

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Squalene synthase (Rattus norvegicus) | BDBM50038096 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of squalene synthase in rat liver. | Bioorg Med Chem Lett 3: 2029-2034 (1993) Article DOI: 10.1016/S0960-894X(01)81008-8 BindingDB Entry DOI: 10.7270/Q22J6BSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50003020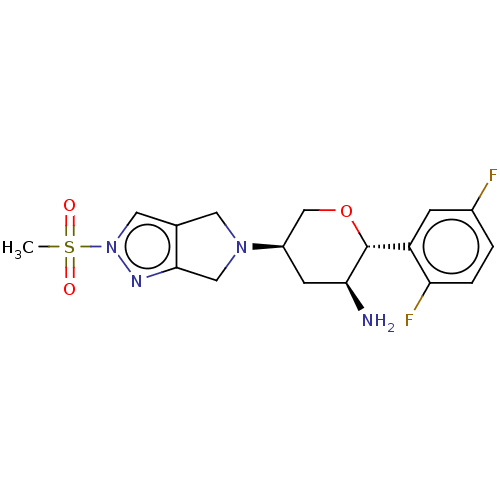 (MK-3102 | OMARIGLIPTIN | US10155775, Omarigliptin ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Competitive reversible inhibition of DPP4 (unknown origin) | J Med Chem 57: 3205-12 (2014) Article DOI: 10.1021/jm401992e BindingDB Entry DOI: 10.7270/Q2WD423H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50277511 (3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human NK3 receptor | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50277511 (3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human NK2 receptor | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Homo sapiens (Human)) | BDBM50047420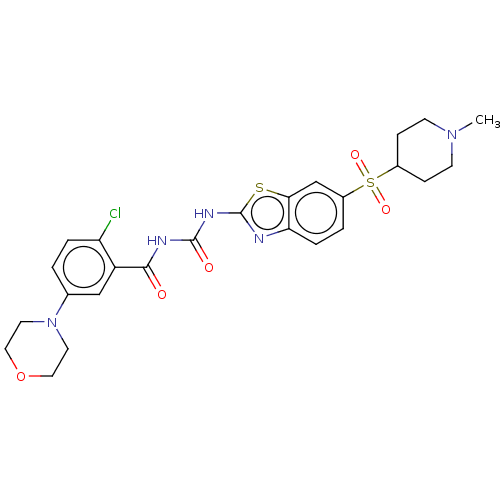 (CHEMBL3319405) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of alpha4 nAChR (unknown origin) | J Med Chem 57: 6128-40 (2014) Article DOI: 10.1021/jm500610n BindingDB Entry DOI: 10.7270/Q279469X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50047420 (CHEMBL3319405) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of DAT (unknown origin) | J Med Chem 57: 6128-40 (2014) Article DOI: 10.1021/jm500610n BindingDB Entry DOI: 10.7270/Q279469X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50234162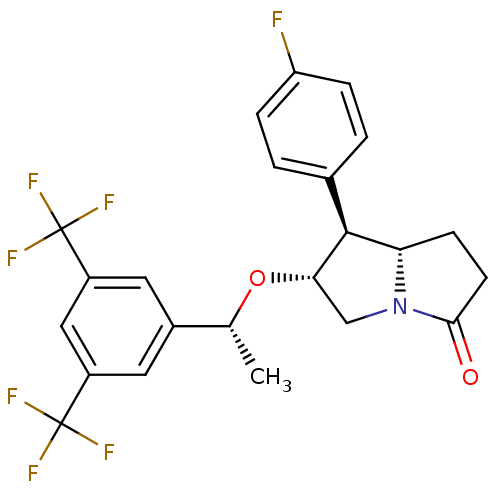 ((6R,7S,7aS)-6-((R)-1-(3,5-bis(trifluoromethyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]L-703606 from human NK1 expressed in CHO cells | Bioorg