

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50235302 (CHEMBL4099771) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Competitive inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50075098 (CHEMBL3414626 | US10143704, Compound A2 | US944606...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Competitive inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]Spiperone from human recombinant dopamine D2S receptor expressed in CHO cells after 2 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50435127 (CHEMBL2392022) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [125I]Peptide YY from neuropeptide Y receptor type 2 in human KAN-TS cells after 2 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM85035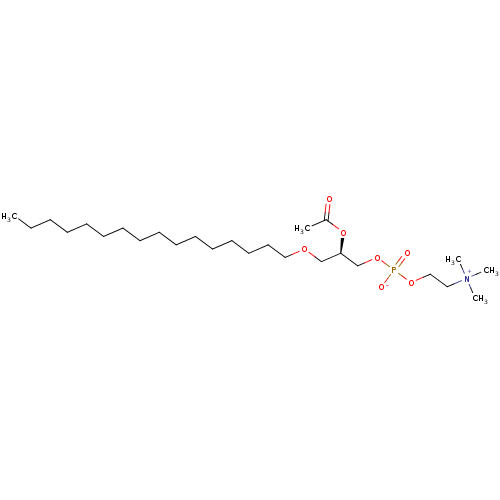 (CAS_65154-06-5 | PAF | bloodplatelet-activatingfac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]PAF from platelet activating factor receptor in human platelets after 3 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50015490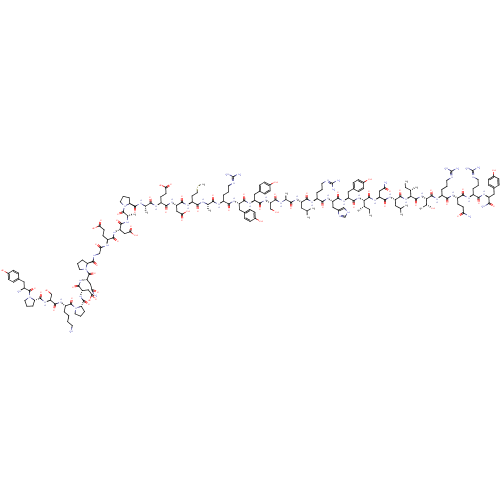 (CHEMBL438945 | H-YPSKPDNPGEDAPAEDMARYYSALRHYINLITR...