 Found 706 hits with Last Name = 'elrod' and Initial = 'k'
Found 706 hits with Last Name = 'elrod' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50103651

(2-(3'-Amino-5-chloro-2-hydroxy-biphenyl-3-yl)-1H-b...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(Cl)cc(-c2cccc(N)c2)c1O Show InChI InChI=1S/C20H16ClN5O/c21-12-8-14(10-2-1-3-13(22)6-10)18(27)15(9-12)20-25-16-5-4-11(19(23)24)7-17(16)26-20/h1-9,27H,22H2,(H3,23,24)(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for factor VIIa/TF |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50103651

(2-(3'-Amino-5-chloro-2-hydroxy-biphenyl-3-yl)-1H-b...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(Cl)cc(-c2cccc(N)c2)c1O Show InChI InChI=1S/C20H16ClN5O/c21-12-8-14(10-2-1-3-13(22)6-10)18(27)15(9-12)20-25-16-5-4-11(19(23)24)7-17(16)26-20/h1-9,27H,22H2,(H3,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50180517
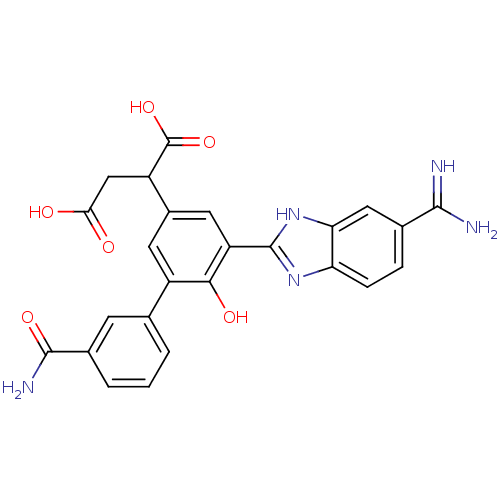
(2-[5-(5-carbamimidoyl-1H-benzoimidazol-2-yl)-3'-ca...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(cc(-c2cccc(c2)C(N)=O)c1O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C25H21N5O6/c26-22(27)12-4-5-18-19(9-12)30-24(29-18)17-8-14(16(25(35)36)10-20(31)32)7-15(21(17)33)11-2-1-3-13(6-11)23(28)34/h1-9,16,33H,10H2,(H3,26,27)(H2,28,34)(H,29,30)(H,31,32)(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to plasma kallikrein |
Bioorg Med Chem Lett 16: 2034-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.060
BindingDB Entry DOI: 10.7270/Q2P55N3N |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50103651

(2-(3'-Amino-5-chloro-2-hydroxy-biphenyl-3-yl)-1H-b...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(Cl)cc(-c2cccc(N)c2)c1O Show InChI InChI=1S/C20H16ClN5O/c21-12-8-14(10-2-1-3-13(22)6-10)18(27)15(9-12)20-25-16-5-4-11(19(23)24)7-17(16)26-20/h1-9,27H,22H2,(H3,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Coagulation factor X |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101871

(3-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(CCC(O)=O)cc(Br)c1O Show InChI InChI=1S/C25H22BrN3O3/c26-20-12-15(6-9-22(30)31)11-19(24(20)32)23-18(10-14-4-2-1-3-5-14)17-13-16(25(27)28)7-8-21(17)29-23/h1-5,7-8,11-13,29,32H,6,9-10H2,(H3,27,28)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101882
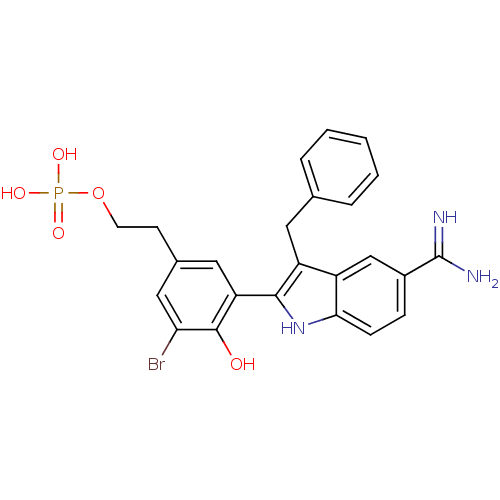
(CHEMBL53829 | Phosphoric acid mono-{2-[3-(3-benzyl...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(CCOP(O)(O)=O)cc(Br)c1O Show InChI InChI=1S/C24H23BrN3O5P/c25-20-12-15(8-9-33-34(30,31)32)11-19(23(20)29)22-18(10-14-4-2-1-3-5-14)17-13-16(24(26)27)6-7-21(17)28-22/h1-7,11-13,28-29H,8-10H2,(H3,26,27)(H2,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101881
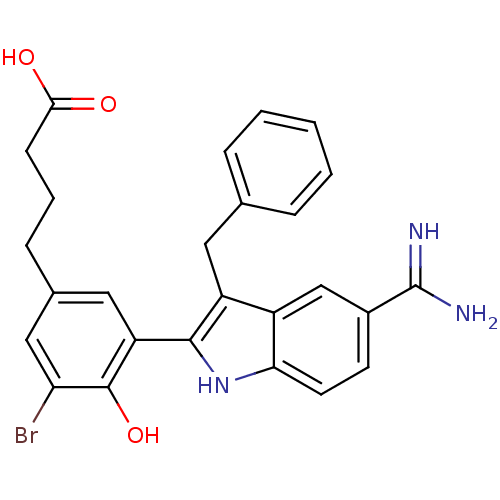
(4-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(CCCC(O)=O)cc(Br)c1O Show InChI InChI=1S/C26H24BrN3O3/c27-21-13-16(7-4-8-23(31)32)12-20(25(21)33)24-19(11-15-5-2-1-3-6-15)18-14-17(26(28)29)9-10-22(18)30-24/h1-3,5-6,9-10,12-14,30,33H,4,7-8,11H2,(H3,28,29)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM16303
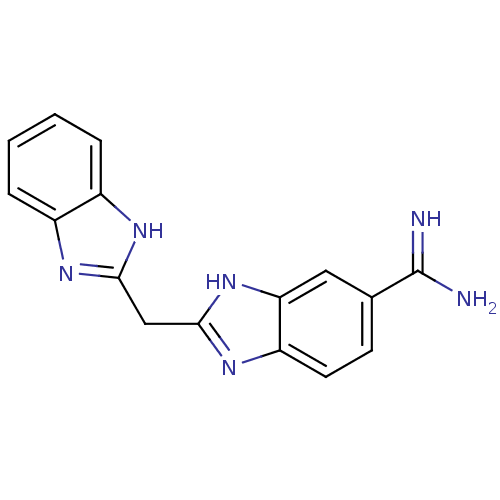
(2-(1H-1,3-benzodiazol-2-ylmethyl)-1H-1,3-benzodiaz...)Show InChI InChI=1S/C16H14N6/c17-16(18)9-5-6-12-13(7-9)22-15(21-12)8-14-19-10-3-1-2-4-11(10)20-14/h1-7H,8H2,(H3,17,18)(H,19,20)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to FXa in presence of Zn2+ |
Bioorg Med Chem Lett 16: 710-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.023
BindingDB Entry DOI: 10.7270/Q2VM4BSJ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50176251

