 Found 58 hits with Last Name = 'fanwick' and Initial = 'pe'
Found 58 hits with Last Name = 'fanwick' and Initial = 'pe' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363378
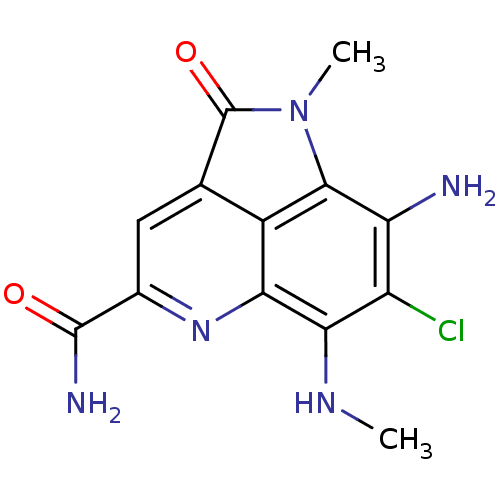
(CHEMBL1945729)Show InChI InChI=1S/C13H12ClN5O2/c1-17-10-7(14)8(15)11-6-4(13(21)19(11)2)3-5(12(16)20)18-9(6)10/h3,17H,15H2,1-2H3,(H2,16,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363366

(CHEMBL1945406)Show InChI InChI=1S/C12H10ClN5O2/c1-18-10-5-3(12(18)20)2-4(11(16)19)17-9(5)7(14)6(13)8(10)15/h2H,14-15H2,1H3,(H2,16,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM2809

(Alkenyldiarylmethanes (ADAM) 6b | CHEMBL105859 | M...)Show SMILES COC(=O)CCC\C=C(\c1ccc(OC)c(OC)c1)c1cc(C)c(OC)c(c1)C(=O)OC Show InChI InChI=1S/C25H30O7/c1-16-13-18(14-20(24(16)31-5)25(27)32-6)19(9-7-8-10-23(26)30-4)17-11-12-21(28-2)22(15-17)29-3/h9,11-15H,7-8,10H2,1-6H3/b19-9- | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV-1 reverse transcriptase activity |
J Med Chem 48: 6140-55 (2005)
Article DOI: 10.1021/jm050452s
BindingDB Entry DOI: 10.7270/Q2TB16F6 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363373
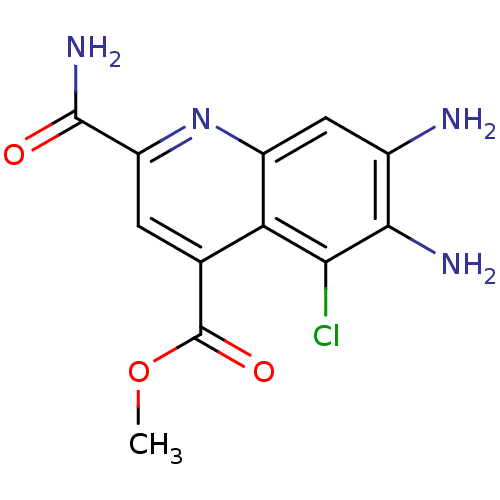
(CHEMBL1945627)Show InChI InChI=1S/C12H11ClN4O3/c1-20-12(19)4-2-7(11(16)18)17-6-3-5(14)10(15)9(13)8(4)6/h2-3H,14-15H2,1H3,(H2,16,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363372
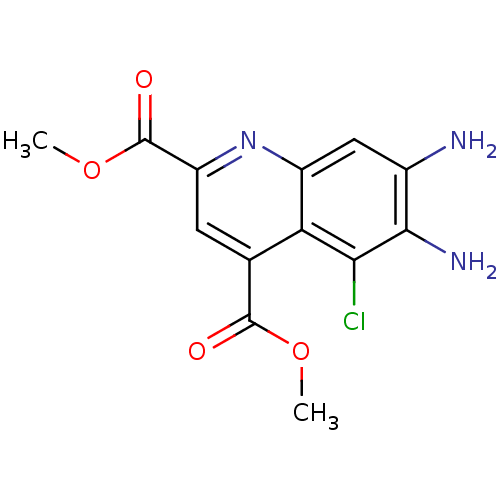
(CHEMBL1945626)Show InChI InChI=1S/C13H12ClN3O4/c1-20-12(18)5-3-8(13(19)21-2)17-7-4-6(15)11(16)10(14)9(5)7/h3-4H,15-16H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM2803

(Alkenyldiarylmethanes (ADAM) 50a | CHEMBL131761 | ...)Show SMILES [#6]-[#8]-[#6](=O)-c1cc(cc(Br)c1-[#8]-[#6])-[#6](=[#6]/[#6]-[#6]-[#7]-1-[#6]-[#6]-[#8]-[#6]-1=O)\c1cc(Br)c(-[#8]-[#6])c(c1)-[#6](=O)-[#8]-[#6] Show InChI InChI=1S/C25H25Br2NO8/c1-32-21-17(23(29)34-3)10-14(12-19(21)26)16(6-5-7-28-8-9-36-25(28)31)15-11-18(24(30)35-4)22(33-2)20(27)13-15/h6,10-13H,5,7-9H2,1-4H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 499 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV-1 reverse transcriptase activity |
J Med Chem 48: 6140-55 (2005)
Article DOI: 10.1021/jm050452s
BindingDB Entry DOI: 10.7270/Q2TB16F6 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain
(Bos taurus) | BDBM50369013
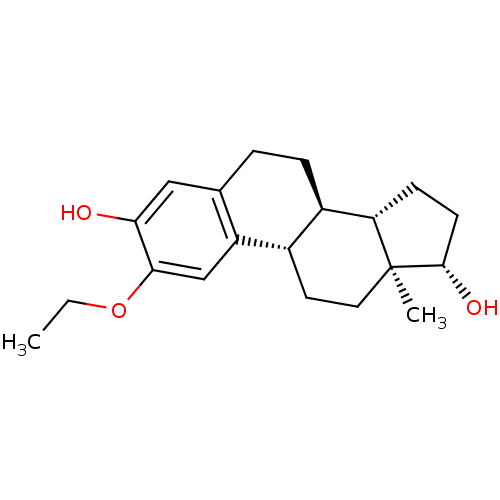
(CHEMBL1627442)Show SMILES CCOc1cc2[C@H]3CC[C@]4(C)[C@@H](O)CC[C@H]4[C@@H]3CCc2cc1O Show InChI InChI=1S/C20H28O3/c1-3-23-18-11-15-12(10-17(18)21)4-5-14-13(15)8-9-20(2)16(14)6-7-19(20)22/h10-11,13-14,16,19,21-22H,3-9H2,1-2H3/t13-,14+,16-,19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of polymerization of purified bovine tubulin |
J Med Chem 43: 2419-29 (2000)
BindingDB Entry DOI: 10.7270/Q2B56KD4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50173819

