 Found 219 hits with Last Name = 'fleck' and Initial = 'c'
Found 219 hits with Last Name = 'fleck' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50111776

(CHEMBL3605361)Show SMILES [I-].[I-].C[n+]1ccc2n(CCCCCCCCCn3c4ccccc4c4c[n+](C)ccc34)c3ccccc3c2c1 Show InChI InChI=1S/C29H34N4O10/c30-13-21-24(36)25(37)28(42-21)43-22(20-12-19(34)27(41-20)33-11-10-23(35)32-29(33)39)14-31-26(38)17-6-8-18(9-7-17)40-15-16-4-2-1-3-5-16/h1-11,19-22,24-25,27-28,34,36-37H,12-15,30H2,(H,31,38)(H,32,35,39)/t19-,20+,21-,22+,24-,25-,27-,28+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay |
J Med Chem 58: 6710-5 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00958
BindingDB Entry DOI: 10.7270/Q2QZ2CRZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50111774
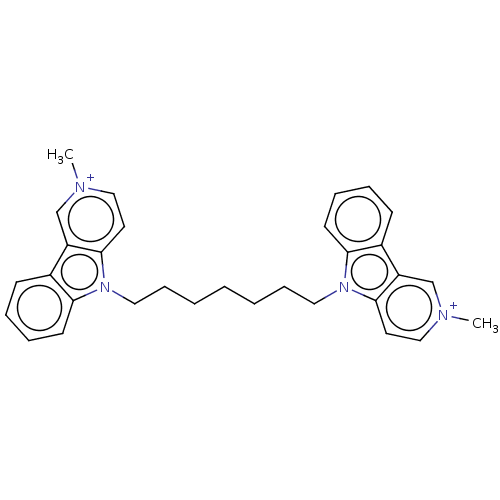
(CHEMBL3605359)Show SMILES [I-].[I-].C[n+]1ccc2n(CCCCCCCn3c4ccccc4c4c[n+](C)ccc34)c3ccccc3c2c1 Show InChI InChI=1S/C24H34N4O9/c1-27(11-13-3-5-14(34-2)6-4-13)12-18(37-23-21(32)20(31)17(10-25)36-23)16-9-15(29)22(35-16)28-8-7-19(30)26-24(28)33/h3-8,15-18,20-23,29,31-32H,9-12,25H2,1-2H3,(H,26,30,33)/t15-,16+,17-,18+,20-,21-,22-,23+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay |
J Med Chem 58: 6710-5 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00958
BindingDB Entry DOI: 10.7270/Q2QZ2CRZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50317179
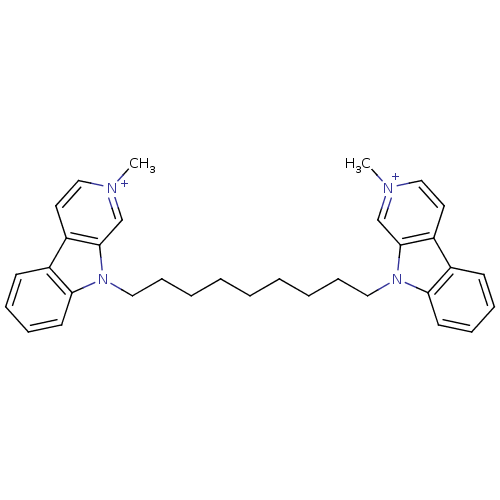
(2-Methyl-9-[9-(2-methyl-beta-carboline-9-yl)nonyl]...)Show SMILES C[n+]1ccc2c(c1)n(CCCCCCCCCn1c3ccccc3c3cc[n+](C)cc13)c1ccccc21 Show InChI InChI=1S/C33H38N4/c1-34-22-18-28-26-14-8-10-16-30(26)36(32(28)24-34)20-12-6-4-3-5-7-13-21-37-31-17-11-9-15-27(31)29-19-23-35(2)25-33(29)37/h8-11,14-19,22-25H,3-7,12-13,20-21H2,1-2H3/q+2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay |
J Med Chem 58: 6710-5 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00958
BindingDB Entry DOI: 10.7270/Q2QZ2CRZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50111775
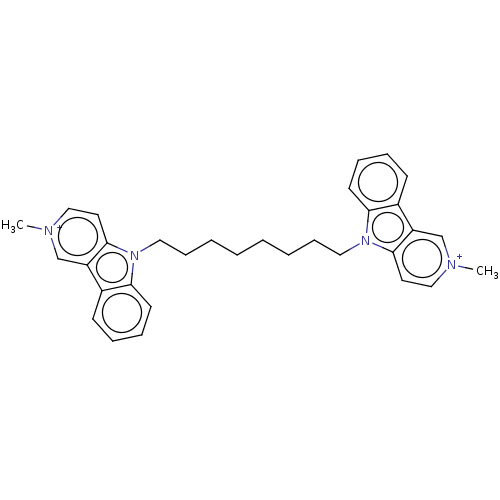
(CHEMBL3605360)Show SMILES [I-].[I-].C[n+]1ccc2n(CCCCCCCCn3c4ccccc4c4c[n+](C)ccc34)c3ccccc3c2c1 Show InChI InChI=1S/C28H38N4O9/c29-13-20-23(35)24(36)27(40-20)41-21(19-12-18(33)26(39-19)32-11-10-22(34)31-28(32)38)14-30-25(37)17-8-6-16(7-9-17)15-4-2-1-3-5-15/h6-11,15,18-21,23-24,26-27,33,35-36H,1-5,12-14,29H2,(H,30,37)(H,31,34,38)/t18-,19+,20-,21+,23-,24-,26-,27+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay |
J Med Chem 58: 6710-5 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00958
BindingDB Entry DOI: 10.7270/Q2QZ2CRZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50396135
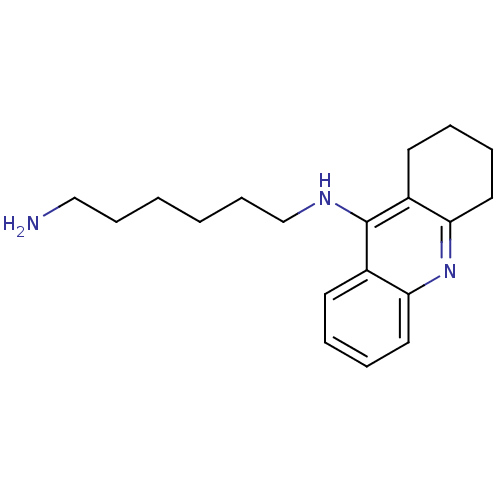
(CHEMBL2171328)Show InChI InChI=1S/C19H27N3/c20-13-7-1-2-8-14-21-19-15-9-3-5-11-17(15)22-18-12-6-4-10-16(18)19/h3,5,9,11H,1-2,4,6-8,10,12-14,20H2,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of horse AChE by colorimetric Ellman assay |
J Med Chem 55: 5231-42 (2012)
Article DOI: 10.1021/jm300246n
BindingDB Entry DOI: 10.7270/Q2X06868 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50396135