(2-((6-chloro-1H-benzo[d]imidazol-2-yl)methyl)-1H-b...)Show SMILES NC(=N)c1ccc2nc(Cc3nc4ccc(Cl)cc4[nH]3)[nH]c2c1 Show InChI InChI=1S/C16H13ClN6/c17-9-2-4-11-13(6-9)23-15(21-11)7-14-20-10-3-1-8(16(18)19)5-12(10)22-14/h1-6H,7H2,(H3,18,19)(H,20,22)(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to FXa in presence of Zn2+ |
Bioorg Med Chem Lett 16: 710-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.023
BindingDB Entry DOI: 10.7270/Q2VM4BSJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM16303

(2-(1H-1,3-benzodiazol-2-ylmethyl)-1H-1,3-benzodiaz...)Show InChI InChI=1S/C16H14N6/c17-16(18)9-5-6-12-13(7-9)22-15(21-12)8-14-19-10-3-1-2-4-11(10)20-14/h1-7H,8H2,(H3,17,18)(H,19,20)(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to thrombin in presence of Zn2+ |
Bioorg Med Chem Lett 16: 710-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.023
BindingDB Entry DOI: 10.7270/Q2VM4BSJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50176252
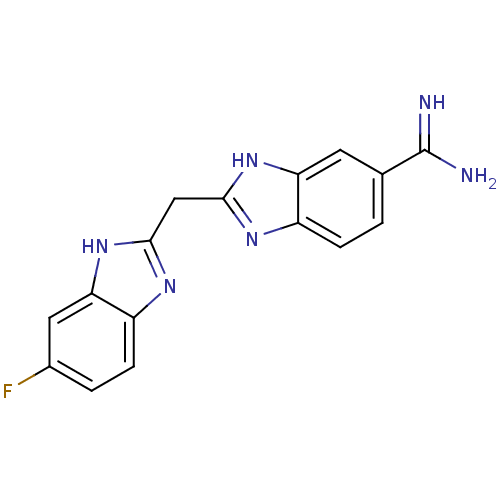
(2-((6-fluoro-1H-benzo[d]imidazol-2-yl)methyl)-1H-b...)Show SMILES NC(=N)c1ccc2nc(Cc3nc4ccc(F)cc4[nH]3)[nH]c2c1 Show InChI InChI=1S/C16H13FN6/c17-9-2-4-11-13(6-9)23-15(21-11)7-14-20-10-3-1-8(16(18)19)5-12(10)22-14/h1-6H,7H2,(H3,18,19)(H,20,22)(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to FXa in presence of Zn2+ |
Bioorg Med Chem Lett 16: 710-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.023
BindingDB Entry DOI: 10.7270/Q2VM4BSJ |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM14338
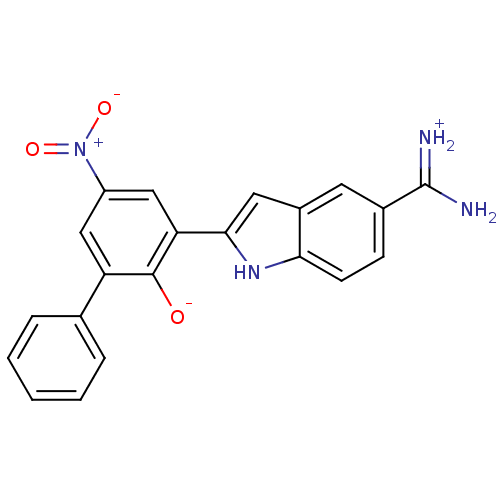
(2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-nit...)Show SMILES NC(=[NH2+])c1ccc2[nH]c(cc2c1)-c1cc(cc(-c2ccccc2)c1[O-])[N+]([O-])=O Show InChI InChI=1S/C21H16N4O3/c22-21(23)13-6-7-18-14(8-13)9-19(24-18)17-11-15(25(27)28)10-16(20(17)26)12-4-2-1-3-5-12/h1-11,24,26H,(H3,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50176257
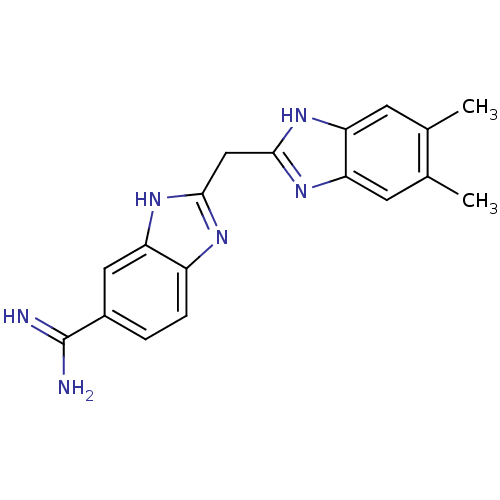
(2-((5,6-dimethyl-1H-benzo[d]imidazol-2-yl)methyl)-...)Show SMILES Cc1cc2nc(Cc3nc4ccc(cc4[nH]3)C(N)=N)[nH]c2cc1C Show InChI InChI=1S/C18H18N6/c1-9-5-13-14(6-10(9)2)23-17(22-13)8-16-21-12-4-3-11(18(19)20)7-15(12)24-16/h3-7H,8H2,1-2H3,(H3,19,20)(H,21,24)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to FXa in presence of Zn2+ |