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [125I]Peptide YY from neuropeptide Y receptor type 1 in human SK-N-MC cells after 60 mins | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156454 (CHEMBL264100 | des-Arg10-Kallidin) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H](Des-Arg10)-Kallidin from bradykinin B1 receptor in human IMR90 cells after 60 mins | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50268107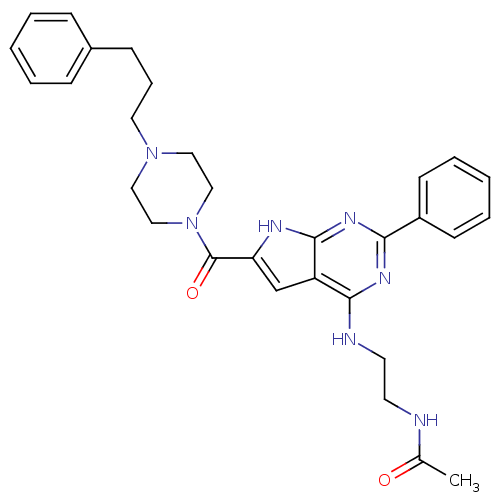 (CHEMBL485862 | CHEMBL500634 | N-(2-(2-phenyl-6-(4-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human A2B receptor | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01431 BindingDB Entry DOI: 10.7270/Q2JQ14S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074509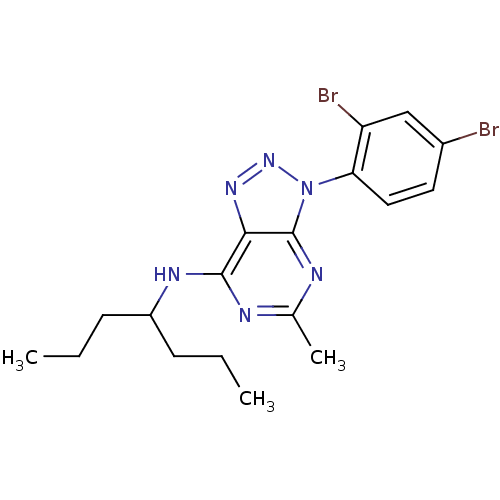 (CHEMBL354886 | [3-(2,4-Dibromo-phenyl)-5-methyl-3H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074501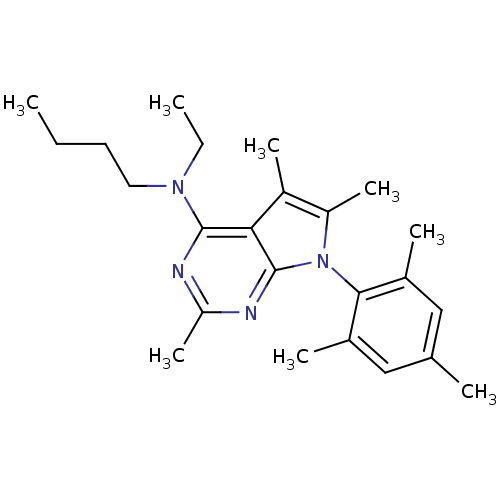 (Butyl-ethyl-[2,5,6-trimethyl-7-(2,4,6-trimethyl-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074492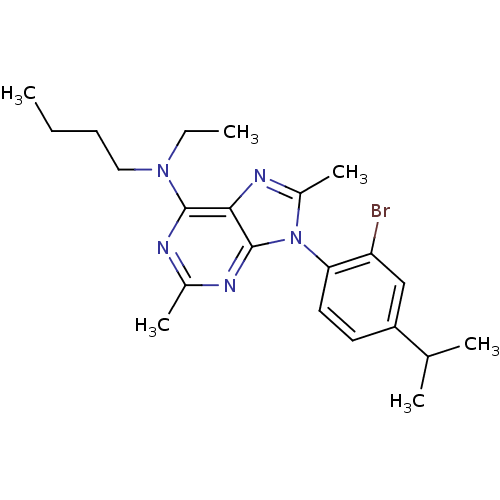 (CHEMBL168554 | [9-(2-Bromo-4-isopropyl-phenyl)-2,8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50074439 (1-(2-Bromo-4,6-dimethoxy-phenyl)-4,6-dimethyl-1H-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity was determined against corticotropin