(3-Chloro-5-[(E)-1-(3-cyano-phenyl)-5-methoxycarbon...)Show SMILES COC(=O)CCC\C=C(/c1cccc(c1)C#N)c1cc(Cl)c(OC)c(c1)C(=O)OC Show InChI InChI=1S/C23H22ClNO5/c1-28-21(26)10-5-4-9-18(16-8-6-7-15(11-16)14-25)17-12-19(23(27)30-3)22(29-2)20(24)13-17/h6-9,11-13H,4-5,10H2,1-3H3/b18-9+ | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV-1 reverse transcriptase activity |
J Med Chem 48: 6140-55 (2005)
Article DOI: 10.1021/jm050452s
BindingDB Entry DOI: 10.7270/Q2TB16F6 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50173813
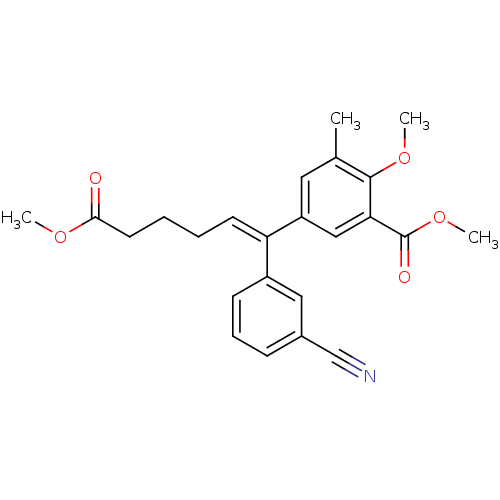
((E)-5-[1-(3-cyanophenyl)-5-methoxycarbonylpent-1-e...)Show SMILES COC(=O)CCC\C=C(/c1cccc(c1)C#N)c1cc(C)c(OC)c(c1)C(=O)OC Show InChI InChI=1S/C24H25NO5/c1-16-12-19(14-21(23(16)29-3)24(27)30-4)20(10-5-6-11-22(26)28-2)18-9-7-8-17(13-18)15-25/h7-10,12-14H,5-6,11H2,1-4H3/b20-10+ | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV-1 reverse transcriptase activity |
J Med Chem 48: 6140-55 (2005)
Article DOI: 10.1021/jm050452s
BindingDB Entry DOI: 10.7270/Q2TB16F6 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain
(Bos taurus) | BDBM50089336

(9-Ethoxy-12a-methyl-1,2,3,3a,3b,4,10b,11,12,12a-de...)Show SMILES CCOc1cc2C3CC[C@]4(C)[C@@H](O)CCC4C3CC=Cc2cc1O |c:20| Show InChI InChI=1S/C21H28O3/c1-3-24-19-12-16-13(11-18(19)22)5-4-6-15-14(16)9-10-21(2)17(15)7-8-20(21)23/h4-5,11-12,14-15,17,20,22-23H,3,6-10H2,1-2H3/t14?,15?,17?,20-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of polymerization of purified bovine tubulin |
J Med Chem 43: 2419-29 (2000)
BindingDB Entry DOI: 10.7270/Q2B56KD4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50173820
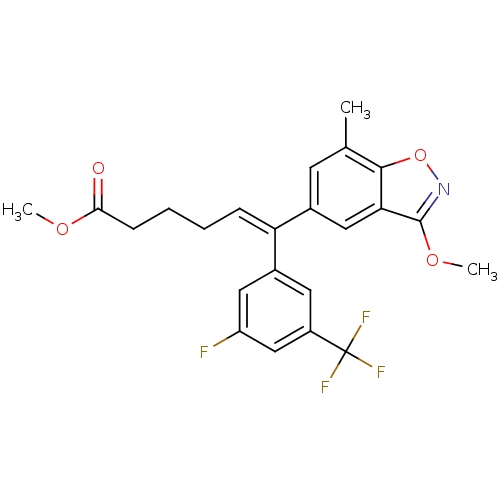
((Z)-6-(3-Fluoro-5-trifluoromethyl-phenyl)-6-(3-met...)Show SMILES COC(=O)CCC\C=C(/c1cc(F)cc(c1)C(F)(F)F)c1cc(C)c2onc(OC)c2c1 Show InChI InChI=1S/C23H21F4NO4/c1-13-8-14(11-19-21(13)32-28-22(19)31-3)18(6-4-5-7-20(29)30-2)15-9-16(23(25,26)27)12-17(24)10-15/h6,8-12H,4-5,7H2,1-3H3/b18-6- | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV-1 reverse transcriptase activity |
J Med Chem 48: 6140-55 (2005)
Article DOI: 10.1021/jm050452s
BindingDB Entry DOI: 10.7270/Q2TB16F6 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain
(Bos taurus) | BDBM50089339