Med Chem 16: 2156-70 (2008) Article DOI: 10.1016/j.bmc.2007.11.081 BindingDB Entry DOI: 10.7270/Q2BK1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50372480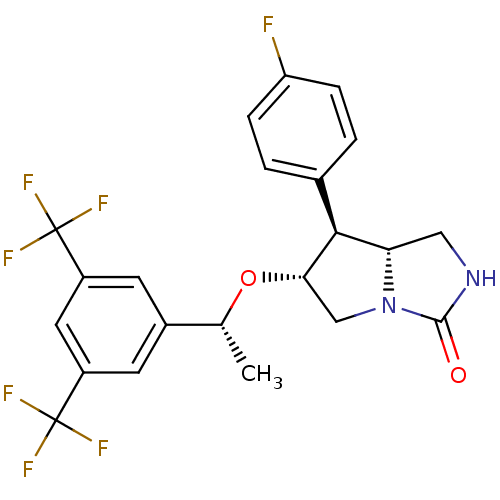 (CHEMBL270090) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]L-703606 from human NK1 expressed in CHO cells | Bioorg Med Chem 16: 2156-70 (2008) Article DOI: 10.1016/j.bmc.2007.11.081 BindingDB Entry DOI: 10.7270/Q2BK1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50277511 (3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]substance P from human NK1 receptor expressed in CHO cells | J Med Chem 56: 5940-8 (2014) Article DOI: 10.1021/jm400751p BindingDB Entry DOI: 10.7270/Q2ZW1N9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50437205 (CHEMBL2402572) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]substance P from human NK1 receptor expressed in CHO cells | J Med Chem 56: 5940-8 (2014) Article DOI: 10.1021/jm400751p BindingDB Entry DOI: 10.7270/Q2ZW1N9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50277511 (3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in absence of human serum albumin | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50047485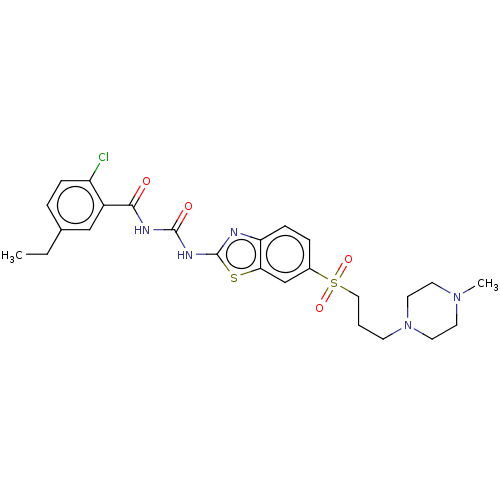 (CHEMBL3319217) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method | J Med Chem 57: 6128-40 (2014) Article DOI: 10.1021/jm500610n BindingDB Entry DOI: 10.7270/Q279469X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50277568 ((3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in absence of human serum albumin | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50373120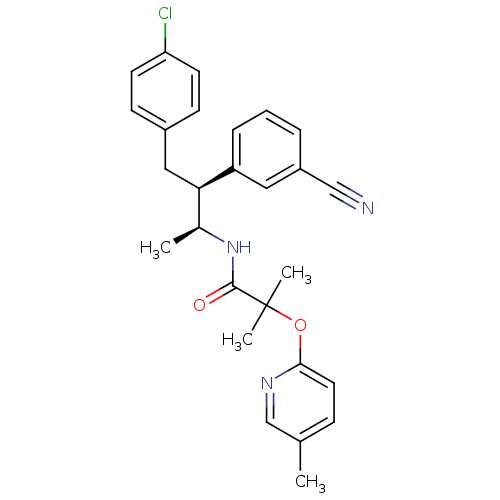 (CHEMBL260977 | MK-0364) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR-141716 from human wild type CB1R expressed in