releasing factor receptor 1 | J Med Chem 42: 819-32 (1999) Article DOI: 10.1021/jm980223o BindingDB Entry DOI: 10.7270/Q22V2GT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50049949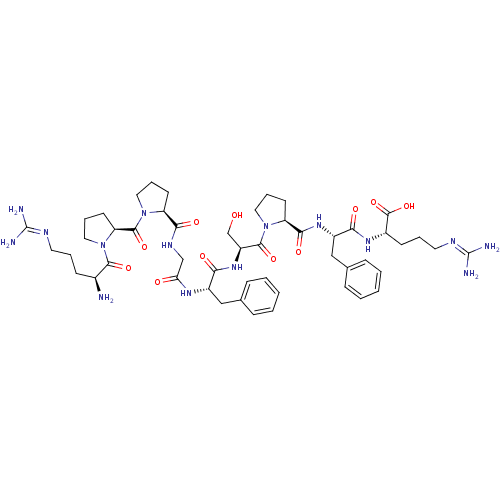 ((BK) H-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg-OH | (b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]Bradykinin from human recombinant bradykinin B2 receptor expressed in CHEM1 cells after 60 mins | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074465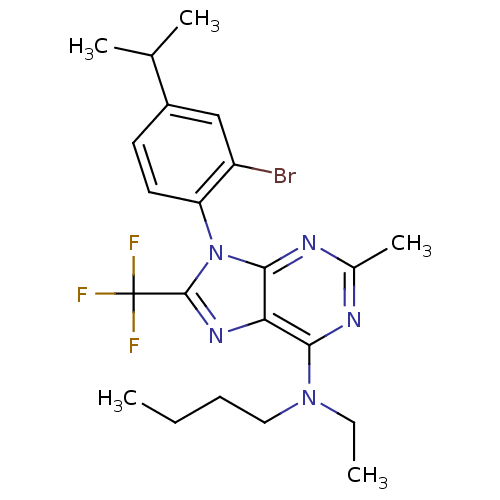 (CHEMBL354982 | [9-(2-Bromo-4-isopropyl-phenyl)-2-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074500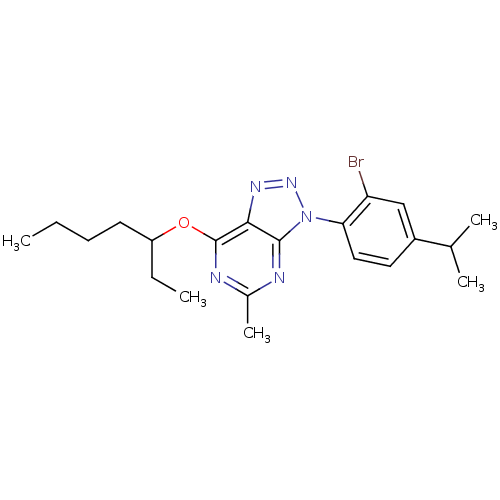 (3-(2-Bromo-4-isopropyl-phenyl)-7-(1-ethyl-pentylox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM22567 (3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]Pyrilamine from human recombinant histamine H1 receptor expressed in CHOK1 cells after 3 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50058163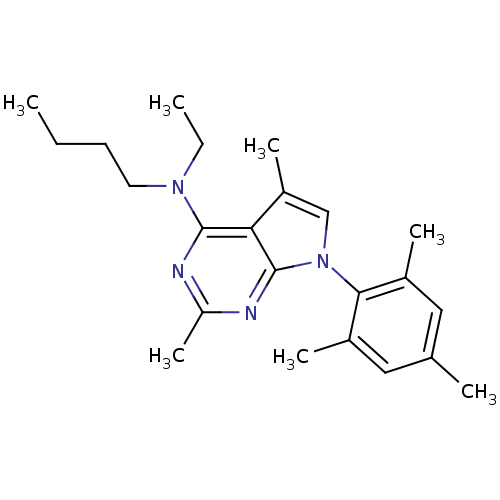 (Butyl-[2,5-dimethyl-7-(2,4,6-trimethyl-phenyl)-7H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM21190 (4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]ZM2421385 from adenosine A2A receptor expressed in human HeLa cell membranes incubated for 30 mins by scintillation counting meth... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01431 BindingDB Entry DOI: 10.7270/Q2JQ14S5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074470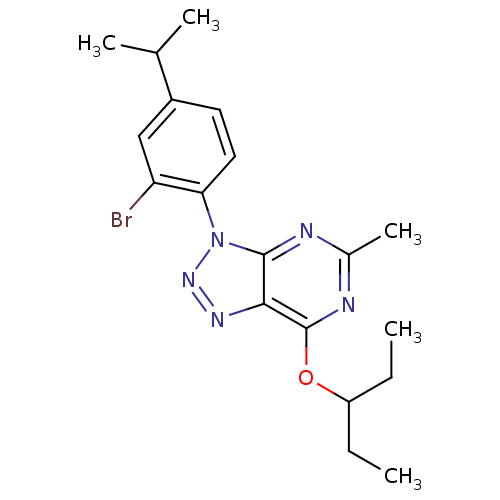 (3-(2-Bromo-4-isopropyl-phenyl)-7-(1-ethyl-propoxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074482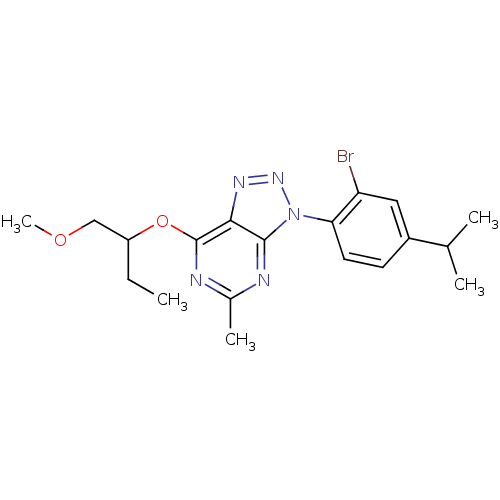 (3-(2-Bromo-4-isopropyl-phenyl)-7-(1-methoxymethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50086170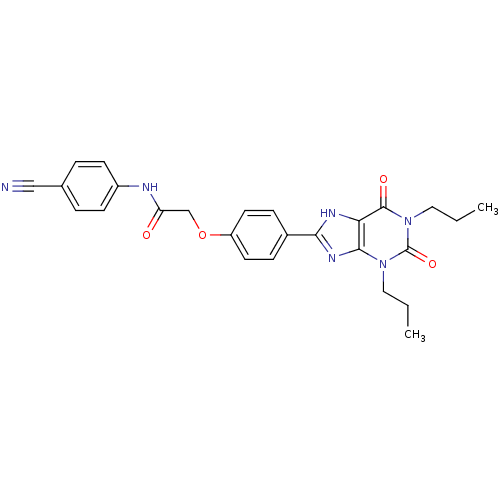 ((4-Cyano-phenyl)-carbamic acid 4-(2,6-dioxo-1,3-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human A2B receptor | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01431 BindingDB Entry DOI: 10.7270/Q2JQ14S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18207 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]Dexamethasone from glucocorticoid receptor in human HeLaS3 cells after 2 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membranes incubated for 60 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01431 BindingDB Entry DOI: 