(9-Ethoxy-12a-methyl-1,2,3,3a,3b,4,5,6,10b,11,12,12...)Show SMILES CCOc1cc2C3CC[C@]4(C)[C@@H](O)CCC4C3CCCc2cc1O Show InChI InChI=1S/C21H30O3/c1-3-24-19-12-16-13(11-18(19)22)5-4-6-15-14(16)9-10-21(2)17(15)7-8-20(21)23/h11-12,14-15,17,20,22-23H,3-10H2,1-2H3/t14?,15?,17?,20-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of polymerization of purified bovine tubulin |
J Med Chem 43: 2419-29 (2000)
BindingDB Entry DOI: 10.7270/Q2B56KD4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50173810
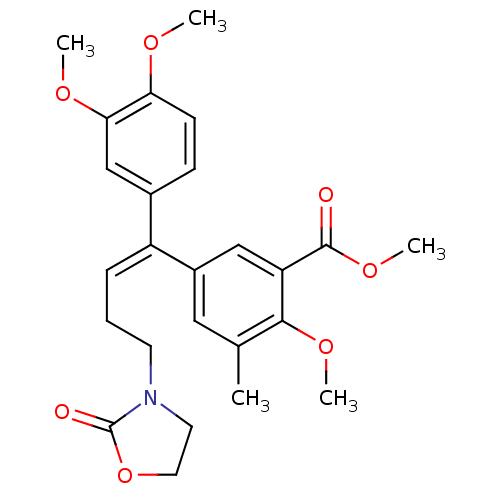
(5-[(E)-1-(3,4-Dimethoxy-phenyl)-4-(2-oxo-oxazolidi...)Show SMILES COC(=O)c1cc(cc(C)c1OC)C(=C/CCN1CCOC1=O)\c1ccc(OC)c(OC)c1 Show InChI InChI=1S/C25H29NO7/c1-16-13-18(14-20(23(16)31-4)24(27)32-5)19(7-6-10-26-11-12-33-25(26)28)17-8-9-21(29-2)22(15-17)30-3/h7-9,13-15H,6,10-12H2,1-5H3/b19-7- | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV-1 reverse transcriptase activity |
J Med Chem 48: 6140-55 (2005)
Article DOI: 10.1021/jm050452s
BindingDB Entry DOI: 10.7270/Q2TB16F6 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363369
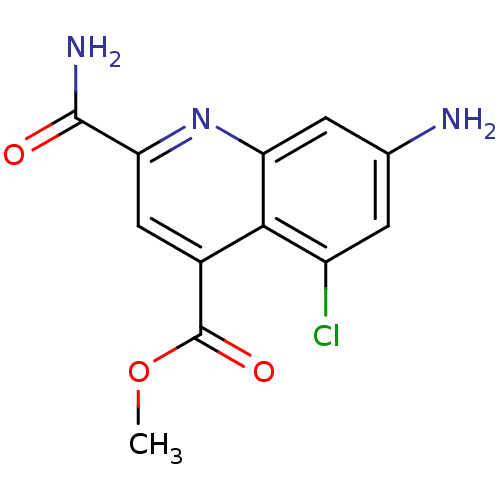
(CHEMBL1945620)Show InChI InChI=1S/C12H10ClN3O3/c1-19-12(18)6-4-9(11(15)17)16-8-3-5(14)2-7(13)10(6)8/h2-4H,14H2,1H3,(H2,15,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain
(Bos taurus) | BDBM50060957
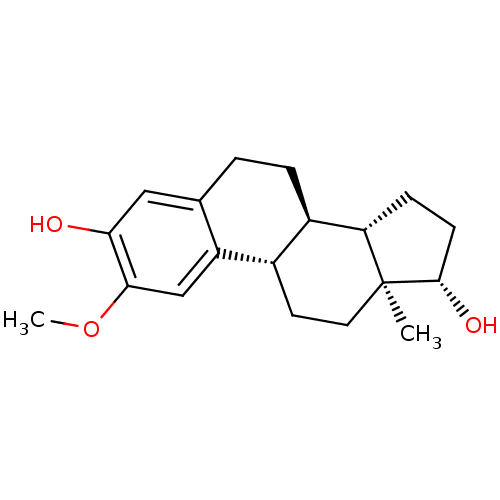
(2-Hydroxyestradol 2-methyl ether | 2-methoxy-17bet...)Show SMILES COc1cc2[C@H]3CC[C@]4(C)[C@@H](O)CC[C@H]4[C@@H]3CCc2cc1O |r| Show InChI InChI=1S/C19H26O3/c1-19-8-7-12-13(15(19)5-6-18(19)21)4-3-11-9-16(20)17(22-2)10-14(11)12/h9-10,12-13,15,18,20-21H,3-8H2,1-2H3/t12-,13+,15-,18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of polymerization of purified bovine tubulin |
J Med Chem 43: 2419-29 (2000)
BindingDB Entry DOI: 10.7270/Q2B56KD4 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363370
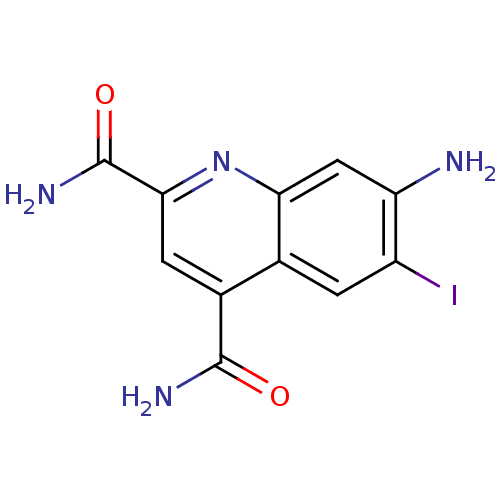
(CHEMBL1945624)Show InChI InChI=1S/C11H9IN4O2/c12-6-1-4-5(10(14)17)2-9(11(15)18)16-8(4)3-7(6)13/h1-3H,13H2,(H2,14,17)(H2,15,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363365
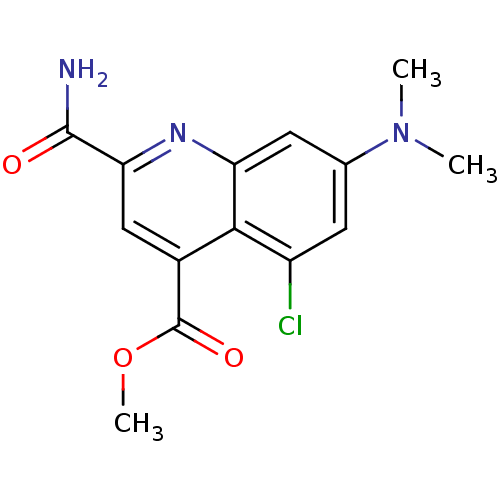
(CHEMBL1945623)Show InChI InChI=1S/C14H14ClN3O3/c1-18(2)7-4-9(15)12-8(14(20)21-3)6-11(13(16)19)17-10(12)5-7/h4-6H,1-3H3,(H2,16,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363371
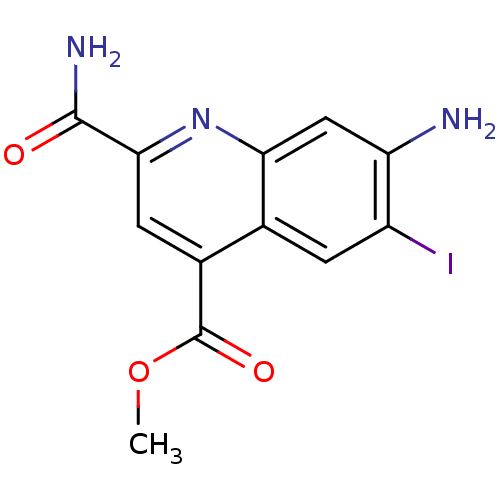
(CHEMBL1945625)Show InChI InChI=1S/C12H10IN3O3/c1-19-12(18)6-3-10(11(15)17)16-9-4-8(14)7(13)2-5(6)9/h2-4H,14H2,1H3,(H2,15,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain
(Bos taurus) | BDBM50369281
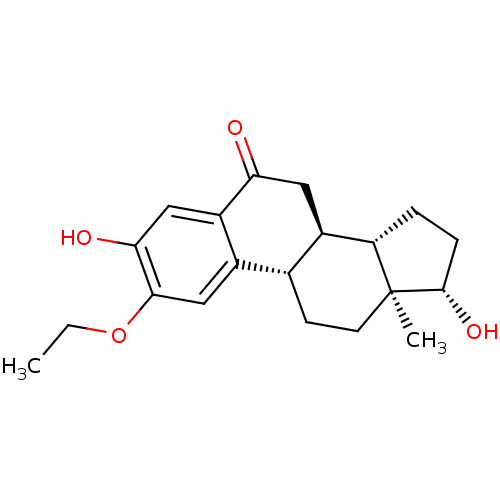
(CHEMBL1627880)Show SMILES CCOc1cc2[C@H]3CC[C@]4(C)[C@@H](O)CC[C@H]4[C@@H]3CC(=O)c2cc1O Show InChI InChI=1S/C20H26O4/c1-3-24-18-10-12-11-6-7-20(2)15(4-5-19(20)23)13(11)8-16(21)14(12)9-17(18)22/h9-11,13,15,19,22-23H,3-8H2,1-2H3/t11-,13-,15+,19+,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of polymerization of purified bovine tubulin |
J Med Chem 43: 2419-29 (2000)
BindingDB Entry DOI: 10.7270/Q2B56KD4 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363367

