 Found 1708 hits with Last Name = 'fairlie' and Initial = 'd'
Found 1708 hits with Last Name = 'fairlie' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM578

((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...)Show SMILES CC(C)[C@H](N1CCCNC1=O)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)COc1c(C)cccc1C)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N4O5/c1-25(2)34(41-20-12-19-38-37(41)45)36(44)39-30(21-28-15-7-5-8-16-28)23-32(42)31(22-29-17-9-6-10-18-29)40-33(43)24-46-35-26(3)13-11-14-27(35)4/h5-11,13-18,25,30-32,34,42H,12,19-24H2,1-4H3,(H,38,45)(H,39,44)(H,40,43)/t30-,31-,32-,34-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against ritonavir-resistant strains. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against human cathepsin D |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036477

(2,6-Dichloro-3-(2-morpholin-4-yl-ethoxy)-benzoic a...)Show SMILES COc1cc2c(C(=O)N(COC(=O)c3c(Cl)ccc(OCCN4CCOCC4)c3Cl)S2(=O)=O)c(c1)C(C)C Show InChI InChI=1S/C25H28Cl2N2O8S/c1-15(2)17-12-16(34-3)13-20-21(17)24(30)29(38(20,32)33)14-37-25(31)22-18(26)4-5-19(23(22)27)36-11-8-28-6-9-35-10-7-28/h4-5,12-13,15H,6-11,14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against Elastase. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM729

((2S,4R)-N-tert-butyl-1-[(2R,3S)-2-hydroxy-3-[(2S)-...)Show SMILES CC(C)[C@H](NC(=O)c1ccc2ccccc2n1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN1CC[C@H](C[C@H]1C(=O)NC(C)(C)C)OCc1ccncc1 |r| Show InChI InChI=1S/C41H52N6O5/c1-27(2)37(45-38(49)33-16-15-30-13-9-10-14-32(30)43-33)40(51)44-34(23-28-11-7-6-8-12-28)36(48)25-47-22-19-31(52-26-29-17-20-42-21-18-29)24-35(47)39(50)46-41(3,4)5/h6-18,20-21,27,31,34-37,48H,19,22-26H2,1-5H3,(H,44,51)(H,45,49)(H,46,50)/t31-,34+,35+,36-,37+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against HIV-1 protease . |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-13 (MMP-13). |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50057826

((S)-1-((R)-2-Amino-3,3-dicyclohexyl-propionyl)-pyr...)Show SMILES N[C@H](C(C1CCCCC1)C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)NCC1CCC(N)CC1 |wU:21.24,wD:1.0,(8.57,-5.29,;9.89,-4.53,;9.89,-2.99,;11.24,-2.22,;12.55,-2.99,;13.9,-2.22,;13.9,-.68,;12.58,.09,;11.23,-.68,;8.57,-2.21,;7.23,-2.98,;5.9,-2.21,;5.9,-.67,;7.23,.1,;8.57,-.67,;11.24,-5.3,;11.24,-6.84,;12.72,-4.89,;13.26,-3.44,;14.8,-3.51,;15.22,-5,;13.92,-5.85,;13.86,-7.39,;12.49,-8.1,;15.15,-8.21,;16.52,-7.51,;17.82,-8.32,;17.78,-9.66,;18.86,-11.05,;17.78,-12.17,;18.86,-13.25,;17.85,-10.92,;16.76,-9.44,)| Show InChI InChI=1S/C27H48N4O2/c28-22-15-13-19(14-16-22)18-30-26(32)23-12-7-17-31(23)27(33)25(29)24(20-8-3-1-4-9-20)21-10-5-2-6-11-21/h19-25H,1-18,28-29H2,(H,30,32)/t19?,22?,23-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against Thrombin. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-2 (MMP-2). |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Bcl-2-related protein A1
(Homo sapiens (Human)) | BDBM50210070
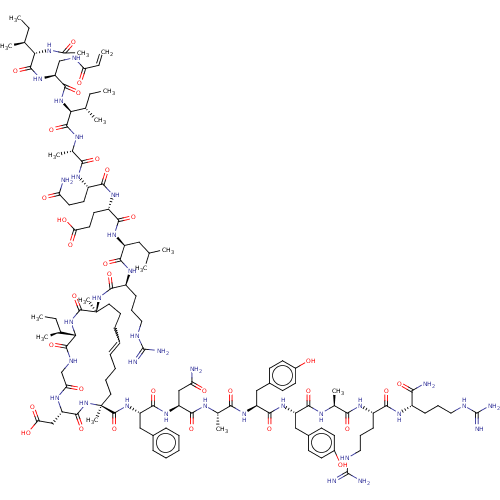
(CHEMBL3883565)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@@H](CNC(=O)C=C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@]1(C)CCCC=CCCC[C@](C)(NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](NC1=O)[C@@H](C)CC)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| Show InChI InChI=1S/C120H186N34O31/c1-16-63(7)93-111(181)134-61-90(161)139-85(59-92(164)165)110(180)154-119(14,114(184)150-83(55-70-32-25-24-26-33-70)107(177)148-84(58-88(122)159)104(174)136-67(11)99(169)145-82(57-72-39-43-74(157)44-40-72)106(176)147-81(56-71-37-41-73(156)42-38-71)103(173)135-66(10)97(167)141-76(35-30-52-131-117(126)127)100(170)140-75(96(123)166)34-29-51-130-116(124)125)49-27-22-20-21-23-28-50-120(15,115(185)152-93)153-109(179)77(36-31-53-132-118(128)129)143-105(175)80(54-62(5)6)146-102(172)79(46-48-91(162)163)144-101(171)78(45-47-87(121)158)142-98(168)68(12)137-112(182)95(65(9)18-3)151-108(178)86(60-133-89(160)19-4)149-113(183)94(64(8)17-2)138-69(13)155/h19-21,24-26,32-33,37-44,62-68,75-86,93-95,156-157H,4,16-18,22-23,27-31,34-36,45-61H2,1-3,5-15H3,(H2,121,158)(H2,122,159)(H2,123,166)(H,133,160)(H,134,181)(H,135,173)(H,136,174)(H,137,182)(H,138,155)(H,139,161)(H,140,170)(H,141,167)(H,142,168)(H,143,175)(H,144,171)(H,145,169)(H,146,172)(H,147,176)(H,148,177)(H,149,183)(H,150,184)(H,151,178)(H,152,185)(H,153,179)(H,154,180)(H,162,163)(H,164,165)(H4,124,125,130)(H4,126,127,131)(H4,128,129,132)/t63-,64-,65-,66-,67-,68-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,93-,94-,95-,119-,120-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of FITC-betaA-DIIRNIARHLAQVGDSMRSI-NH2 binding to recombinant human Bcl2A1 (1 to 152 residues) BH3 binding site expressed in Escherichia c... |
ACS Med Chem Lett 8: 22-26 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00395
BindingDB Entry DOI: 10.7270/Q2NS0WWV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50084664

(({3-(5-Carbamimidoyl-2-hydroxy-phenoxy)-2,6-difluo...)Show SMILES CN(CC(O)=O)c1c(F)c(Oc2cccc(c2)C2=NCCN2C)cc(Oc2cc(ccc2O)C(N)=N)c1F |t:18| Show InChI InChI=1S/C26H25F2N5O5/c1-32-9-8-31-26(32)15-4-3-5-16(10-15)37-19-12-20(23(28)24(22(19)27)33(2)13-21(35)36)38-18-11-14(25(29)30)6-7-17(18)34/h3-7,10-12,34H,8-9,13H2,1-2H3,(H3,29,30)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against Coagulation factor X |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM50231952
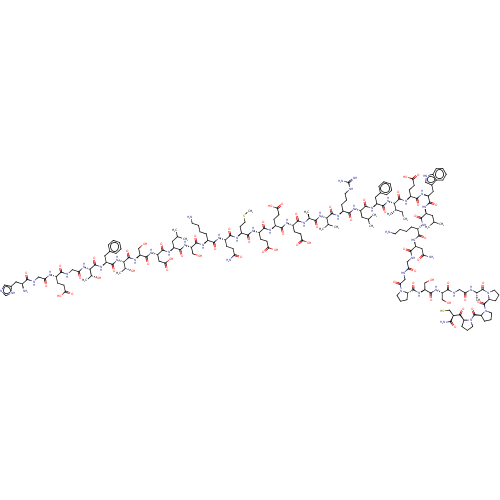
(CHEMBL4081554)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H](N)Cc1cnc[nH]1)[C@@H](C)O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)NCC(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)[C@H](CS)C(N)=O |r| Show InChI InChI=1S/C184H281N49O59S2/c1-16-94(10)147(178(287)213-115(52-58-144(255)256)164(273)218-122(73-101-77-195-106-39-24-23-38-103(101)106)169(278)215-117(68-90(2)3)166(275)205-108(41-26-28-61-186)159(268)219-123(75-134(189)241)155(264)198-79-135(242)196-83-139(246)230-63-31-44-130(230)176(285)224-127(86-236)175(284)222-125(84-234)156(265)200-80-136(243)202-96(12)181(290)232-65-32-45-131(232)183(292)233-66-33-46-132(233)182(291)231-64-30-43-129(231)150(259)104(88-293)151(190)260)228-171(280)120(71-99-34-19-17-20-35-99)217-167(276)118(69-91(4)5)214-160(269)109(42-29-62-194-184(191)192)212-177(286)146(93(8)9)227-152(261)95(11)203-157(266)112(49-55-141(249)250)208-162(271)113(50-56-142(251)252)209-163(272)114(51-57-143(253)254)210-165(274)116(59-67-294-15)211-161(270)111(47-53-133(188)240)207-158(267)107(40-25-27-60-185)206-173(282)126(85-235)223-168(277)119(70-92(6)7)216-170(279)124(76-145(257)258)220-174(283)128(87-237)225-180(289)149(98(14)239)229-172(281)121(72-100-36-21-18-22-37-100)221-179(288)148(97(13)238)226-138(245)82-199-154(263)110(48-54-140(247)248)204-137(244)81-197-153(262)105(187)74-102-78-193-89-201-102/h17-24,34-39,77-78,89-98,104-105,107-132,146-149,195,234-239,293H,16,25-33,40-76,79-88,185-187H2,1-15H3,(H2,188,240)(H2,189,241)(H2,190,260)(H,193,201)(H,196,242)(H,197,262)(H,198,264)(H,199,263)(H,200,265)(H,202,243)(H,203,266)(H,204,244)(H,205,275)(H,206,282)(H,207,267)(H,208,271)(H,209,272)(H,210,274)(H,211,270)(H,212,286)(H,213,287)(H,214,269)(H,215,278)(H,216,279)(H,217,276)(H,218,273)(H,219,268)(H,220,283)(H,221,288)(H,222,284)(H,223,277)(H,224,285)(H,225,289)(H,226,245)(H,227,261)(H,228,280)(H,229,281)(H,247,248)(H,249,250)(H,251,252)(H,253,254)(H,255,256)(H,257,258)(H4,191,192,194)/t94-,95-,96-,97+,98+,104-,105-,107-,108-,109-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,146-,147-,148-,149-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]-GLP (7 to 36 residues) from human GLP1R expressed in CHO cell membranes incubated for 30 mins measured after 10 hrs by scintil... |
Eur J Med Chem 127: 703-714 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.044
BindingDB Entry DOI: 10.7270/Q2T155W1 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM729

