/Q2T152NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50063281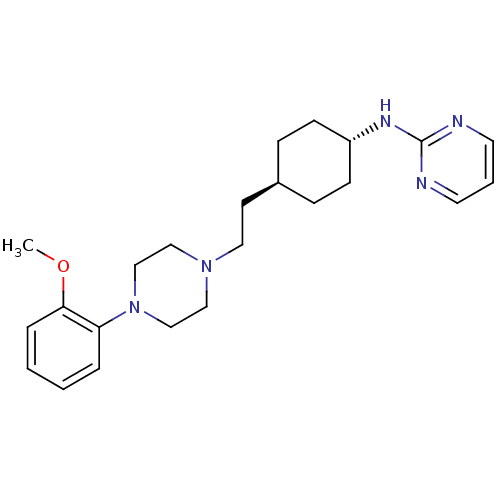 ((4-{2-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-ethyl}...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description The binding affinity of the compound was determined by measuring its ability to displace [3H]-8-OH-DPAT radioligand in 5-hydroxytryptamine 1A recepto... | J Med Chem 41: 760-71 (1998) Article DOI: 10.1021/jm9707378 BindingDB Entry DOI: 10.7270/Q20G3J97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (MOUSE) | BDBM50240845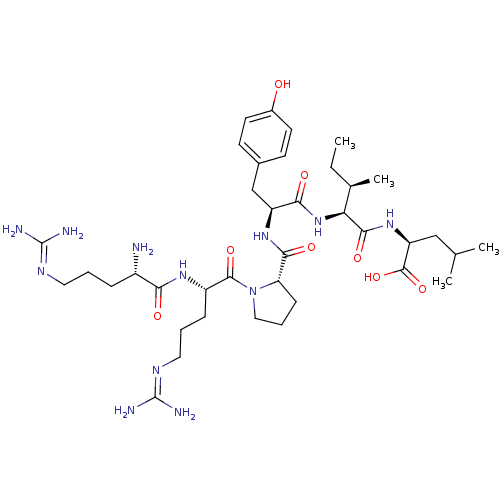 ((S)-2-{(2S,3R)-2-[(S)-2-({(S)-1-[(S)-2-((S)-2-Amin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Neurotensin Receptor | Bioorg Med Chem Lett 5: 997-1002 (1995) Article DOI: 10.1016/0960-894X(95)00155-M BindingDB Entry DOI: 10.7270/Q2GQ6Z76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50031191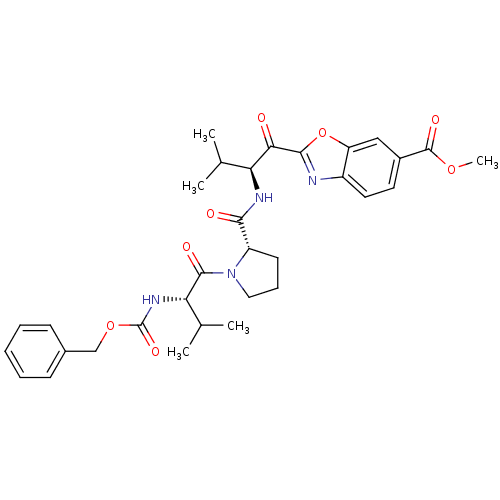 (2-((S)-2-{[(S)-1-((S)-2-Benzyloxycarbonylamino-3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 38: 3972-82 (1995) BindingDB Entry DOI: 10.7270/Q2T152NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036049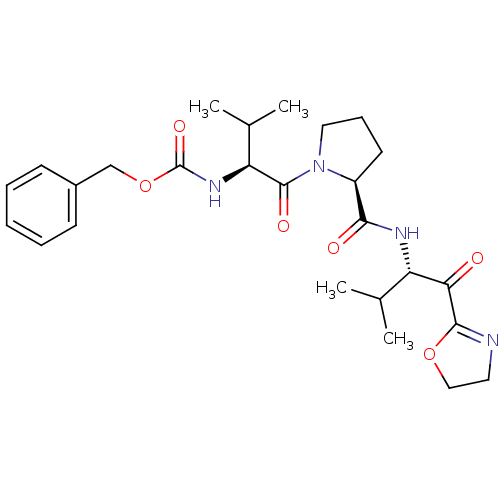 (((S)-1-{(S)-2-[(S)-1-(4,5-Dihydro-oxazole-2-carbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036047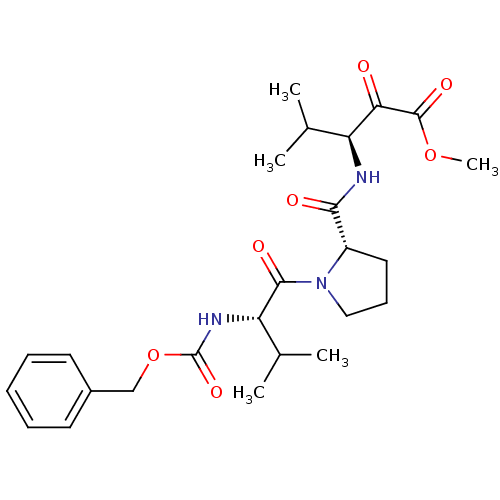 ((S)-3-{[(S)-1-((S)-2-Benzyloxycarbonylamino-3-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50031193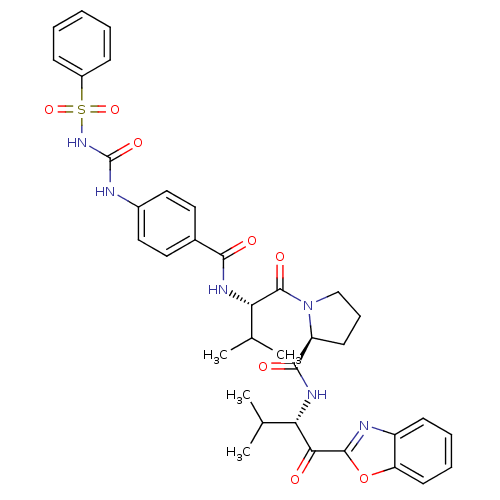 (1-[2-(4-Benzenesulfonamido-carbamide-benzoylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 38: 3972-82 (1995) BindingDB Entry DOI: 10.7270/Q2T152NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 46754 total ) | Next | Last >> |