(CHEMBL1945407)Show InChI InChI=1S/C13H11ClN2O4/c1-19-12(17)7-5-10(13(18)20-2)16-9-4-6(15)3-8(14)11(7)9/h3-5H,15H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363364
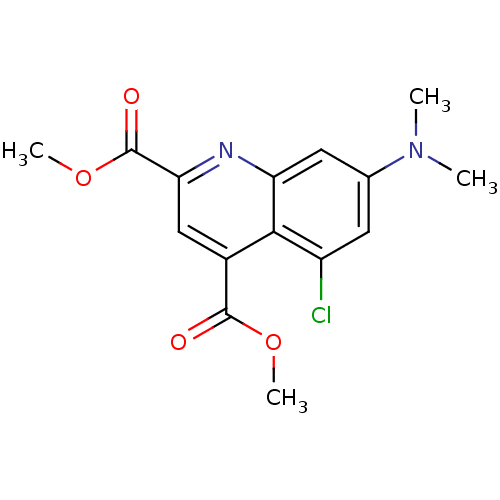
(CHEMBL1944648)Show InChI InChI=1S/C15H15ClN2O4/c1-18(2)8-5-10(16)13-9(14(19)21-3)7-12(15(20)22-4)17-11(13)6-8/h5-7H,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50060957

(2-Hydroxyestradol 2-methyl ether | 2-methoxy-17bet...)Show SMILES COc1cc2[C@H]3CC[C@]4(C)[C@@H](O)CC[C@H]4[C@@H]3CCc2cc1O |r| Show InChI InChI=1S/C19H26O3/c1-19-8-7-12-13(15(19)5-6-18(19)21)4-3-11-9-16(20)17(22-2)10-14(11)12/h9-10,12-13,15,18,20-21H,3-8H2,1-2H3/t12-,13+,15-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against bovine brain tubulin polymerization |
J Med Chem 47: 5126-39 (2004)
Article DOI: 10.1021/jm049647a
BindingDB Entry DOI: 10.7270/Q2CZ37Z8 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain
(Bos taurus) | BDBM50089349
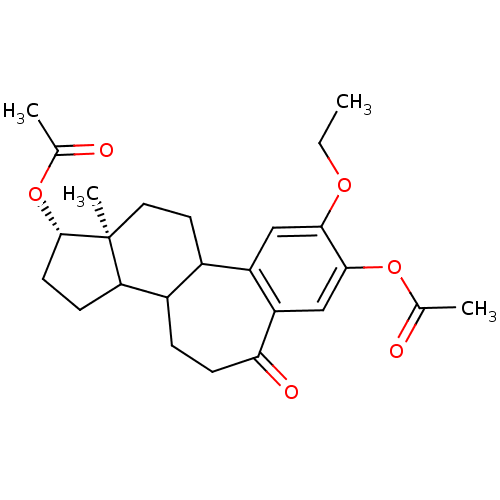
(Acetic acid 1-acetoxy-9-ethoxy-12a-methyl-6-oxo-1,...)Show SMILES CCOc1cc2C3CC[C@]4(C)[C@H](CCC4C3CCC(=O)c2cc1OC(C)=O)OC(C)=O Show InChI InChI=1S/C25H32O6/c1-5-29-22-12-18-16-10-11-25(4)20(7-9-24(25)31-15(3)27)17(16)6-8-21(28)19(18)13-23(22)30-14(2)26/h12-13,16-17,20,24H,5-11H2,1-4H3/t16?,17?,20?,24-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of polymerization of purified bovine tubulin |
J Med Chem 43: 2419-29 (2000)
BindingDB Entry DOI: 10.7270/Q2B56KD4 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363368
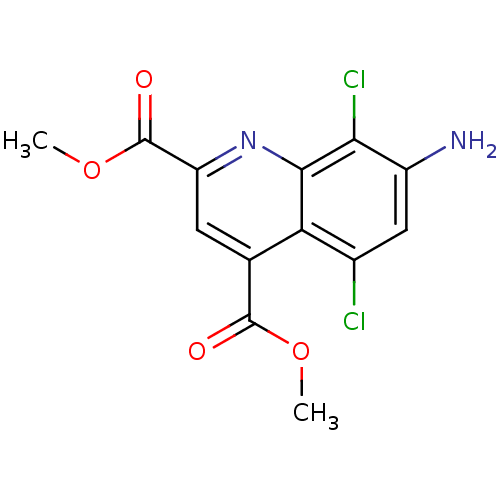
(CHEMBL1945619)Show InChI InChI=1S/C13H10Cl2N2O4/c1-20-12(18)5-3-8(13(19)21-2)17-11-9(5)6(14)4-7(16)10(11)15/h3-4H,16H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50173808
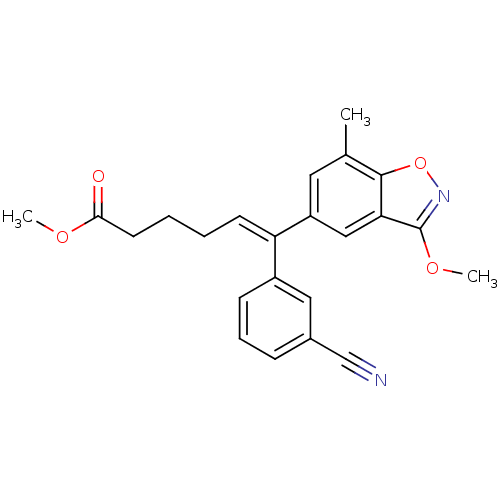
((E)-6-(3-Cyano-phenyl)-6-(3-methoxy-7-methyl-benzo...)Show SMILES COC(=O)CCC\C=C(/c1cccc(c1)C#N)c1cc(C)c2onc(OC)c2c1 Show InChI InChI=1S/C23H22N2O4/c1-15-11-18(13-20-22(15)29-25-23(20)28-3)19(9-4-5-10-21(26)27-2)17-8-6-7-16(12-17)14-24/h6-9,11-13H,4-5,10H2,1-3H3/b19-9+ | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV-1 reverse transcriptase activity |
J Med Chem 48: 6140-55 (2005)
Article DOI: 10.1021/jm050452s
BindingDB Entry DOI: 10.7270/Q2TB16F6 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363375