CHO cells | J Med Chem 51: 2108-14 (2008) Article DOI: 10.1021/jm7014974 BindingDB Entry DOI: 10.7270/Q27H1KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50277571 (3-[(4R,5S)-5-{(1R)-1-[3,5-Bis(trifluoromethyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in absence of human serum albumin | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50277569 ((4R,5S)-2-Acetyl-5-{(1R)-1-[3,5-bis(trifluoromethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in absence of human serum albumin | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50277572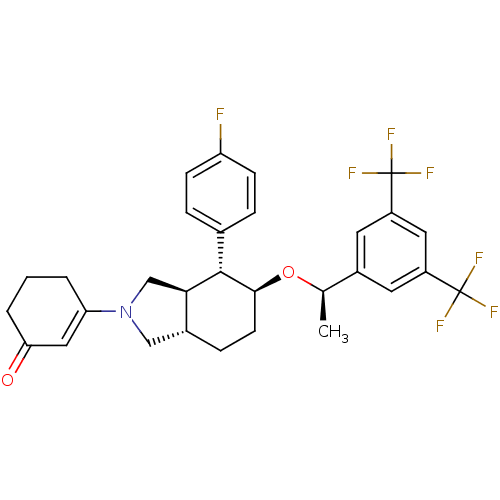 (3-[(4R,5S)-5-{(1R)-1-[3,5-Bis(trifluoromethyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in absence of human serum albumin | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50110088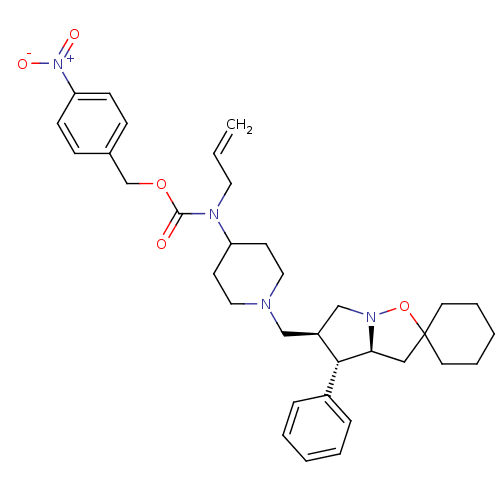 (4N-allyl-4N-[4-nitrobenzyloxycarboyl]-1-[4'-phenyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]-labeled MIP-1alpha from the C-C chemokine receptor type 5 expressed on CHO cell membranes | Bioorg Med Chem Lett 12: 677-9 (2002) BindingDB Entry DOI: 10.7270/Q2DF6QHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50372485 (CHEMBL409520) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]L-703606 from human NK1 expressed in CHO cells | Bioorg Med Chem 16: 2156-70 (2008) Article DOI: 10.1016/j.bmc.2007.11.081 BindingDB Entry DOI: 10.7270/Q2BK1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50277510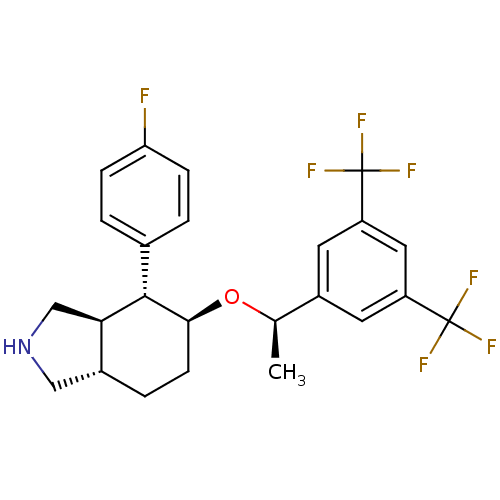 ((3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in absence of human serum albumin | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50047481 (CHEMBL3319221) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method | J Med Chem 57: 6128-40 (2014) Article DOI: 10.1021/jm500610n BindingDB Entry DOI: 10.7270/Q279469X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50042565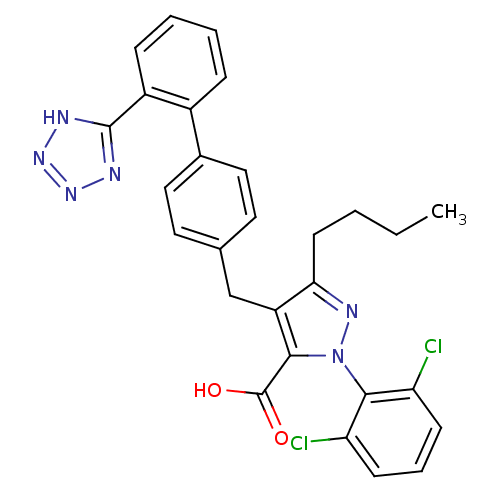 (5-Butyl-2-(2,6-dichloro-phenyl)-4-[2'-(1H-tetrazol...