Bioorg Med Chem Lett 16: 710-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.023
BindingDB Entry DOI: 10.7270/Q2VM4BSJ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101883
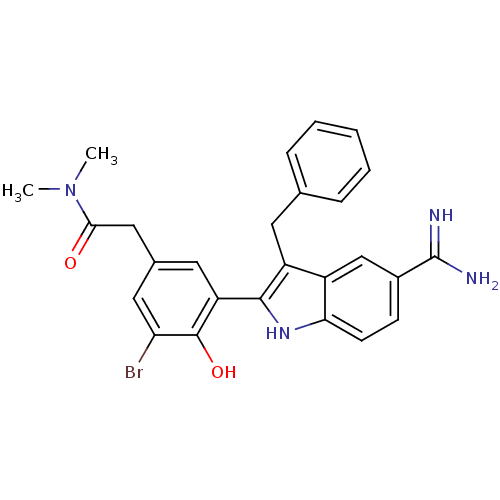
(2-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...)Show SMILES CN(C)C(=O)Cc1cc(Br)c(O)c(c1)-c1[nH]c2ccc(cc2c1Cc1ccccc1)C(N)=N Show InChI InChI=1S/C26H25BrN4O2/c1-31(2)23(32)13-16-11-20(25(33)21(27)12-16)24-19(10-15-6-4-3-5-7-15)18-14-17(26(28)29)8-9-22(18)30-24/h3-9,11-12,14,30,33H,10,13H2,1-2H3,(H3,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101885
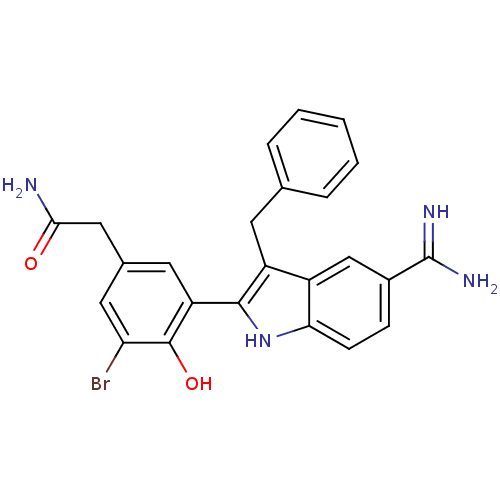
(2-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...)Show SMILES NC(=O)Cc1cc(Br)c(O)c(c1)-c1[nH]c2ccc(cc2c1Cc1ccccc1)C(N)=N Show InChI InChI=1S/C24H21BrN4O2/c25-19-10-14(11-21(26)30)9-18(23(19)31)22-17(8-13-4-2-1-3-5-13)16-12-15(24(27)28)6-7-20(16)29-22/h1-7,9-10,12,29,31H,8,11H2,(H2,26,30)(H3,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50180400
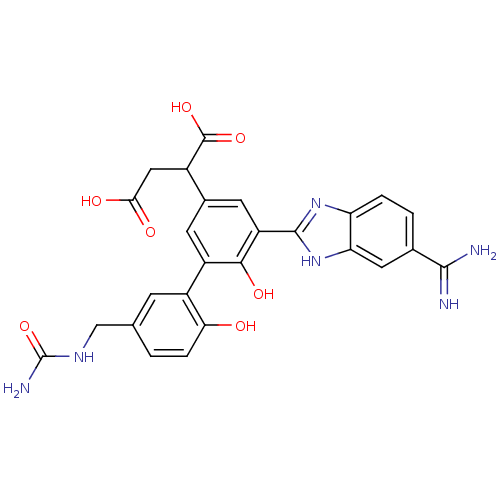
(2-[5-(5-carbamimidoyl-1H-benzoimidazol-2-yl)-6,2'-...)Show SMILES NC(=O)NCc1ccc(O)c(c1)-c1cc(cc(-c2nc3ccc(cc3[nH]2)C(N)=N)c1O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C26H24N6O7/c27-23(28)12-2-3-18-19(8-12)32-24(31-18)17-7-13(14(25(37)38)9-21(34)35)6-16(22(17)36)15-5-11(1-4-20(15)33)10-30-26(29)39/h1-8,14,33,36H,9-10H2,(H3,27,28)(H,31,32)(H,34,35)(H,37,38)(H3,29,30,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to F7a/TF complex |
Bioorg Med Chem Lett 16: 2037-41 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.059
BindingDB Entry DOI: 10.7270/Q2639PBP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14352
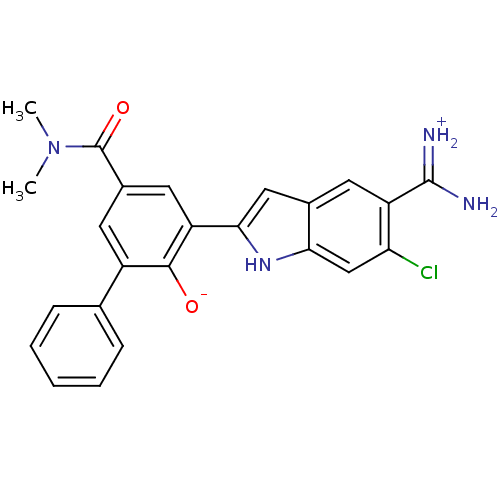
(2-{5-[amino(iminiumyl)methyl]-6-chloro-1H-indol-2-...)Show SMILES CN(C)C(=O)c1cc(-c2cc3cc(C(N)=[NH2+])c(Cl)cc3[nH]2)c([O-])c(c1)-c1ccccc1 Show InChI InChI=1S/C24H21ClN4O2/c1-29(2)24(31)15-9-16(13-6-4-3-5-7-13)22(30)18(10-15)21-11-14-8-17(23(26)27)19(25)12-20(14)28-21/h3-12,28,30H,1-2H3,(H3,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14351