((2S,4R)-N-tert-butyl-1-[(2R,3S)-2-hydroxy-3-[(2S)-...)Show SMILES CC(C)[C@H](NC(=O)c1ccc2ccccc2n1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN1CC[C@H](C[C@H]1C(=O)NC(C)(C)C)OCc1ccncc1 |r| Show InChI InChI=1S/C41H52N6O5/c1-27(2)37(45-38(49)33-16-15-30-13-9-10-14-32(30)43-33)40(51)44-34(23-28-11-7-6-8-12-28)36(48)25-47-22-19-31(52-26-29-17-20-42-21-18-29)24-35(47)39(50)46-41(3,4)5/h6-18,20-21,27,31,34-37,48H,19,22-26H2,1-5H3,(H,44,51)(H,45,49)(H,46,50)/t31-,34+,35+,36-,37+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against HIV-1 protease . |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50084682

(1-[3-(4-Carbamimidoyl-phenyl)-2-(2-methyl-1,2,3,4-...)Show SMILES Cc1cc(ccc1C(=O)N[C@@H](CCCNC(N)=N)B1O[C@@H]2CC3CC(C3(C)C)[C@]2(C)O1)-c1ccccc1S(=O)(=O)NC(C)(C)C |TLB:30:28:25:23| Show InChI InChI=1S/C33H48BN5O5S/c1-20-17-21(24-11-8-9-12-25(24)45(41,42)39-31(2,3)4)14-15-23(20)29(40)38-28(13-10-16-37-30(35)36)34-43-27-19-22-18-26(32(22,5)6)33(27,7)44-34/h8-9,11-12,14-15,17,22,26-28,39H,10,13,16,18-19H2,1-7H3,(H,38,40)(H4,35,36,37)/t22?,26?,27-,28+,33+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against Thrombin. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM11863

(4-({[4-(4-chlorophenoxy)benzene]sulfonyl}methyl)-N...)Show SMILES ONC(=O)C1(CS(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)CCOCC1 Show InChI InChI=1S/C19H20ClNO6S/c20-14-1-3-15(4-2-14)27-16-5-7-17(8-6-16)28(24,25)13-19(18(22)21-23)9-11-26-12-10-19/h1-8,23H,9-13H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-2 (MMP-2). |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM50231900
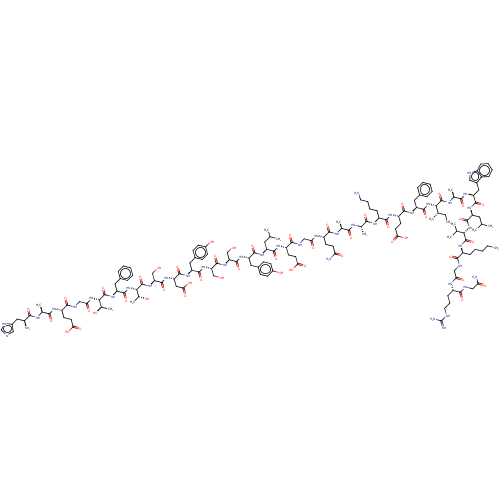
(CHEMBL4060480)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)Cc1cnc[nH]1)[C@@H](C)O)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C155H229N41O47/c1-15-79(8)125(152(241)174-83(12)131(220)182-110(63-90-66-165-96-34-23-22-33-94(90)96)143(232)184-105(58-77(4)5)145(234)194-124(78(6)7)151(240)181-98(35-24-26-54-156)134(223)167-69-117(206)175-97(37-28-56-164-155(161)162)133(222)166-68-116(160)205)195-146(235)108(59-86-29-18-16-19-30-86)185-139(228)103(49-53-122(213)214)180-138(227)99(36-25-27-55-157)177-129(218)81(10)171-128(217)80(9)173-137(226)102(46-50-115(159)204)176-118(207)70-168-136(225)101(48-52-121(211)212)179-140(229)104(57-76(2)3)183-141(230)106(61-88-38-42-92(202)43-39-88)187-148(237)112(72-197)191-150(239)113(73-198)190-142(231)107(62-89-40-44-93(203)45-41-89)186-144(233)111(65-123(215)216)188-149(238)114(74-199)192-154(243)127(85(14)201)196-147(236)109(60-87-31-20-17-21-32-87)189-153(242)126(84(13)200)193-119(208)71-169-135(224)100(47-51-120(209)210)178-130(219)82(11)172-132(221)95(158)64-91-67-163-75-170-91/h16-23,29-34,38-45,66-67,75-85,95,97-114,124-127,165,197-203H,15,24-28,35-37,46-65,68-74,156-158H2,1-14H3,(H2,159,204)(H2,160,205)(H,163,170)(H,166,222)(H,167,223)(H,168,225)(H,169,224)(H,171,217)(H,172,221)(H,173,226)(H,174,241)(H,175,206)(H,176,207)(H,177,218)(H,178,219)(H,179,229)(H,180,227)(H,181,240)(H,182,220)(H,183,230)(H,184,232)(H,185,228)(H,186,233)(H,187,237)(H,188,238)(H,189,242)(H,190,231)(H,191,239)(H,192,243)(H,193,208)(H,194,234)(H,195,235)(H,196,236)(H,209,210)(H,211,212)(H,213,214)(H,215,216)(H4,161,162,164)/t79-,80-,81-,82-,83-,84+,85+,95-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,112-,113-,114-,124-,125-,126-,127-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity at human GLP1R expressed in CHO cells assessed as cAMP accumulation incubated for 30 mins by LANCE assay |
Eur J Med Chem 127: 703-714 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.044
BindingDB Entry DOI: 10.7270/Q2T155W1 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-3 (MMP-3). |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50369584
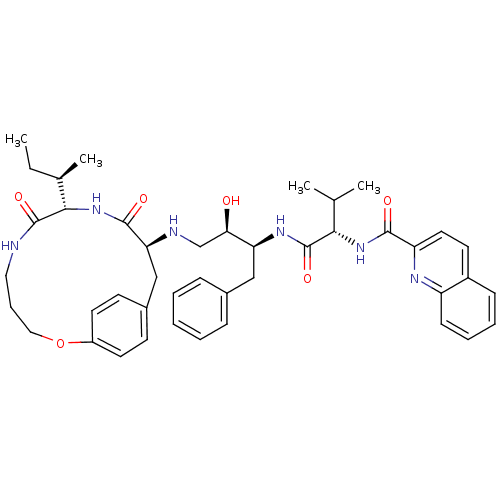
(CHEMBL1790230)Show SMILES CC[C@@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(OCCCNC1=O)cc2)NC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)c1ccc2ccccc2n1)C(C)C Show InChI InChI=1S/C43H54N6O6/c1-5-28(4)39-42(53)44-22-11-23-55-32-19-16-30(17-20-32)25-36(41(52)49-39)45-26-37(50)35(24-29-12-7-6-8-13-29)47-43(54)38(27(2)3)48-40(51)34-21-18-31-14-9-10-15-33(31)46-34/h6-10,12-21,27-28,35-39,45,50H,5,11,22-26H2,1-4H3,(H,44,53)(H,47,54)(H,48,51)(H,49,52)/t28-,35+,36+,37-,38+,39+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 protease |
J Med Chem 43: 3495-504 (2000)
BindingDB Entry DOI: 10.7270/Q2KH0P1S |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50037991

(CHEMBL325166 | NAPSAGATRAN | Ro-46-6240 | {[(S)-3-...)Show SMILES NC(=N)N1CCC[C@@H](CNC(=O)C[C@H](NS(=O)(=O)c2ccc3ccccc3c2)C(=O)N(CC(O)=O)C2CC2)C1 Show InChI InChI=1S/C26H34N6O6S/c27-26(28)31-11-3-4-17(15-31)14-29-23(33)13-22(25(36)32(16-24(34)35)20-8-9-20)30-39(37,38)21-10-7-18-5-1-2-6-19(18)12-21/h1-2,5-7,10,12,17,20,22,30H,3-4,8-9,11,13-16H2,(H3,27,28)(H,29,33)(H,34,35)/t17-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against Thrombin. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50369584