(CHEMBL2171328)Show InChI InChI=1S/C19H27N3/c20-13-7-1-2-8-14-21-19-15-9-3-5-11-17(15)22-18-12-6-4-10-16(18)19/h3,5,9,11H,1-2,4,6-8,10,12-14,20H2,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.04 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of horse AChE by colorimetric Ellman assay |
J Med Chem 55: 5231-42 (2012)
Article DOI: 10.1021/jm300246n
BindingDB Entry DOI: 10.7270/Q2X06868 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50111778
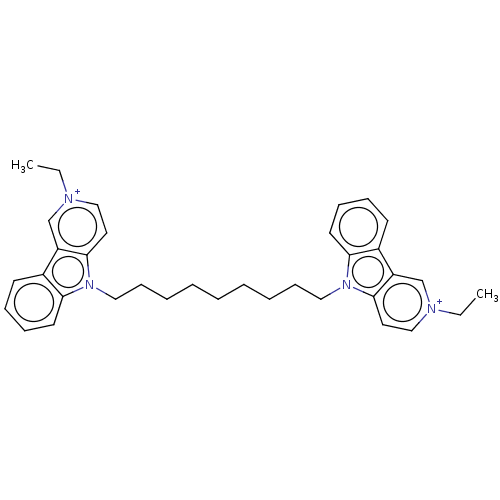
(CHEMBL3605363)Show SMILES [I-].[I-].CC[n+]1ccc2n(CCCCCCCCCn3c4ccccc4c4c[n+](CC)ccc34)c3ccccc3c2c1 Show InChI InChI=1S/C24H31F3N4O8/c25-24(26,27)13-3-1-2-12(8-13)4-6-29-11-17(39-22-20(35)19(34)16(10-28)38-22)15-9-14(32)21(37-15)31-7-5-18(33)30-23(31)36/h1-3,5,7-8,14-17,19-22,29,32,34-35H,4,6,9-11,28H2,(H,30,33,36)/t14-,15+,16-,17+,19-,20-,21-,22+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay |
J Med Chem 58: 6710-5 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00958
BindingDB Entry DOI: 10.7270/Q2QZ2CRZ |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM15581

(CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...)Show InChI InChI=1S/C13H15Cl2NO/c1-3-7-16(2)8-4-9-17-13-6-5-11(14)10-12(13)15/h1,5-6,10H,4,7-9H2,2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOA |
J Med Chem 58: 6710-5 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00958
BindingDB Entry DOI: 10.7270/Q2QZ2CRZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50111780
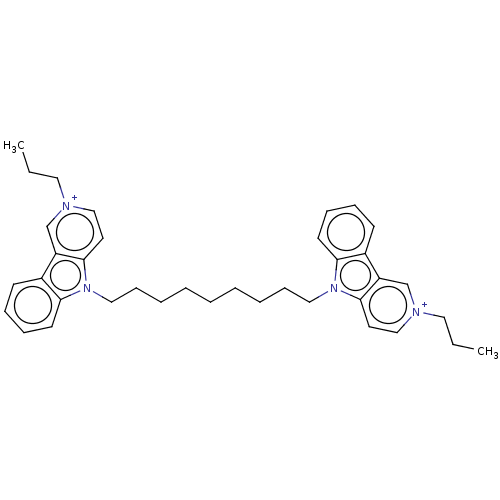
(CHEMBL3605365)Show SMILES [I-].[I-].CCC[n+]1ccc2n(CCCCCCCCCn3c4ccccc4c4c[n+](CCC)ccc34)c3ccccc3c2c1 Show InChI InChI=1S/C22H31N5O8/c23-10-15-18(30)19(31)21(34-15)35-16(11-25-7-3-12-1-5-24-6-2-12)14-9-13(28)20(33-14)27-8-4-17(29)26-22(27)32/h1-2,4-6,8,13-16,18-21,25,28,30-31H,3,7,9-11,23H2,(H,26,29,32)/t13-,14+,15-,16+,18-,19-,20-,21+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay |
J Med Chem 58: 6710-5 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00958
BindingDB Entry DOI: 10.7270/Q2QZ2CRZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of horse AChE by colorimetric Ellman assay |
J Med Chem 55: 5231-42 (2012)
Article DOI: 10.1021/jm300246n
BindingDB Entry DOI: 10.7270/Q2X06868 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50111777
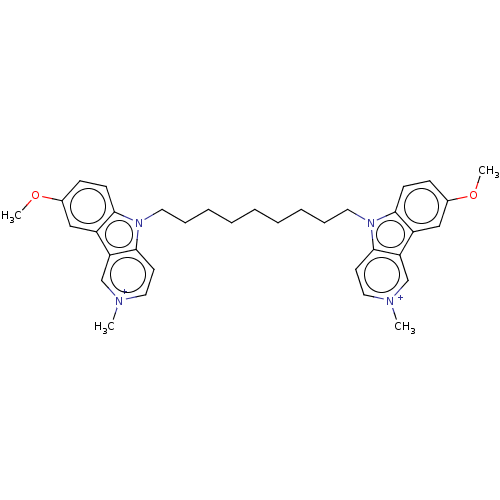
(CHEMBL3605362)Show SMILES [I-].[I-].COc1ccc2n(CCCCCCCCCn3c4ccc(OC)cc4c4c[n+](C)ccc34)c3cc[n+](C)cc3c2c1 Show InChI InChI=1S/C28H39N5O9/c29-13-20-23(36)24(37)26(41-20)42-21(19-12-18(34)25(40-19)33-11-10-22(35)32-28(33)39)14-30-27(38)31-17-8-6-16(7-9-17)15-4-2-1-3-5-15/h6-11,15,18-21,23-26,34,36-37H,1-5,12-14,29H2,(H2,30,31,38)(H,32,35,39)/t18-,19+,20-,21+,23-,24-,25-,26+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay |
J Med Chem 58: 6710-5 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00958
BindingDB Entry DOI: 10.7270/Q2QZ2CRZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena
Curated by ChEMBL
| Assay Description
Inhibition of BChE in equine serum using butyrylthiocholine iodide substrate by Ellman assay |
J Med Chem 58: 6710-5 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00958
BindingDB Entry DOI: 10.7270/Q2QZ2CRZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Equus caballus (Horse)) | BDBM50415462