10.7270/Q2JQ14S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074468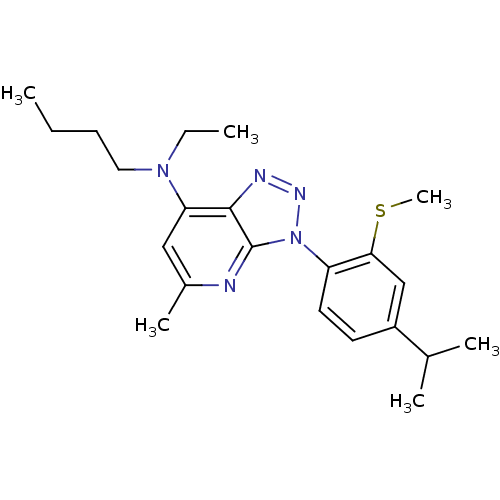 (Butyl-ethyl-[3-(4-isopropyl-2-methylsulfanyl-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM82561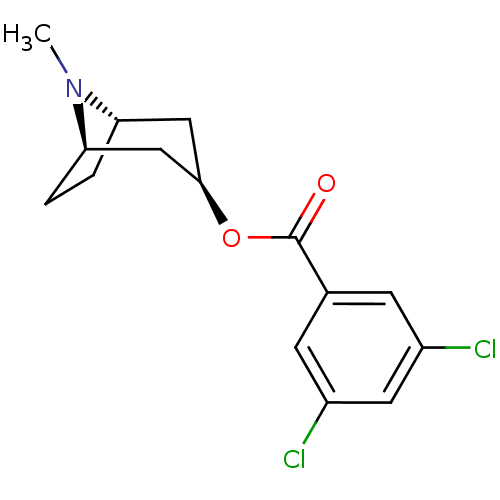 (CAS_40796-97-2 | TROPANYL 3,5-DICHLOROBENZOATE | T...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]GR65630 from human recombinant 5HT3 receptor expressed in HEK293 cells after 60 mins | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50435126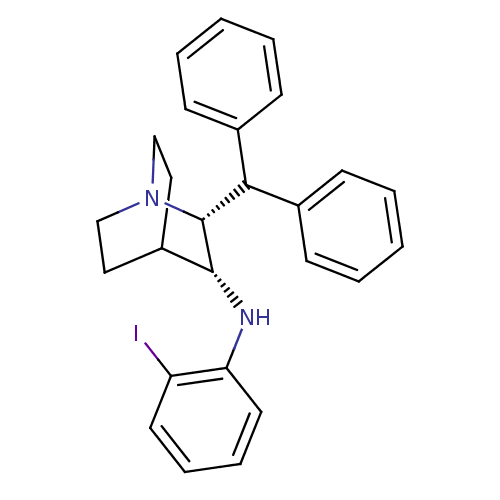 (CHEMBL2392023) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]Substance P from human recombinant substance P receptor expressed in CHO cells after 90 mins | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074474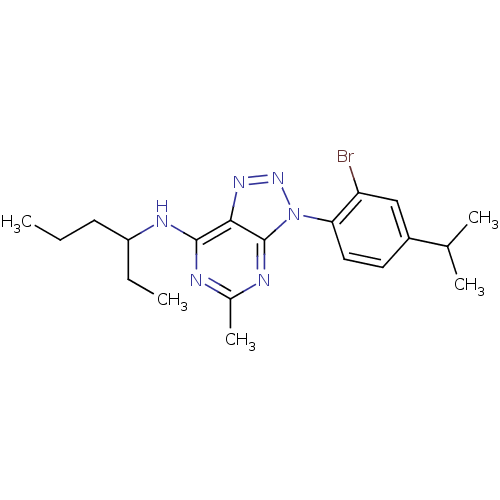 (CHEMBL166669 | [3-(2-Bromo-4-isopropyl-phenyl)-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid type B receptor subunit 1 (Homo sapiens (Human)) | BDBM50435128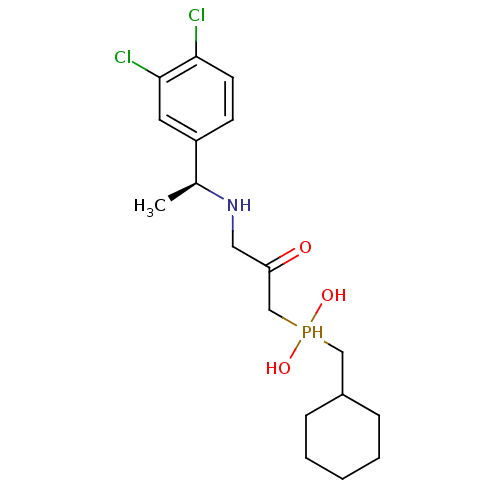 (CHEMBL2391908) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]CGP54626 from human recombinant GABAB1A receptor expressed in CHO cells after 3 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074459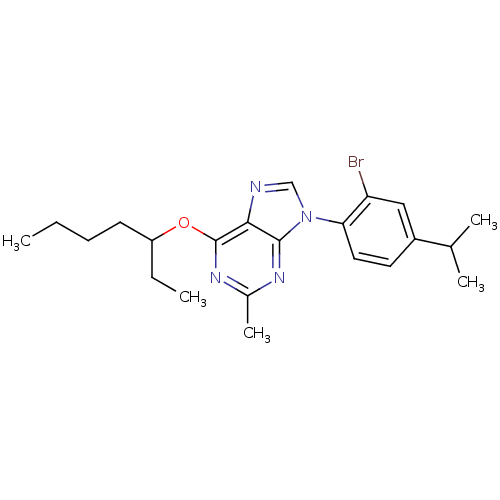 (9-(2-Bromo-4-isopropyl-phenyl)-6-(1-ethyl-pentylox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074458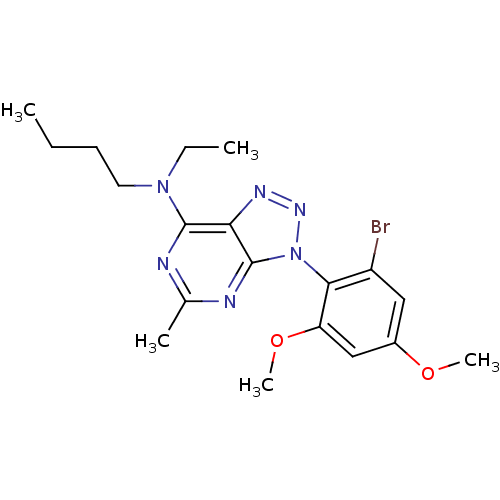 (CHEMBL9633 | [3-(2-Bromo-4,6-dimethoxy-phenyl)-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074452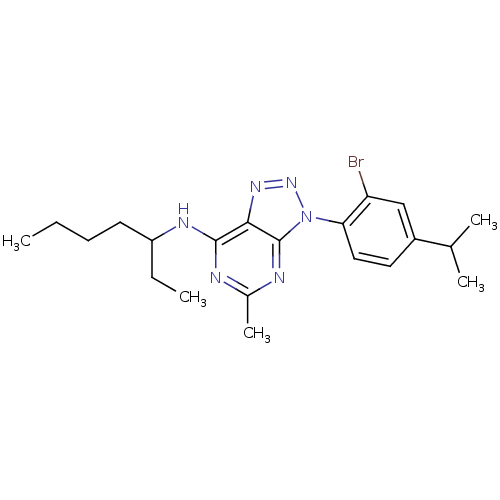 (CHEMBL169952 | [3-(2-Bromo-4-isopropyl-phenyl)-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074453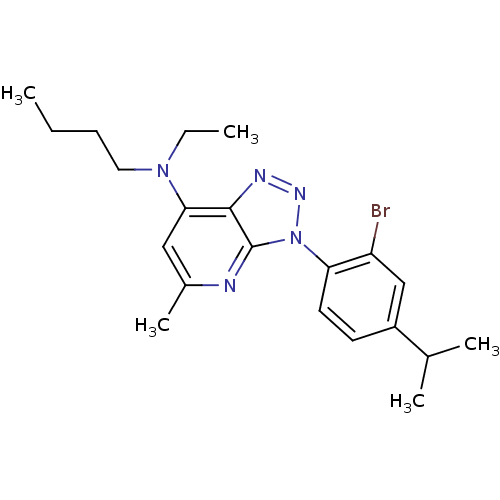 (CHEMBL171202 | [3-(2-Bromo-4-isopropyl-phenyl)-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50159488 (CHEMBL3787197) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human adenosine A2B receptor expressed in HEK293 cell membranes incubated for 30 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01431 BindingDB Entry DOI: 10.7270/Q2JQ14S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074496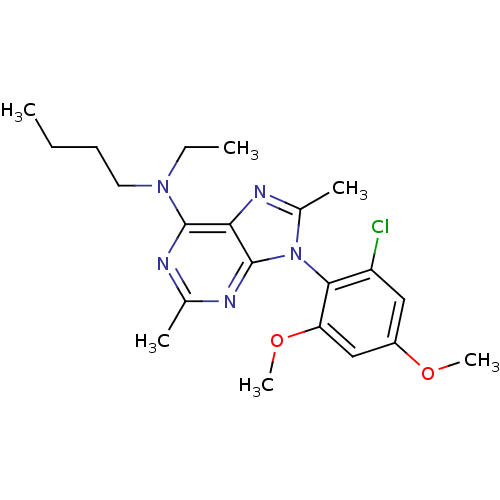 (Butyl-[9-(2-chloro-4,6-dimethoxy-phenyl)-2,8-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074542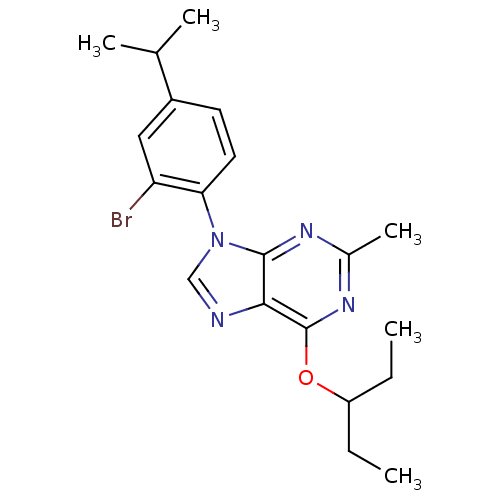 (9-(2-Bromo-4-isopropyl-phenyl)-6-(1-ethyl-propoxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074522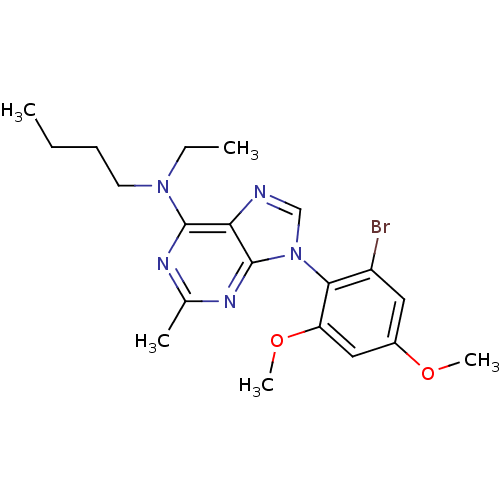 (CHEMBL354940 | [9-(2-Bromo-4,6-dimethoxy-phenyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074546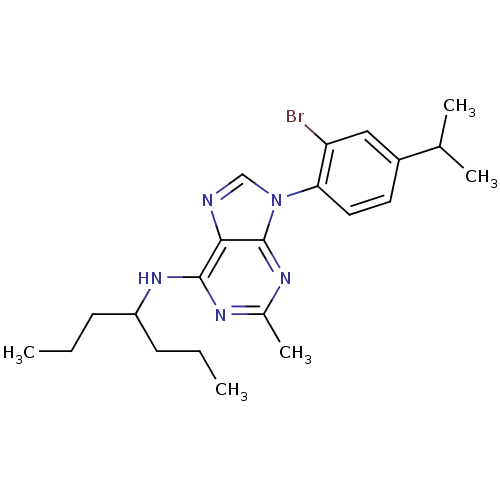 (CHEMBL169430 | [9-(2-Bromo-4-isopropyl-phenyl)-2-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074456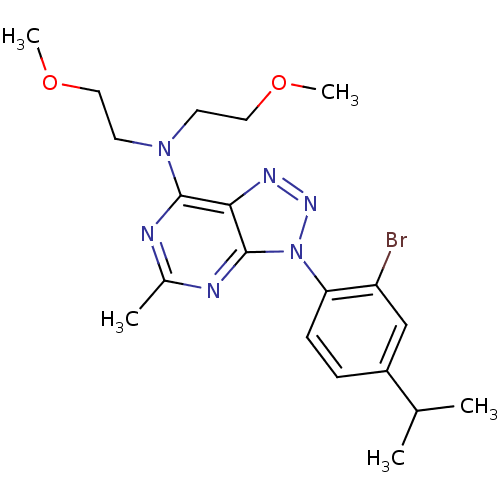 (CHEMBL10504 | SC241 | [3-(2-Bromo-4-isopropyl-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074495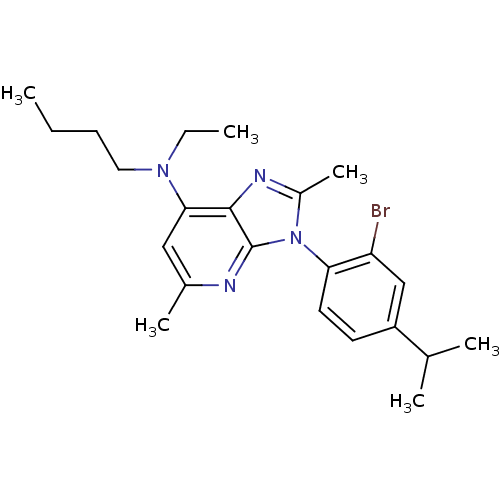 (CHEMBL355049 | [3-(2-Bromo-4-isopropyl-phenyl)-2,5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074525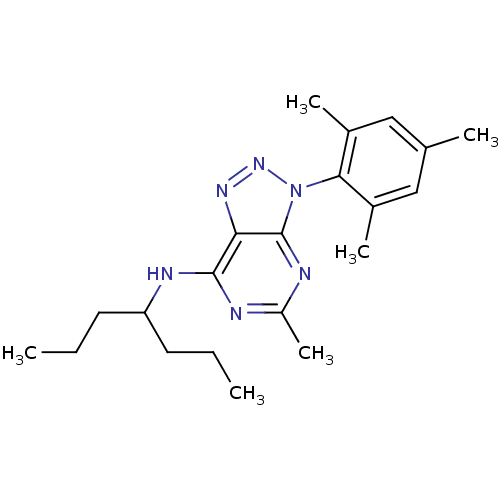 (CHEMBL355689 | [5-Methyl-3-(2,4,6-trimethyl-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074520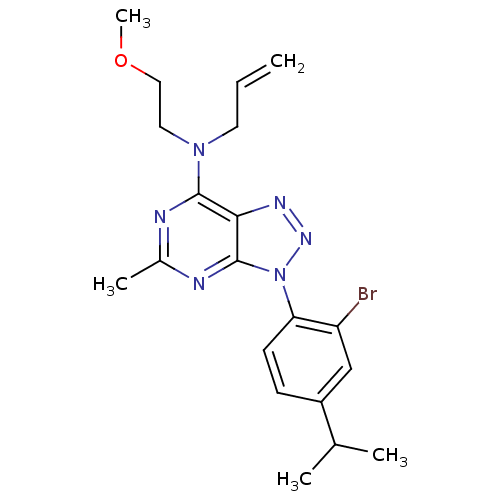 (Allyl-[3-(2-bromo-4-isopropyl-phenyl)-5-methyl-3H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM8885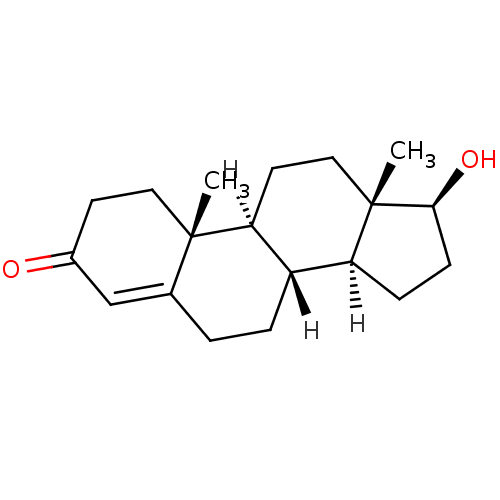 ((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]Mibolerone from rat recombinant androgen receptor expressed in Escherichia coli after 4 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074472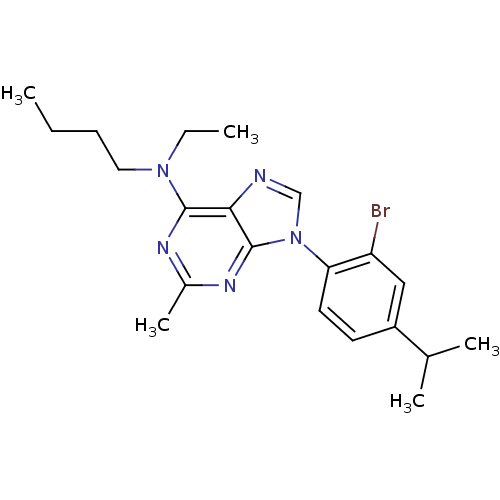 (CHEMBL352340 | [9-(2-Bromo-4-isopropyl-phenyl)-2-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074447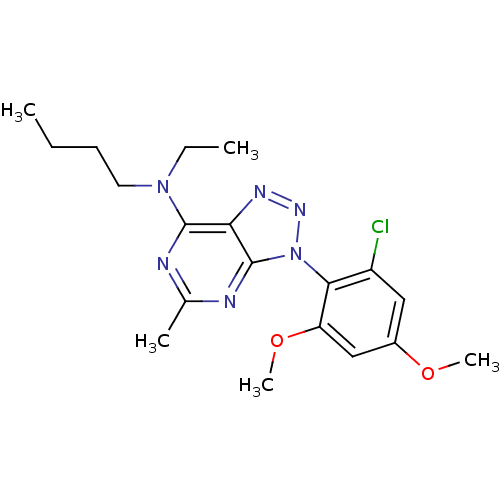 (Butyl-[3-(2-chloro-4,6-dimethoxy-phenyl)-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074534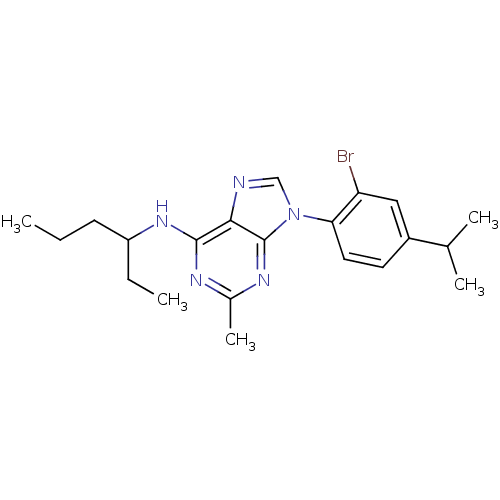 (CHEMBL367110 | [9-(2-Bromo-4-isopropyl-phenyl)-2-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074454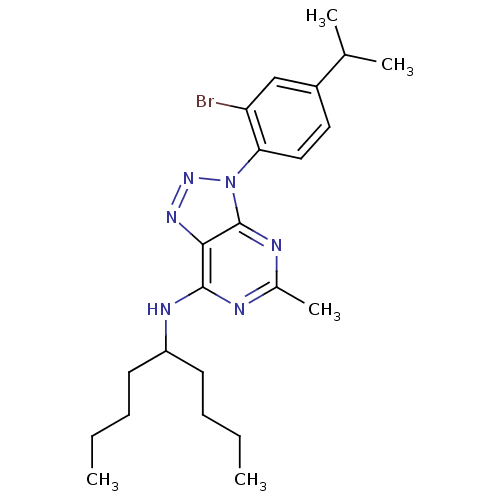 (CHEMBL169781 | [3-(2-Bromo-4-isopropyl-phenyl)-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074457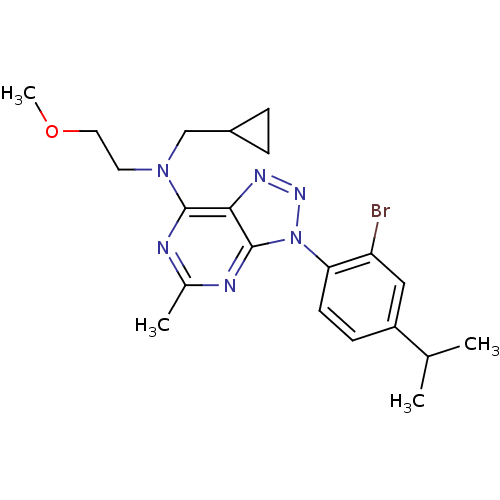 (CHEMBL355329 | [3-(2-Bromo-4-isopropyl-phenyl)-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074503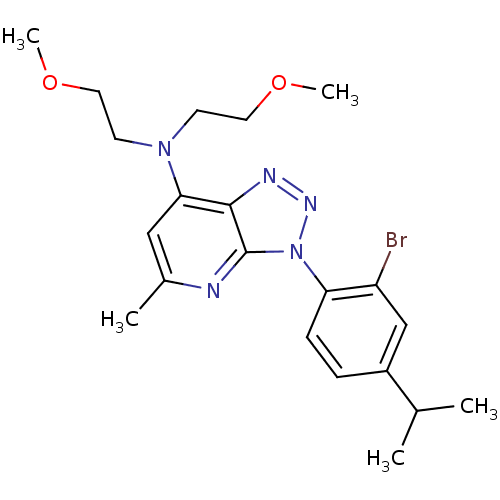 (CHEMBL169561 | [3-(2-Bromo-4-isopropyl-phenyl)-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074449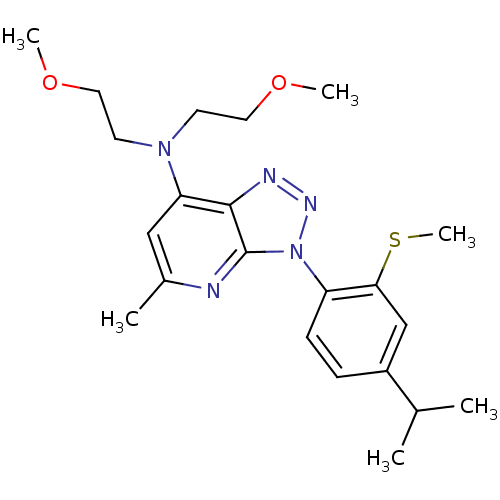 (CHEMBL352353 | [3-(4-Isopropyl-2-methylsulfanyl-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50074411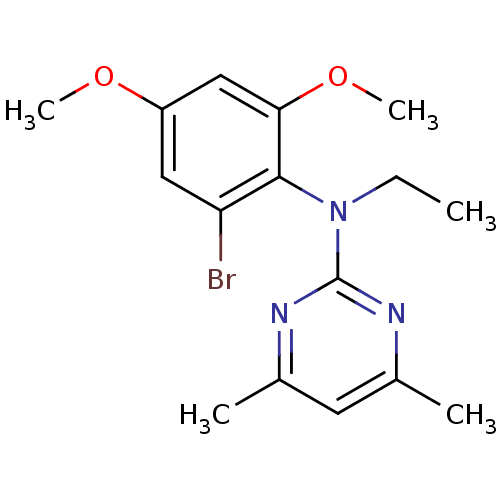 ((2-Bromo-4,6-dimethoxy-phenyl)-(4,6-dimethyl-pyrim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity was determined against corticotropin releasing factor receptor 1 | J Med Chem 42: 819-32 (1999) Article DOI: 10.1021/jm980223o BindingDB Entry DOI: 10.7270/Q22V2GT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 778 total ) | Next | Last >> |