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluation of Angiotensin II antagonistic activity by displacement of [125I]-Sar Ile-AII at the rabbit aorta Angiotensin II receptor, type 1 | J Med Chem 36: 3595-605 (1994) BindingDB Entry DOI: 10.7270/Q25Q4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50042571 (2-Benzyl-5-butyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluation of Angiotensin II antagonistic activity by displacement of [125I]-Sar Ile-AII at the rabbit aorta Angiotensin II receptor, type 1 | J Med Chem 36: 3595-605 (1994) BindingDB Entry DOI: 10.7270/Q25Q4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50042578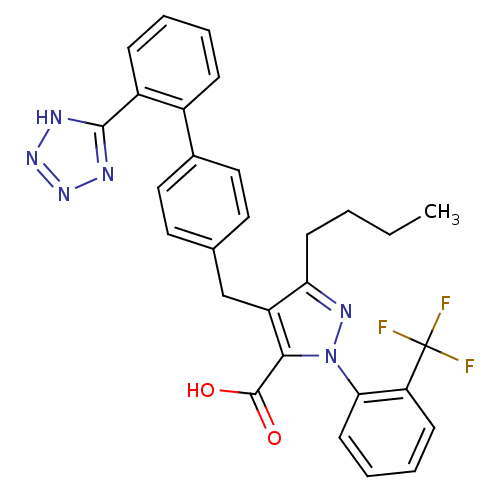 (5-Butyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluation of Angiotensin II antagonistic activity by displacement of [125I]-Sar Ile-AII at the rabbit aorta Angiotensin II receptor, type 1 | J Med Chem 36: 3595-605 (1994) BindingDB Entry DOI: 10.7270/Q25Q4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50047429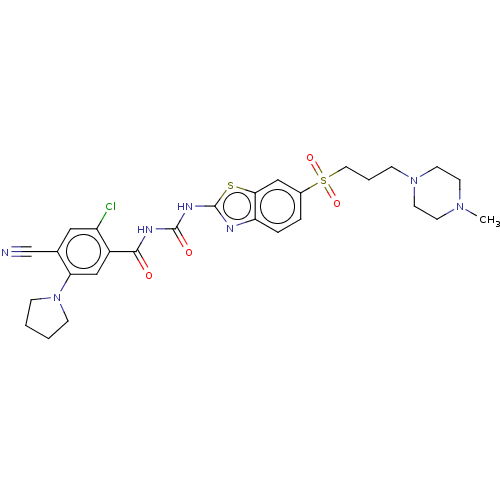 (CHEMBL3319398) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method | J Med Chem 57: 6128-40 (2014) Article DOI: 10.1021/jm500610n BindingDB Entry DOI: 10.7270/Q279469X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50042570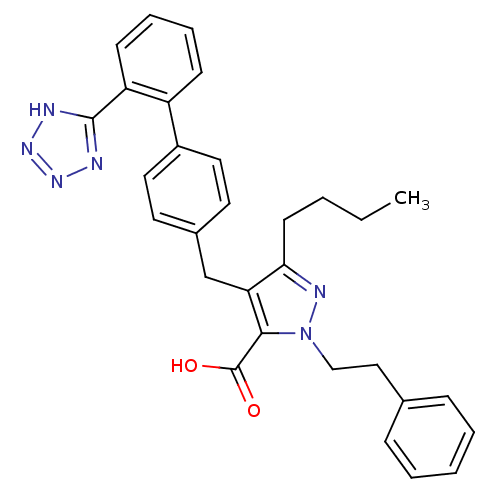 (5-Butyl-2-phenethyl-4-[2'-(1H-tetrazol-5-yl)-biphe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluation of Angiotensin II antagonistic activity by displacement of [125I]-Sar Ile-AII at the rabbit aorta Angiotensin II receptor, type 1 | J Med Chem 36: 3595-605 (1994) BindingDB