(CHEMBL1790230)Show SMILES CC[C@@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(OCCCNC1=O)cc2)NC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)c1ccc2ccccc2n1)C(C)C Show InChI InChI=1S/C43H54N6O6/c1-5-28(4)39-42(53)44-22-11-23-55-32-19-16-30(17-20-32)25-36(41(52)49-39)45-26-37(50)35(24-29-12-7-6-8-13-29)47-43(54)38(27(2)3)48-40(51)34-21-18-31-14-9-10-15-33(31)46-34/h6-10,12-21,27-28,35-39,45,50H,5,11,22-26H2,1-4H3,(H,44,53)(H,47,54)(H,48,51)(H,49,52)/t28-,35+,36+,37-,38+,39+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Tested for inhibition of synthetic HIV-1 protease by using fluorometric assay |
J Med Chem 47: 1641-51 (2004)
Article DOI: 10.1021/jm030337m
BindingDB Entry DOI: 10.7270/Q2JD4XJK |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [491-589,Q7K,L33I,C67B,C95B]
(Human immunodeficiency virus type 1) | BDBM798
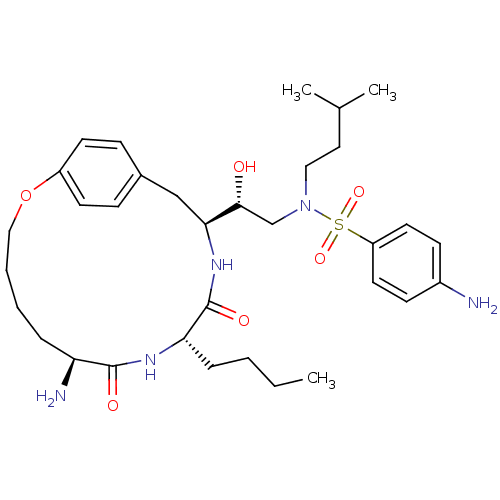
((2R)-2-[(7S,10S,13S)-7-amino-10-butyl-8,11-dioxo-2...)Show SMILES CCCC[C@@H]1NC(=O)[C@@H](N)CCCCOc2ccc(C[C@H](NC1=O)[C@H](O)CN(CCC(C)C)S(=O)(=O)c1ccc(N)cc1)cc2 |r| Show InChI InChI=1S/C33H51N5O6S/c1-4-5-9-29-33(41)37-30(21-24-10-14-26(15-11-24)44-20-7-6-8-28(35)32(40)36-29)31(39)22-38(19-18-23(2)3)45(42,43)27-16-12-25(34)13-17-27/h10-17,23,28-31,39H,4-9,18-22,34-35H2,1-3H3,(H,36,40)(H,37,41)/t28-,29-,30-,31+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | -56.5 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Queensland
| Assay Description
Inhibition constants were determined by a fluorometric assay. Ki values were calculated from either Dixon plots or Henderson plots in cases where the... |
J Med Chem 45: 371-81 (2002)
Article DOI: 10.1021/jm010414i
BindingDB Entry DOI: 10.7270/Q2FQ9TSJ |
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM50261506
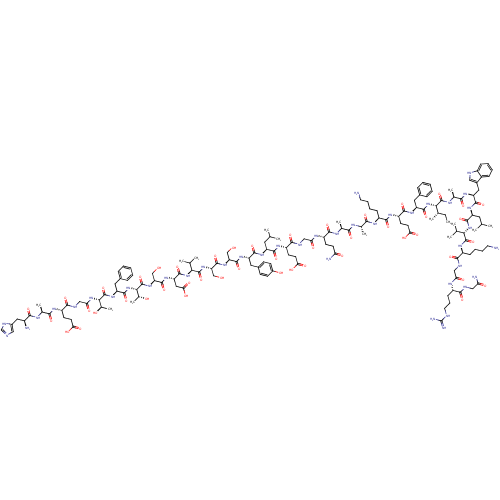
(CHEMBL499930 | HAEGTFTSDVSSYLEGQAAKEFIAWLVKGRG-NH2)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)Cc1cnc[nH]1)[C@@H](C)O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C151H229N41O46/c1-17-77(10)121(148(236)170-81(14)127(215)178-105(60-87-63-161-92-36-25-24-35-90(87)92)138(226)180-101(56-74(4)5)139(227)189-119(75(6)7)146(234)177-94(37-26-28-52-152)130(218)163-66-112(201)171-93(39-30-54-160-151(157)158)129(217)162-65-111(156)200)191-140(228)103(57-84-31-20-18-21-32-84)181-135(223)99(47-51-117(208)209)176-134(222)95(38-27-29-53-153)173-125(213)79(12)167-124(212)78(11)169-133(221)98(44-48-110(155)199)172-113(202)67-164-132(220)97(46-50-116(206)207)175-136(224)100(55-73(2)3)179-137(225)102(59-86-40-42-89(198)43-41-86)182-143(231)107(69-193)185-145(233)109(71-195)186-147(235)120(76(8)9)190-142(230)106(62-118(210)211)183-144(232)108(70-194)187-150(238)123(83(16)197)192-141(229)104(58-85-33-22-19-23-34-85)184-149(237)122(82(15)196)188-114(203)68-165-131(219)96(45-49-115(204)205)174-126(214)80(13)168-128(216)91(154)61-88-64-159-72-166-88/h18-25,31-36,40-43,63-64,72-83,91,93-109,119-123,161,193-198H,17,26-30,37-39,44-62,65-71,152-154H2,1-16H3,(H2,155,199)(H2,156,200)(H,159,166)(H,162,217)(H,163,218)(H,164,220)(H,165,219)(H,167,212)(H,168,216)(H,169,221)(H,170,236)(H,171,201)(H,172,202)(H,173,213)(H,174,214)(H,175,224)(H,176,222)(H,177,234)(H,178,215)(H,179,225)(H,180,226)(H,181,223)(H,182,231)(H,183,232)(H,184,237)(H,185,233)(H,186,235)(H,187,238)(H,188,203)(H,189,227)(H,190,230)(H,191,228)(H,192,229)(H,204,205)(H,206,207)(H,208,209)(H,210,211)(H4,157,158,160)/t77-,78-,79-,80-,81-,82+,83+,91-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,119-,120-,121-,122-,123-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity at human GLP1R expressed in CHO cells assessed as cAMP accumulation incubated for 30 mins by LANCE assay |
Eur J Med Chem 127: 703-714 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.044
BindingDB Entry DOI: 10.7270/Q2T155W1 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [491-589,Q7K,L33I,C67B,C95B]
(Human immunodeficiency virus type 1) | BDBM797
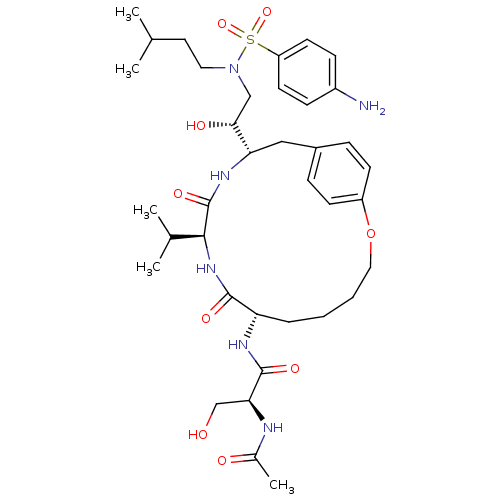
((2S)-N-[(7S,10S,13S)-13-[(1R)-2-[(4-aminobenzene)(...)Show SMILES CC(C)CCN(C[C@@H](O)[C@@H]1Cc2ccc(OCCCC[C@H](NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](C(C)C)C(=O)N1)cc2)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C37H56N6O9S/c1-23(2)17-18-43(53(50,51)29-15-11-27(38)12-16-29)21-33(46)31-20-26-9-13-28(14-10-26)52-19-7-6-8-30(40-36(48)32(22-44)39-25(5)45)35(47)42-34(24(3)4)37(49)41-31/h9-16,23-24,30-34,44,46H,6-8,17-22,38H2,1-5H3,(H,39,45)(H,40,48)(H,41,49)(H,42,47)/t30-,31-,32-,33+,34-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | -56.2 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Queensland
| Assay Description
Inhibition constants were determined by a fluorometric assay. Ki values were calculated from either Dixon plots or Henderson plots in cases where the... |
J Med Chem 45: 371-81 (2002)
Article DOI: 10.1021/jm010414i
BindingDB Entry DOI: 10.7270/Q2FQ9TSJ |
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM50231888
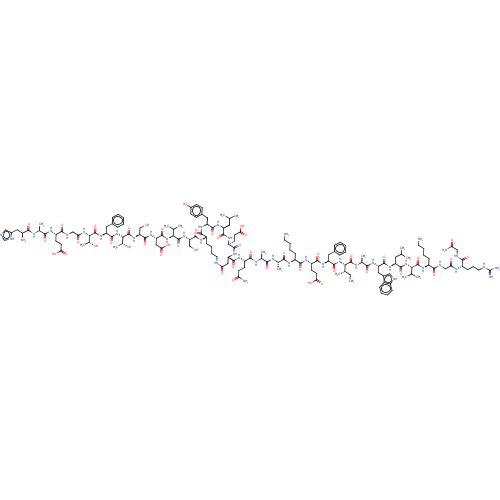
(CHEMBL4081357)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)Cc2cnc[nH]2)[C@@H](C)O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N1)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C156H236N42O46/c1-17-80(10)125(153(242)175-84(14)131(220)184-109(64-90-68-167-95-38-25-24-37-93(90)95)143(232)186-105(60-77(4)5)145(234)195-123(78(6)7)151(240)183-97(39-26-29-55-157)134(223)169-71-117(207)176-96(42-32-58-166-156(162)163)133(222)168-70-115(161)205)197-146(235)107(61-87-33-20-18-21-34-87)188-139(228)102(49-53-120(211)212)180-137(226)98(40-27-30-56-158)177-129(218)82(12)172-128(217)81(11)174-136(225)101(47-51-114(160)204)181-144(233)110-66-116(206)165-57-31-28-41-99(138(227)187-106(63-89-43-45-92(203)46-44-89)142(231)185-104(59-76(2)3)141(230)182-103(140(229)189-110)50-54-121(213)214)179-149(238)112(73-199)192-152(241)124(79(8)9)196-148(237)111(67-122(215)216)190-150(239)113(74-200)193-155(244)127(86(16)202)198-147(236)108(62-88-35-22-19-23-36-88)191-154(243)126(85(15)201)194-118(208)72-170-135(224)100(48-52-119(209)210)178-130(219)83(13)173-132(221)94(159)65-91-69-164-75-171-91/h18-25,33-38,43-46,68-69,75-86,94,96-113,123-127,167,199-203H,17,26-32,39-42,47-67,70-74,157-159H2,1-16H3,(H2,160,204)(H2,161,205)(H,164,171)(H,165,206)(H,168,222)(H,169,223)(H,170,224)(H,172,217)(H,173,221)(H,174,225)(H,175,242)(H,176,207)(H,177,218)(H,178,219)(H,179,238)(H,180,226)(H,181,233)(H,182,230)(H,183,240)(H,184,220)(H,185,231)(H,186,232)(H,187,227)(H,188,228)(H,189,229)(H,190,239)(H,191,243)(H,192,241)(H,193,244)(H,194,208)(H,195,234)(H,196,237)(H,197,235)(H,198,236)(H,209,210)(H,211,212)(H,213,214)(H,215,216)(H4,162,163,166)/t80-,81-,82-,83-,84-,85+,86+,94-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,112-,113-,123-,124-,125-,126-,127-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]-GLP (7 to 36 residues) from human GLP1R expressed in CHO cell membranes incubated for 30 mins measured after 10 hrs by scintil... |
Eur J Med Chem 127: 703-714 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.044
BindingDB Entry DOI: 10.7270/Q2T155W1 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50068972
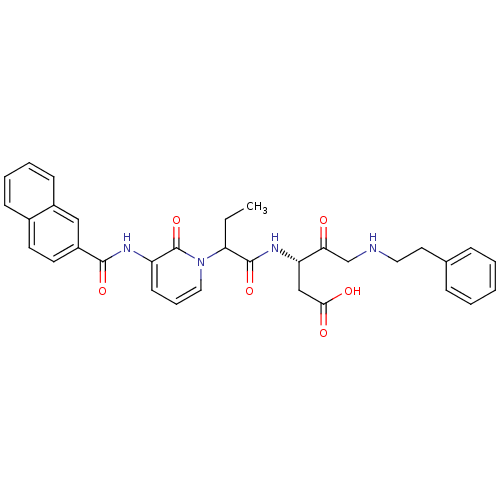
((S)-3-(2-{3-[(Naphthalene-2-carbonyl)-amino]-2-oxo...)Show SMILES CCC(C(=O)N[C@@H](CC(O)=O)C(=O)CNCCc1ccccc1)n1cccc(NC(=O)c2ccc3ccccc3c2)c1=O Show InChI InChI=1S/C33H34N4O6/c1-2-28(32(42)36-27(20-30(39)40)29(38)21-34-17-16-22-9-4-3-5-10-22)37-18-8-13-26(33(37)43)35-31(41)25-15-14-23-11-6-7-12-24(23)19-25/h3-15,18-19,27-28,34H,2,16-17,20-21H2,1H3,(H,35,41)(H,36,42)(H,39,40)/t27-,28?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
The binding affinity against IL-1 beta converting enzyme |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50142990
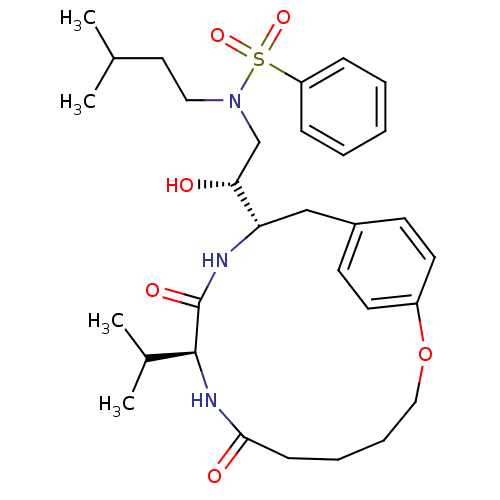
(CHEMBL288836 | N-[(R)-2-Hydroxy-2-((9S,12S)-9-isop...)Show SMILES CC(C)CCN(C[C@@H](O)[C@@H]1Cc2ccc(OCCCCC(=O)N[C@@H](C(C)C)C(=O)N1)cc2)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C31H45N3O6S/c1-22(2)17-18-34(41(38,39)26-10-6-5-7-11-26)21-28(35)27-20-24-13-15-25(16-14-24)40-19-9-8-12-29(36)33-30(23(3)4)31(37)32-27/h5-7,10-11,13-16,22-23,27-28,30,35H,8-9,12,17-21H2,1-4H3,(H,32,37)(H,33,36)/t27-,28+,30-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Tested for inhibition of synthetic HIV-1 protease by using fluorometric assay |
J Med Chem 47: 1641-51 (2004)
Article DOI: 10.1021/jm030337m
BindingDB Entry DOI: 10.7270/Q2JD4XJK |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50058491