(CHEMBL589384)Show SMILES CN1CCC=C(C1)c1nsnc1OCCCOCCCC(=O)NCCCCCCCCNc1c2CCCCc2nc2ccccc12 |c:4| Show InChI InChI=1S/C36H52N6O3S/c1-42-23-12-15-28(27-42)34-36(41-46-40-34)45-26-14-25-44-24-13-20-33(43)37-21-10-4-2-3-5-11-22-38-35-29-16-6-8-18-31(29)39-32-19-9-7-17-30(32)35/h6,8,15-16,18H,2-5,7,9-14,17,19-27H2,1H3,(H,37,43)(H,38,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BChE from equine serum by Ellman's method |
J Med Chem 53: 2094-103 (2010)
Article DOI: 10.1021/jm901616h
BindingDB Entry DOI: 10.7270/Q2KW5H8J |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Schiller University Jena
Curated by ChEMBL
| Assay Description
Inhibition of BuChE from equine serum |
J Med Chem 51: 7666-9 (2008)
Article DOI: 10.1021/jm801131a
BindingDB Entry DOI: 10.7270/Q2TT4QSV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BuChE by Ellman's assay |
J Med Chem 51: 713-6 (2008)
Article DOI: 10.1021/jm701491k
BindingDB Entry DOI: 10.7270/Q2XP75SX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Equus caballus (Horse)) | BDBM50415464
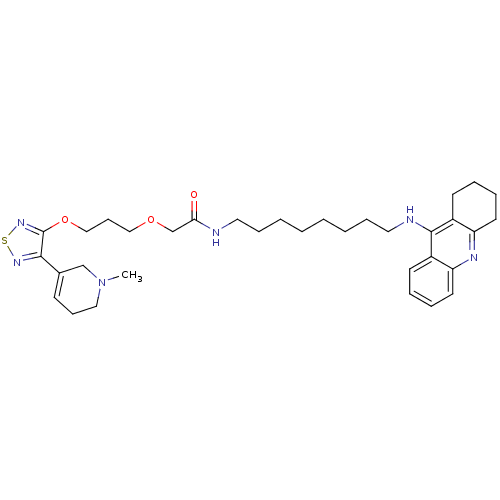
(CHEMBL589405)Show SMILES CN1CCC=C(C1)c1nsnc1OCCCOCC(=O)NCCCCCCCCNc1c2CCCCc2nc2ccccc12 |c:4| Show InChI InChI=1S/C34H48N6O3S/c1-40-21-12-14-26(24-40)32-34(39-44-38-32)43-23-13-22-42-25-31(41)35-19-10-4-2-3-5-11-20-36-33-27-15-6-8-17-29(27)37-30-18-9-7-16-28(30)33/h6,8,14-15,17H,2-5,7,9-13,16,18-25H2,1H3,(H,35,41)(H,36,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.13 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BChE from equine serum by Ellman's method |
J Med Chem 53: 2094-103 (2010)
Article DOI: 10.1021/jm901616h
BindingDB Entry DOI: 10.7270/Q2KW5H8J |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.13 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BChE from equine serum by Ellman's method |
J Med Chem 53: 2094-103 (2010)
Article DOI: 10.1021/jm901616h
BindingDB Entry DOI: 10.7270/Q2KW5H8J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.13 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of horse AChE by colorimetric Ellman assay |
J Med Chem 55: 5231-42 (2012)
Article DOI: 10.1021/jm300246n
BindingDB Entry DOI: 10.7270/Q2X06868 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50371550

(CHEMBL272229)Show InChI InChI=1S/C18H24N4O3/c23-22(24)25-13-12-19-10-5-11-20-18-14-6-1-3-8-16(14)21-17-9-4-2-7-15(17)18/h1,3,6,8,19H,2,4-5,7,9-13H2,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BuChE by Ellman's assay |
J Med Chem 51: 713-6 (2008)
Article DOI: 10.1021/jm701491k
BindingDB Entry DOI: 10.7270/Q2XP75SX |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50317179

(2-Methyl-9-[9-(2-methyl-beta-carboline-9-yl)nonyl]...)Show SMILES C[n+]1ccc2c(c1)n(CCCCCCCCCn1c3ccccc3c3cc[n+](C)cc13)c1ccccc21 Show InChI InChI=1S/C33H38N4/c1-34-22-18-28-26-14-8-10-16-30(26)36(32(28)24-34)20-12-6-4-3-5-7-13-21-37-31-17-11-9-15-27(31)29-19-23-35(2)25-33(29)37/h8-11,14-19,22-25H,3-7,12-13,20-21H2,1-2H3/q+2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena
Curated by ChEMBL
| Assay Description
Inhibition of BChE in equine serum using butyrylthiocholine iodide substrate by Ellman assay |
J Med Chem 58: 6710-5 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00958
BindingDB Entry DOI: 10.7270/Q2QZ2CRZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50371559
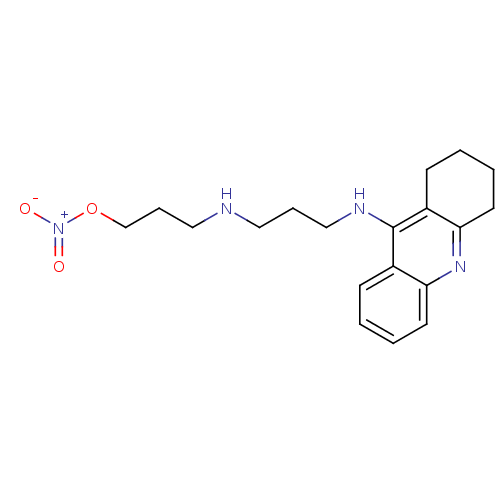
(CHEMBL270491)Show InChI InChI=1S/C19H26N4O3/c24-23(25)26-14-6-12-20-11-5-13-21-19-15-7-1-3-9-17(15)22-18-10-4-2-8-16(18)19/h1,3,7,9,20H,2,4-6,8,10-14H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BuChE by Ellman's assay |
J Med Chem 51: 713-6 (2008)
Article DOI: 10.1021/jm701491k
BindingDB Entry DOI: 10.7270/Q2XP75SX |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50371549