Entry DOI: 10.7270/Q25Q4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50277567 ((4R,5S)-5-{(1R)-1-[3,5-Bis(trifluoromethyl)phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.255 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in absence of human serum albumin | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50372486 (CHEMBL272569) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]L-703606 from human NK1 expressed in CHO cells | Bioorg Med Chem 16: 2156-70 (2008) Article DOI: 10.1016/j.bmc.2007.11.081 BindingDB Entry DOI: 10.7270/Q2BK1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50372479 (CHEMBL259251) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]L-703606 from human NK1 expressed in CHO cells | Bioorg Med Chem 16: 2156-70 (2008) Article DOI: 10.1016/j.bmc.2007.11.081 BindingDB Entry DOI: 10.7270/Q2BK1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50047480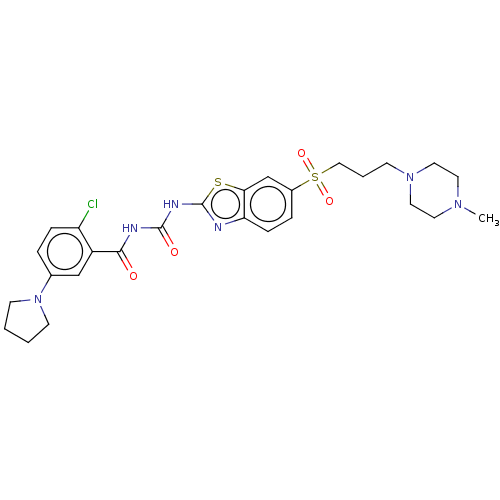 (CHEMBL3319222) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method | J Med Chem 57: 6128-40 (2014) Article DOI: 10.1021/jm500610n BindingDB Entry DOI: 10.7270/Q279469X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50047431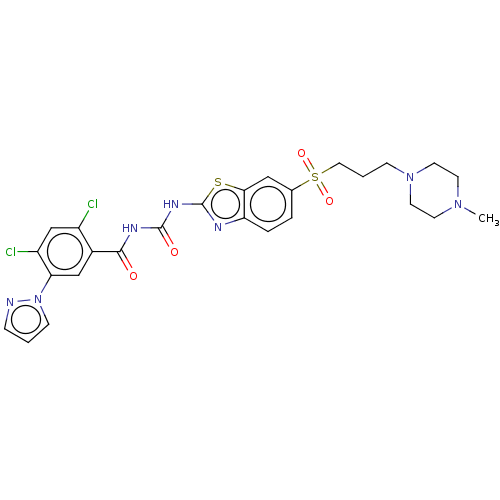 (CHEMBL3319397) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method | J Med Chem 57: 6128-40 (2014) Article DOI: 10.1021/jm500610n BindingDB Entry DOI: 10.7270/Q279469X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50047482 (CHEMBL3319220) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method | J Med Chem 57: 6128-40 (2014) Article DOI: 10.1021/jm500610n BindingDB Entry DOI: 10.7270/Q279469X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21279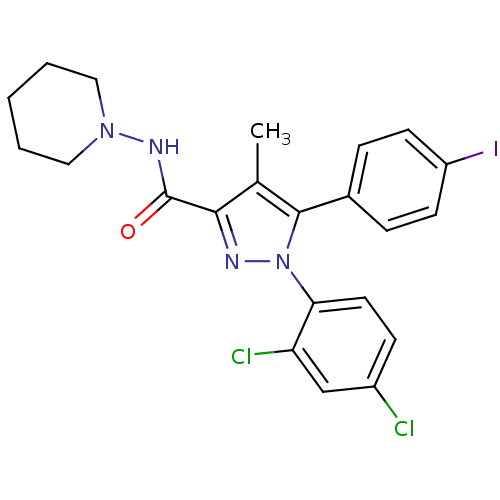 (1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR-141716 