(3-{4-[2-(4-{1-[4-(2-Carboxy-2-methyl-propane-1-sul...)Show SMILES CC(C)(CS(=O)(=O)c1ccc(OC(=O)C(C)(C)c2ccc(cc2)C(C)(C)C(=O)Oc2ccc(cc2)S(=O)(=O)CC(C)(C)C(O)=O)cc1)C(O)=O Show InChI InChI=1S/C36H42O12S2/c1-33(2,29(37)38)21-49(43,44)27-17-13-25(14-18-27)47-31(41)35(5,6)23-9-11-24(12-10-23)36(7,8)32(42)48-26-15-19-28(20-16-26)50(45,46)22-34(3,4)30(39)40/h9-20H,21-22H2,1-8H3,(H,37,38)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against Elastase. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50084686
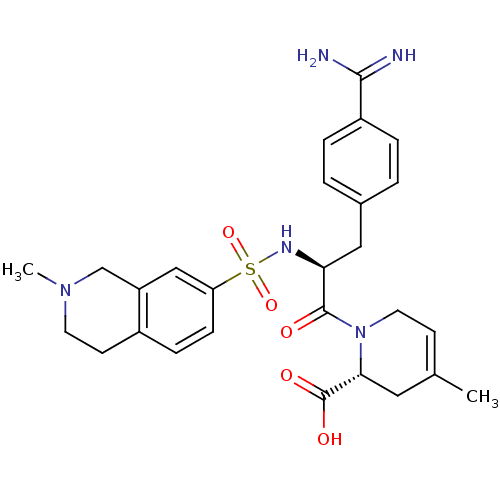
(1-[3-(4-Carbamimidoyl-phenyl)-2-(2-methyl-1,2,3,4-...)Show SMILES CN1CCc2ccc(cc2C1)S(=O)(=O)N[C@@H](Cc1ccc(cc1)C(N)=N)C(=O)N1CC=C(C)C[C@@H]1C(O)=O |t:33| Show InChI InChI=1S/C27H33N5O5S/c1-17-9-12-32(24(13-17)27(34)35)26(33)23(14-18-3-5-20(6-4-18)25(28)29)30-38(36,37)22-8-7-19-10-11-31(2)16-21(19)15-22/h3-9,15,23-24,30H,10-14,16H2,1-2H3,(H3,28,29)(H,34,35)/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against Trypsin |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM50231942