(2-{5-[amino(iminiumyl)methyl]-6-chloro-1H-indol-2-...)Show SMILES COc1cc(-c2cc3cc(C(N)=[NH2+])c(Cl)cc3[nH]2)c([O-])c(c1)-c1ccccc1 Show InChI InChI=1S/C22H18ClN3O2/c1-28-14-9-15(12-5-3-2-4-6-12)21(27)17(10-14)20-8-13-7-16(22(24)25)18(23)11-19(13)26-20/h2-11,26-27H,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50176254

(2-((5,7-dichloro-1H-benzo[d]imidazol-2-yl)methyl)-...)Show SMILES NC(=N)c1ccc2nc(Cc3nc4cc(Cl)cc(Cl)c4[nH]3)[nH]c2c1 Show InChI InChI=1S/C16H12Cl2N6/c17-8-4-9(18)15-12(5-8)23-14(24-15)6-13-21-10-2-1-7(16(19)20)3-11(10)22-13/h1-5H,6H2,(H3,19,20)(H,21,22)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to FXa in presence of Zn2+ |
Bioorg Med Chem Lett 16: 710-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.023
BindingDB Entry DOI: 10.7270/Q2VM4BSJ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50176250
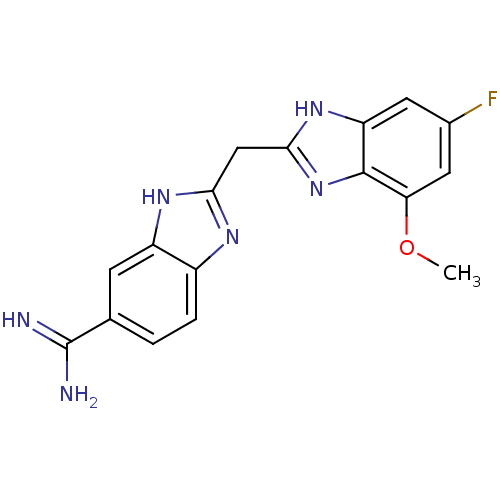
(2-((5-fluoro-7-methoxy-1H-benzo[d]imidazol-2-yl)me...)Show SMILES COc1cc(F)cc2[nH]c(Cc3nc4ccc(cc4[nH]3)C(N)=N)nc12 Show InChI InChI=1S/C17H15FN6O/c1-25-13-6-9(18)5-12-16(13)24-15(23-12)7-14-21-10-3-2-8(17(19)20)4-11(10)22-14/h2-6H,7H2,1H3,(H3,19,20)(H,21,22)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to FXa in presence of Zn2+ |
Bioorg Med Chem Lett 16: 710-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.023
BindingDB Entry DOI: 10.7270/Q2VM4BSJ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101879
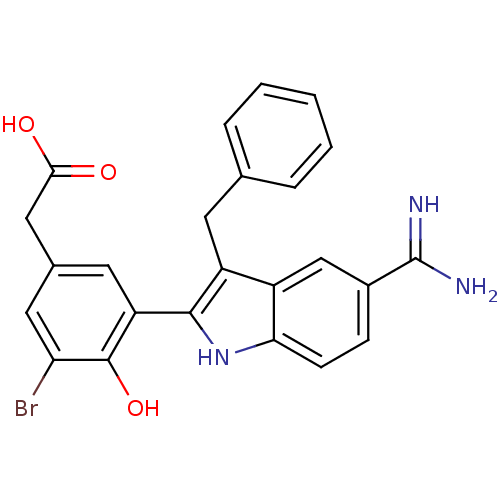
(CHEMBL50924 | [3-(3-Benzyl-5-carbamimidoyl-1H-indo...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(CC(O)=O)cc(Br)c1O Show InChI InChI=1S/C24H20BrN3O3/c25-19-10-14(11-21(29)30)9-18(23(19)31)22-17(8-13-4-2-1-3-5-13)16-12-15(24(26)27)6-7-20(16)28-22/h1-7,9-10,12,28,31H,8,11H2,(H3,26,27)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101880
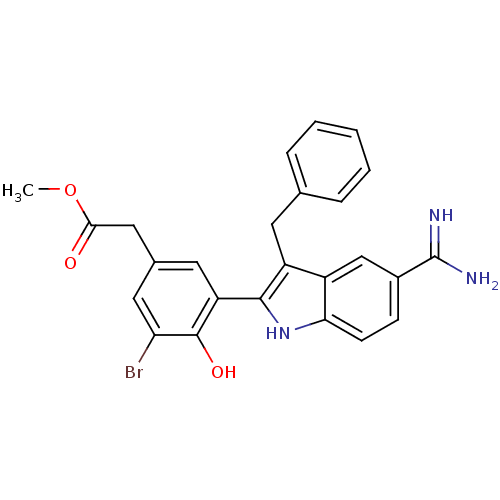
(CHEMBL416127 | [3-(3-Benzyl-5-carbamimidoyl-1H-ind...)Show SMILES COC(=O)Cc1cc(Br)c(O)c(c1)-c1[nH]c2ccc(cc2c1Cc1ccccc1)C(N)=N Show InChI InChI=1S/C25H22BrN3O3/c1-32-22(30)12-15-10-19(24(31)20(26)11-15)23-18(9-14-5-3-2-4-6-14)17-13-16(25(27)28)7-8-21(17)29-23/h2-8,10-11,13,29,31H,9,12H2,1H3,(H3,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM14921
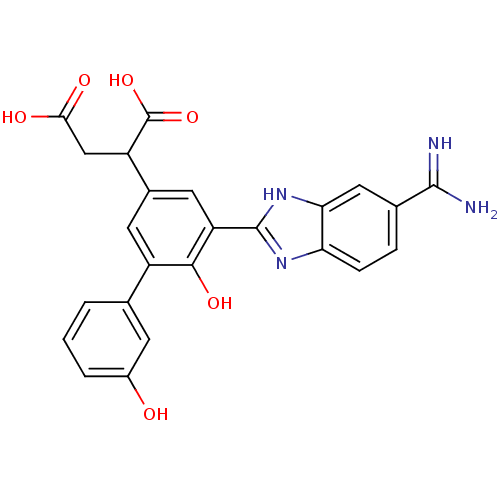
(2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-4-h...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(cc(-c2cccc(O)c2)c1O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C24H20N4O6/c25-22(26)12-4-5-18-19(9-12)28-23(27-18)17-8-13(16(24(33)34)10-20(30)31)7-15(21(17)32)11-2-1-3-14(29)6-11/h1-9,16,29,32H,10H2,(H3,25,26)(H,27,28)(H,30,31)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to plasma kallikrein |
Bioorg Med Chem Lett 16: 2034-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.060
BindingDB Entry DOI: 10.7270/Q2P55N3N |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50103655
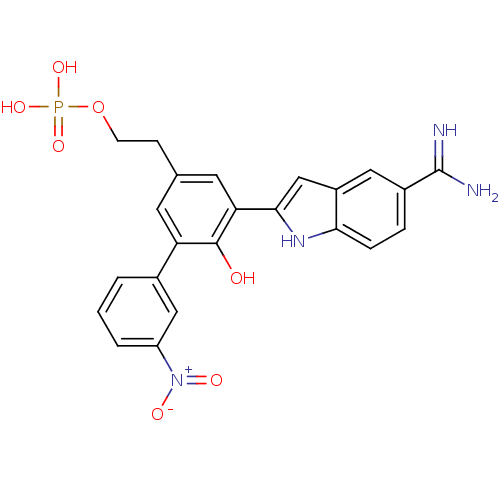
(CHEMBL72231 | Phosphoric acid mono-{2-[5-(5-carbam...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(CCO[P+](O)(O)[O-])cc(c1O)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C23H21N4O7P/c24-23(25)15-4-5-20-16(10-15)12-21(26-20)19-9-13(6-7-34-35(31,32)33)8-18(22(19)28)14-2-1-3-17(11-14)27(29)30/h1-5,8-12,26,28H,6-7H2,(H3,24,25)(H2,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for factor VIIa/TF |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM13778

