

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50066789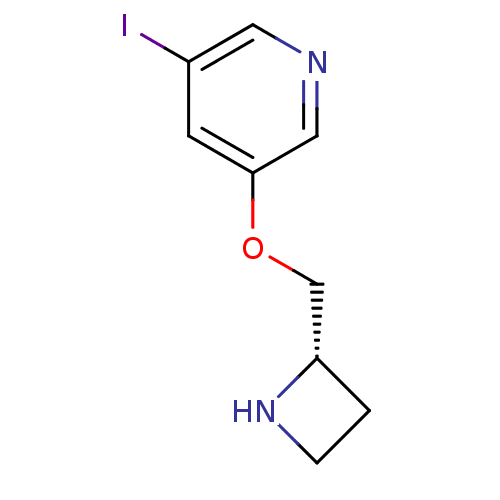 (3-((S)-1-Azetidin-2-ylmethoxy)-5-iodo-pyridine | A...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California - Irvine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat brain homogenate by scintillation counting | Bioorg Med Chem Lett 22: 7610-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.012 BindingDB Entry DOI: 10.7270/Q20V8GNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50486116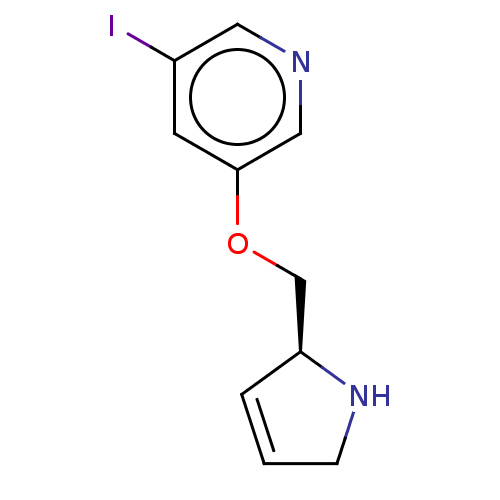 (Niodene) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California - Irvine Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from alpha4beta2 nAChR in Sprague-Dawley rat brain homogenate after 75 mins by scintillation counting | Bioorg Med Chem Lett 22: 7610-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.012 BindingDB Entry DOI: 10.7270/Q20V8GNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50483134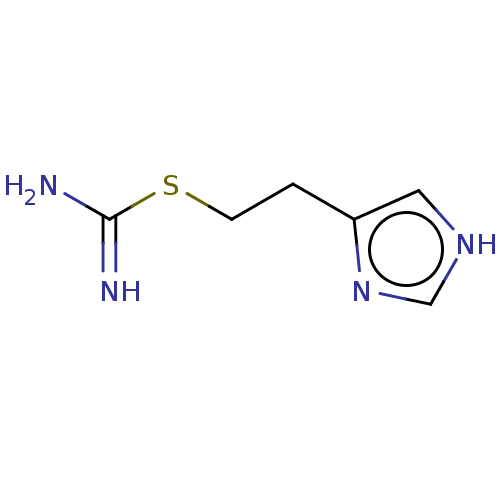 (CHEBI:64156 | Imetit) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50486115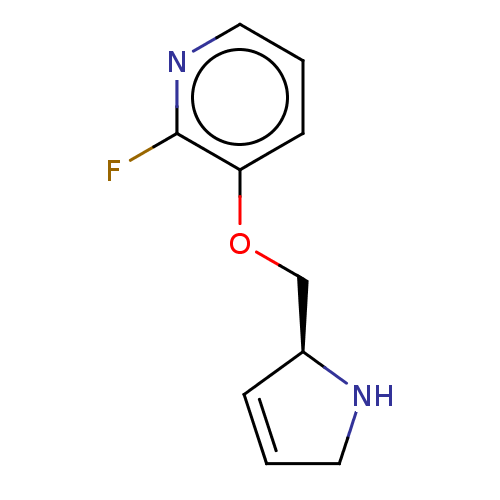 (CHEMBL2207676) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California - Irvine Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from alpha4beta2 nAChR in rat brain homogenate | Bioorg Med Chem Lett 22: 7610-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.012 BindingDB Entry DOI: 10.7270/Q20V8GNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517508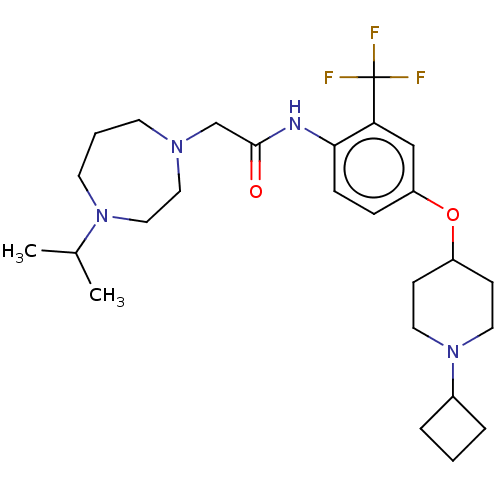 (CHEMBL4594107) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28661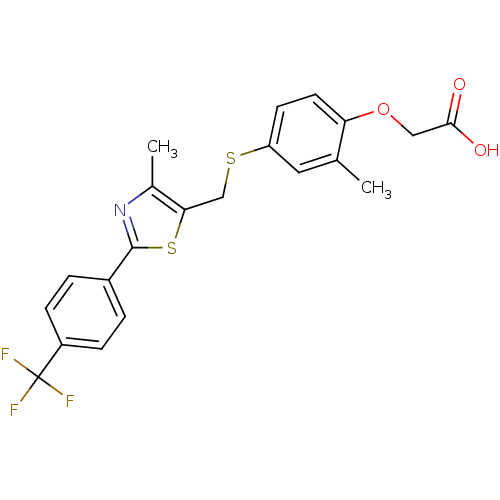 (2-{2-methyl-4-[({4-methyl-2-[4-(trifluoromethyl)ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jnatprod.2c00791 BindingDB Entry DOI: 10.7270/Q2G44VDK | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517531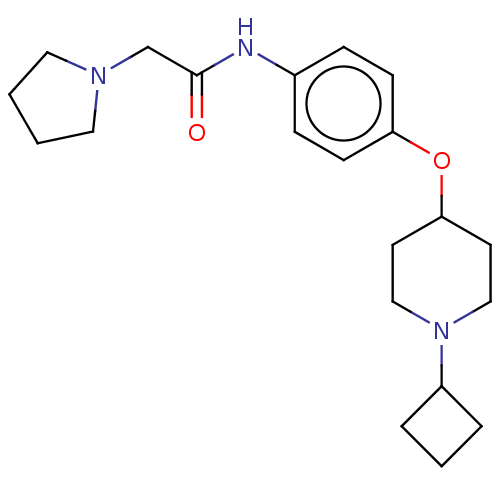 (CHEMBL4445638) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517534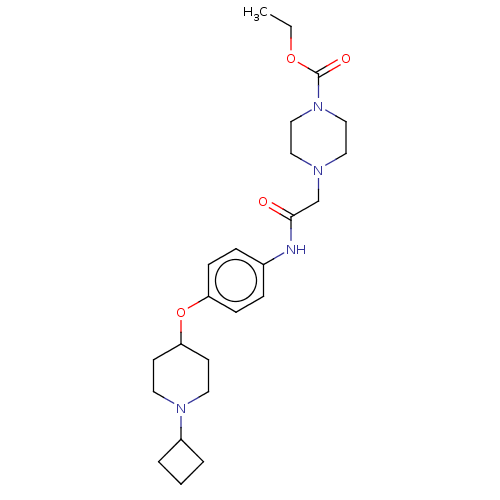 (CHEMBL4476066) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517529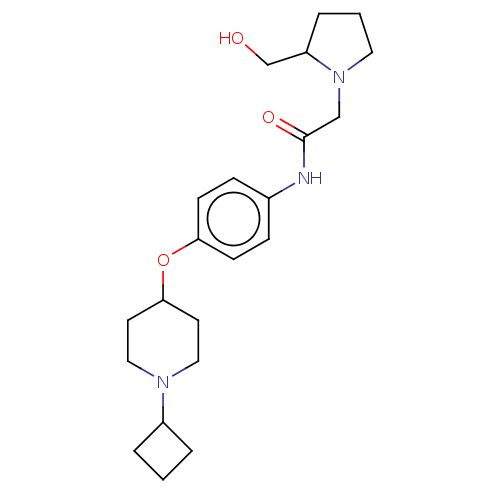 (CHEMBL4587541) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50066788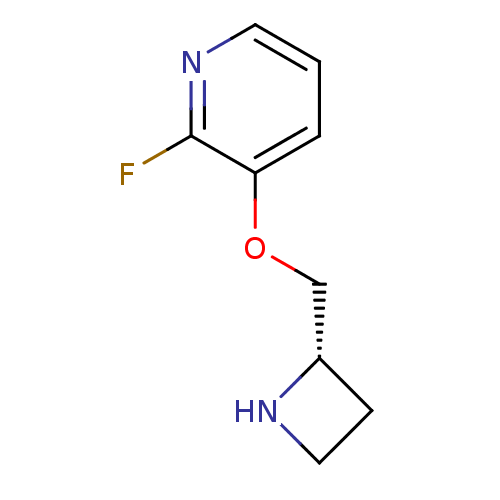 ((S)-3-(azetidin-2-ylmethoxy)-2-fluoropyridine | 3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California - Irvine Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat brain homogenate by scintillation counting | Bioorg Med Chem Lett 22: 7610-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.012 BindingDB Entry DOI: 10.7270/Q20V8GNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517507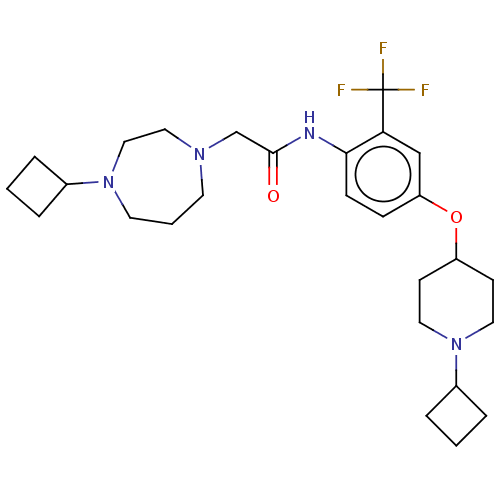 (CHEMBL4456037) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16047 ((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant beta-secretase 1 | Bioorg Med Chem Lett 22: 5460-5 (2012) Article DOI: 10.1016/j.bmcl.2012.07.043 BindingDB Entry DOI: 10.7270/Q2BV7HQM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517523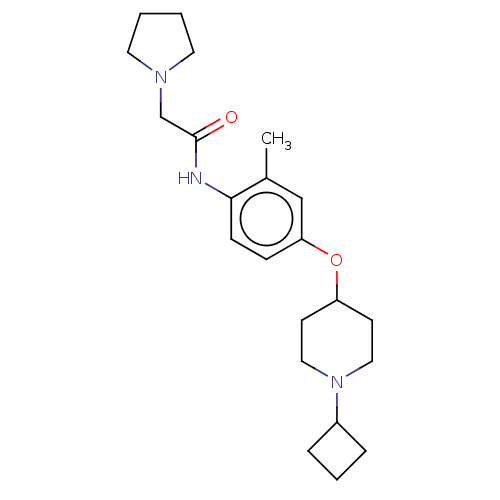 (CHEMBL4518550) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50085048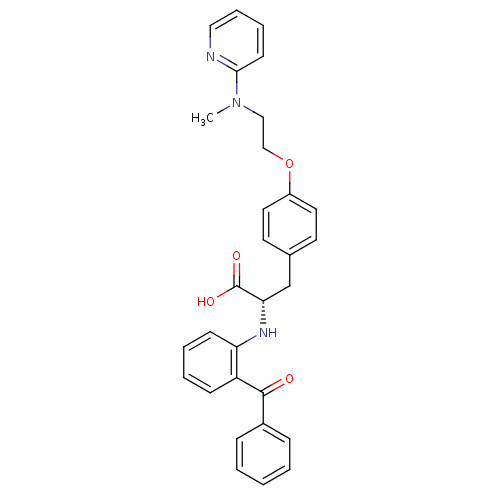 ((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(methyl-pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jnatprod.2c00791 BindingDB Entry DOI: 10.7270/Q2G44VDK | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517505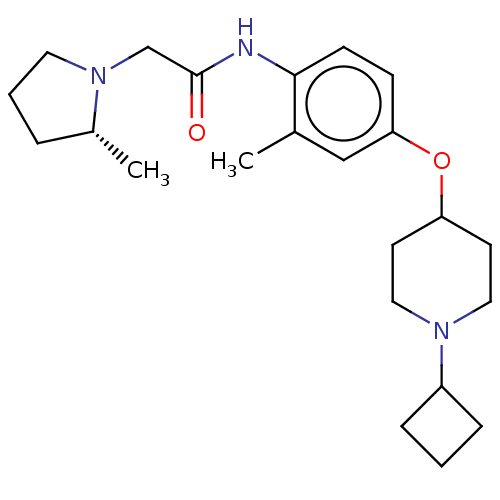 (CHEMBL4521909) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517521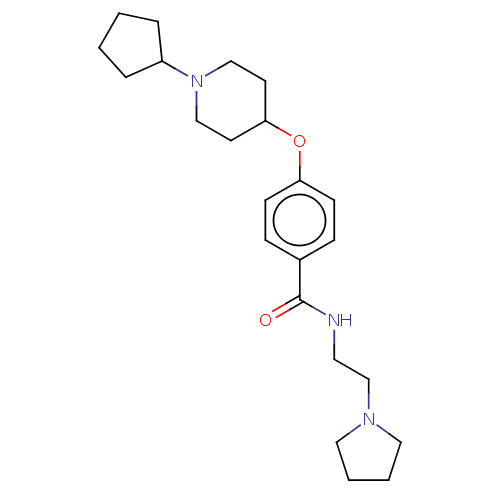 (CHEMBL4574463) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517522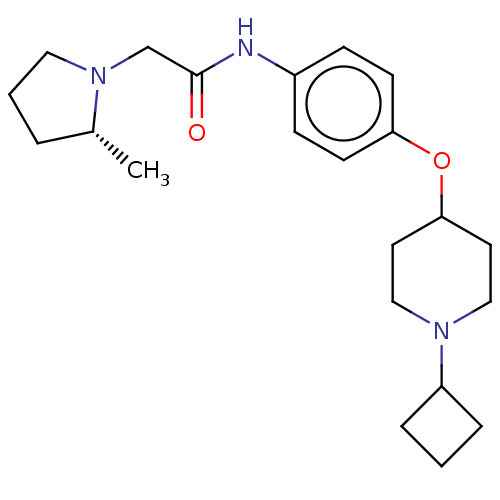 (CHEMBL4534815) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517502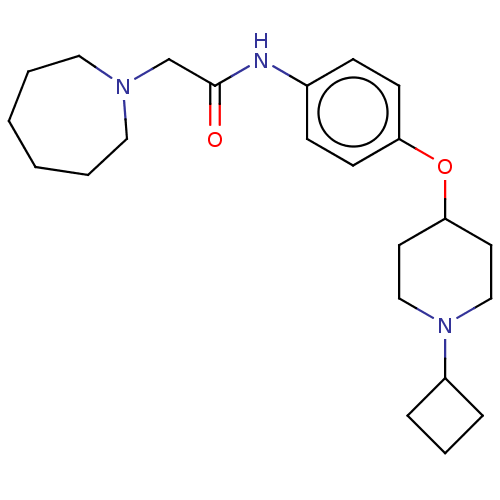 (CHEMBL4439392) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM82070 (CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California - Irvine Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from alpha4beta2 nAChR in Sprague-Dawley rat brain homogenate after 75 mins by scintillation counting | Bioorg Med Chem Lett 22: 7610-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.012 BindingDB Entry DOI: 10.7270/Q20V8GNK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517540 (CHEMBL4452215) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517527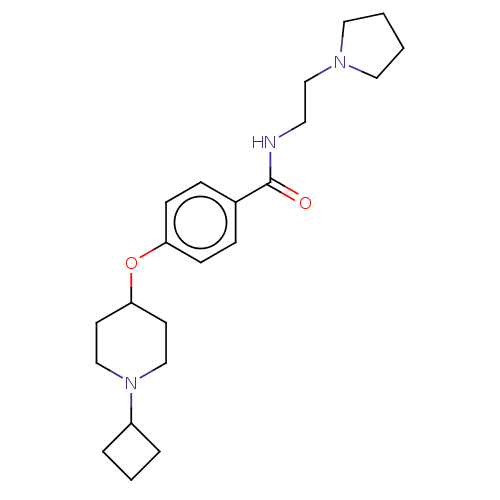 (CHEMBL4576724) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517491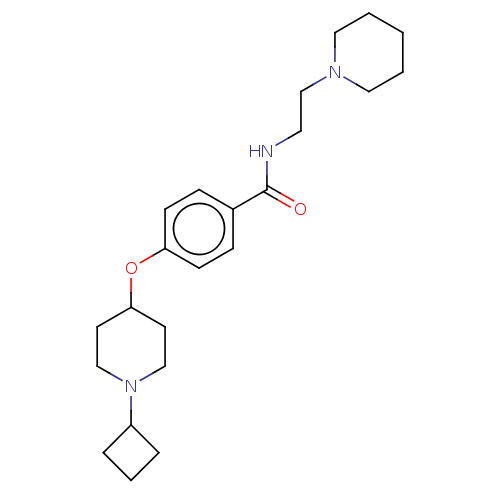 (CHEMBL4468778) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517506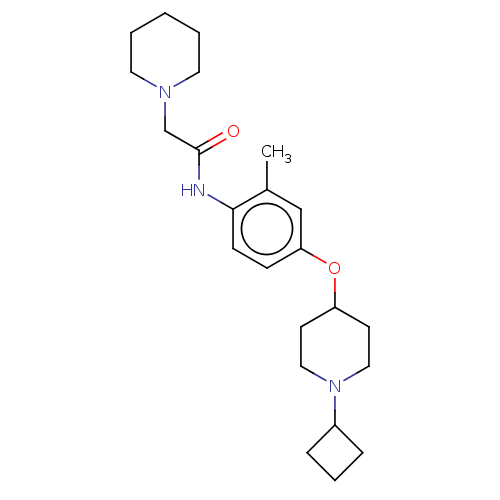 (CHEMBL4476420) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50456614 (CHEMBL4208912) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma1 receptor in guinea pig brain cortex membranes after 120 mins by microbeta