(CHEMBL4065403)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)Cc1cnc[nH]1)[C@@H](C)O)[C@@H](C)O)C(=O)N[C@H]1CCCCNC(=O)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(C)C)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C157H229N41O48/c1-15-79(8)126(154(244)182-99-36-25-27-55-165-118(208)65-111(136(226)170-70-119(209)176-97(37-28-56-166-157(162)163)134(224)168-69-117(161)207)191-153(243)125(78(6)7)196-147(237)105(58-77(4)5)184-145(235)110(188-140(99)230)63-90-67-167-96-34-23-22-33-94(90)96)197-148(238)108(59-86-29-18-16-19-30-86)185-141(231)103(49-53-123(215)216)180-139(229)98(35-24-26-54-158)177-130(220)81(10)172-129(219)80(9)174-137(227)101(46-50-116(160)206)179-132(222)83(12)175-138(228)102(48-52-122(213)214)181-142(232)104(57-76(2)3)183-143(233)106(61-88-38-42-92(204)43-39-88)187-150(240)113(72-199)193-152(242)114(73-200)192-144(234)107(62-89-40-44-93(205)45-41-89)186-146(236)112(66-124(217)218)189-151(241)115(74-201)194-156(246)128(85(14)203)198-149(239)109(60-87-31-20-17-21-32-87)190-155(245)127(84(13)202)195-120(210)71-169-135(225)100(47-51-121(211)212)178-131(221)82(11)173-133(223)95(159)64-91-68-164-75-171-91/h16-23,29-34,38-45,67-68,75-85,95,97-115,125-128,167,199-205H,15,24-28,35-37,46-66,69-74,158-159H2,1-14H3,(H2,160,206)(H2,161,207)(H,164,171)(H,165,208)(H,168,224)(H,169,225)(H,170,226)(H,172,219)(H,173,223)(H,174,227)(H,175,228)(H,176,209)(H,177,220)(H,178,221)(H,179,222)(H,180,229)(H,181,232)(H,182,244)(H,183,233)(H,184,235)(H,185,231)(H,186,236)(H,187,240)(H,188,230)(H,189,241)(H,190,245)(H,191,243)(H,192,234)(H,193,242)(H,194,246)(H,195,210)(H,196,237)(H,197,238)(H,198,239)(H,211,212)(H,213,214)(H,215,216)(H,217,218)(H4,162,163,166)/t79-,80-,81-,82-,83-,84+,85+,95-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,112-,113-,114-,115-,125-,126-,127-,128-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]-GLP (7 to 36 residues) from human GLP1R expressed in CHO cell membranes incubated for 30 mins measured after 10 hrs by scintil... |
Eur J Med Chem 127: 703-714 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.044
BindingDB Entry DOI: 10.7270/Q2T155W1 |
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM50231949
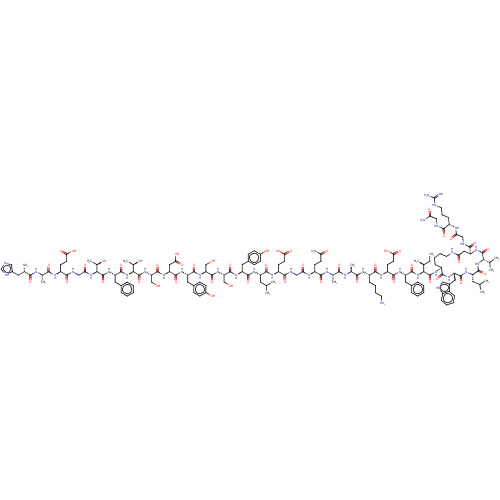
(CHEMBL4069162)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)Cc1cnc[nH]1)[C@@H](C)O)[C@@H](C)O)C(=O)N[C@H]1CCCCNC(=O)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(C)C)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C156H227N41O48/c1-14-79(8)126(153(243)181-98-35-24-26-54-164-117(207)64-110(136(226)170-70-118(208)175-96(36-27-55-165-156(161)162)133(223)167-68-116(160)206)190-152(242)125(78(6)7)195-146(236)104(57-77(4)5)183-144(234)109(187-139(98)229)62-89-66-166-95-33-22-21-32-93(89)95)196-147(237)107(58-85-28-17-15-18-29-85)184-140(230)102(48-52-123(215)216)180-138(228)97(34-23-25-53-157)177-130(220)81(10)172-129(219)80(9)174-137(227)101(45-49-115(159)205)176-119(209)69-168-135(225)100(47-51-122(213)214)179-141(231)103(56-76(2)3)182-142(232)105(60-87-37-41-91(203)42-38-87)186-149(239)112(72-198)192-151(241)113(73-199)191-143(233)106(61-88-39-43-92(204)44-40-88)185-145(235)111(65-124(217)218)188-150(240)114(74-200)193-155(245)128(84(13)202)197-148(238)108(59-86-30-19-16-20-31-86)189-154(244)127(83(12)201)194-120(210)71-169-134(224)99(46-50-121(211)212)178-131(221)82(11)173-132(222)94(158)63-90-67-163-75-171-90/h15-22,28-33,37-44,66-67,75-84,94,96-114,125-128,166,198-204H,14,23-27,34-36,45-65,68-74,157-158H2,1-13H3,(H2,159,205)(H2,160,206)(H,163,171)(H,164,207)(H,167,223)(H,168,225)(H,169,224)(H,170,226)(H,172,219)(H,173,222)(H,174,227)(H,175,208)(H,176,209)(H,177,220)(H,178,221)(H,179,231)(H,180,228)(H,181,243)(H,182,232)(H,183,234)(H,184,230)(H,185,235)(H,186,239)(H,187,229)(H,188,240)(H,189,244)(H,190,242)(H,191,233)(H,192,241)(H,193,245)(H,194,210)(H,195,236)(H,196,237)(H,197,238)(H,211,212)(H,213,214)(H,215,216)(H,217,218)(H4,161,162,165)/t79-,80-,81-,82-,83+,84+,94-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,112-,113-,114-,125-,126-,127-,128-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]-GLP (7 to 36 residues) from human GLP1R expressed in CHO cell membranes incubated for 30 mins measured after 10 hrs by scintil... |
Eur J Med Chem 127: 703-714 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.044
BindingDB Entry DOI: 10.7270/Q2T155W1 |
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM50231943
(CHEMBL4093072)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H](N)Cc1cnc[nH]1)[C@@H](C)O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)NCC(N)=O |r| Show InChI InChI=1S/C150H231N41O48S/c1-15-77(10)121(147(237)177-96(43-49-118(210)211)135(225)182-103(60-83-64-161-87-34-23-22-33-85(83)87)140(230)179-98(55-73(2)3)137(227)169-89(36-25-27-52-152)130(220)183-104(62-109(155)197)127(217)164-67-111(199)162-66-110(156)198)190-142(232)101(58-81-29-18-16-19-30-81)181-138(228)99(56-74(4)5)178-131(221)90(37-28-53-160-150(157)158)176-146(236)120(76(8)9)189-124(214)78(11)167-128(218)93(40-46-115(204)205)172-133(223)94(41-47-116(206)207)173-134(224)95(42-48-117(208)209)174-136(226)97(50-54-240-14)175-132(222)92(38-44-108(154)196)171-129(219)88(35-24-26-51-151)170-144(234)106(70-192)186-139(229)100(57-75(6)7)180-141(231)105(63-119(212)213)184-145(235)107(71-193)187-149(239)123(80(13)195)191-143(233)102(59-82-31-20-17-21-32-82)185-148(238)122(79(12)194)188-113(201)69-165-126(216)91(39-45-114(202)203)168-112(200)68-163-125(215)86(153)61-84-65-159-72-166-84/h16-23,29-34,64-65,72-80,86,88-107,120-123,161,192-195H,15,24-28,35-63,66-71,151-153H2,1-14H3,(H2,154,196)(H2,155,197)(H2,156,198)(H,159,166)(H,162,199)(H,163,215)(H,164,217)(H,165,216)(H,167,218)(H,168,200)(H,169,227)(H,170,234)(H,171,219)(H,172,223)(H,173,224)(H,174,226)(H,175,222)(H,176,236)(H,177,237)(H,178,221)(H,179,230)(H,180,231)(H,181,228)(H,182,225)(H,183,220)(H,184,235)(H,185,238)(H,186,229)(H,187,239)(H,188,201)(H,189,214)(H,190,232)(H,191,233)(H,202,203)(H,204,205)(H,206,207)(H,208,209)(H,210,211)(H,212,213)(H4,157,158,160)/t77-,78-,79+,80+,86-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,120-,121-,122-,123-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]-GLP (7 to 36 residues) from human GLP1R expressed in CHO cell membranes incubated for 30 mins measured after 10 hrs by scintil... |
Eur J Med Chem 127: 703-714 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.044
BindingDB Entry DOI: 10.7270/Q2T155W1 |
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM50231887
(CHEMBL4091638)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)Cc2cnc[nH]2)[C@@H](C)O)[C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C150H224N42O47/c1-13-75(6)120(147(237)170-79(10)126(216)179-103(59-85-63-161-90-34-21-20-33-88(85)90)137(227)180-99(55-73(2)3)140(230)190-119(74(4)5)146(236)178-92(35-22-25-51-151)129(219)163-66-112(202)171-91(38-28-54-160-150(156)157)128(218)162-65-110(155)200)191-141(231)101(56-82-29-16-14-17-30-82)181-135(225)98(46-50-117(209)210)177-133(223)93(36-23-26-52-152)173-124(214)77(8)167-123(213)76(7)169-132(222)97(43-47-109(154)199)172-113(203)67-164-131(221)96(45-49-116(207)208)176-138(228)104-61-111(201)159-53-27-24-37-94(134(224)186-107(70-194)145(235)187-106(69-193)143(233)182-100(136(226)183-104)58-84-39-41-87(198)42-40-84)175-139(229)105(62-118(211)212)184-144(234)108(71-195)188-149(239)122(81(12)197)192-142(232)102(57-83-31-18-15-19-32-83)185-148(238)121(80(11)196)189-114(204)68-165-130(220)95(44-48-115(205)206)174-125(215)78(9)168-127(217)89(153)60-86-64-158-72-166-86/h14-21,29-34,39-42,63-64,72-81,89,91-108,119-122,161,193-198H,13,22-28,35-38,43-62,65-71,151-153H2,1-12H3,(H2,154,199)(H2,155,200)(H,158,166)(H,159,201)(H,162,218)(H,163,219)(H,164,221)(H,165,220)(H,167,213)(H,168,217)(H,169,222)(H,170,237)(H,171,202)(H,172,203)(H,173,214)(H,174,215)(H,175,229)(H,176,228)(H,177,223)(H,178,236)(H,179,216)(H,180,227)(H,181,225)(H,182,233)(H,183,226)(H,184,234)(H,185,238)(H,186,224)(H,187,235)(H,188,239)(H,189,204)(H,190,230)(H,191,231)(H,192,232)(H,205,206)(H,207,208)(H,209,210)(H,211,212)(H4,156,157,160)/t75-,76-,77-,78-,79-,80+,81+,89-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,119-,120-,121-,122-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]-GLP (7 to 36 residues) from human GLP1R expressed in CHO cell membranes incubated for 30 mins measured after 10 hrs by scintil... |
Eur J Med Chem 127: 703-714 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.044
BindingDB Entry DOI: 10.7270/Q2T155W1 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM11863

(4-({[4-(4-chlorophenoxy)benzene]sulfonyl}methyl)-N...)Show SMILES ONC(=O)C1(CS(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)CCOCC1 Show InChI InChI=1S/C19H20ClNO6S/c20-14-1-3-15(4-2-14)27-16-5-7-17(8-6-16)28(24,25)13-19(18(22)21-23)9-11-26-12-10-19/h1-8,23H,9-13H2,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-13 (MMP-13). |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM517

((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(Cc2cccnc2)CCN1C[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against HIV-1 protease enzyme. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM50086603