(CHEMBL270349)Show SMILES CC(C)(CO[N+]([O-])=O)C(=O)NCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C21H28N4O4/c1-21(2,14-29-25(27)28)20(26)23-13-7-12-22-19-15-8-3-5-10-17(15)24-18-11-6-4-9-16(18)19/h3,5,8,10H,4,6-7,9,11-14H2,1-2H3,(H,22,24)(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BuChE by Ellman's assay |
J Med Chem 51: 713-6 (2008)
Article DOI: 10.1021/jm701491k
BindingDB Entry DOI: 10.7270/Q2XP75SX |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50233374
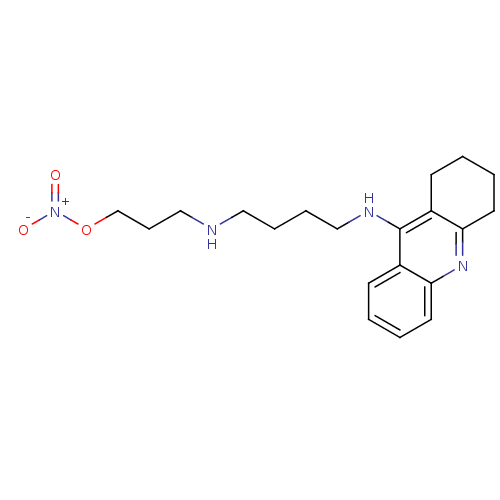
(9-[N-gamma-(3'-(nitrooxy)propyl)-gamma-aminobutyla...)Show InChI InChI=1S/C20H28N4O3/c25-24(26)27-15-7-13-21-12-5-6-14-22-20-16-8-1-3-10-18(16)23-19-11-4-2-9-17(19)20/h1,3,8,10,21H,2,4-7,9,11-15H2,(H,22,23) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Schiller University Jena
Curated by ChEMBL
| Assay Description
Inhibition of Electric eel AChE |
J Med Chem 51: 7666-9 (2008)
Article DOI: 10.1021/jm801131a
BindingDB Entry DOI: 10.7270/Q2TT4QSV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50233374

(9-[N-gamma-(3'-(nitrooxy)propyl)-gamma-aminobutyla...)Show InChI InChI=1S/C20H28N4O3/c25-24(26)27-15-7-13-21-12-5-6-14-22-20-16-8-1-3-10-18(16)23-19-11-4-2-9-17(19)20/h1,3,8,10,21H,2,4-7,9,11-15H2,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of AChE by Ellman's assay |
J Med Chem 51: 713-6 (2008)
Article DOI: 10.1021/jm701491k
BindingDB Entry DOI: 10.7270/Q2XP75SX |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50415465
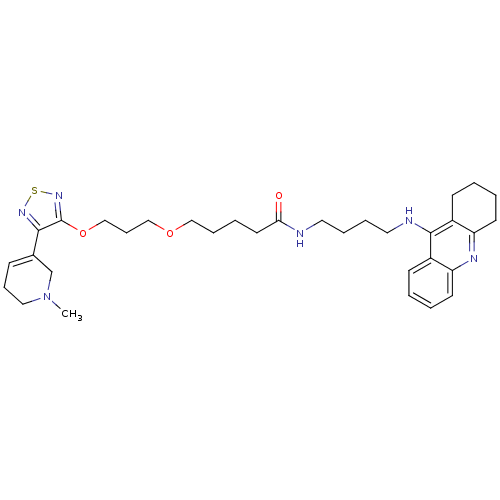
(CHEMBL589409)Show SMILES CN1CCC=C(C1)c1nsnc1OCCCOCCCCC(=O)NCCCCNc1c2CCCCc2nc2ccccc12 |c:4| Show InChI InChI=1S/C33H46N6O3S/c1-39-20-10-12-25(24-39)31-33(38-43-37-31)42-23-11-22-41-21-9-6-17-30(40)34-18-7-8-19-35-32-26-13-2-4-15-28(26)36-29-16-5-3-14-27(29)32/h2,4,12-13,15H,3,5-11,14,16-24H2,1H3,(H,34,40)(H,35,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.89 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BChE from equine serum by Ellman's method |
J Med Chem 53: 2094-103 (2010)
Article DOI: 10.1021/jm901616h
BindingDB Entry DOI: 10.7270/Q2KW5H8J |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50415461
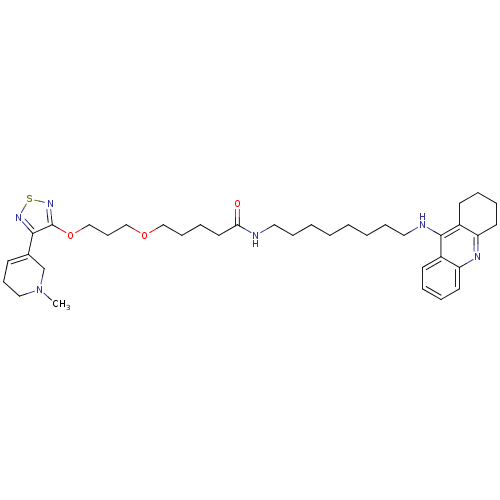
(CHEMBL589352)Show SMILES CN1CCC=C(C1)c1nsnc1OCCCOCCCCC(=O)NCCCCCCCCNc1c2CCCCc2nc2ccccc12 |c:4| Show InChI InChI=1S/C37H54N6O3S/c1-43-24-14-16-29(28-43)35-37(42-47-41-35)46-27-15-26-45-25-13-10-21-34(44)38-22-11-4-2-3-5-12-23-39-36-30-17-6-8-19-32(30)40-33-20-9-7-18-31(33)36/h6,8,16-17,19H,2-5,7,9-15,18,20-28H2,1H3,(H,38,44)(H,39,40) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.89 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BChE from equine serum by Ellman's method |
J Med Chem 53: 2094-103 (2010)
Article DOI: 10.1021/jm901616h
BindingDB Entry DOI: 10.7270/Q2KW5H8J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50415461