(2-[3-(5-carbamimidoyl-1H-indol-2-yl)-4-hydroxy-5-(...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(CC(O)=O)cc(c1O)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C23H18N4O5/c24-23(25)14-4-5-19-15(9-14)11-20(26-19)18-7-12(8-21(28)29)6-17(22(18)30)13-2-1-3-16(10-13)27(31)32/h1-7,9-11,26,30H,8H2,(H3,24,25)(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for factor VIIa/TF |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM14917
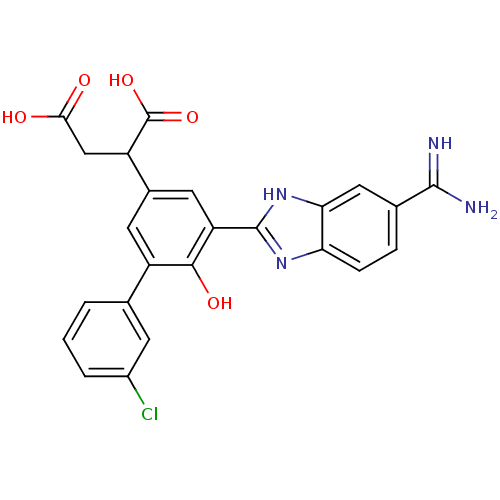
(2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-5-(...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(cc(-c2cccc(Cl)c2)c1O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C24H19ClN4O5/c25-14-3-1-2-11(6-14)15-7-13(16(24(33)34)10-20(30)31)8-17(21(15)32)23-28-18-5-4-12(22(26)27)9-19(18)29-23/h1-9,16,32H,10H2,(H3,26,27)(H,28,29)(H,30,31)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to plasma kallikrein |
Bioorg Med Chem Lett 16: 2034-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.060
BindingDB Entry DOI: 10.7270/Q2P55N3N |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14354
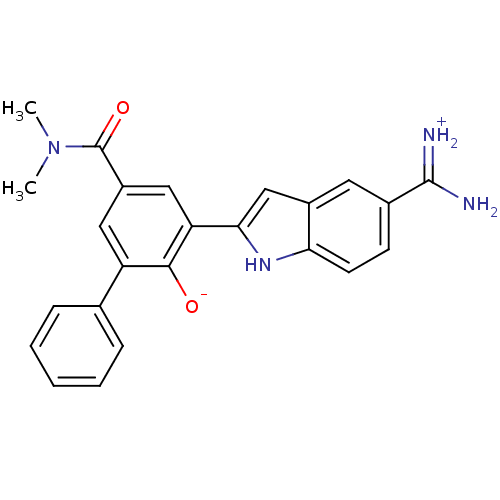
(2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-(di...)Show SMILES CN(C)C(=O)c1cc(-c2cc3cc(ccc3[nH]2)C(N)=[NH2+])c([O-])c(c1)-c1ccccc1 Show InChI InChI=1S/C24H22N4O2/c1-28(2)24(30)17-11-18(14-6-4-3-5-7-14)22(29)19(12-17)21-13-16-10-15(23(25)26)8-9-20(16)27-21/h3-13,27,29H,1-2H3,(H3,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50103651

(2-(3'-Amino-5-chloro-2-hydroxy-biphenyl-3-yl)-1H-b...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(Cl)cc(-c2cccc(N)c2)c1O Show InChI InChI=1S/C20H16ClN5O/c21-12-8-14(10-2-1-3-13(22)6-10)18(27)15(9-12)20-25-16-5-4-11(19(23)24)7-17(16)26-20/h1-9,27H,22H2,(H3,23,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Plasmin |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101870
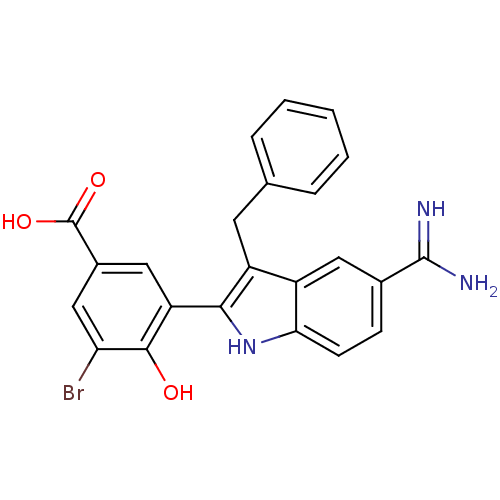
(3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-bromo...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(cc(Br)c1O)C(O)=O Show InChI InChI=1S/C23H18BrN3O3/c24-18-11-14(23(29)30)10-17(21(18)28)20-16(8-12-4-2-1-3-5-12)15-9-13(22(25)26)6-7-19(15)27-20/h1-7,9-11,27-28H,8H2,(H3,25,26)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM14914

(2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-4-h...)Show SMILES Cc1cccc(c1)-c1cc(cc(-c2nc3ccc(cc3[nH]2)C(N)=N)c1O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C25H22N4O5/c1-12-3-2-4-13(7-12)16-8-15(17(25(33)34)11-21(30)31)9-18(22(16)32)24-28-19-6-5-14(23(26)27)10-20(19)29-24/h2-10,17,32H,11H2,1H3,(H3,26,27)(H,28,29)(H,30,31)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to plasma kallikrein |
Bioorg Med Chem Lett 16: 2034-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.060
BindingDB Entry DOI: 10.7270/Q2P55N3N |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM14898
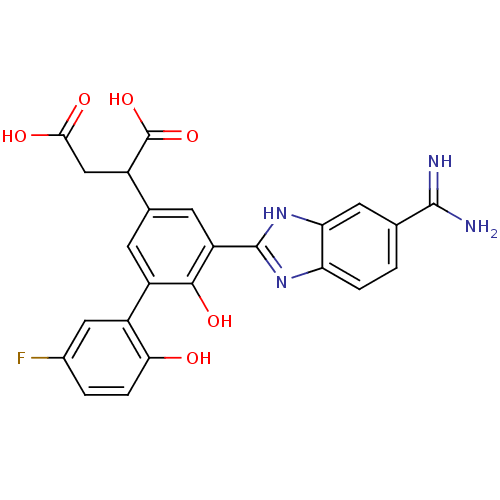
(2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-5-(...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(cc(c1O)-c1cc(F)ccc1O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C24H19FN4O6/c25-12-2-4-19(30)14(8-12)15-5-11(13(24(34)35)9-20(31)32)6-16(21(15)33)23-28-17-3-1-10(22(26)27)7-18(17)29-23/h1-8,13,30,33H,9H2,(H3,26,27)(H,28,29)(H,31,32)(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to F7a/TF complex |
Bioorg Med Chem Lett 16: 2037-41 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.059
BindingDB Entry DOI: 10.7270/Q2639PBP |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50103651

(2-(3'-Amino-5-chloro-2-hydroxy-biphenyl-3-yl)-1H-b...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(Cl)cc(-c2cccc(N)c2)c1O Show InChI InChI=1S/C20H16ClN5O/c21-12-8-14(10-2-1-3-13(22)6-10)18(27)15(9-12)20-25-16-5-4-11(19(23)24)7-17(16)26-20/h1-9,27H,22H2,(H3,23,24)(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Coagulation factor II |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50103651