(CHEMBL3426241)Show SMILES CCc1cc(OC)ccc1-c1ccc(C[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@](C)(Cc2ccccc2F)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)C(C)(C)NC(=O)[C@@H](N)Cc2cnc[nH]2)[C@@H](C)O)[C@@H](C)O)C(=O)N[C@@H](CCCc2ccccc2)C(N)=O)cc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]-17 from human GLP-1R expressed in CHO cell membranes incubated for 120 mins by scintillation counting based radioligand bindin... |
J Med Chem 58: 4080-5 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00166
BindingDB Entry DOI: 10.7270/Q2DB83KP |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM11863

(4-({[4-(4-chlorophenoxy)benzene]sulfonyl}methyl)-N...)Show SMILES ONC(=O)C1(CS(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)CCOCC1 Show InChI InChI=1S/C19H20ClNO6S/c20-14-1-3-15(4-2-14)27-16-5-7-17(8-6-16)28(24,25)13-19(18(22)21-23)9-11-26-12-10-19/h1-8,23H,9-13H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-9 (MMP-9). |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50370377
(CHEMBL1790231)Show SMILES CC[C@@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(OCCCNC1=O)cc2)NC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(C)=O)C(C)C Show InChI InChI=1S/C41H62N6O7/c1-8-27(6)37-40(52)42-19-12-20-54-31-17-15-30(16-18-31)23-33(38(50)47-37)43-24-35(49)32(22-29-13-10-9-11-14-29)45-41(53)36(26(4)5)46-39(51)34(21-25(2)3)44-28(7)48/h9-11,13-18,25-27,32-37,43,49H,8,12,19-24H2,1-7H3,(H,42,52)(H,44,48)(H,45,53)(H,46,51)(H,47,50)/t27-,32+,33+,34+,35-,36+,37+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Tested for inhibition of synthetic HIV-1 protease by using fluorometric assay |
J Med Chem 47: 1641-51 (2004)
Article DOI: 10.1021/jm030337m
BindingDB Entry DOI: 10.7270/Q2JD4XJK |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288192
(CHEMBL83186 | N-[2-Hydroxy-2-((S)-8-isopropyl-6,9-...)Show SMILES CC(C)CCN(C[C@@H](O)[C@H]1Cc2ccc(OCCCC(=O)N[C@@H](CC(N)=O)C(=O)N1)cc2)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C29H40N4O7S/c1-20(2)14-15-33(41(38,39)23-7-4-3-5-8-23)19-26(34)24-17-21-10-12-22(13-11-21)40-16-6-9-28(36)31-25(18-27(30)35)29(37)32-24/h3-5,7-8,10-13,20,24-26,34H,6,9,14-19H2,1-2H3,(H2,30,35)(H,31,36)(H,32,37)/t24-,25+,26-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease. |
Bioorg Med Chem Lett 6: 2531-2536 (1996)
Article DOI: 10.1016/0960-894X(96)00468-4
BindingDB Entry DOI: 10.7270/Q2S1830C |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM577

((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...)Show SMILES CC(C)CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)O[C@H]1CCOC1)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C25H35N3O6S/c1-18(2)15-28(35(31,32)22-10-8-20(26)9-11-22)16-24(29)23(14-19-6-4-3-5-7-19)27-25(30)34-21-12-13-33-17-21/h3-11,18,21,23-24,29H,12-17,26H2,1-2H3,(H,27,30)/t21-,23-,24+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against HIV-protease |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM13928
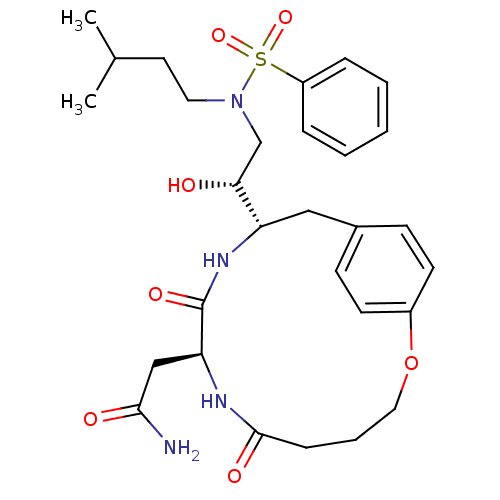
(2-[(8S,11S)-11-[(1R)-2-[benzene(3-methylbutyl)sulf...)Show SMILES CC(C)CCN(C[C@@H](O)[C@@H]1Cc2ccc(OCCCC(=O)N[C@@H](CC(N)=O)C(=O)N1)cc2)S(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C29H40N4O7S/c1-20(2)14-15-33(41(38,39)23-7-4-3-5-8-23)19-26(34)24-17-21-10-12-22(13-11-21)40-16-6-9-28(36)31-25(18-27(30)35)29(37)32-24/h3-5,7-8,10-13,20,24-26,34H,6,9,14-19H2,1-2H3,(H2,30,35)(H,31,36)(H,32,37)/t24-,25-,26+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.600 | -54.8 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Queensland
| Assay Description
he Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in fl... |
Biochemistry 38: 7978-88 (1999)
Article DOI: 10.1021/bi990174x
BindingDB Entry DOI: 10.7270/Q20000B3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50092152
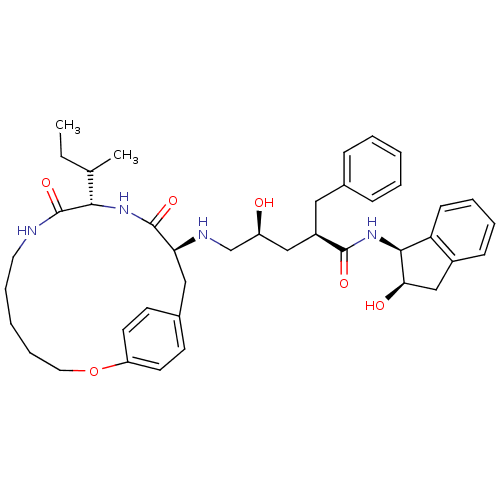
(2-Benzyl-5-(10-sec-butyl-9,12-dioxo-2-oxa-8,11-dia...)Show SMILES CCC(C)[C@@H]1NC(=O)[C@H](Cc2ccc(OCCCCCNC1=O)cc2)NC[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 Show InChI InChI=1S/C41H54N4O6/c1-3-27(2)37-41(50)42-20-10-5-11-21-51-33-18-16-29(17-19-33)23-35(40(49)44-37)43-26-32(46)24-31(22-28-12-6-4-7-13-28)39(48)45-38-34-15-9-8-14-30(34)25-36(38)47/h4,6-9,12-19,27,31-32,35-38,43,46-47H,3,5,10-11,20-26H2,1-2H3,(H,42,50)(H,44,49)(H,45,48)/t27?,31-,32+,35+,36-,37+,38+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 protease |
J Med Chem 43: 3495-504 (2000)
BindingDB Entry DOI: 10.7270/Q2KH0P1S |
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM50301953
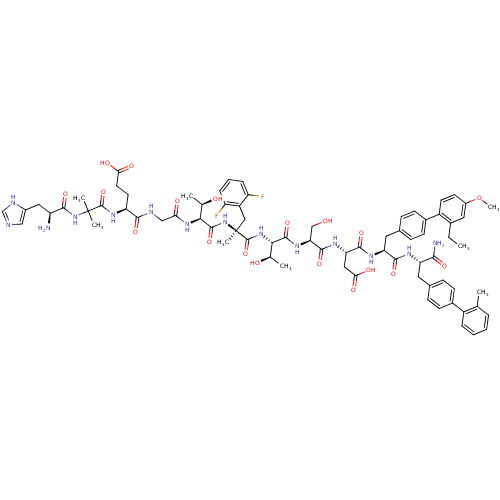
((3S,6S,9S,12S,15S,21S)-21-(2-((S)-2-amino-3-(1H-im...)Show SMILES CCc1cc(OC)ccc1-c1ccc(C[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@](C)(Cc2c(F)cccc2F)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)C(C)(C)NC(=O)[C@@H](N)Cc2cnc[nH]2)[C@@H](C)O)[C@@H](C)O)C(=O)N[C@@H](Cc2ccc(cc2)-c2ccccc2C)C(N)=O)cc1 |r| Show InChI InChI=1S/C76H94F2N14O19/c1-9-44-31-48(111-8)25-26-50(44)46-23-19-43(20-24-46)30-57(68(104)84-56(65(80)101)29-42-17-21-45(22-18-42)49-14-11-10-13-39(49)2)85-69(105)58(33-62(99)100)86-70(106)59(37-93)87-71(107)63(40(3)94)90-74(110)76(7,34-51-52(77)15-12-16-53(51)78)92-72(108)64(41(4)95)89-60(96)36-82-67(103)55(27-28-61(97)98)88-73(109)75(5,6)91-66(102)54(79)32-47-35-81-38-83-47/h10-26,31,35,38,40-41,54-59,63-64,93-95H,9,27-30,32-34,36-37,79H2,1-8H3,(H2,80,101)(H,81,83)(H,82,103)(H,84,104)(H,85,105)(H,86,106)(H,87,107)(H,88,109)(H,89,96)(H,90,110)(H,91,102)(H,92,108)(H,97,98)(H,99,100)/t40-,41-,54+,55+,56+,57+,58+,59+,63+,64+,76+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]-17 from human GLP-1R expressed in CHO cell membranes incubated for 120 mins by scintillation counting based radioligand bindin... |
J Med Chem 58: 4080-5 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00166
BindingDB Entry DOI: 10.7270/Q2DB83KP |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50084682