(CHEMBL1945629)Show InChI InChI=1S/C12H7Cl2N3O2/c1-17-10-6(14)3-5(13)9-8(10)4(12(17)19)2-7(16-9)11(15)18/h2-3H,1H3,(H2,15,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain
(Bos taurus) | BDBM50089346
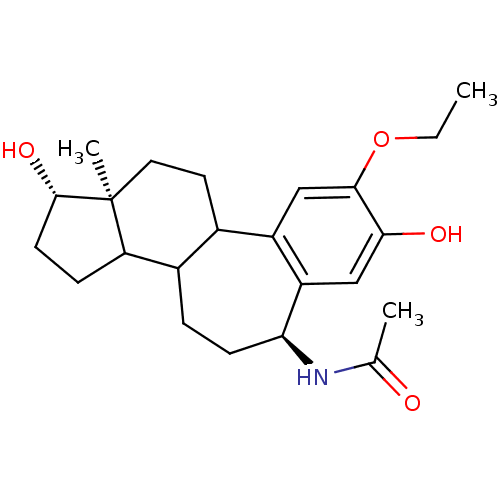
(CHEMBL310453 | N-(9-Ethoxy-1,8-dihydroxy-12a-methy...)Show SMILES CCOc1cc2C3CC[C@]4(C)[C@@H](O)CCC4C3CC[C@H](NC(C)=O)c2cc1O Show InChI InChI=1S/C23H33NO4/c1-4-28-21-12-16-14-9-10-23(3)18(6-8-22(23)27)15(14)5-7-19(24-13(2)25)17(16)11-20(21)26/h11-12,14-15,18-19,22,26-27H,4-10H2,1-3H3,(H,24,25)/t14?,15?,18?,19-,22-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of polymerization of purified bovine tubulin |
J Med Chem 43: 2419-29 (2000)
BindingDB Entry DOI: 10.7270/Q2B56KD4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50173805
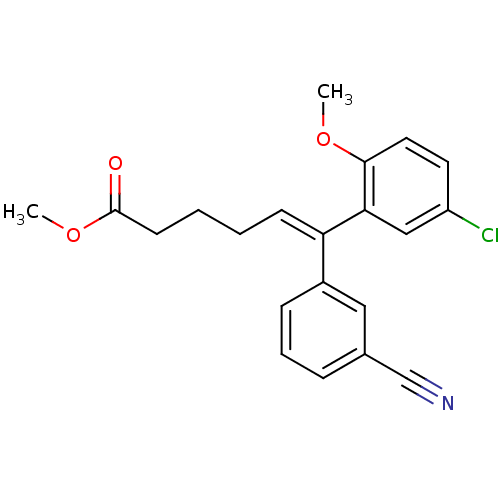
((E)-6-(5-Chloro-2-methoxy-phenyl)-6-(3-cyano-pheny...)Show SMILES COC(=O)CCC\C=C(/c1cccc(c1)C#N)c1cc(Cl)ccc1OC Show InChI InChI=1S/C21H20ClNO3/c1-25-20-11-10-17(22)13-19(20)18(8-3-4-9-21(24)26-2)16-7-5-6-15(12-16)14-23/h5-8,10-13H,3-4,9H2,1-2H3/b18-8+ | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV-1 reverse transcriptase activity |
J Med Chem 48: 6140-55 (2005)
Article DOI: 10.1021/jm050452s
BindingDB Entry DOI: 10.7270/Q2TB16F6 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363376
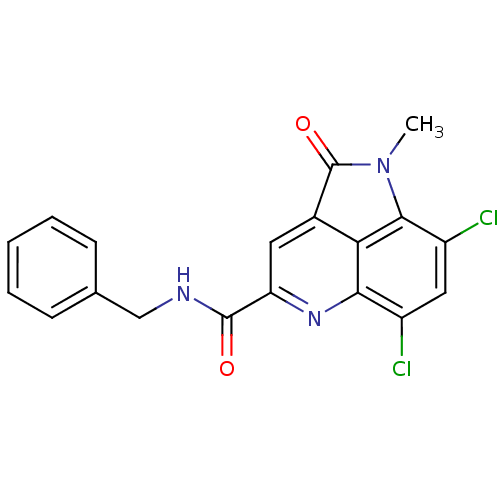
(CHEMBL1945630)Show SMILES CN1C(=O)c2cc(nc3c(Cl)cc(Cl)c1c23)C(=O)NCc1ccccc1 Show InChI InChI=1S/C19H13Cl2N3O2/c1-24-17-13(21)8-12(20)16-15(17)11(19(24)26)7-14(23-16)18(25)22-9-10-5-3-2-4-6-10/h2-8H,9H2,1H3,(H,22,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain
(Bos taurus) | BDBM50089347

(9-Ethoxy-1,8-dihydroxy-12a-methyl-2,3,3a,3b,4,6,10...)Show SMILES CCOc1cc2C3CC[C@]4(C)[C@@H](O)CCC4C3CC(=O)Cc2cc1O Show InChI InChI=1S/C21H28O4/c1-3-25-19-11-15-12(9-18(19)23)8-13(22)10-16-14(15)6-7-21(2)17(16)4-5-20(21)24/h9,11,14,16-17,20,23-24H,3-8,10H2,1-2H3/t14?,16?,17?,20-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of polymerization of purified bovine tubulin |
J Med Chem 43: 2419-29 (2000)
BindingDB Entry DOI: 10.7270/Q2B56KD4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50173811

((Z)-6-(5-Chloro-2-methoxy-phenyl)-6-(2,7-dimethyl-...)Show SMILES COC(=O)CCC\C=C(\c1cc(C)c2on(C)c(=O)c2c1)c1cc(Cl)ccc1OC Show InChI InChI=1S/C23H24ClNO5/c1-14-11-15(12-19-22(14)30-25(2)23(19)27)17(7-5-6-8-21(26)29-4)18-13-16(24)9-10-20(18)28-3/h7,9-13H,5-6,8H2,1-4H3/b17-7- | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV-1 reverse transcriptase activity |
J Med Chem 48: 6140-55 (2005)
Article DOI: 10.1021/jm050452s
BindingDB Entry DOI: 10.7270/Q2TB16F6 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363363
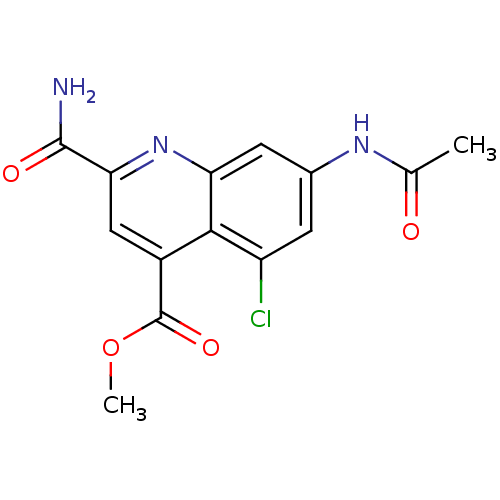
(CHEMBL1945622)Show InChI InChI=1S/C14H12ClN3O4/c1-6(19)17-7-3-9(15)12-8(14(21)22-2)5-11(13(16)20)18-10(12)4-7/h3-5H,1-2H3,(H2,16,20)(H,17,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50173818
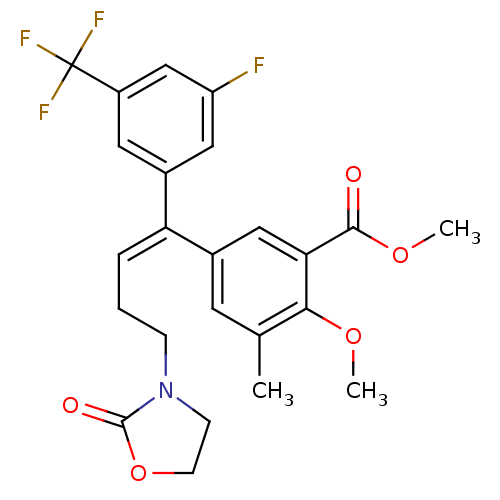
(5-[(E)-1-(3-Fluoro-5-trifluoromethyl-phenyl)-4-(2-...)Show SMILES COC(=O)c1cc(cc(C)c1OC)C(=C/CCN1CCOC1=O)\c1cc(F)cc(c1)C(F)(F)F Show InChI InChI=1S/C24H23F4NO5/c1-14-9-15(12-20(21(14)32-2)22(30)33-3)19(5-4-6-29-7-8-34-23(29)31)16-10-17(24(26,27)28)13-18(25)11-16/h5,9-13H,4,6-8H2,1-3H3/b19-5+ | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV-1 reverse transcriptase activity |
J Med Chem 48: 6140-55 (2005)
Article DOI: 10.1021/jm050452s
BindingDB Entry DOI: 10.7270/Q2TB16F6 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363374
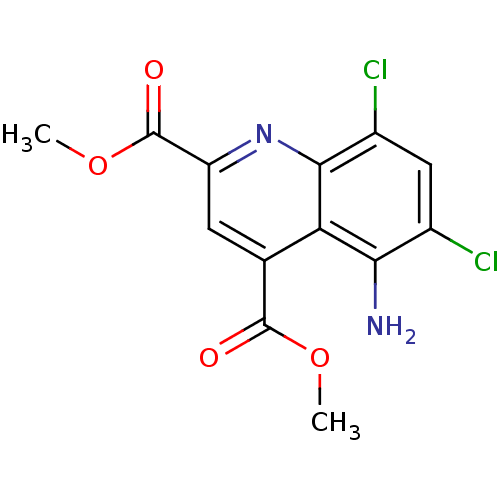
(CHEMBL1945628)Show InChI InChI=1S/C13H10Cl2N2O4/c1-20-12(18)5-3-8(13(19)21-2)17-11-7(15)4-6(14)10(16)9(5)11/h3-4H,16H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363377

