

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50218166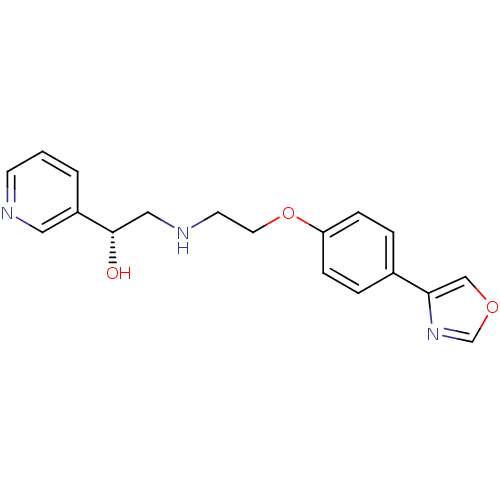 ((R)-2-(2-(4-(oxazol-4-yl)phenoxy)ethylamino)-1-(py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50218165 ((R)-1-(pyridin-3-yl)-2-(2-(4-(thiazol-4-yl)phenoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50218173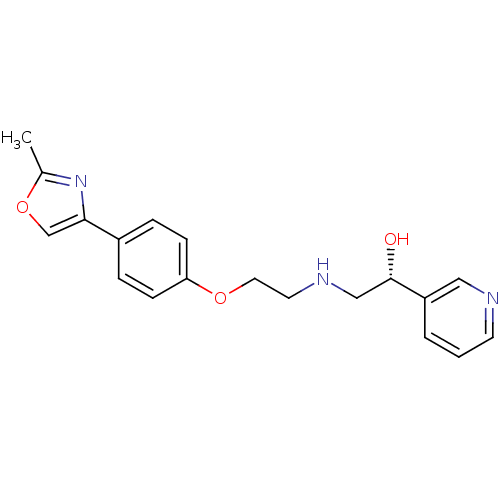 ((R)-2-(2-(4-(2-methyloxazol-4-yl)phenoxy)ethylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50218170 ((R)-2-(2-(4-(2-(hydroxymethyl)oxazol-4-yl)phenoxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218180 ((R)-2-(2-(4-(2-ethylthiazol-4-yl)phenoxy)ethylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50218168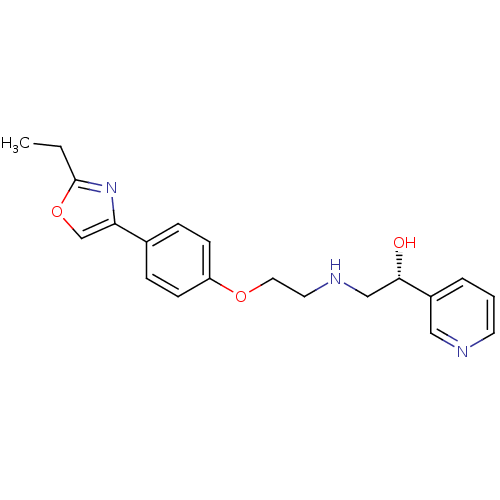 ((R)-2-(2-(4-(2-ethyloxazol-4-yl)phenoxy)ethylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50218167 ((R)-2-(2-(4-(2-isopropyloxazol-4-yl)phenoxy)ethyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218177 ((R)-2-(2-(4-(2-(benzyloxymethyl)oxazol-4-yl)phenox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218176 ((R)-1-(pyridin-3-yl)-2-(2-(4-(2-(pyridin-4-yl)thia...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218164 ((R)-2-(2-(4-(2-methylthiazol-4-yl)phenoxy)ethylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218182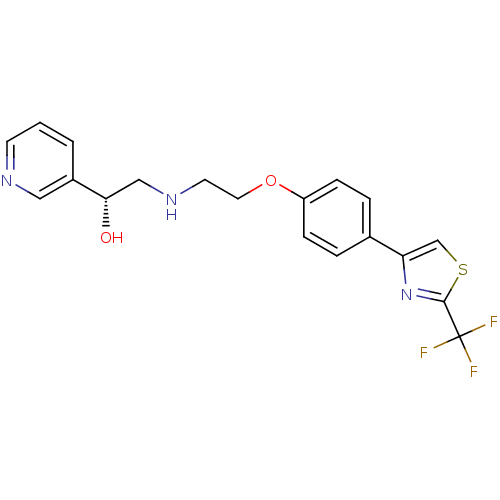 ((R)-1-(pyridin-3-yl)-2-(2-(4-(2-(trifluoromethyl)t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50218164 ((R)-2-(2-(4-(2-methylthiazol-4-yl)phenoxy)ethylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218185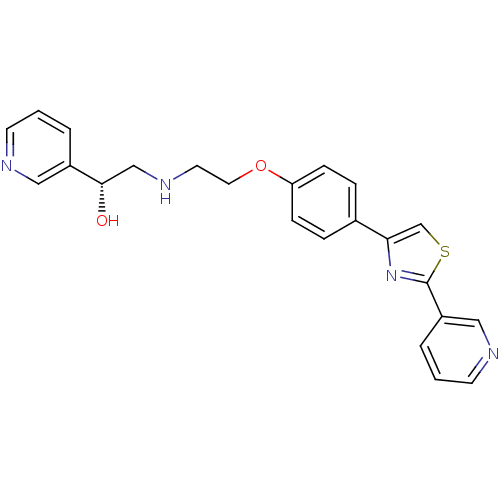 ((1R)-1-(pyridin-3-yl)-2-(2-(4-(2-(pyridin-3-yl)thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218172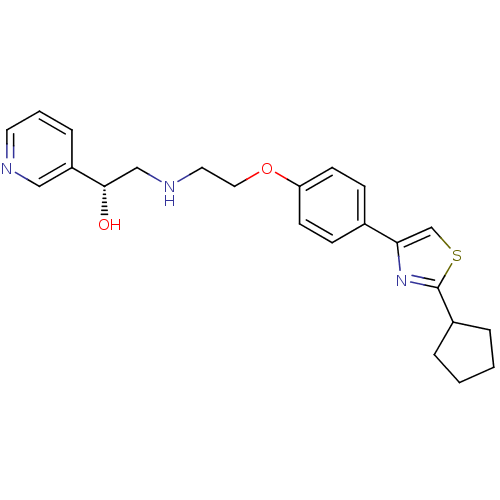 ((R)-2-(2-(4-(2-cyclopentylthiazol-4-yl)phenoxy)eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218165 ((R)-1-(pyridin-3-yl)-2-(2-(4-(thiazol-4-yl)phenoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218174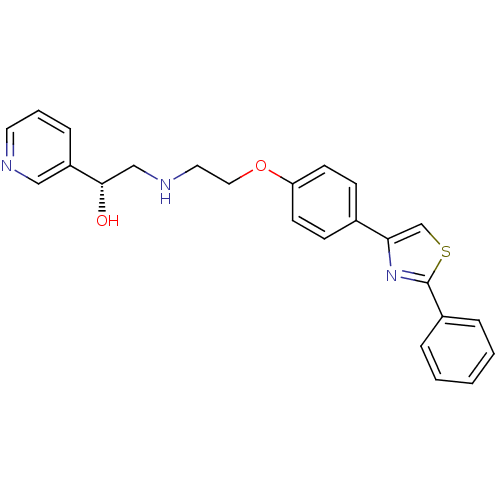 ((R)-2-(2-(4-(2-phenylthiazol-4-yl)phenoxy)ethylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218181 ((R)-2-(2-(4-(5-methyloxazol-4-yl)phenoxy)ethylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218166 ((R)-2-(2-(4-(oxazol-4-yl)phenoxy)ethylamino)-1-(py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218171 ((R)-2-(2-(4-(2-(methoxymethyl)oxazol-4-yl)phenoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218170 ((R)-2-(2-(4-(2-(hydroxymethyl)oxazol-4-yl)phenoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218167 ((R)-2-(2-(4-(2-isopropyloxazol-4-yl)phenoxy)ethyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218168 ((R)-2-(2-(4-(2-ethyloxazol-4-yl)phenoxy)ethylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218173 ((R)-2-(2-(4-(2-methyloxazol-4-yl)phenoxy)ethylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50218174 ((R)-2-(2-(4-(2-phenylthiazol-4-yl)phenoxy)ethylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218179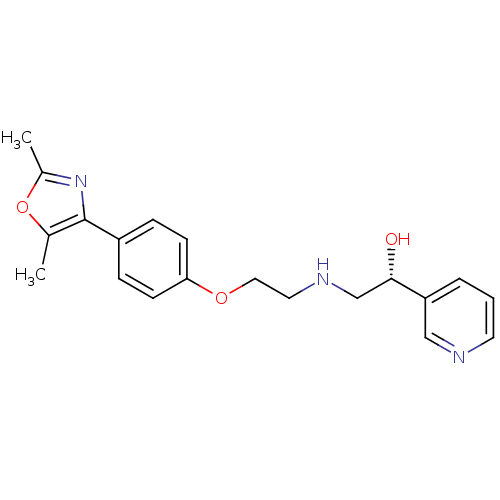 ((R)-2-(2-(4-(2,5-dimethyloxazol-4-yl)phenoxy)ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Sus scrofa) | BDBM50561618 (CHEMBL4746439) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity pig brain GABA-AT assessed as inactivation constant using GABA as substrate in presence of NADP+ and alpha-ketoglutarate by SSDH cou... | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00672 BindingDB Entry DOI: 10.7270/Q2QZ2FPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Sus scrofa) | BDBM50561615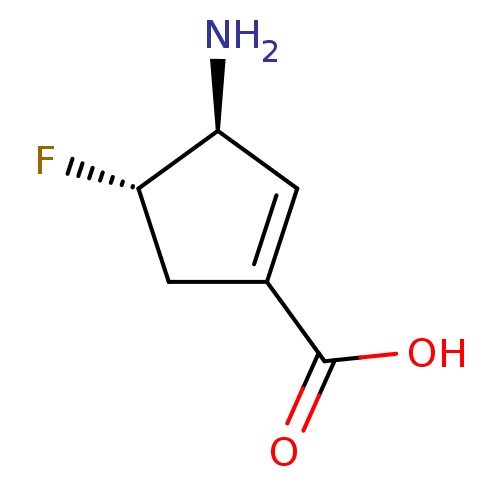 (CHEMBL4747359) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity pig brain GABA-AT assessed as