(1-[3-(4-Carbamimidoyl-phenyl)-2-(2-methyl-1,2,3,4-...)Show SMILES Cc1cc(ccc1C(=O)N[C@@H](CCCNC(N)=N)B1O[C@@H]2CC3CC(C3(C)C)[C@]2(C)O1)-c1ccccc1S(=O)(=O)NC(C)(C)C |TLB:30:28:25:23| Show InChI InChI=1S/C33H48BN5O5S/c1-20-17-21(24-11-8-9-12-25(24)45(41,42)39-31(2,3)4)14-15-23(20)29(40)38-28(13-10-16-37-30(35)36)34-43-27-19-22-18-26(32(22,5)6)33(27,7)44-34/h8-9,11-12,14-15,17,22,26-28,39H,10,13,16,18-19H2,1-7H3,(H,38,40)(H4,35,36,37)/t22?,26?,27-,28+,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against Trypsin |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50084670
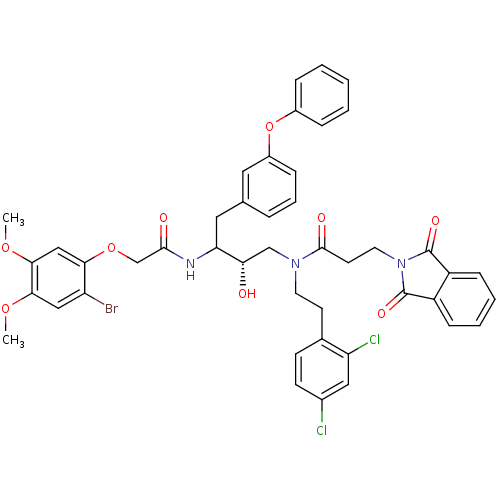
(CHEMBL323806 | N-[3-[2-(2-Bromo-4,5-dimethoxy-phen...)Show SMILES COc1cc(Br)c(OCC(=O)NC(Cc2cccc(Oc3ccccc3)c2)[C@@H](O)CN(CCc2ccc(Cl)cc2Cl)C(=O)CCN2C(=O)c3ccccc3C2=O)cc1OC Show InChI InChI=1S/C45H42BrCl2N3O9/c1-57-40-24-35(46)39(25-41(40)58-2)59-27-42(53)49-37(22-28-9-8-12-32(21-28)60-31-10-4-3-5-11-31)38(52)26-50(19-17-29-15-16-30(47)23-36(29)48)43(54)18-20-51-44(55)33-13-6-7-14-34(33)45(51)56/h3-16,21,23-25,37-38,52H,17-20,22,26-27H2,1-2H3,(H,49,53)/t37?,38-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against aspartic proteases |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17941
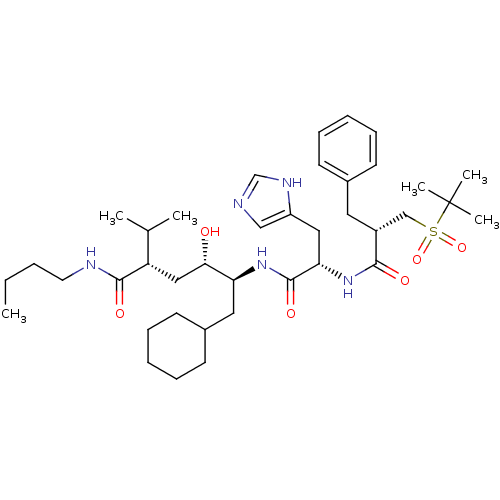
((2S,4S,5S)-5-[(2S)-2-[(2S)-2-benzyl-3-[(2-methylpr...)Show SMILES CCCCNC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)CS(=O)(=O)C(C)(C)C)C(C)C |r| Show InChI InChI=1S/C39H63N5O6S/c1-7-8-19-41-37(47)32(27(2)3)23-35(45)33(21-29-17-13-10-14-18-29)43-38(48)34(22-31-24-40-26-42-31)44-36(46)30(20-28-15-11-9-12-16-28)25-51(49,50)39(4,5)6/h9,11-12,15-16,24,26-27,29-30,32-35,45H,7-8,10,13-14,17-23,25H2,1-6H3,(H,40,42)(H,41,47)(H,43,48)(H,44,46)/t30-,32+,33+,34+,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against aspartic protease renin. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50077669

((S)-2-Benzyl-N-[(S)-1-((1S,2R,3S)-1-cyclohexylmeth...)Show SMILES CC(C)(C)S(=O)(=O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)C1CC1 Show InChI InChI=1S/C33H50N4O6S/c1-33(2,3)44(42,43)20-25(16-22-10-6-4-7-11-22)31(40)37-28(18-26-19-34-21-35-26)32(41)36-27(17-23-12-8-5-9-13-23)30(39)29(38)24-14-15-24/h4,6-7,10-11,19,21,23-25,27-30,38-39H,5,8-9,12-18,20H2,1-3H3,(H,34,35)(H,36,41)(H,37,40)/t25-,27+,28+,29+,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against aspartic protease renin. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM50231894