(2-(3'-Amino-5-chloro-2-hydroxy-biphenyl-3-yl)-1H-b...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(Cl)cc(-c2cccc(N)c2)c1O Show InChI InChI=1S/C20H16ClN5O/c21-12-8-14(10-2-1-3-13(22)6-10)18(27)15(9-12)20-25-16-5-4-11(19(23)24)7-17(16)26-20/h1-9,27H,22H2,(H3,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Trypsin |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50103652
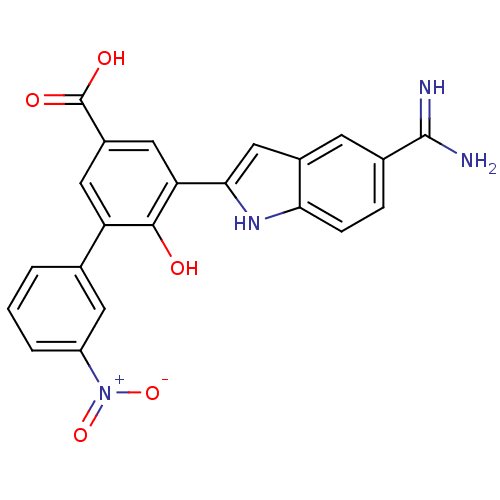
(5-(5-Carbamimidoyl-1H-indol-2-yl)-6-hydroxy-3'-nit...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(cc(c1O)-c1cccc(c1)[N+]([O-])=O)C(O)=O Show InChI InChI=1S/C22H16N4O5/c23-21(24)12-4-5-18-13(6-12)10-19(25-18)17-9-14(22(28)29)8-16(20(17)27)11-2-1-3-15(7-11)26(30)31/h1-10,25,27H,(H3,23,24)(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for factor VIIa/TF |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50180516
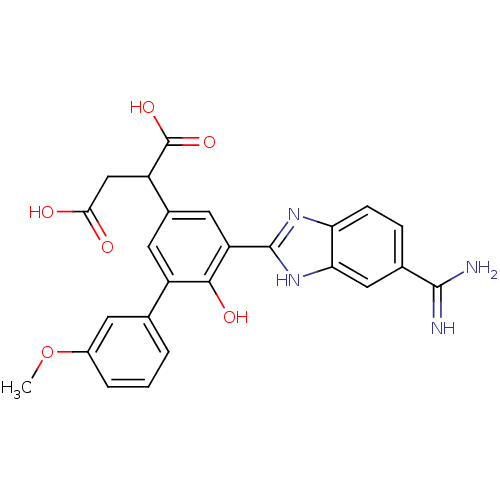
(2-[5-(5-carbamimidoyl-1H-benzoimidazol-2-yl)-6-hyd...)Show SMILES COc1cccc(c1)-c1cc(cc(-c2nc3ccc(cc3[nH]2)C(N)=N)c1O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C25H22N4O6/c1-35-15-4-2-3-12(7-15)16-8-14(17(25(33)34)11-21(30)31)9-18(22(16)32)24-28-19-6-5-13(23(26)27)10-20(19)29-24/h2-10,17,32H,11H2,1H3,(H3,26,27)(H,28,29)(H,30,31)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to plasma kallikrein |
Bioorg Med Chem Lett 16: 2034-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.060
BindingDB Entry DOI: 10.7270/Q2P55N3N |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM14939
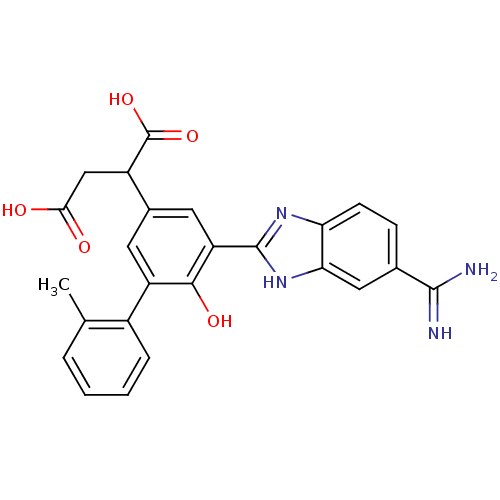
(2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-4-h...)Show SMILES Cc1ccccc1-c1cc(cc(-c2nc3ccc(cc3[nH]2)C(N)=N)c1O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C25H22N4O5/c1-12-4-2-3-5-15(12)17-8-14(16(25(33)34)11-21(30)31)9-18(22(17)32)24-28-19-7-6-13(23(26)27)10-20(19)29-24/h2-10,16,32H,11H2,1H3,(H3,26,27)(H,28,29)(H,30,31)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to plasma kallikrein |
Bioorg Med Chem Lett 16: 2034-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.060
BindingDB Entry DOI: 10.7270/Q2P55N3N |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50176256