(CHEMBL589352)Show SMILES CN1CCC=C(C1)c1nsnc1OCCCOCCCCC(=O)NCCCCCCCCNc1c2CCCCc2nc2ccccc12 |c:4| Show InChI InChI=1S/C37H54N6O3S/c1-43-24-14-16-29(28-43)35-37(42-47-41-35)46-27-15-26-45-25-13-10-21-34(44)38-22-11-4-2-3-5-12-23-39-36-30-17-6-8-19-32(30)40-33-20-9-7-18-31(33)36/h6,8,16-17,19H,2-5,7,9-15,18,20-28H2,1H3,(H,38,44)(H,39,40) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.17 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
J Med Chem 53: 2094-103 (2010)
Article DOI: 10.1021/jm901616h
BindingDB Entry DOI: 10.7270/Q2KW5H8J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50371558

(CHEMBL261384)Show SMILES [O-][N+](=O)OCCCCCCNCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C22H32N4O3/c27-26(28)29-17-8-2-1-7-14-23-15-9-16-24-22-18-10-3-5-12-20(18)25-21-13-6-4-11-19(21)22/h3,5,10,12,23H,1-2,4,6-9,11,13-17H2,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of AChE by Ellman's assay |
J Med Chem 51: 713-6 (2008)
Article DOI: 10.1021/jm701491k
BindingDB Entry DOI: 10.7270/Q2XP75SX |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50371559

(CHEMBL270491)Show InChI InChI=1S/C19H26N4O3/c24-23(25)26-14-6-12-20-11-5-13-21-19-15-7-1-3-9-17(15)22-18-10-4-2-8-16(18)19/h1,3,7,9,20H,2,4-6,8,10-14H2,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of AChE by Ellman's assay |
J Med Chem 51: 713-6 (2008)
Article DOI: 10.1021/jm701491k
BindingDB Entry DOI: 10.7270/Q2XP75SX |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50415462

(CHEMBL589384)Show SMILES CN1CCC=C(C1)c1nsnc1OCCCOCCCC(=O)NCCCCCCCCNc1c2CCCCc2nc2ccccc12 |c:4| Show InChI InChI=1S/C36H52N6O3S/c1-42-23-12-15-28(27-42)34-36(41-46-40-34)45-26-14-25-44-24-13-20-33(43)37-21-10-4-2-3-5-11-22-38-35-29-16-6-8-18-31(29)39-32-19-9-7-17-30(32)35/h6,8,15-16,18H,2-5,7,9-14,17,19-27H2,1H3,(H,37,43)(H,38,39) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.46 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
J Med Chem 53: 2094-103 (2010)
Article DOI: 10.1021/jm901616h
BindingDB Entry DOI: 10.7270/Q2KW5H8J |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50111772
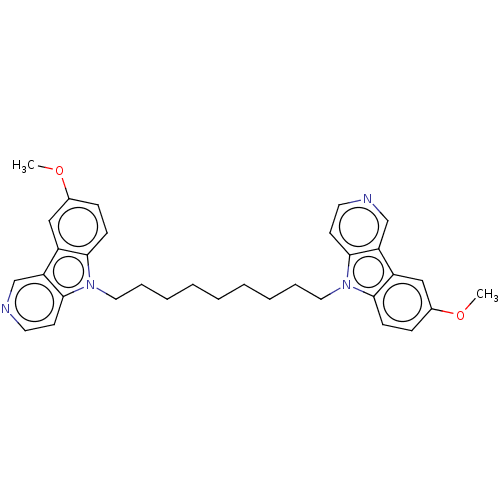
(CHEMBL3605357)Show SMILES COc1ccc2n(CCCCCCCCCn3c4ccncc4c4cc(OC)ccc34)c3ccncc3c2c1 Show InChI InChI=1S/C22H37N5O8/c1-26-7-2-3-12(26)4-6-24-11-16(35-21-19(31)18(30)15(10-23)34-21)14-9-13(28)20(33-14)27-8-5-17(29)25-22(27)32/h5,8,12-16,18-21,24,28,30-31H,2-4,6-7,9-11,23H2,1H3,(H,25,29,32)/t12?,13-,14+,15-,16+,18-,19-,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena
Curated by ChEMBL
| Assay Description
Inhibition of BChE in equine serum using butyrylthiocholine iodide substrate by Ellman assay |
J Med Chem 58: 6710-5 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00958
BindingDB Entry DOI: 10.7270/Q2QZ2CRZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50415464