(CHEMBL4100325)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCCCNC(=O)CC[C@H](NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)Cc2cnc[nH]2)[C@@H](C)O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N1)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C157H238N42O46/c1-17-81(10)126(154(243)176-85(14)132(221)186-111(66-91-69-168-96-38-25-24-37-94(91)96)145(234)188-107(62-78(4)5)146(235)196-124(79(6)7)152(241)185-98(39-26-29-57-158)135(224)170-72-118(208)177-97(42-32-60-167-157(163)164)134(223)169-71-116(162)206)198-147(236)109(63-88-33-20-18-21-34-88)190-142(231)105(51-56-122(214)215)182-138(227)99(40-27-30-58-159)178-130(219)83(12)173-129(218)82(11)175-137(226)102(47-52-115(161)205)181-139(228)100-41-28-31-59-166-117(207)53-48-103(141(230)189-108(65-90-43-45-93(204)46-44-90)144(233)187-106(61-77(2)3)143(232)183-104(140(229)180-100)50-55-121(212)213)184-150(239)113(74-200)193-153(242)125(80(8)9)197-149(238)112(68-123(216)217)191-151(240)114(75-201)194-156(245)128(87(16)203)199-148(237)110(64-89-35-22-19-23-36-89)192-155(244)127(86(15)202)195-119(209)73-171-136(225)101(49-54-120(210)211)179-131(220)84(13)174-133(222)95(160)67-92-70-165-76-172-92/h18-25,33-38,43-46,69-70,76-87,95,97-114,124-128,168,200-204H,17,26-32,39-42,47-68,71-75,158-160H2,1-16H3,(H2,161,205)(H2,162,206)(H,165,172)(H,166,207)(H,169,223)(H,170,224)(H,171,225)(H,173,218)(H,174,222)(H,175,226)(H,176,243)(H,177,208)(H,178,219)(H,179,220)(H,180,229)(H,181,228)(H,182,227)(H,183,232)(H,184,239)(H,185,241)(H,186,221)(H,187,233)(H,188,234)(H,189,230)(H,190,231)(H,191,240)(H,192,244)(H,193,242)(H,194,245)(H,195,209)(H,196,235)(H,197,238)(H,198,236)(H,199,237)(H,210,211)(H,212,213)(H,214,215)(H,216,217)(H4,163,164,167)/t81-,82-,83-,84-,85-,86+,87+,95-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,112-,113-,114-,124-,125-,126-,127-,128-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]-GLP (7 to 36 residues) from human GLP1R expressed in CHO cell membranes incubated for 30 mins measured after 10 hrs by scintil... |
Eur J Med Chem 127: 703-714 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.044
BindingDB Entry DOI: 10.7270/Q2T155W1 |
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM50231902
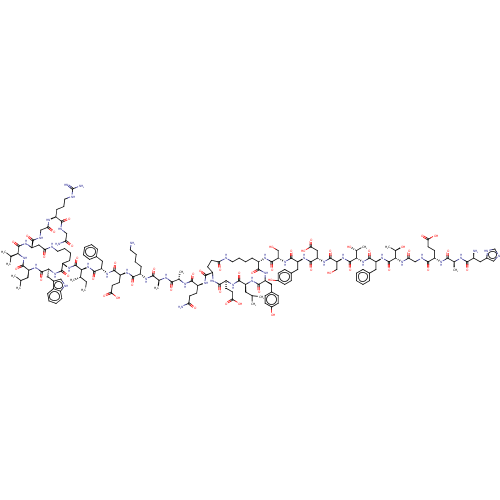
(CHEMBL4096416)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCC(=O)NCCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)Cc2cnc[nH]2)[C@@H](C)O)[C@@H](C)O)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N1)C(=O)N[C@H]1CCCCNC(=O)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(C)C)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C162H236N42O48/c1-14-83(8)131(159(250)189-103-38-25-28-60-171-123(214)70-116(140(231)176-75-124(215)181-100(39-29-61-172-162(167)168)138(229)174-74-121(166)212)198-158(249)130(82(6)7)202-153(244)110(63-81(4)5)191-151(242)115(195-144(103)235)68-93-72-173-99-35-22-21-34-97(93)99)203-154(245)113(64-89-30-17-15-18-31-89)193-147(238)108(52-57-128(221)222)187-142(233)101(36-23-26-58-163)182-135(226)85(10)178-134(225)84(9)180-141(232)105(48-53-120(165)211)185-145(236)106-49-54-122(213)170-59-27-24-37-102(143(234)192-111(66-91-40-44-95(209)45-41-91)149(240)190-109(62-80(2)3)148(239)188-107(146(237)186-106)51-56-127(219)220)184-156(247)118(77-205)199-150(241)112(67-92-42-46-96(210)47-43-92)194-152(243)117(71-129(223)224)196-157(248)119(78-206)200-161(252)133(88(13)208)204-155(246)114(65-90-32-19-16-20-33-90)197-160(251)132(87(12)207)201-125(216)76-175-139(230)104(50-55-126(217)218)183-136(227)86(11)179-137(228)98(164)69-94-73-169-79-177-94/h15-22,30-35,40-47,72-73,79-88,98,100-119,130-133,173,205-210H,14,23-29,36-39,48-71,74-78,163-164H2,1-13H3,(H2,165,211)(H2,166,212)(H,169,177)(H,170,213)(H,171,214)(H,174,229)(H,175,230)(H,176,231)(H,178,225)(H,179,228)(H,180,232)(H,181,215)(H,182,226)(H,183,227)(H,184,247)(H,185,236)(H,186,237)(H,187,233)(H,188,239)(H,189,250)(H,190,240)(H,191,242)(H,192,234)(H,193,238)(H,194,243)(H,195,235)(H,196,248)(H,197,251)(H,198,249)(H,199,241)(H,200,252)(H,201,216)(H,202,244)(H,203,245)(H,204,246)(H,217,218)(H,219,220)(H,221,222)(H,223,224)(H4,167,168,172)/t83-,84-,85-,86-,87+,88+,98-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,130-,131-,132-,133-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]-GLP (7 to 36 residues) from human GLP1R expressed in CHO cell membranes incubated for 30 mins measured after 10 hrs by scintil... |
Eur J Med Chem 127: 703-714 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.044
BindingDB Entry DOI: 10.7270/Q2T155W1 |
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM50231962
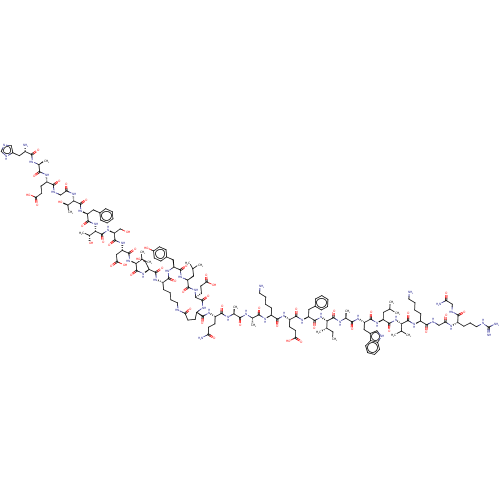
(CHEMBL4099379)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCC(=O)NCCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)Cc2cnc[nH]2)[C@@H](C)O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N1)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C157H238N42O46/c1-17-81(10)126(154(243)176-85(14)132(221)186-111(66-91-69-168-96-38-25-24-37-94(91)96)145(234)188-107(62-78(4)5)146(235)196-124(79(6)7)152(241)185-98(39-26-29-57-158)135(224)170-72-118(208)177-97(42-32-60-167-157(163)164)134(223)169-71-116(162)206)198-147(236)109(63-88-33-20-18-21-34-88)190-142(231)105(51-56-122(214)215)183-138(227)99(40-27-30-58-159)178-130(219)83(12)173-129(218)82(11)175-137(226)102(47-52-115(161)205)181-140(229)103-48-53-117(207)166-59-31-28-41-100(139(228)189-108(65-90-43-45-93(204)46-44-90)144(233)187-106(61-77(2)3)143(232)184-104(141(230)182-103)50-55-121(212)213)180-150(239)113(74-200)193-153(242)125(80(8)9)197-149(238)112(68-123(216)217)191-151(240)114(75-201)194-156(245)128(87(16)203)199-148(237)110(64-89-35-22-19-23-36-89)192-155(244)127(86(15)202)195-119(209)73-171-136(225)101(49-54-120(210)211)179-131(220)84(13)174-133(222)95(160)67-92-70-165-76-172-92/h18-25,33-38,43-46,69-70,76-87,95,97-114,124-128,168,200-204H,17,26-32,39-42,47-68,71-75,158-160H2,1-16H3,(H2,161,205)(H2,162,206)(H,165,172)(H,166,207)(H,169,223)(H,170,224)(H,171,225)(H,173,218)(H,174,222)(H,175,226)(H,176,243)(H,177,208)(H,178,219)(H,179,220)(H,180,239)(H,181,229)(H,182,230)(H,183,227)(H,184,232)(H,185,241)(H,186,221)(H,187,233)(H,188,234)(H,189,228)(H,190,231)(H,191,240)(H,192,244)(H,193,242)(H,194,245)(H,195,209)(H,196,235)(H,197,238)(H,198,236)(H,199,237)(H,210,211)(H,212,213)(H,214,215)(H,216,217)(H4,163,164,167)/t81-,82-,83-,84-,85-,86+,87+,95-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,112-,113-,114-,124-,125-,126-,127-,128-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]-GLP (7 to 36 residues) from human GLP1R expressed in CHO cell membranes incubated for 30 mins measured after 10 hrs by scintil... |
Eur J Med Chem 127: 703-714 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.044
BindingDB Entry DOI: 10.7270/Q2T155W1 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [491-589,Q7K,L33I,C67B,C95B]
(Human immunodeficiency virus type 1) | BDBM795
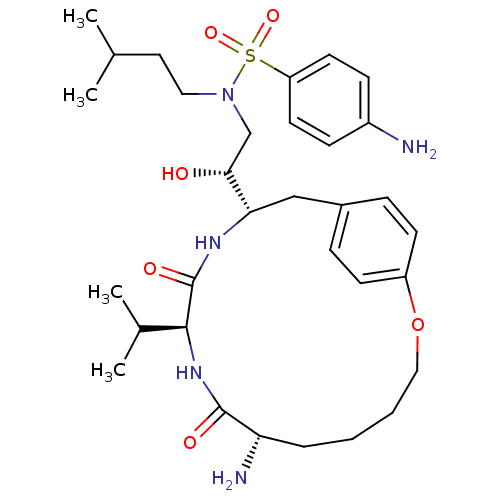
((2R)-2-[(7S,10S,13S)-7-amino-8,11-dioxo-10-(propan...)Show SMILES CC(C)CCN(C[C@@H](O)[C@@H]1Cc2ccc(OCCCC[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N1)cc2)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C32H49N5O6S/c1-21(2)16-17-37(44(41,42)26-14-10-24(33)11-15-26)20-29(38)28-19-23-8-12-25(13-9-23)43-18-6-5-7-27(34)31(39)36-30(22(3)4)32(40)35-28/h8-15,21-22,27-30,38H,5-7,16-20,33-34H2,1-4H3,(H,35,40)(H,36,39)/t27-,28-,29+,30-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | -53.7 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Queensland
| Assay Description
Inhibition constants were determined by a fluorometric assay. Ki values were calculated from either Dixon plots or Henderson plots in cases where the... |
J Med Chem 45: 371-81 (2002)
Article DOI: 10.1021/jm010414i
BindingDB Entry DOI: 10.7270/Q2FQ9TSJ |
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM50231896
(CHEMBL4084829)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)Cc2cnc[nH]2)[C@@H](C)O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N1)C(=O)N[C@H]1CCCCNC(=O)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(C)C)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C157H234N42O47/c1-16-80(10)126(154(244)183-98-40-27-30-55-165-116(207)65-109(135(225)171-71-118(209)176-95(41-31-57-167-157(162)163)133(223)169-70-115(161)206)192-152(242)124(78(6)7)196-146(236)104(59-77(4)5)185-144(234)108(188-139(98)229)63-89-68-168-94-37-24-23-36-92(89)94)198-147(237)106(60-86-32-19-17-20-33-86)187-140(230)101(48-52-121(213)214)180-137(227)96(38-25-28-54-158)177-130(220)82(12)173-129(219)81(11)175-136(226)100(46-50-114(160)205)181-145(235)110-66-117(208)166-56-29-26-39-97(138(228)186-105(62-88-42-44-91(204)45-43-88)143(233)184-103(58-76(2)3)142(232)182-102(141(231)189-110)49-53-122(215)216)179-150(240)112(73-200)193-153(243)125(79(8)9)197-149(239)111(67-123(217)218)190-151(241)113(74-201)194-156(246)128(85(15)203)199-148(238)107(61-87-34-21-18-22-35-87)191-155(245)127(84(14)202)195-119(210)72-170-134(224)99(47-51-120(211)212)178-131(221)83(13)174-132(222)93(159)64-90-69-164-75-172-90/h17-24,32-37,42-45,68-69,75-85,93,95-113,124-128,168,200-204H,16,25-31,38-41,46-67,70-74,158-159H2,1-15H3,(H2,160,205)(H2,161,206)(H,164,172)(H,165,207)(H,166,208)(H,169,223)(H,170,224)(H,171,225)(H,173,219)(H,174,222)(H,175,226)(H,176,209)(H,177,220)(H,178,221)(H,179,240)(H,180,227)(H,181,235)(H,182,232)(H,183,244)(H,184,233)(H,185,234)(H,186,228)(H,187,230)(H,188,229)(H,189,231)(H,190,241)(H,191,245)(H,192,242)(H,193,243)(H,194,246)(H,195,210)(H,196,236)(H,197,239)(H,198,237)(H,199,238)(H,211,212)(H,213,214)(H,215,216)(H,217,218)(H4,162,163,167)/t80-,81-,82-,83-,84+,85+,93-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,112-,113-,124-,125-,126-,127-,128-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]-GLP (7 to 36 residues) from human GLP1R expressed in CHO cell membranes incubated for 30 mins measured after 10 hrs by scintil... |
Eur J Med Chem 127: 703-714 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.044
BindingDB Entry DOI: 10.7270/Q2T155W1 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50041291
(2-[(R)-3-(1,1-Dimethyl-2-phenyl-ethyl)-2,4,8-triox...)Show SMILES CC(C)C(NC(=O)Cn1c(cc2c([nH]c(=O)n(c2=O)C(C)(C)Cc2ccccc2)c1=O)-c1ccccc1)C(=O)C(F)(F)F Show InChI InChI=1S/C31H31F3N4O5/c1-18(2)24(26(40)31(32,33)34)35-23(39)17-37-22(20-13-9-6-10-14-20)15-21-25(28(37)42)36-29(43)38(27(21)41)30(3,4)16-19-11-7-5-8-12-19/h5-15,18,24H,16-17H2,1-4H3,(H,35,39)(H,36,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against Elastase. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
























































