

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM9012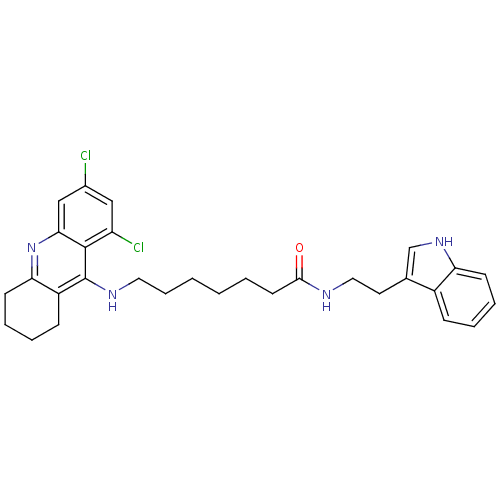 (7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM9017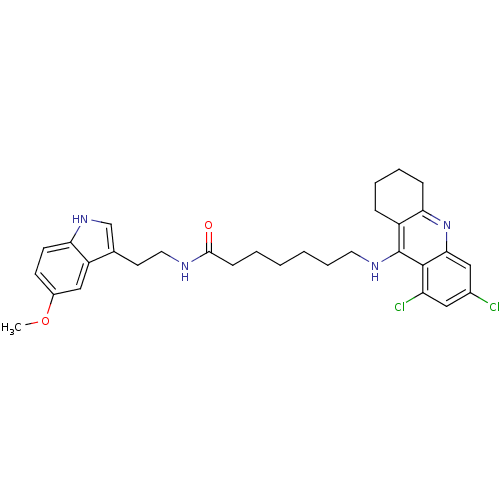 (7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM9009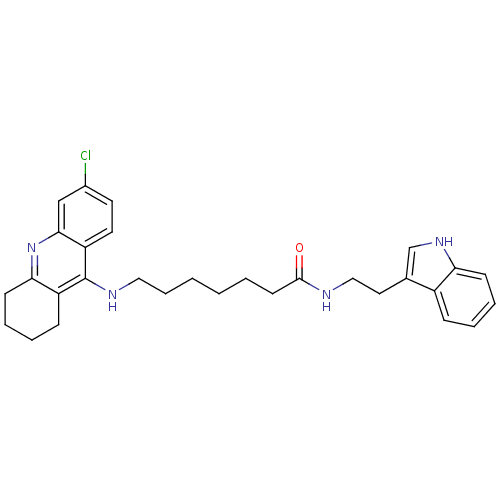 (7-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9009 (7-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM9018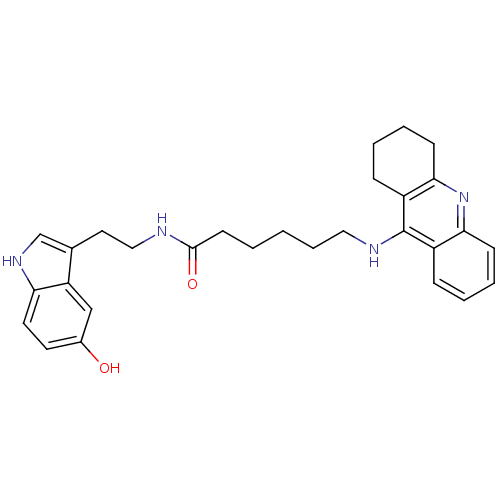 (6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM9007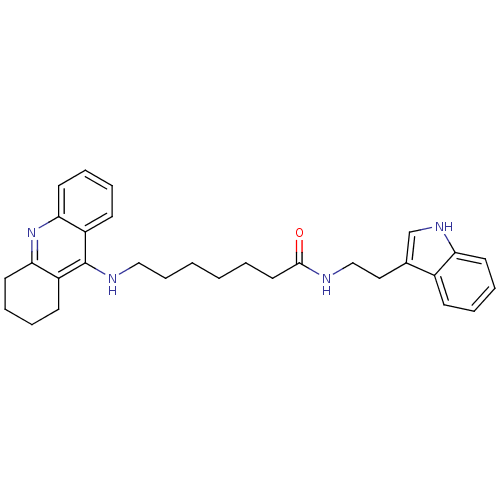 (7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptanoic...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM9014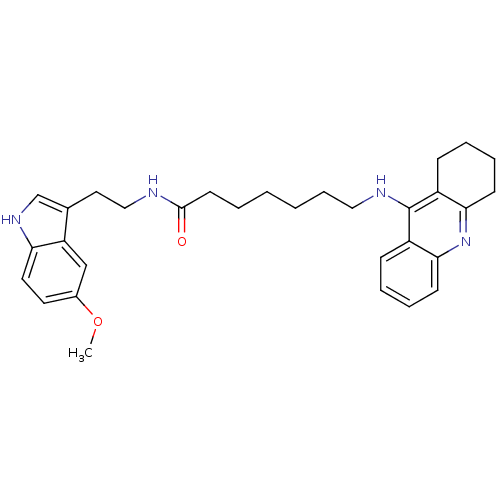 (7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptanoic...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM9010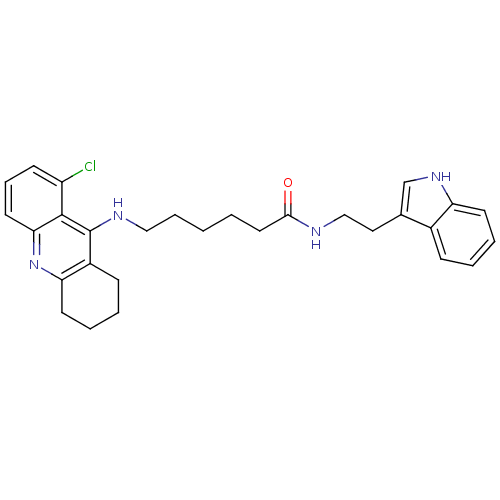 (6-(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM9007 (7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptanoic...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9007 (7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptanoic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9018 (6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9008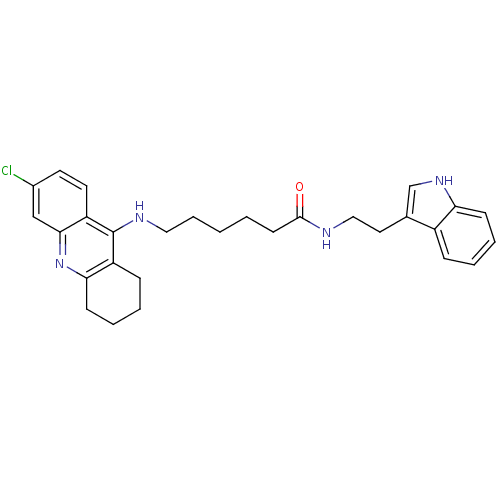 (6-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM9013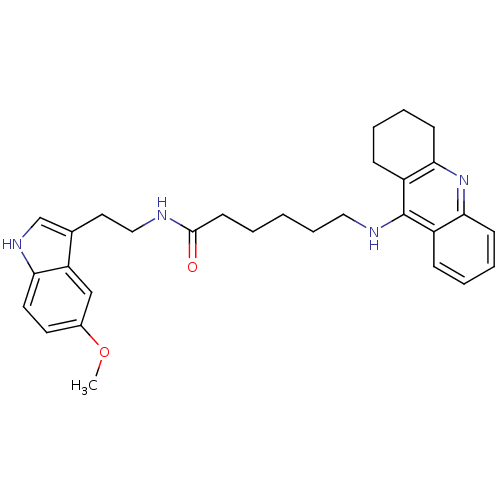 (6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9012 (7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9014 (7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptanoic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM9014 (7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptanoic...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9014 (7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptanoic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM9018 (6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9011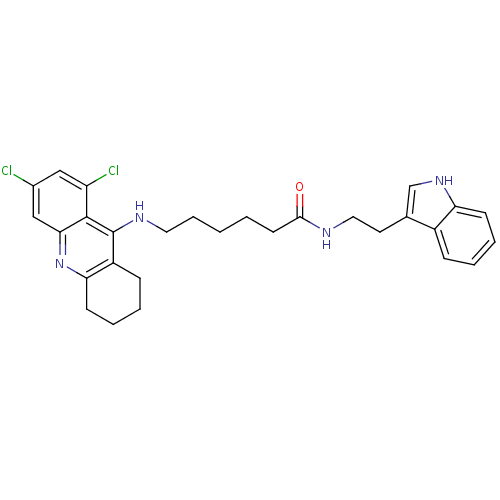 (6-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9006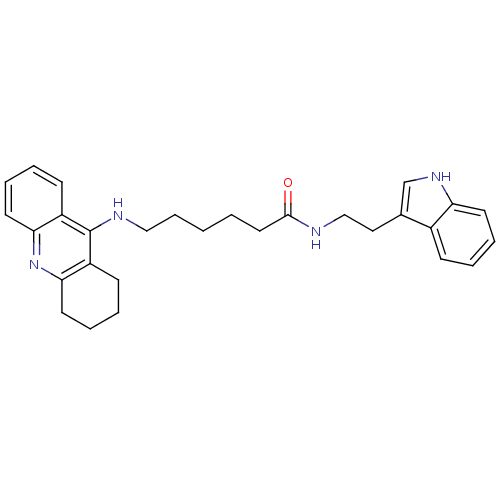 (6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9017 (7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM9008 (6-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9007 (7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptanoic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9012 (7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM9009 (7-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM9011 (6-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylami...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9013 (6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9015 (6-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM9006 (6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM9010 (6-(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM9016 (6-(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9010 (6-(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9017 (7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9016 (6-(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9009 (7-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9018 (6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM9015 (6-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9010 (6-(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM9012 (7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylami...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM9017 (7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylami...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC) | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 459-62 (2006) Article DOI: 10.1021/jm050746d BindingDB Entry DOI: 10.7270/Q2VD6WN2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||