(CHEMBL589405)Show SMILES CN1CCC=C(C1)c1nsnc1OCCCOCC(=O)NCCCCCCCCNc1c2CCCCc2nc2ccccc12 |c:4| Show InChI InChI=1S/C34H48N6O3S/c1-40-21-12-14-26(24-40)32-34(39-44-38-32)43-23-13-22-42-25-31(41)35-19-10-4-2-3-5-11-20-36-33-27-15-6-8-17-29(27)37-30-18-9-7-16-28(30)33/h6,8,14-15,17H,2-5,7,9-13,16,18-25H2,1H3,(H,35,41)(H,36,37) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.61 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
J Med Chem 53: 2094-103 (2010)
Article DOI: 10.1021/jm901616h
BindingDB Entry DOI: 10.7270/Q2KW5H8J |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50415476
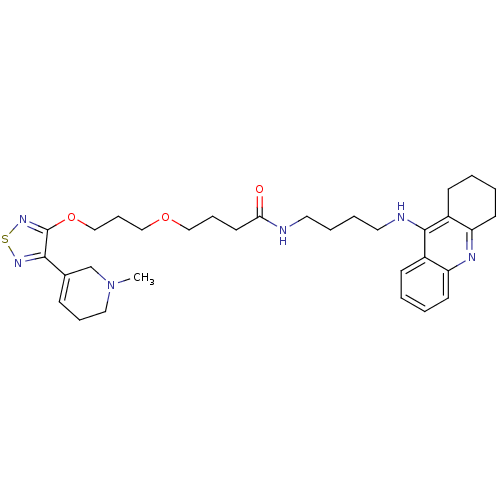
(CHEMBL589496)Show SMILES CN1CCC=C(C1)c1nsnc1OCCCOCCCC(=O)NCCCCNc1c2CCCCc2nc2ccccc12 |c:4| Show InChI InChI=1S/C32H44N6O3S/c1-38-19-8-11-24(23-38)30-32(37-42-36-30)41-22-10-21-40-20-9-16-29(39)33-17-6-7-18-34-31-25-12-2-4-14-27(25)35-28-15-5-3-13-26(28)31/h2,4,11-12,14H,3,5-10,13,15-23H2,1H3,(H,33,39)(H,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.76 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BChE from equine serum by Ellman's method |
J Med Chem 53: 2094-103 (2010)
Article DOI: 10.1021/jm901616h
BindingDB Entry DOI: 10.7270/Q2KW5H8J |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50273429
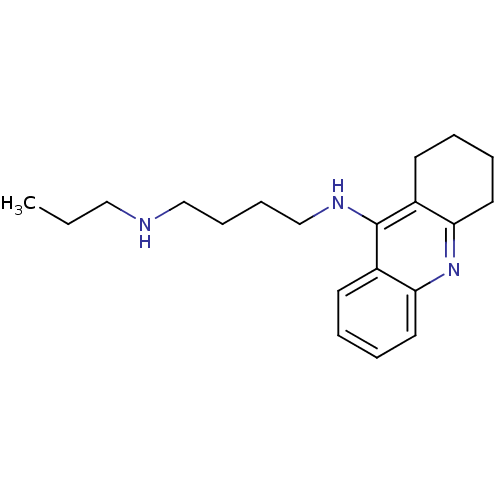
(9-[N(delta)-propyl -delta-aminobutylamino]-1,2,3,4...)Show InChI InChI=1S/C20H29N3/c1-2-13-21-14-7-8-15-22-20-16-9-3-5-11-18(16)23-19-12-6-4-10-17(19)20/h3,5,9,11,21H,2,4,6-8,10,12-15H2,1H3,(H,22,23) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Schiller University Jena
Curated by ChEMBL
| Assay Description
Inhibition of BuChE from equine serum |
J Med Chem 51: 7666-9 (2008)
Article DOI: 10.1021/jm801131a
BindingDB Entry DOI: 10.7270/Q2TT4QSV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50111779
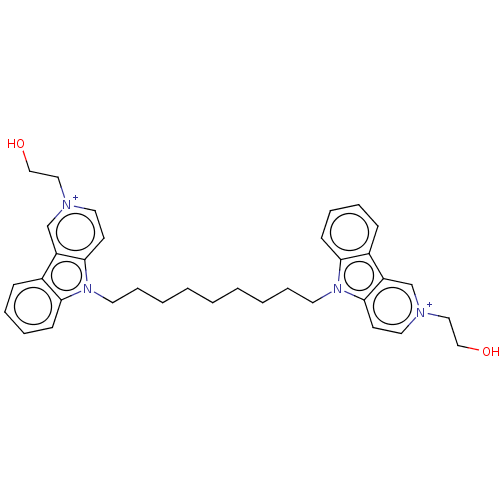
(CHEMBL3605364)Show SMILES [I-].[I-].OCC[n+]1ccc2n(CCCCCCCCCn3c4ccccc4c4c[n+](CCO)ccc34)c3ccccc3c2c1 Show InChI InChI=1S/C29H52N4O8/c1-2-3-4-5-6-7-8-9-10-11-12-13-15-31-19-23(41-28-26(37)25(36)22(18-30)40-28)21-17-20(34)27(39-21)33-16-14-24(35)32-29(33)38/h14,16,20-23,25-28,31,34,36-37H,2-13,15,17-19,30H2,1H3,(H,32,35,38)/t20-,21+,22-,23+,25-,26-,27-,28+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay |
J Med Chem 58: 6710-5 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00958
BindingDB Entry DOI: 10.7270/Q2QZ2CRZ |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50273578

(9-[Nbeta-(2'-(Nitrooxy)ethyl)-beta-aminoethylamino...)Show InChI InChI=1S/C17H22N4O3/c22-21(23)24-12-11-18-9-10-19-17-13-5-1-3-7-15(13)20-16-8-4-2-6-14(16)17/h1,3,5,7,18H,2,4,6,8-12H2,(H,19,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Schiller University Jena
Curated by ChEMBL
| Assay Description
Inhibition of BuChE from equine serum |
J Med Chem 51: 7666-9 (2008)
Article DOI: 10.1021/jm801131a
BindingDB Entry DOI: 10.7270/Q2TT4QSV |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50371553
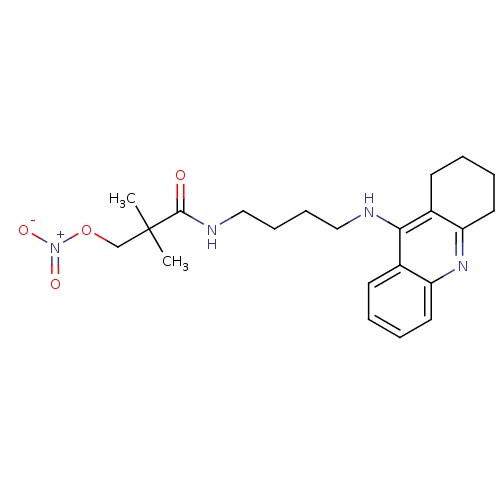
(CHEMBL271746)Show SMILES CC(C)(CO[N+]([O-])=O)C(=O)NCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C22H30N4O4/c1-22(2,15-30-26(28)29)21(27)24-14-8-7-13-23-20-16-9-3-5-11-18(16)25-19-12-6-4-10-17(19)20/h3,5,9,11H,4,6-8,10,12-15H2,1-2H3,(H,23,25)(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BuChE by Ellman's assay |
J Med Chem 51: 713-6 (2008)
Article DOI: 10.1021/jm701491k
BindingDB Entry DOI: 10.7270/Q2XP75SX |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50111785
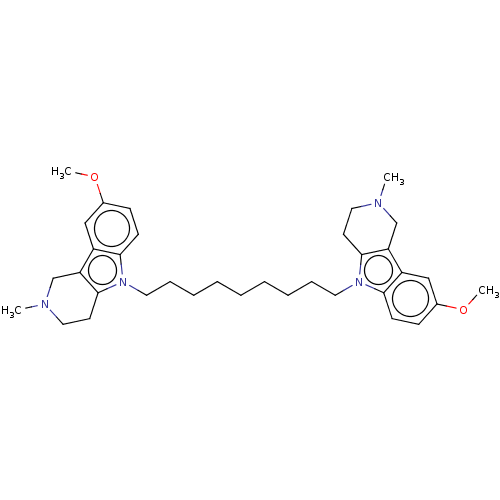
(CHEMBL3605370)Show SMILES COc1ccc2n(CCCCCCCCCn3c4CCN(C)Cc4c4cc(OC)ccc34)c3CCN(C)Cc3c2c1 Show InChI InChI=1S/C27H38N4O8/c28-14-20-23(34)24(35)26(38-20)39-21(15-30-9-6-17(7-10-30)12-16-4-2-1-3-5-16)19-13-18(32)25(37-19)31-11-8-22(33)29-27(31)36/h1-5,8,11,17-21,23-26,32,34-35H,6-7,9-10,12-15,28H2,(H,29,33,36)/t18-,19+,20-,21+,23-,24-,25-,26+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay |
J Med Chem 58: 6710-5 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00958
BindingDB Entry DOI: 10.7270/Q2QZ2CRZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50273380