from human wild type CB1R expressed in CHO cells | J Med Chem 51: 2108-14 (2008) Article DOI: 10.1021/jm7014974 BindingDB Entry DOI: 10.7270/Q27H1KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50372483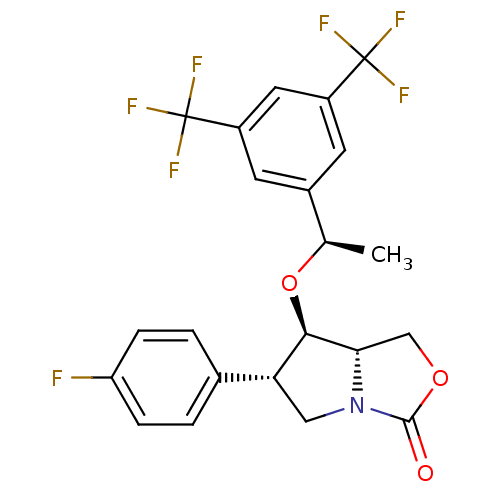 (CHEMBL272469) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]L-703606 from human NK1 expressed in CHO cells | Bioorg Med Chem 16: 2156-70 (2008) Article DOI: 10.1016/j.bmc.2007.11.081 BindingDB Entry DOI: 10.7270/Q2BK1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50047483 (CHEMBL3319219) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method | J Med Chem 57: 6128-40 (2014) Article DOI: 10.1021/jm500610n BindingDB Entry DOI: 10.7270/Q279469X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50042559 (5-Butyl-2-(2-chloro-phenyl)-4-[2'-(1H-tetrazol-5-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluation of Angiotensin II antagonistic activity by displacement of [125I]-Sar Ile-AII at the rabbit aorta Angiotensin II receptor, type 1 | J Med Chem 36: 3595-605 (1994) BindingDB Entry DOI: 10.7270/Q25Q4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50448706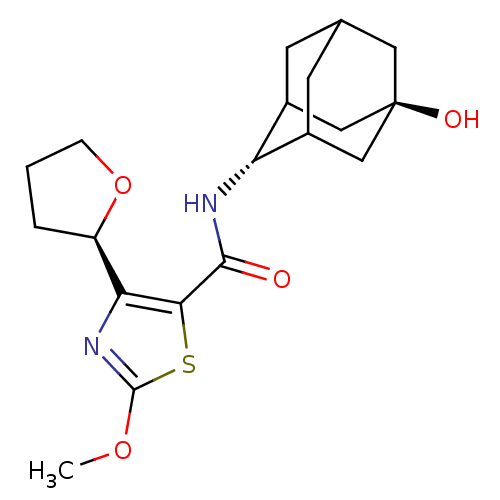 (CHEMBL3127854) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis | J Med Chem 57: 970-86 (2014) Article DOI: 10.1021/jm4016729 BindingDB Entry DOI: 10.7270/Q2Z32149 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50448693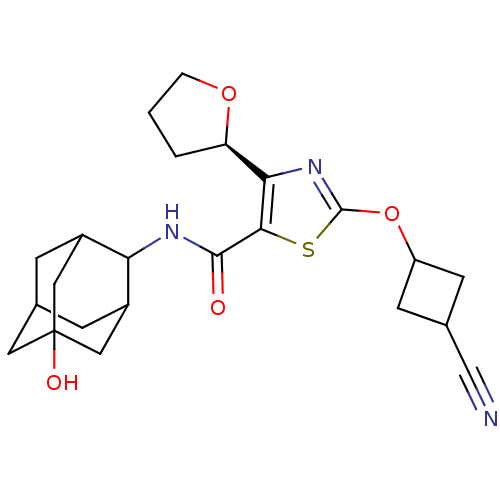 (CHEMBL3127857) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis | J Med Chem 57: 970-86 (2014) Article DOI: 10.1021/jm4016729 BindingDB Entry DOI: 10.7270/Q2Z32149 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50047688 (CHEMBL3319407) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method | J Med Chem 57: 6128-40 (2014) Article DOI: 10.1021/jm500610n BindingDB Entry DOI: 10.7270/Q279469X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50042534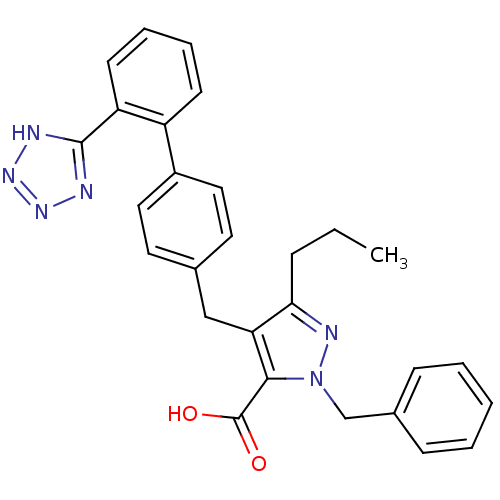 (2-Benzyl-5-propyl-4-[2'-(1H-tetrazol-5-yl)-bipheny...