scintillation counting met... | Eur J Med Chem 138: 552-564 (2017) Article DOI: 10.1016/j.ejmech.2017.06.068 BindingDB Entry DOI: 10.7270/Q2DV1NGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517528 (CHEMBL4476745) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517532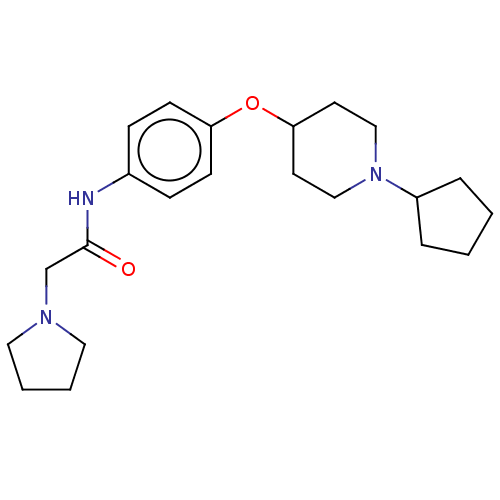 (CHEMBL4450961) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517494 (CHEMBL4561912) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517504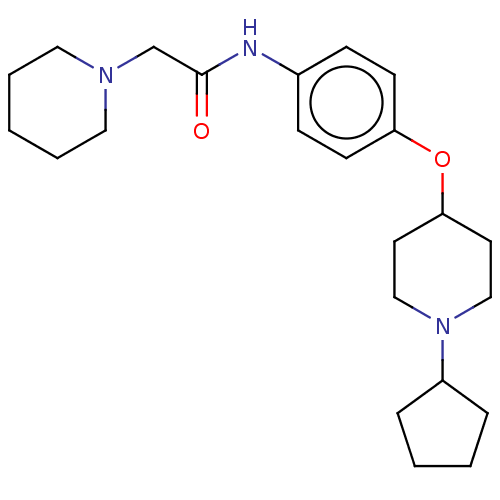 (CHEMBL4464101) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517501 (CHEMBL4578297) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517519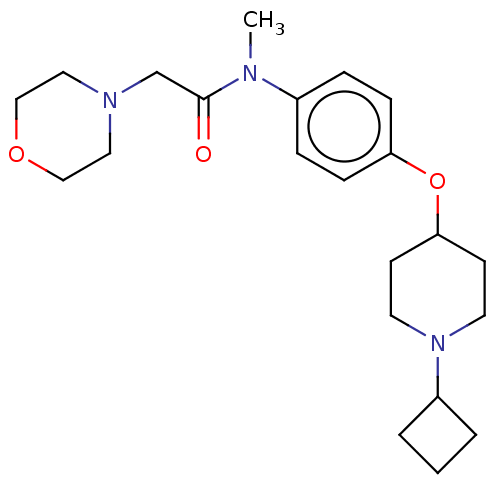 (CHEMBL4527873) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517509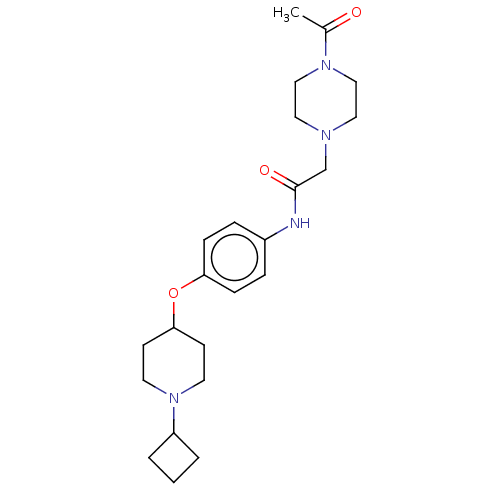 (CHEMBL4515325) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma1 receptor in guinea pig brain cortex membranes after 120 mins by microbeta scintillation counting met... | Eur J Med Chem 138: 552-564 (2017) Article DOI: 10.1016/j.ejmech.2017.06.068 BindingDB Entry DOI: 10.7270/Q2DV1NGG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517499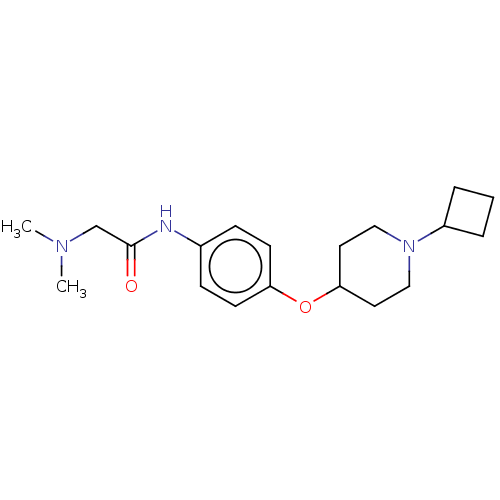 (CHEMBL4465570) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Binding affinity to sigma-1 receptor (unknown origin) by radioligand displacement assay | Bioorg Med Chem Lett 26: 889-93 (2016) Article DOI: 10.1016/j.bmcl.2015.12.067 BindingDB Entry DOI: 10.7270/Q2BZ67W6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-Pentazocine from sigma 1 receptor in guinea pig brain cortex membranes after 120 mins by scintillation counting analysis | Bioorg Med Chem 26: 501-508 (2018) Article DOI: 10.1016/j.bmc.2017.12.010 BindingDB Entry DOI: 10.7270/Q2MG7S3J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517511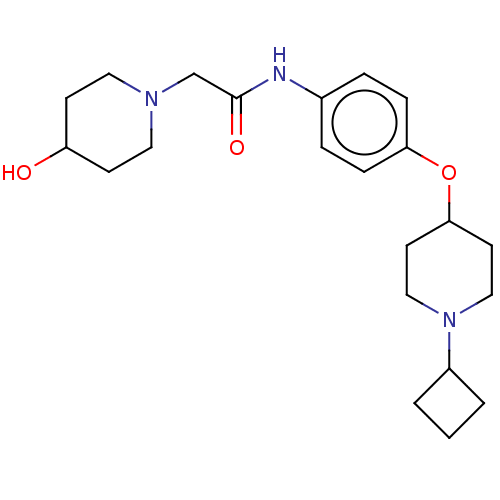 (CHEMBL4439589) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50099491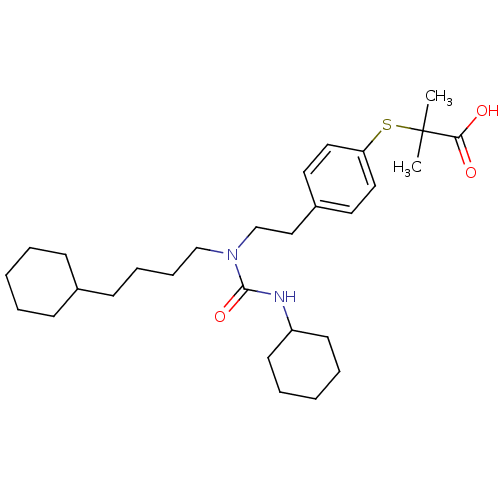 (2-(4-(2-(3-cyclohexyl-1-(4-cyclohexylbutyl)ureido)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jnatprod.2c00791 BindingDB Entry DOI: 10.7270/Q2G44VDK | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517512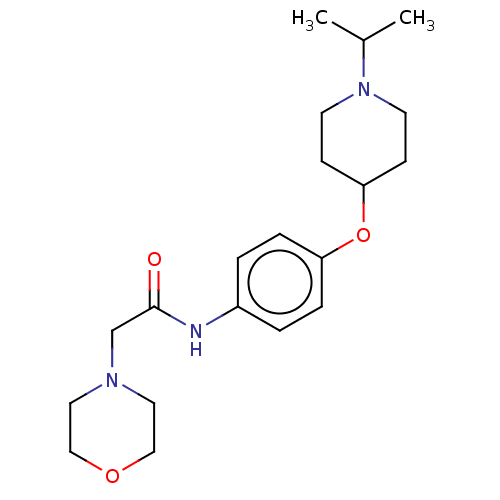 (CHEMBL4564463) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517497 (CHEMBL4593911) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517524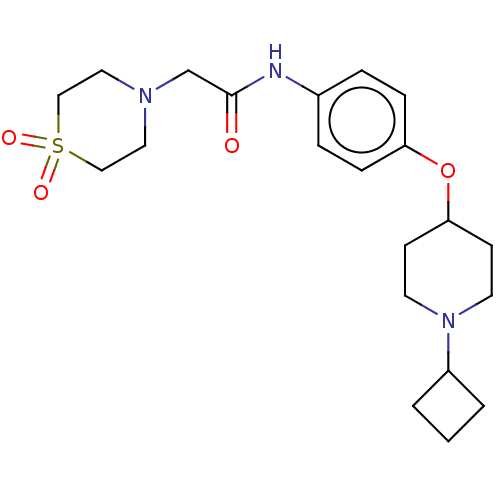 (CHEMBL4520938) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517498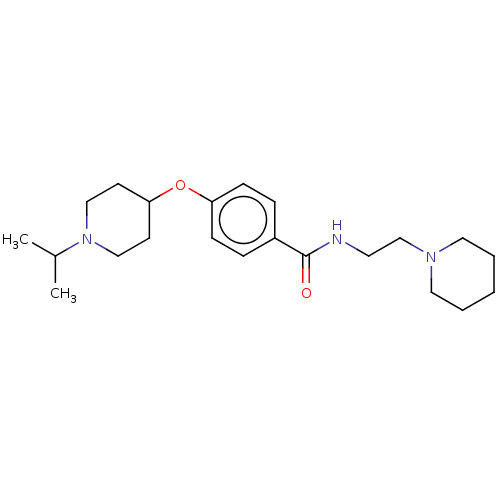 (CHEMBL4470829) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517510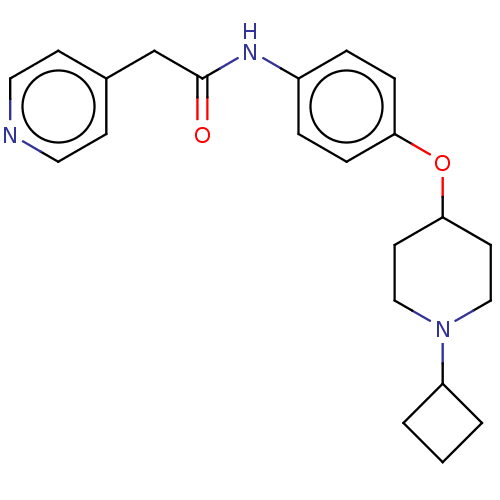 (CHEMBL4440461) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50517497 (CHEMBL4593911) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from rat histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517530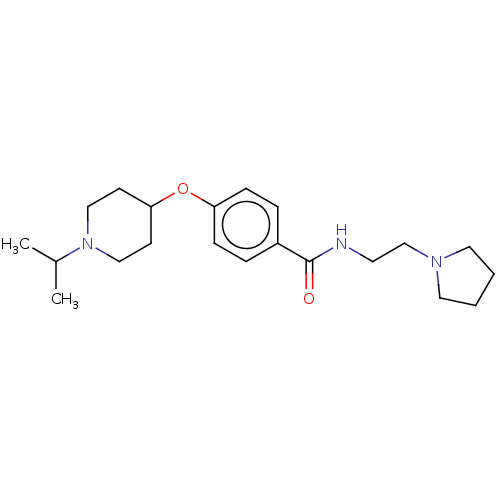 (CHEMBL4545816) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50456626 (CHEMBL4215178) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma1 receptor in guinea pig brain cortex membranes after 120 mins by microbeta scintillation counting met... | Eur J Med Chem 138: 552-564 (2017) Article DOI: 10.1016/j.ejmech.2017.06.068 BindingDB Entry DOI: 10.7270/Q2DV1NGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein, intestinal (Homo sapiens (Human)) | BDBM50448437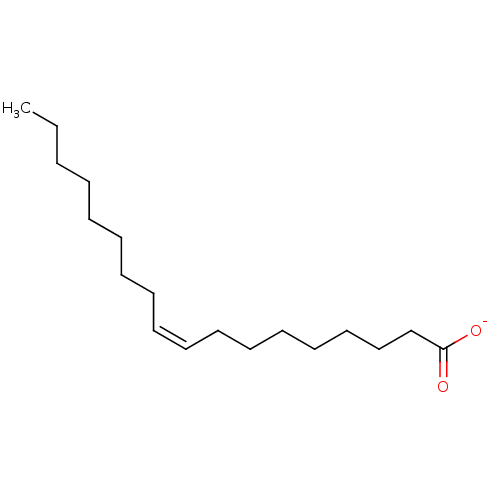 (CHEMBL3122151 | Oleate) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 10 | -86.7 | n/a | n/a | n/a | n/a | n/a | n/a | 293.15 |