(9-[N(theta)-(2'-(Nitrooxy)ethyl)-theta-aminooctyla...)Show SMILES [O-][N+](=O)OCCNCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C23H34N4O3/c28-27(29)30-18-17-24-15-9-3-1-2-4-10-16-25-23-19-11-5-7-13-21(19)26-22-14-8-6-12-20(22)23/h5,7,11,13,24H,1-4,6,8-10,12,14-18H2,(H,25,26) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Schiller University Jena
Curated by ChEMBL
| Assay Description
Inhibition of Electric eel AChE |
J Med Chem 51: 7666-9 (2008)
Article DOI: 10.1021/jm801131a
BindingDB Entry DOI: 10.7270/Q2TT4QSV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50415476

(CHEMBL589496)Show SMILES CN1CCC=C(C1)c1nsnc1OCCCOCCCC(=O)NCCCCNc1c2CCCCc2nc2ccccc12 |c:4| Show InChI InChI=1S/C32H44N6O3S/c1-38-19-8-11-24(23-38)30-32(37-42-36-30)41-22-10-21-40-20-9-16-29(39)33-17-6-7-18-34-31-25-12-2-4-14-27(25)35-28-15-5-3-13-26(28)31/h2,4,11-12,14H,3,5-10,13,15-23H2,1H3,(H,33,39)(H,34,35) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.51 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
J Med Chem 53: 2094-103 (2010)
Article DOI: 10.1021/jm901616h
BindingDB Entry DOI: 10.7270/Q2KW5H8J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50415465

(CHEMBL589409)Show SMILES CN1CCC=C(C1)c1nsnc1OCCCOCCCCC(=O)NCCCCNc1c2CCCCc2nc2ccccc12 |c:4| Show InChI InChI=1S/C33H46N6O3S/c1-39-20-10-12-25(24-39)31-33(38-43-37-31)42-23-11-22-41-21-9-6-17-30(40)34-18-7-8-19-35-32-26-13-2-4-15-28(26)36-29-16-5-3-14-27(29)32/h2,4,12-13,15H,3,5-11,14,16-24H2,1H3,(H,34,40)(H,35,36) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.71 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
J Med Chem 53: 2094-103 (2010)
Article DOI: 10.1021/jm901616h
BindingDB Entry DOI: 10.7270/Q2KW5H8J |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50415468
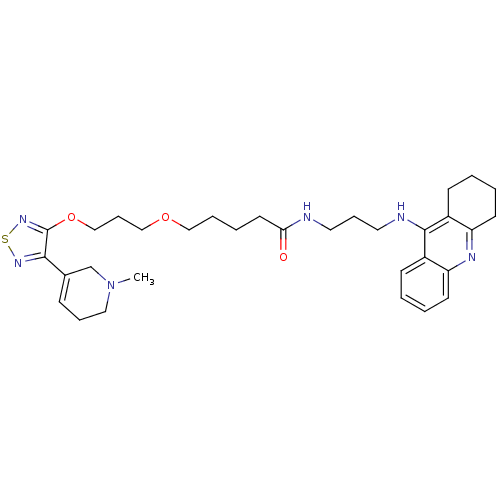
(CHEMBL590059)Show SMILES CN1CCC=C(C1)c1nsnc1OCCCOCCCCC(=O)NCCCNc1c2CCCCc2nc2ccccc12 |c:4| Show InChI InChI=1S/C32H44N6O3S/c1-38-19-8-11-24(23-38)30-32(37-42-36-30)41-22-10-21-40-20-7-6-16-29(39)33-17-9-18-34-31-25-12-2-4-14-27(25)35-28-15-5-3-13-26(28)31/h2,4,11-12,14H,3,5-10,13,15-23H2,1H3,(H,33,39)(H,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.91 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BChE from equine serum by Ellman's method |
J Med Chem 53: 2094-103 (2010)
Article DOI: 10.1021/jm901616h
BindingDB Entry DOI: 10.7270/Q2KW5H8J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50273578