inactivation constant using GABA as substrate in presence of NADP+ and alpha-ketoglutarate by SSDH cou... | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00672 BindingDB Entry DOI: 10.7270/Q2QZ2FPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Sus scrofa) | BDBM50085233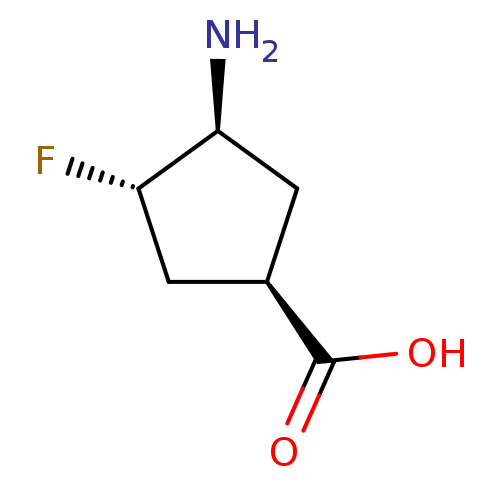 (3-Amino-4-fluoro-cyclopentanecarboxylic acid | CHE...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity pig brain GABA-AT assessed as inactivation constant using GABA as substrate in presence of NADP+ and alpha-ketoglutarate by SSDH cou... | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00672 BindingDB Entry DOI: 10.7270/Q2QZ2FPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Sus scrofa) | BDBM50135932 ((1S,3S)-3-Amino-4-difluoromethylene-cyclopentaneca...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity pig brain GABA-AT assessed as inactivation constant using GABA as substrate in presence of NADP+ and alpha-ketoglutarate by SSDH cou... | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00672 BindingDB Entry DOI: 10.7270/Q2QZ2FPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ornithine aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50561615 (CHEMBL4747359) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to ornithine aminotransferase (unknown origin) assessed as inhibition constant by SSDH coupled enzyme based UV-Vis spectrophotometry | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00672 BindingDB Entry DOI: 10.7270/Q2QZ2FPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Sus scrofa) | BDBM50118886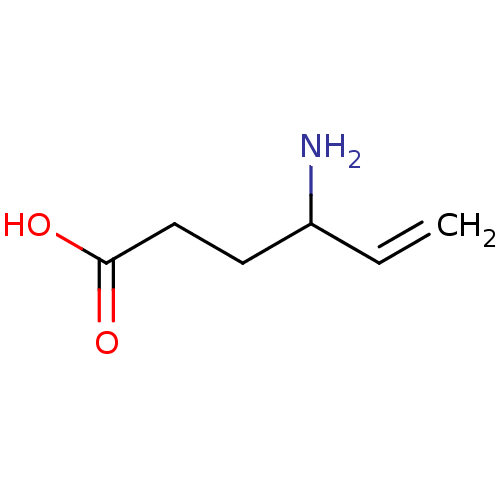 (4-Amino-hex-5-enoic acid | CHEMBL89598 | US1018980...