(2-((5,6-difluoro-1H-benzo[d]imidazol-2-yl)methyl)-...)Show SMILES NC(=N)c1ccc2nc(Cc3nc4cc(F)c(F)cc4[nH]3)[nH]c2c1 Show InChI InChI=1S/C16H12F2N6/c17-8-4-12-13(5-9(8)18)24-15(23-12)6-14-21-10-2-1-7(16(19)20)3-11(10)22-14/h1-5H,6H2,(H3,19,20)(H,21,22)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to FXa in presence of Zn2+ |
Bioorg Med Chem Lett 16: 710-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.023
BindingDB Entry DOI: 10.7270/Q2VM4BSJ |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM14927
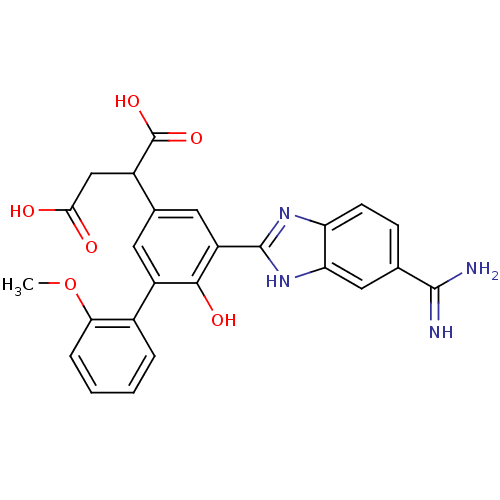
(2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-4-h...)Show SMILES COc1ccccc1-c1cc(cc(-c2nc3ccc(cc3[nH]2)C(N)=N)c1O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C25H22N4O6/c1-35-20-5-3-2-4-14(20)16-8-13(15(25(33)34)11-21(30)31)9-17(22(16)32)24-28-18-7-6-12(23(26)27)10-19(18)29-24/h2-10,15,32H,11H2,1H3,(H3,26,27)(H,28,29)(H,30,31)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to plasma kallikrein |
Bioorg Med Chem Lett 16: 2034-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.060
BindingDB Entry DOI: 10.7270/Q2P55N3N |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM14912
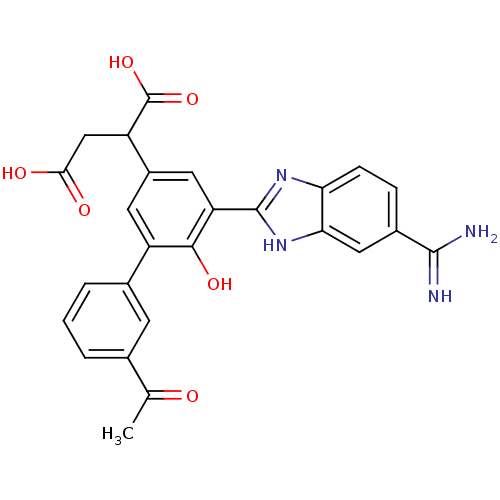
(2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-5-(...)Show SMILES CC(=O)c1cccc(c1)-c1cc(cc(-c2nc3ccc(cc3[nH]2)C(N)=N)c1O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C26H22N4O6/c1-12(31)13-3-2-4-14(7-13)17-8-16(18(26(35)36)11-22(32)33)9-19(23(17)34)25-29-20-6-5-15(24(27)28)10-21(20)30-25/h2-10,18,34H,11H2,1H3,(H3,27,28)(H,29,30)(H,32,33)(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to plasma kallikrein |
Bioorg Med Chem Lett 16: 2034-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.060
BindingDB Entry DOI: 10.7270/Q2P55N3N |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50176255
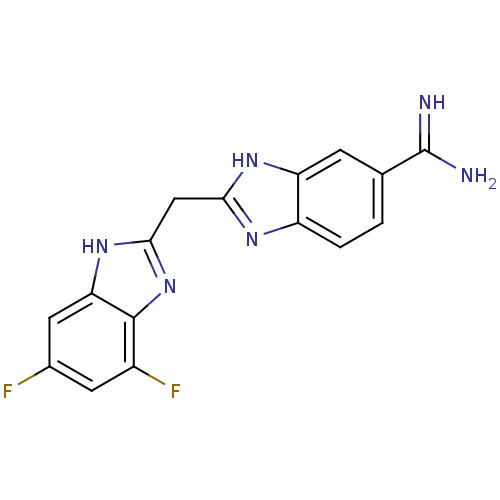
(2-((5,7-difluoro-1H-benzo[d]imidazol-2-yl)methyl)-...)Show SMILES NC(=N)c1ccc2nc(Cc3nc4c(F)cc(F)cc4[nH]3)[nH]c2c1 Show InChI InChI=1S/C16H12F2N6/c17-8-4-9(18)15-12(5-8)23-14(24-15)6-13-21-10-2-1-7(16(19)20)3-11(10)22-13/h1-5H,6H2,(H3,19,20)(H,21,22)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to FXa in presence of Zn2+ |
Bioorg Med Chem Lett 16: 710-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.023
BindingDB Entry DOI: 10.7270/Q2VM4BSJ |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14142
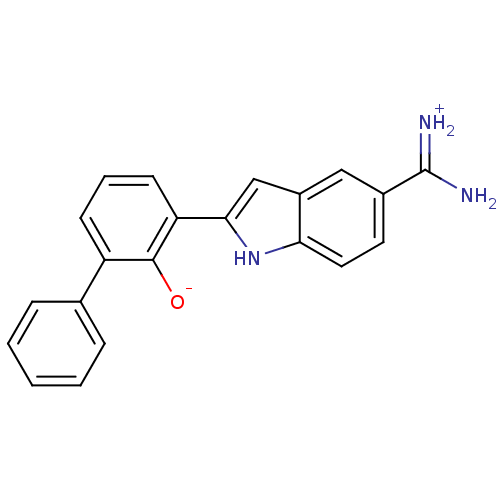
(2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-phe...)Show SMILES NC(=[NH2+])c1ccc2[nH]c(cc2c1)-c1cccc(-c2ccccc2)c1[O-] Show InChI InChI=1S/C21H17N3O/c22-21(23)14-9-10-18-15(11-14)12-19(24-18)17-8-4-7-16(20(17)25)13-5-2-1-3-6-13/h1-12,24-25H,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 8 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM13940
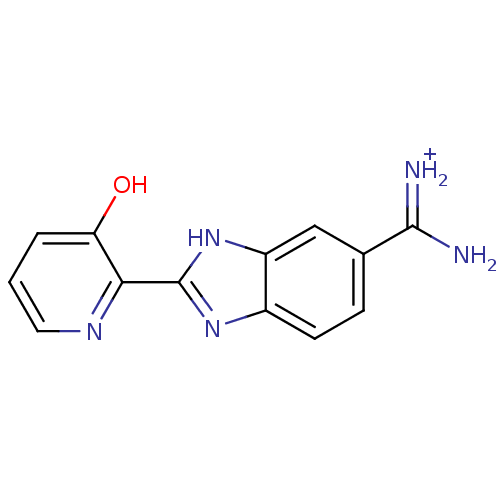
(2-(3-HYDROXY-PYRIDIN-2-YL)-1H-BENZOIMIDAZOLE-5-CAR...)Show InChI InChI=1S/C13H11N5O/c14-12(15)7-3-4-8-9(6-7)18-13(17-8)11-10(19)2-1-5-16-11/h1-6,19H,(H3,14,15)(H,17,18)/p+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Corporation
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 307: 1451-86 (2001)
Article DOI: 10.1006/jmbi.2001.4516
BindingDB Entry DOI: 10.7270/Q2FX77PG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM14897
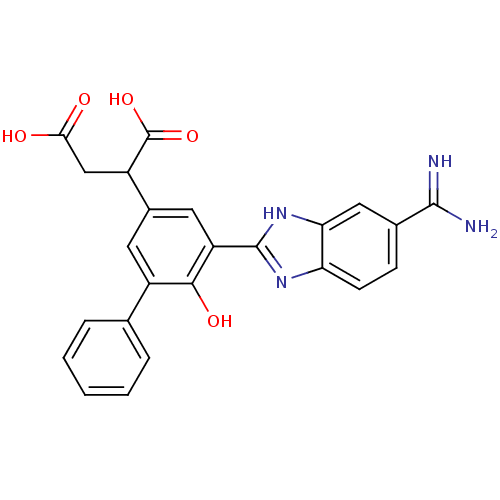
(2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-4-h...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(cc(-c2ccccc2)c1O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C24H20N4O5/c25-22(26)13-6-7-18-19(10-13)28-23(27-18)17-9-14(16(24(32)33)11-20(29)30)8-15(21(17)31)12-4-2-1-3-5-12/h1-10,16,31H,11H2,(H3,25,26)(H,27,28)(H,29,30)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to plasma kallikrein |
Bioorg Med Chem Lett 16: 2034-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.060
BindingDB Entry DOI: 10.7270/Q2P55N3N |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14152
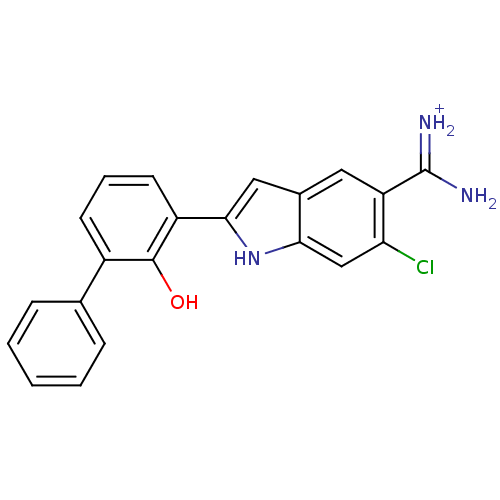
(6-CHLORO-2-(2-HYDROXY-BIPHENYL-3-YL)-1H-INDOLE-5-C...)Show SMILES NC(=[NH2+])c1cc2cc([nH]c2cc1Cl)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C21H16ClN3O/c22-17-11-18-13(9-16(17)21(23)24)10-19(25-18)15-8-4-7-14(20(15)26)12-5-2-1-3-6-12/h1-11,25-26H,(H3,23,24)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 9 | -45.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101869