(CHEMBL1945727)Show SMILES CN(C)C(=O)c1cc2C(=O)N(C)c3c(Cl)cc(Cl)c(n1)c23 Show InChI InChI=1S/C14H11Cl2N3O2/c1-18(2)14(21)9-4-6-10-11(17-9)7(15)5-8(16)12(10)19(3)13(6)20/h4-5H,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain
(Bos taurus) | BDBM50089335

(9-Ethoxy-1,8-dihydroxy-12a-methyl-2,3,3a,3b,4,5,10...)Show SMILES CCOc1cc2C3CC[C@]4(C)[C@@H](O)CCC4C3CC\C(=N/OC)c2cc1O Show InChI InChI=1S/C22H31NO4/c1-4-27-20-12-15-13-9-10-22(2)17(6-8-21(22)25)14(13)5-7-18(23-26-3)16(15)11-19(20)24/h11-14,17,21,24-25H,4-10H2,1-3H3/b23-18+/t13?,14?,17?,21-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of polymerization of purified bovine tubulin |
J Med Chem 43: 2419-29 (2000)
BindingDB Entry DOI: 10.7270/Q2B56KD4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50173812
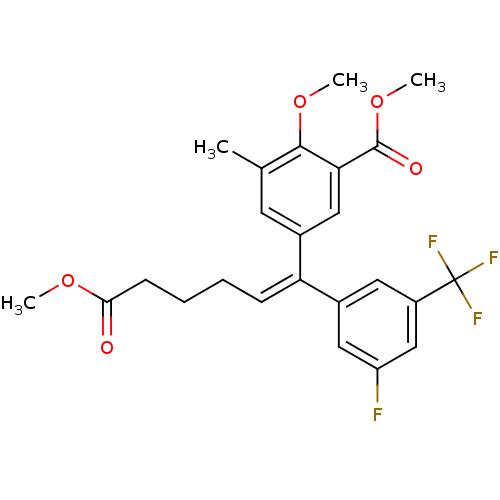
(5-[(E)-1-(3-Fluoro-5-trifluoromethyl-phenyl)-5-met...)Show SMILES COC(=O)CCC\C=C(\c1cc(F)cc(c1)C(F)(F)F)c1cc(C)c(OC)c(c1)C(=O)OC Show InChI InChI=1S/C24H24F4O5/c1-14-9-15(12-20(22(14)32-3)23(30)33-4)19(7-5-6-8-21(29)31-2)16-10-17(24(26,27)28)13-18(25)11-16/h7,9-13H,5-6,8H2,1-4H3/b19-7+ | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV-1 reverse transcriptase activity |
J Med Chem 48: 6140-55 (2005)
Article DOI: 10.1021/jm050452s
BindingDB Entry DOI: 10.7270/Q2TB16F6 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain
(Bos taurus) | BDBM50089340

(9-Ethoxy-1,8-dihydroxy-12a-methyl-2,3,3a,3b,4,5,10...)Show SMILES CCOc1cc2C3CC[C@]4(C)[C@@H](O)CCC4C3CCC(N=O)c2cc1O Show InChI InChI=1S/C21H29NO4/c1-3-26-19-11-14-12-8-9-21(2)16(5-7-20(21)24)13(12)4-6-17(22-25)15(14)10-18(19)23/h10-13,16-17,20,23-24H,3-9H2,1-2H3/t12?,13?,16?,17?,20-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of polymerization of purified bovine tubulin |
J Med Chem 43: 2419-29 (2000)
BindingDB Entry DOI: 10.7270/Q2B56KD4 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain
(Bos taurus) | BDBM50089345

(B-Homo-2-ethoxy-3,17beta-estradiol-7-tosylhydrazon...)Show SMILES CCOc1cc2C3CC[C@]4(C)[C@@H](O)CCC4C3CC(Cc2cc1O)=NNS(=O)(=O)c1ccc(C)cc1 |w:24.28| Show InChI InChI=1S/C28H36N2O5S/c1-4-35-26-16-22-18(14-25(26)31)13-19(29-30-36(33,34)20-7-5-17(2)6-8-20)15-23-21(22)11-12-28(3)24(23)9-10-27(28)32/h5-8,14,16,21,23-24,27,30-32H,4,9-13,15H2,1-3H3/t21?,23?,24?,27-,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of polymerization of purified bovine tubulin |
J Med Chem 43: 2419-29 (2000)
BindingDB Entry DOI: 10.7270/Q2B56KD4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50173809
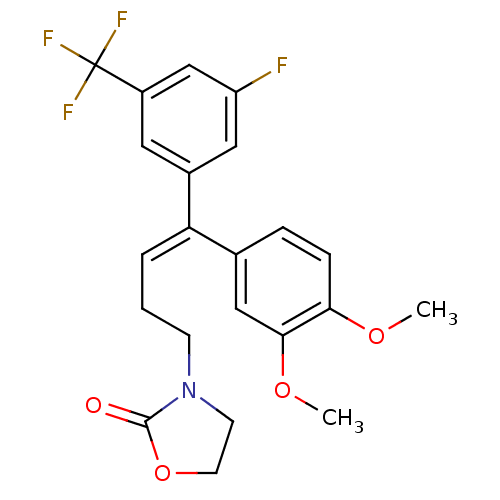
(3-[(E)-4-(3,4-Dimethoxy-phenyl)-4-(3-fluoro-5-trif...)Show SMILES COc1ccc(cc1OC)C(=C/CCN1CCOC1=O)\c1cc(F)cc(c1)C(F)(F)F Show InChI InChI=1S/C22H21F4NO4/c1-29-19-6-5-14(12-20(19)30-2)18(4-3-7-27-8-9-31-21(27)28)15-10-16(22(24,25)26)13-17(23)11-15/h4-6,10-13H,3,7-9H2,1-2H3/b18-4+ | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV-1 reverse transcriptase activity |
J Med Chem 48: 6140-55 (2005)
Article DOI: 10.1021/jm050452s
BindingDB Entry DOI: 10.7270/Q2TB16F6 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1/Tubulin beta-2B chain
(Bos taurus) | BDBM50089351