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity pig brain GABA-AT assessed as inactivation constant using GABA as substrate in presence of NADP+ and alpha-ketoglutarate by SSDH cou... | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00672 BindingDB Entry DOI: 10.7270/Q2QZ2FPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Sus scrofa) | BDBM50561616 (CHEMBL4758952) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.01E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity pig brain GABA-AT assessed as inactivation constant using GABA as substrate in presence of NADP+ and alpha-ketoglutarate by SSDH cou... | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00672 BindingDB Entry DOI: 10.7270/Q2QZ2FPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Sus scrofa) | BDBM50561617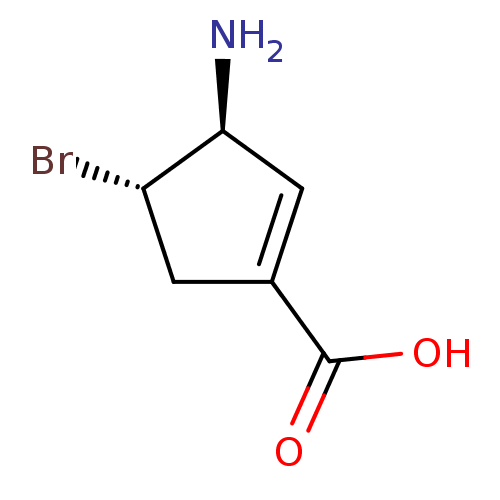 (CHEMBL4741430) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.43E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity pig brain GABA-AT assessed as inactivation constant using GABA as substrate in presence of NADP+ and alpha-ketoglutarate by SSDH cou... | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00672 BindingDB Entry DOI: 10.7270/Q2QZ2FPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50146201 (6-Phenyl-5-[4-(2-pyrrolidin-1-yl-ethoxy)-phenyl]-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor alpha expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50146201 (6-Phenyl-5-[4-(2-pyrrolidin-1-yl-ethoxy)-phenyl]-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor beta expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50347385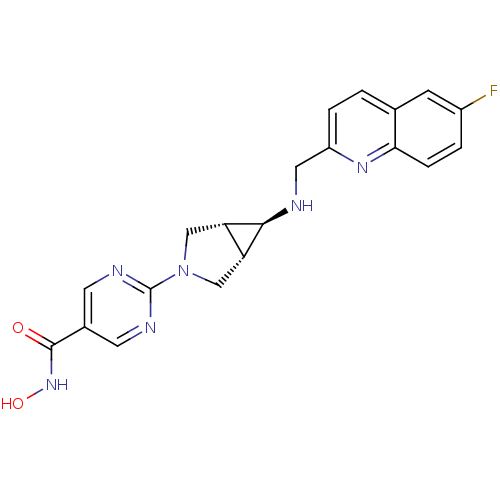 (CHEMBL1801250) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chroma Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using fluor de Lys as substrate by fluorometric analysis | J Med Chem 53: 8663-78 (2010) Article DOI: 10.1021/jm101177s BindingDB Entry DOI: 10.7270/Q2G1616P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50347385 (CHEMBL1801250) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chroma Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 using fluor de Lys as substrate by fluorometric analysis | J Med Chem 53: 8663-78 (2010) Article DOI: 10.1021/jm101177s BindingDB Entry DOI: 10.7270/Q2G1616P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50146206 (2-Benzyl-1-[4-(2-pyrrolidin-1-yl-ethoxy)-phenyl]-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor alpha expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50146200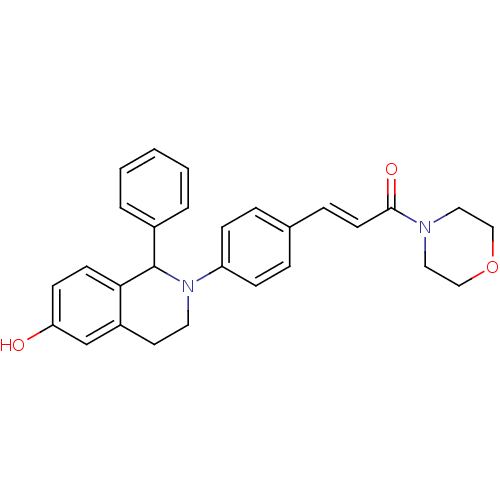 ((E)-3-[4-(6-Hydroxy-1-phenyl-3,4-dihydro-1H-isoqui...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor beta expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50530873 (CHEMBL4456215) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... | J Med Chem 62: 10402-10422 (2019) Article DOI: 10.1021/acs.jmedchem.9b01499 BindingDB Entry DOI: 10.7270/Q27H1P12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50530873 (CHEMBL4456215) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... | J Med Chem 62: 10402-10422 (2019) Article DOI: 10.1021/acs.jmedchem.9b01499 BindingDB Entry DOI: 10.7270/Q27H1P12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50146223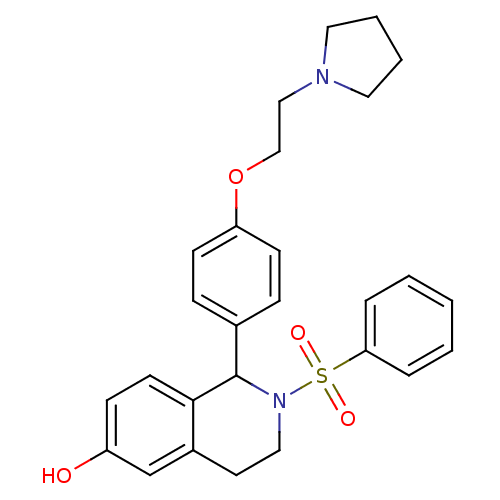 (2-Benzenesulfonyl-1-[4-(2-pyrrolidin-1-yl-ethoxy)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor alpha expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50146216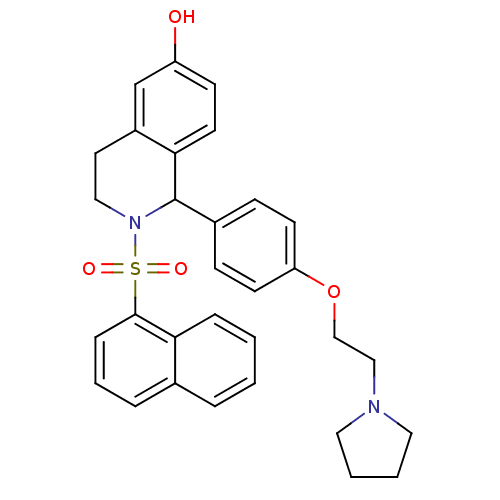 (2-(Naphthalene-1-sulfonyl)-1-[4-(2-pyrrolidin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor alpha expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50146200 ((E)-3-[4-(6-Hydroxy-1-phenyl-3,4-dihydro-1H-isoqui...