Monash University | Assay Description Briefly, steady-state fluorescence spectra of ANS binding was monitored by measuring the increase in fluorescence signal between 450?550 nm following... | ACS Chem Biol 9: 2526-34 (2014) Article DOI: 10.1021/cb5005178 BindingDB Entry DOI: 10.7270/Q2348J4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50452836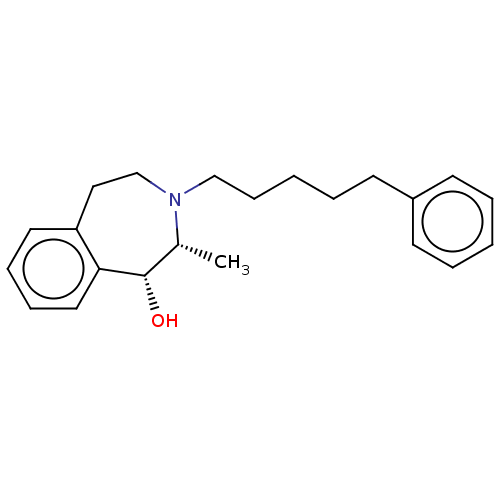 (CHEMBL4218465) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-Pentazocine from sigma 1 receptor in guinea pig brain cortex membranes after 120 mins by scintillation counting analysis | Bioorg Med Chem 26: 501-508 (2018) Article DOI: 10.1016/j.bmc.2017.12.010 BindingDB Entry DOI: 10.7270/Q2MG7S3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517503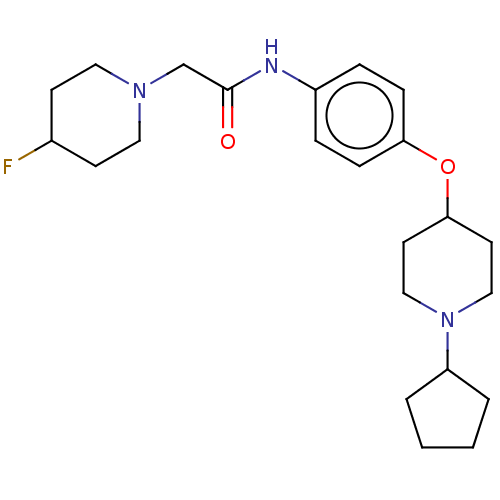 (CHEMBL4515283) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50517520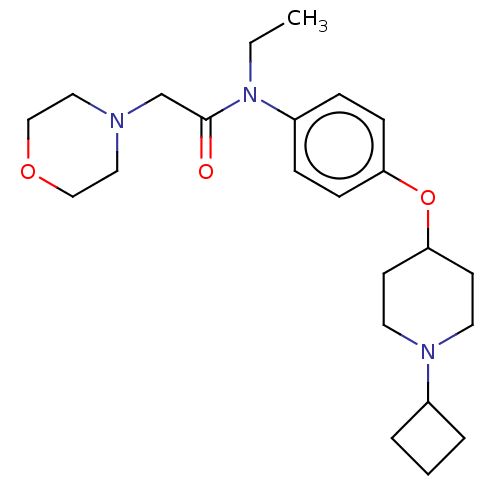 (CHEMBL4441571) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50389518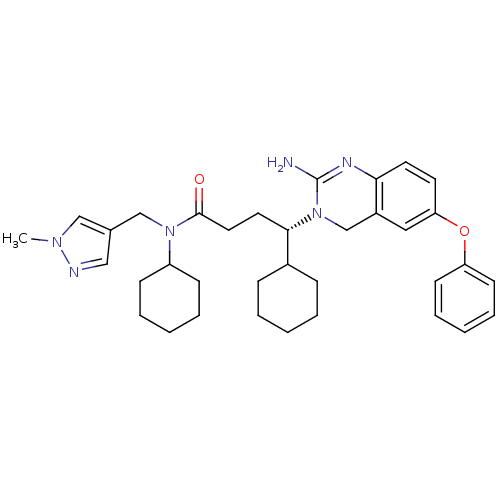 (CHEMBL2063207) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant beta-secretase 1 | Bioorg Med Chem Lett 22: 5460-5 (2012) Article DOI: 10.1016/j.bmcl.2012.07.043 BindingDB Entry DOI: 10.7270/Q2BV7HQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 573 total ) | Next | Last >> |