(9-Ethoxy-1,8-dihydroxy-12a-methyl-2,3,3a,3b,4,6,10...)Show SMILES CCOc1cc2C3CC[C@]4(C)[C@@H](O)CCC4C3CC(Cc2cc1O)N=O Show InChI InChI=1S/C21H29NO4/c1-3-26-19-11-15-12(9-18(19)23)8-13(22-25)10-16-14(15)6-7-21(2)17(16)4-5-20(21)24/h9,11,13-14,16-17,20,23-24H,3-8,10H2,1-2H3/t13?,14?,16?,17?,20-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 9.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of polymerization of purified bovine tubulin |
J Med Chem 43: 2419-29 (2000)
BindingDB Entry DOI: 10.7270/Q2B56KD4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50173814
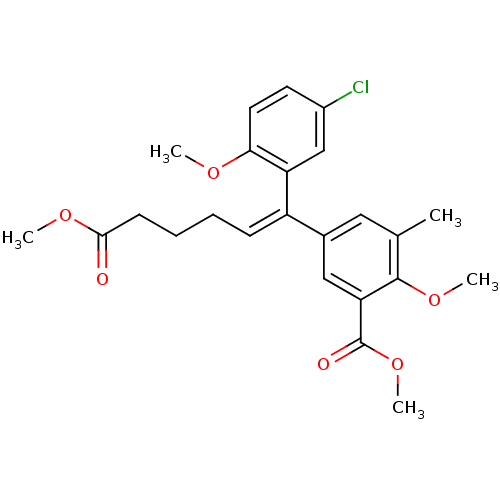
(5-[(Z)-1-(5-Chloro-2-methoxy-phenyl)-5-methoxycarb...)Show SMILES COC(=O)CCC\C=C(\c1cc(C)c(OC)c(c1)C(=O)OC)c1cc(Cl)ccc1OC Show InChI InChI=1S/C24H27ClO6/c1-15-12-16(13-20(23(15)30-4)24(27)31-5)18(8-6-7-9-22(26)29-3)19-14-17(25)10-11-21(19)28-2/h8,10-14H,6-7,9H2,1-5H3/b18-8- | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV-1 reverse transcriptase activity |
J Med Chem 48: 6140-55 (2005)
Article DOI: 10.1021/jm050452s
BindingDB Entry DOI: 10.7270/Q2TB16F6 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50173815
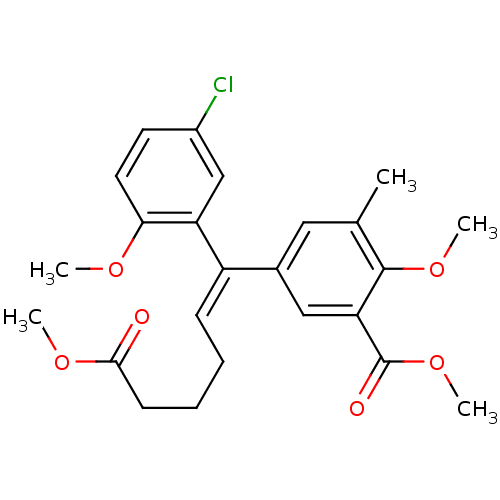
(5-[(E)-1-(5-Chloro-2-methoxy-phenyl)-5-methoxycarb...)Show SMILES COC(=O)CCC\C=C(/c1cc(C)c(OC)c(c1)C(=O)OC)c1cc(Cl)ccc1OC Show InChI InChI=1S/C24H27ClO6/c1-15-12-16(13-20(23(15)30-4)24(27)31-5)18(8-6-7-9-22(26)29-3)19-14-17(25)10-11-21(19)28-2/h8,10-14H,6-7,9H2,1-5H3/b18-8+ | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV-1 reverse transcriptase activity |
J Med Chem 48: 6140-55 (2005)
Article DOI: 10.1021/jm050452s
BindingDB Entry DOI: 10.7270/Q2TB16F6 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50173817