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor alpha expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50347384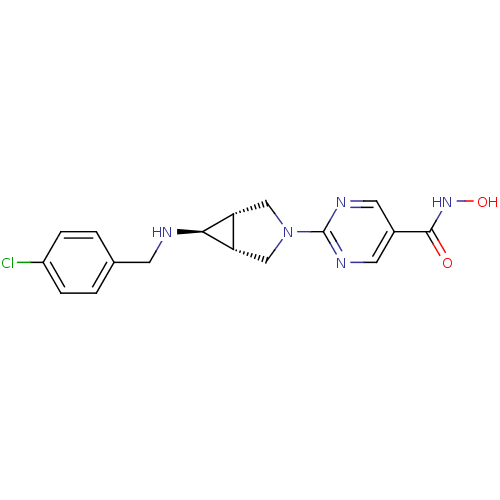 (CHEMBL1801238) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Chroma Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using fluor de Lys as substrate by fluorometric analysis | J Med Chem 53: 8663-78 (2010) Article DOI: 10.1021/jm101177s BindingDB Entry DOI: 10.7270/Q2G1616P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50530894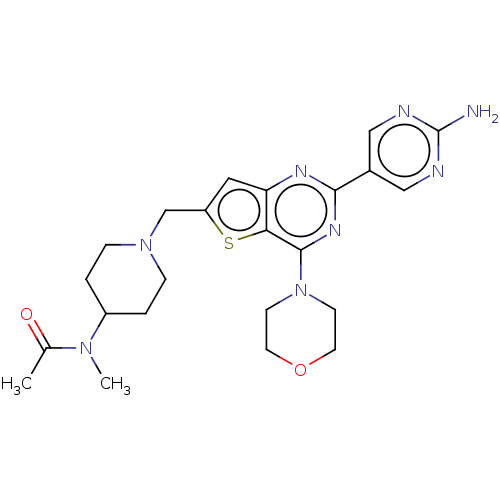 (CHEMBL4437468) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... | J Med Chem 62: 10402-10422 (2019) Article DOI: 10.1021/acs.jmedchem.9b01499 BindingDB Entry DOI: 10.7270/Q27H1P12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50530928 (CHEMBL4534127) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... | J Med Chem 62: 10402-10422 (2019) Article DOI: 10.1021/acs.jmedchem.9b01499 BindingDB Entry DOI: 10.7270/Q27H1P12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50530895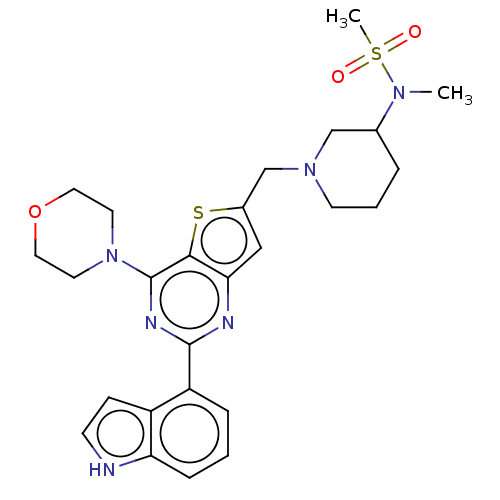 (CHEMBL4590897) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... | J Med Chem 62: 10402-10422 (2019) Article DOI: 10.1021/acs.jmedchem.9b01499 BindingDB Entry DOI: 10.7270/Q27H1P12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50530877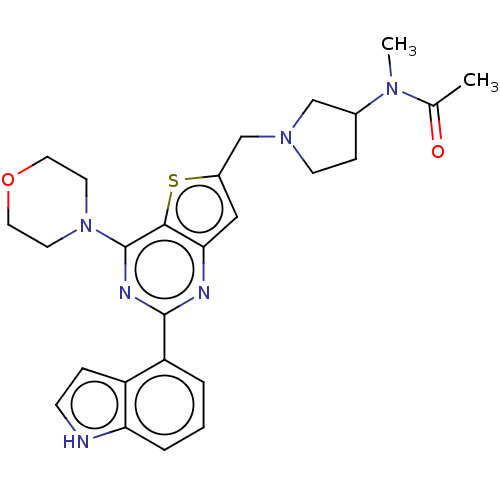 (CHEMBL4580111) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... | J Med Chem 62: 10402-10422 (2019) Article DOI: 10.1021/acs.jmedchem.9b01499 BindingDB Entry DOI: 10.7270/Q27H1P12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50530894 (CHEMBL4437468) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... | J Med Chem 62: 10402-10422 (2019) Article DOI: 10.1021/acs.jmedchem.9b01499 BindingDB Entry DOI: 10.7270/Q27H1P12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 728 total ) | Next | Last >> |