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluation of Angiotensin II antagonistic activity by displacement of [125I]-Sar Ile-AII at the rabbit aorta Angiotensin II receptor, type 1 | J Med Chem 36: 3595-605 (1994) BindingDB Entry DOI: 10.7270/Q25Q4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50448731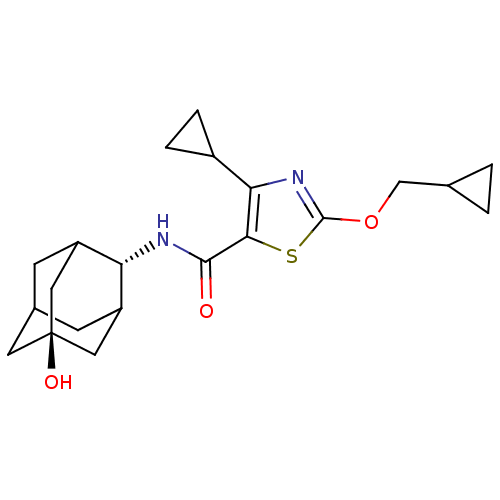 (CHEMBL3127868) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis | J Med Chem 57: 970-86 (2014) Article DOI: 10.1021/jm4016729 BindingDB Entry DOI: 10.7270/Q2Z32149 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50047426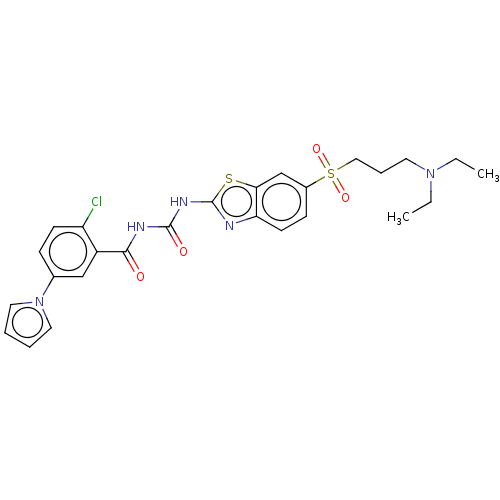 (CHEMBL3319400) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method | J Med Chem 57: 6128-40 (2014) Article DOI: 10.1021/jm500610n BindingDB Entry DOI: 10.7270/Q279469X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50448704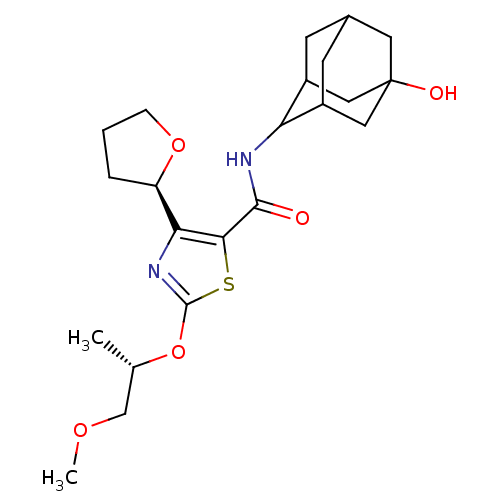 (CHEMBL3127856) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis | J Med Chem 57: 970-86 (2014) Article DOI: 10.1021/jm4016729 BindingDB Entry DOI: 10.7270/Q2Z32149 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50042569 (5-Butyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluation of Angiotensin II antagonistic activity by displacement of [125I]-Sar Ile-AII at the rabbit aorta Angiotensin II receptor, type 1 | J Med Chem 36: 3595-605 (1994) BindingDB Entry DOI: 10.7270/Q25Q4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50047474 (CHEMBL3319228) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method | J Med Chem 57: 6128-40 (2014) Article DOI: 10.1021/jm500610n BindingDB Entry DOI: 10.7270/Q279469X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50448705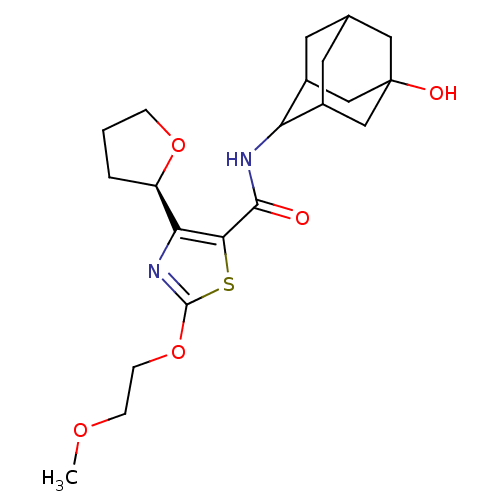 (CHEMBL3127855) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human 11beta-HSD1 using cortisone/[3H]-cortisone as substrate after 5 hrs by reverse-phase HPLC analysis | J Med Chem 57: 970-86 (2014) Article DOI: 10.1021/jm4016729 BindingDB Entry DOI: 10.7270/Q2Z32149 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50042576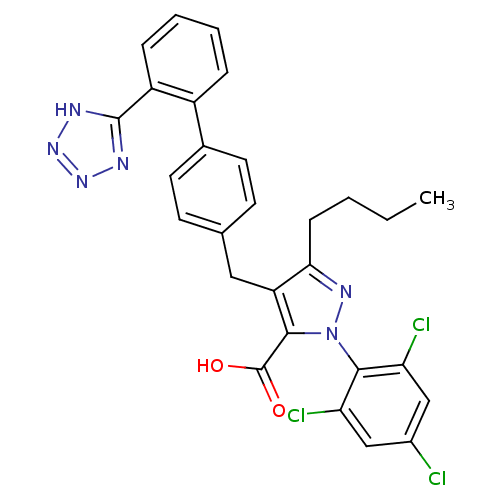 (5-Butyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluation of Angiotensin II antagonistic activity by displacement of [125I]-Sar Ile-AII at the rabbit aorta Angiotensin II receptor, type 1 | J Med Chem 36: 3595-605 (1994) BindingDB Entry DOI: 10.7270/Q25Q4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50042538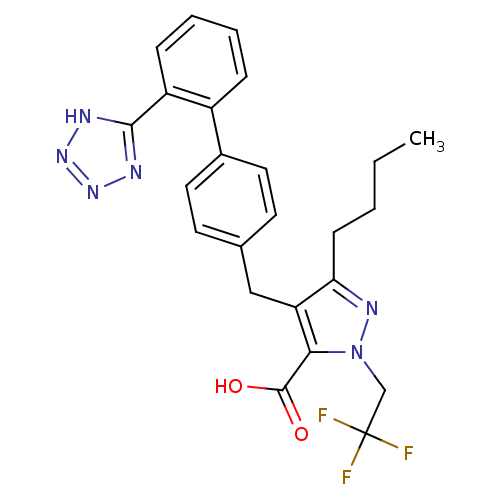 (5-Butyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluation of Angiotensin II antagonistic activity by displacement of [125I]-Sar Ile-AII at the rabbit aorta Angiotensin II receptor, type 1 | J Med Chem 36: 3595-605 (1994) BindingDB Entry DOI: 10.7270/Q25Q4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50277570 ((4R,5S)-5-{(1R)-1-[3,5-Bis(trifluoromethyl)phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.525 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in absence of human serum albumin | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50047475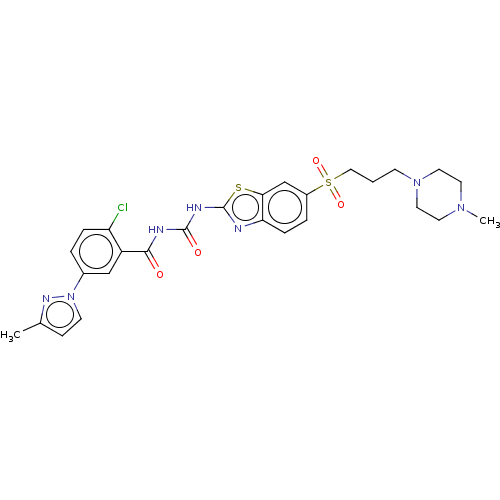 (CHEMBL3319227) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method | J Med Chem 57: 6128-40 (2014) Article DOI: 10.1021/jm500610n BindingDB Entry DOI: 10.7270/Q279469X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1390 total ) | Next | Last >> |