(3-Chloro-5-[(E)-1-(3-fluoro-5-trifluoromethyl-phen...)Show SMILES COC(=O)CCC\C=C(/c1cc(F)cc(c1)C(F)(F)F)c1cc(Cl)c(OC)c(c1)C(=O)OC Show InChI InChI=1S/C23H21ClF4O5/c1-31-20(29)7-5-4-6-17(13-8-15(23(26,27)28)12-16(25)9-13)14-10-18(22(30)33-3)21(32-2)19(24)11-14/h6,8-12H,4-5,7H2,1-3H3/b17-6+ | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV-1 reverse transcriptase activity |
J Med Chem 48: 6140-55 (2005)
Article DOI: 10.1021/jm050452s
BindingDB Entry DOI: 10.7270/Q2TB16F6 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50410542
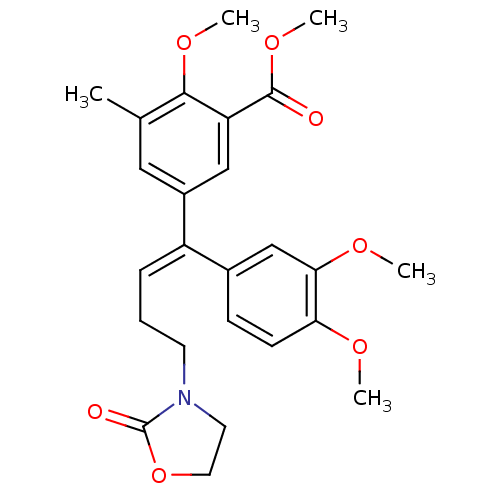
(CHEMBL2096827)Show SMILES COC(=O)c1cc(cc(C)c1OC)C(=C\CCN1CCOC1=O)\c1ccc(OC)c(OC)c1 Show InChI InChI=1S/C25H29NO7/c1-16-13-18(14-20(23(16)31-4)24(27)32-5)19(7-6-10-26-11-12-33-25(26)28)17-8-9-21(29-2)22(15-17)30-3/h7-9,13-15H,6,10-12H2,1-5H3/b19-7+ | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV-1 reverse transcriptase activity |
J Med Chem 48: 6140-55 (2005)
Article DOI: 10.1021/jm050452s
BindingDB Entry DOI: 10.7270/Q2TB16F6 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50173806
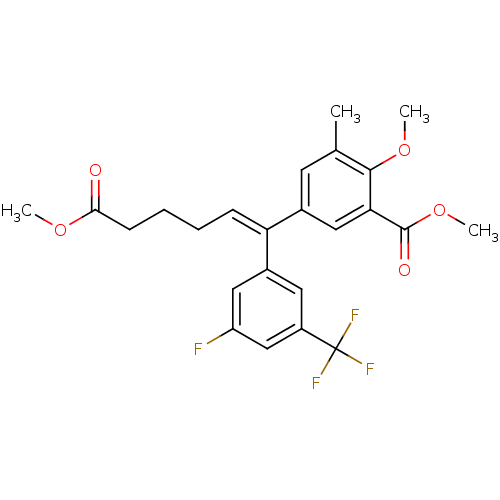
(5-[(Z)-1-(3-Fluoro-5-trifluoromethyl-phenyl)-5-met...)Show SMILES COC(=O)CCC\C=C(/c1cc(F)cc(c1)C(F)(F)F)c1cc(C)c(OC)c(c1)C(=O)OC Show InChI InChI=1S/C24H24F4O5/c1-14-9-15(12-20(22(14)32-3)23(30)33-4)19(7-5-6-8-21(29)31-2)16-10-17(24(26,27)28)13-18(25)11-16/h7,9-13H,5-6,8H2,1-4H3/b19-7- | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV-1 reverse transcriptase activity |
J Med Chem 48: 6140-55 (2005)
Article DOI: 10.1021/jm050452s
BindingDB Entry DOI: 10.7270/Q2TB16F6 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363362
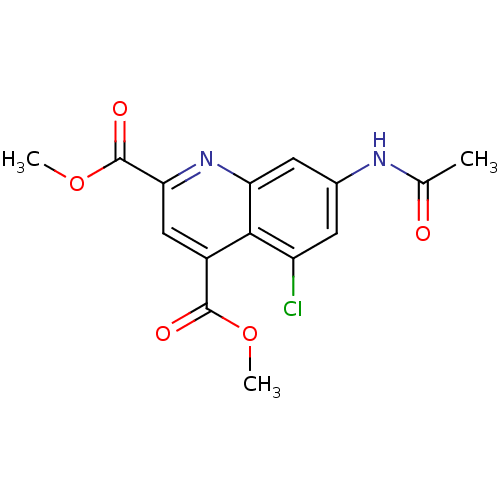
(CHEMBL1945621)Show SMILES COC(=O)c1cc(C(=O)OC)c2c(Cl)cc(NC(C)=O)cc2n1 Show InChI InChI=1S/C15H13ClN2O5/c1-7(19)17-8-4-10(16)13-9(14(20)22-2)6-12(15(21)23-3)18-11(13)5-8/h4-6H,1-3H3,(H,17,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50173804
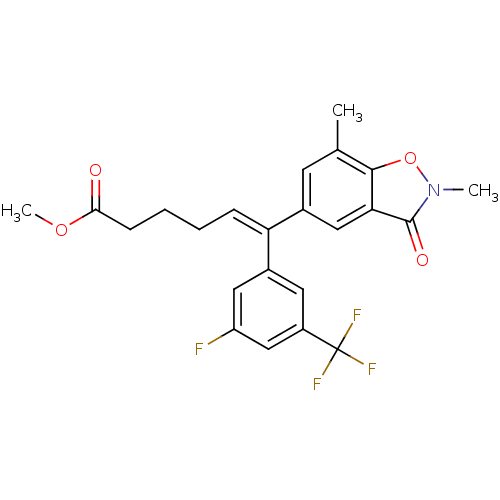
((Z)-6-(2,7-Dimethyl-3-oxo-2,3-dihydro-benzo[d]isox...)Show SMILES COC(=O)CCC\C=C(/c1cc(F)cc(c1)C(F)(F)F)c1cc(C)c2on(C)c(=O)c2c1 Show InChI InChI=1S/C23H21F4NO4/c1-13-8-14(11-19-21(13)32-28(2)22(19)30)18(6-4-5-7-20(29)31-3)15-9-16(23(25,26)27)12-17(24)10-15/h6,8-12H,4-5,7H2,1-3H3/b18-6- | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV-1 reverse transcriptase activity |
J Med Chem 48: 6140-55 (2005)
Article DOI: 10.1021/jm050452s
BindingDB Entry DOI: 10.7270/Q2TB16F6 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50173807
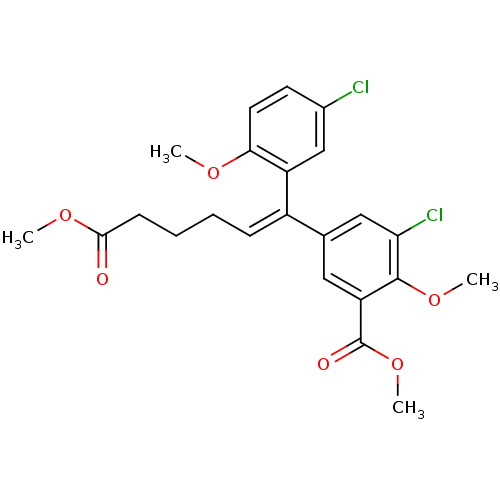
(3-Chloro-5-[(Z)-1-(5-chloro-2-methoxy-phenyl)-5-me...)Show SMILES COC(=O)CCC\C=C(\c1cc(Cl)c(OC)c(c1)C(=O)OC)c1cc(Cl)ccc1OC Show InChI InChI=1S/C23H24Cl2O6/c1-28-20-10-9-15(24)13-17(20)16(7-5-6-8-21(26)29-2)14-11-18(23(27)31-4)22(30-3)19(25)12-14/h7,9-13H,5-6,8H2,1-4H3/b16-7- | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV-1 reverse transcriptase activity |
J Med Chem 48: 6140-55 (2005)
Article DOI: 10.1021/jm050452s
BindingDB Entry DOI: 10.7270/Q2TB16F6 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50173816
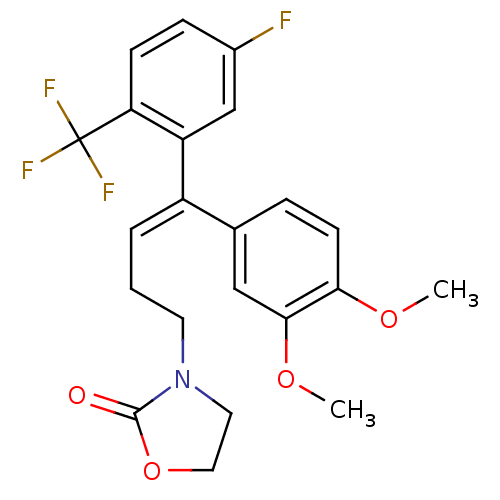
(3-[(E)-4-(3,4-Dimethoxy-phenyl)-4-(5-fluoro-2-trif...)Show SMILES COc1ccc(cc1OC)C(=C/CCN1CCOC1=O)\c1cc(F)ccc1C(F)(F)F Show InChI InChI=1S/C22H21F4NO4/c1-29-19-8-5-14(12-20(19)30-2)16(4-3-9-27-10-11-31-21(27)28)17-13-15(23)6-7-18(17)22(24,25)26/h4-8,12-13H,3,9-11H2,1-2H3/b16-4+ | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV-1 reverse transcriptase activity |
J Med Chem 48: 6140-55 (2005)
Article DOI: 10.1021/jm050452s
BindingDB Entry DOI: 10.7270/Q2TB16F6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data