(2-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...)Show SMILES CC(C)(C(O)=O)c1cc(Br)c(O)c(c1)-c1[nH]c2ccc(cc2c1Cc1ccccc1)C(N)=N Show InChI InChI=1S/C26H24BrN3O3/c1-26(2,25(32)33)16-12-19(23(31)20(27)13-16)22-18(10-14-6-4-3-5-7-14)17-11-15(24(28)29)8-9-21(17)30-22/h3-9,11-13,30-31H,10H2,1-2H3,(H3,28,29)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM14920
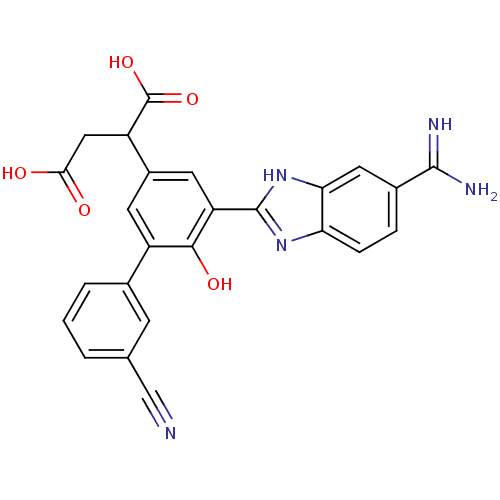
(2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-5-(...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(cc(-c2cccc(c2)C#N)c1O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C25H19N5O5/c26-11-12-2-1-3-13(6-12)16-7-15(17(25(34)35)10-21(31)32)8-18(22(16)33)24-29-19-5-4-14(23(27)28)9-20(19)30-24/h1-9,17,33H,10H2,(H3,27,28)(H,29,30)(H,31,32)(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to plasma kallikrein |
Bioorg Med Chem Lett 16: 2034-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.060
BindingDB Entry DOI: 10.7270/Q2P55N3N |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101867

(3-Benzyl-2-[3-bromo-2-hydroxy-5-(2H-tetrazol-5-yl)...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(cc(Br)c1O)-c1nnn[nH]1 Show InChI InChI=1S/C23H18BrN7O/c24-18-11-14(23-28-30-31-29-23)10-17(21(18)32)20-16(8-12-4-2-1-3-5-12)15-9-13(22(25)26)6-7-19(15)27-20/h1-7,9-11,27,32H,8H2,(H3,25,26)(H,28,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM14142

(2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-phe...)Show SMILES NC(=[NH2+])c1ccc2[nH]c(cc2c1)-c1cccc(-c2ccccc2)c1[O-] Show InChI InChI=1S/C21H17N3O/c22-21(23)14-9-10-18-15(11-14)12-19(24-18)17-8-4-7-16(20(17)25)13-5-2-1-3-6-13/h1-12,24-25H,(H3,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 11 | -45.0 | n/a | n/a | n/a | n/a | n/a | 7.45 | 22 |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
J Mol Biol 329: 93-120 (2003)
Article DOI: 10.1016/s0022-2836(03)00399-1
BindingDB Entry DOI: 10.7270/Q2R78CGQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14149
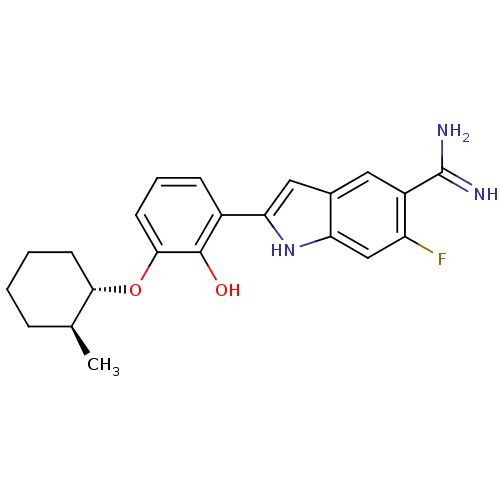
(6-fluoro-2-(2-hydroxy-3-{[(1S,2S)-2-methylcyclohex...)Show SMILES C[C@H]1CCCC[C@@H]1Oc1cccc(-c2cc3cc(C(N)=N)c(F)cc3[nH]2)c1O |r| Show InChI InChI=1S/C22H24FN3O2/c1-12-5-2-3-7-19(12)28-20-8-4-6-14(21(20)27)18-10-13-9-15(22(24)25)16(23)11-17(13)26-18/h4,6,8-12,19,26-27H,2-3,5,7H2,1H3,(H3,24,25)/t12-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 11 | -45.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50180403

(2-[5-(5-carbamimidoyl-1H-benzoimidazol-2-yl)-5'-ca...)Show SMILES NC(=O)Cc1ccc(O)c(c1)-c1cc(cc(-c2nc3ccc(cc3[nH]2)C(N)=N)c1O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C26H23N5O7/c27-21(33)6-11-1-4-20(32)15(5-11)16-7-13(14(26(37)38)10-22(34)35)8-17(23(16)36)25-30-18-3-2-12(24(28)29)9-19(18)31-25/h1-5,7-9,14,32,36H,6,10H2,(H2,27,33)(H3,28,29)(H,30,31)(H,34,35)(H,37,38) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to F7a/TF complex |
Bioorg Med Chem Lett 16: 2037-41 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.059
BindingDB Entry DOI: 10.7270/Q2639PBP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data