(9-[Nbeta-(2'-(Nitrooxy)ethyl)-beta-aminoethylamino...)Show InChI InChI=1S/C17H22N4O3/c22-21(23)24-12-11-18-9-10-19-17-13-5-1-3-7-15(13)20-16-8-4-2-6-14(16)17/h1,3,5,7,18H,2,4,6,8-12H2,(H,19,20) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Schiller University Jena
Curated by ChEMBL
| Assay Description
Inhibition of Electric eel AChE |
J Med Chem 51: 7666-9 (2008)
Article DOI: 10.1021/jm801131a
BindingDB Entry DOI: 10.7270/Q2TT4QSV |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50415466
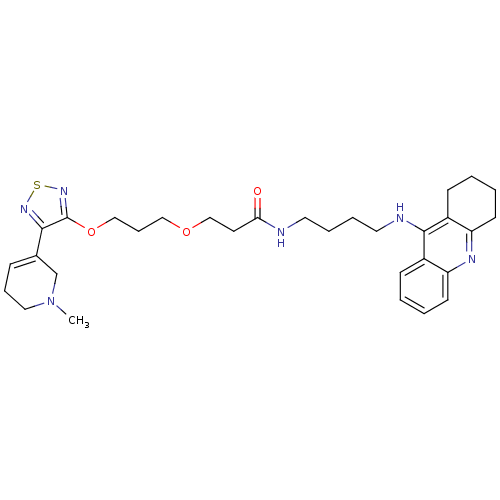
(CHEMBL589419)Show SMILES CN1CCC=C(C1)c1nsnc1OCCCOCCC(=O)NCCCCNc1c2CCCCc2nc2ccccc12 |c:4| Show InChI InChI=1S/C31H42N6O3S/c1-37-18-8-10-23(22-37)29-31(36-41-35-29)40-20-9-19-39-21-15-28(38)32-16-6-7-17-33-30-24-11-2-4-13-26(24)34-27-14-5-3-12-25(27)30/h2,4,10-11,13H,3,5-9,12,14-22H2,1H3,(H,32,38)(H,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.12 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BChE from equine serum by Ellman's method |
J Med Chem 53: 2094-103 (2010)
Article DOI: 10.1021/jm901616h
BindingDB Entry DOI: 10.7270/Q2KW5H8J |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50415471

(CHEMBL599652)Show SMILES CN1CCC=C(C1)c1nsnc1OCCCOCC(=O)NCCCNc1c2CCCCc2nc2ccccc12 |c:4| Show InChI InChI=1S/C29H38N6O3S/c1-35-16-6-9-21(19-35)27-29(34-39-33-27)38-18-8-17-37-20-26(36)30-14-7-15-31-28-22-10-2-4-12-24(22)32-25-13-5-3-11-23(25)28/h2,4,9-10,12H,3,5-8,11,13-20H2,1H3,(H,30,36)(H,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.77 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BChE from equine serum by Ellman's method |
J Med Chem 53: 2094-103 (2010)
Article DOI: 10.1021/jm901616h
BindingDB Entry DOI: 10.7270/Q2KW5H8J |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50233374

(9-[N-gamma-(3'-(nitrooxy)propyl)-gamma-aminobutyla...)Show InChI InChI=1S/C20H28N4O3/c25-24(26)27-15-7-13-21-12-5-6-14-22-20-16-8-1-3-10-18(16)23-19-11-4-2-9-17(19)20/h1,3,8,10,21H,2,4-7,9,11-15H2,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BuChE by Ellman's assay |
J Med Chem 51: 713-6 (2008)
Article DOI: 10.1021/jm701491k
BindingDB Entry DOI: 10.7270/Q2XP75SX |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50233374

(9-[N-gamma-(3'-(nitrooxy)propyl)-gamma-aminobutyla...)Show InChI InChI=1S/C20H28N4O3/c25-24(26)27-15-7-13-21-12-5-6-14-22-20-16-8-1-3-10-18(16)23-19-11-4-2-9-17(19)20/h1,3,8,10,21H,2,4-7,9,11-15H2,(H,22,23) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Schiller University Jena
Curated by ChEMBL
| Assay Description
Inhibition of BuChE from equine serum |
J Med Chem 51: 7666-9 (2008)
Article DOI: 10.1021/jm801131a
BindingDB Entry DOI: 10.7270/Q2TT4QSV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50371550

(CHEMBL272229)Show InChI InChI=1S/C18H24N4O3/c23-22(24)25-13-12-19-10-5-11-20-18-14-6-1-3-8-16(14)21-17-9-4-2-7-15(17)18/h1,3,6,8,19H,2,4-5,7,9-13H2,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of AChE by Ellman's assay |
J Med Chem 51: 713-6 (2008)
Article DOI: 10.1021/jm701491k
BindingDB Entry DOI: 10.7270/Q2XP75SX |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50415469
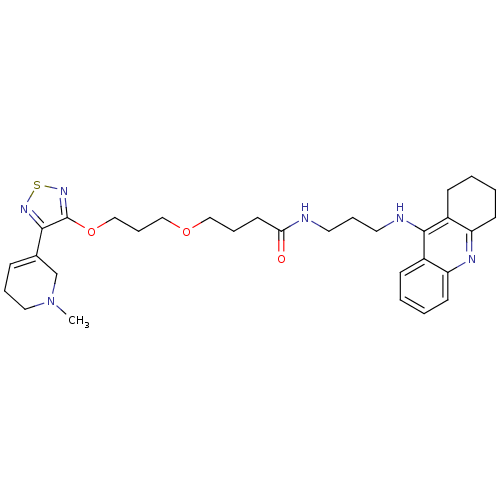
(CHEMBL590055)Show SMILES CN1CCC=C(C1)c1nsnc1OCCCOCCCC(=O)NCCCNc1c2CCCCc2nc2ccccc12 |c:4| Show InChI InChI=1S/C31H42N6O3S/c1-37-18-6-10-23(22-37)29-31(36-41-35-29)40-21-9-20-39-19-7-15-28(38)32-16-8-17-33-30-24-11-2-4-13-26(24)34-27-14-5-3-12-25(27)30/h2,4,10-11,13H,3,5-9,12,14-22H2,1H3,(H,32,38)(H,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BChE from equine serum by Ellman's method |
J Med Chem 53: 2094-103 (2010)
Article DOI: 10.1021/jm901616h
BindingDB Entry DOI: 10.7270/Q2KW5H8J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50415468

(CHEMBL590059)Show SMILES CN1CCC=C(C1)c1nsnc1OCCCOCCCCC(=O)NCCCNc1c2CCCCc2nc2ccccc12 |c:4| Show InChI InChI=1S/C32H44N6O3S/c1-38-19-8-11-24(23-38)30-32(37-42-36-30)41-22-10-21-40-20-7-6-16-29(39)33-17-9-18-34-31-25-12-2-4-14-27(25)35-28-15-5-3-13-26(28)31/h2,4,11-12,14H,3,5-10,13,15-23H2,1H3,(H,33,39)(H,34,35) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
J Med Chem 53: 2094-103 (2010)
Article DOI: 10.1021/jm901616h
BindingDB Entry DOI: 10.7270/Q2KW5H8J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data