

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50586159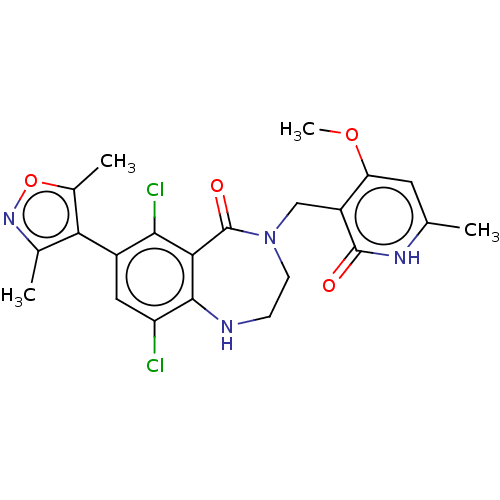 (CHEMBL5087917) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00448 BindingDB Entry DOI: 10.7270/Q2J10735 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433063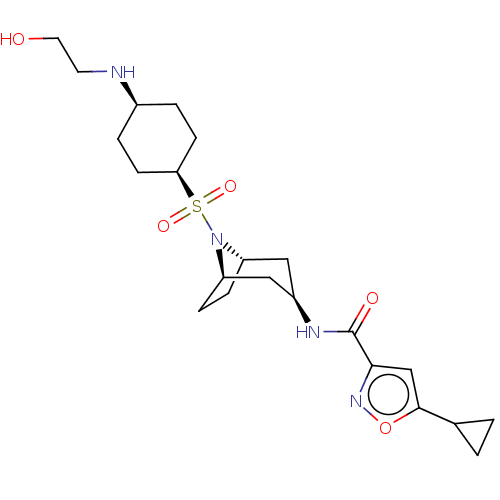 (5-cyclopropyl-N-((1R,3R,5S)-8-(((1S,4S)- 4-((2- hy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378442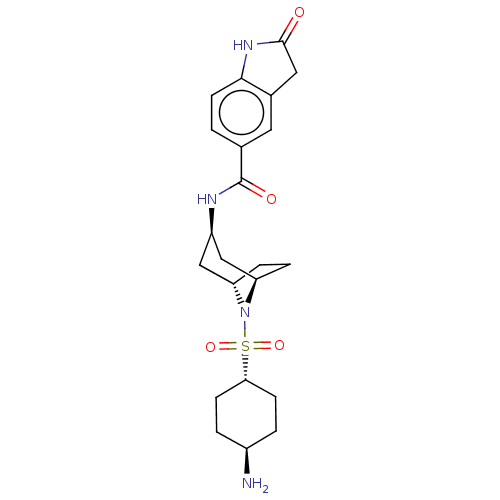 (N-((1R,3R,5S)-8-(((1r,4R)-4-aminocyclohexyl)sulfon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378443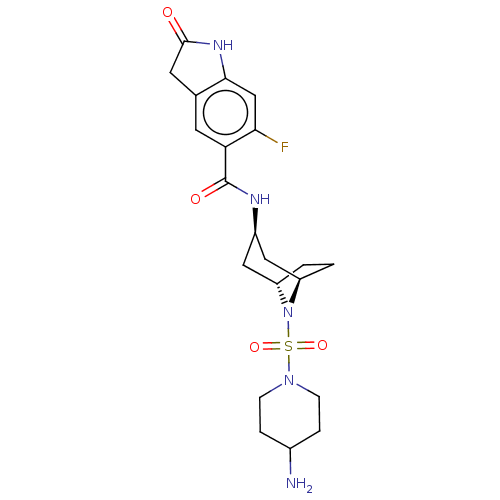 (N-((1R,3r,5S)-8-((4-aminopiperidin-1-yl)sulfonyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432825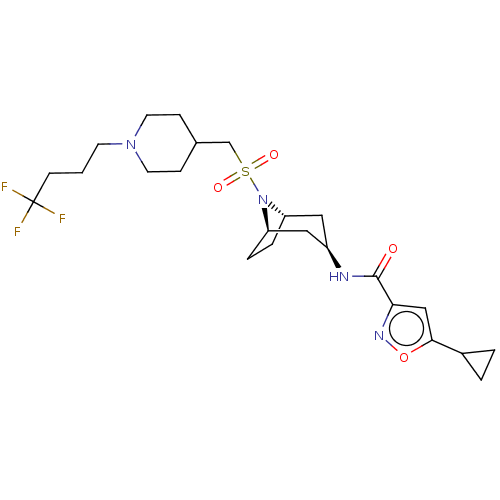 (5-cyclopropyl-N-((1R,3r,5S)-8-(((1-(4,4,4- trifluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378444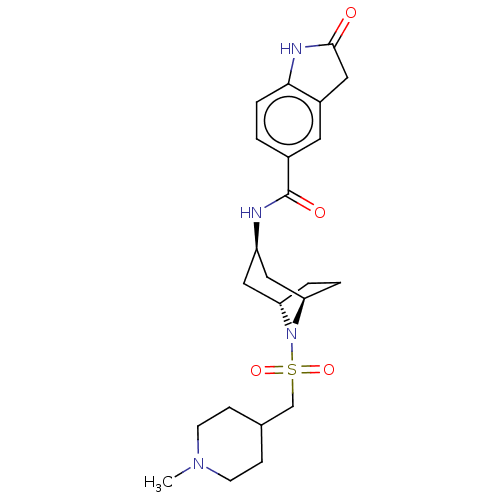 (N-((1R,3r,5S)-8-(((1-methylpiperidin-4-yl)methyl)s...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378445 (N-((1R,3r,5S)-8-((4-aminopiperidin-1-yl)sulfonyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433064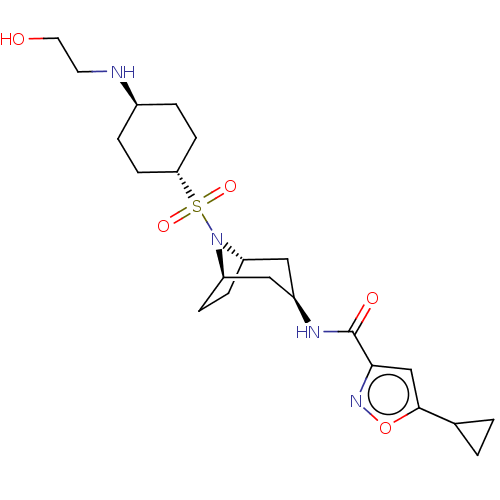 (5-cyclopropyl-N-((1R,3R,5S)-8-(((1R,4R)- 4-((2- hy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433065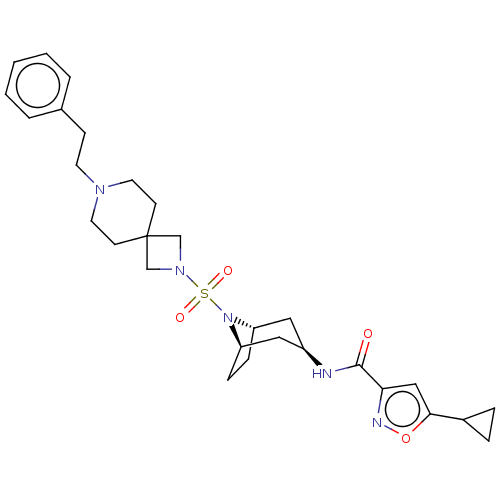 (5-cyclopropyl-N-((1R,3r,5S)-8-((7- phenethyl-2,7-d...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432822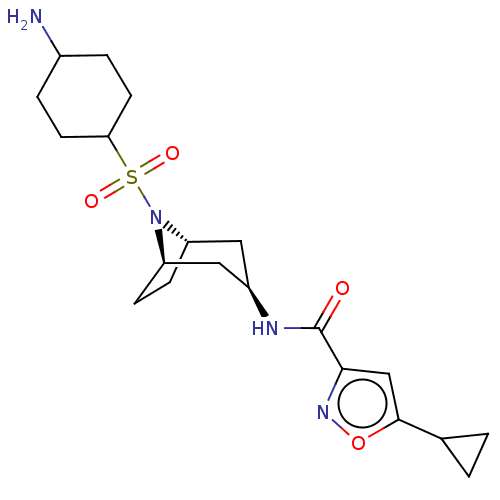 (N-((1R,3r,5S)-8-((4- aminocyclohexyl)sulfonyl)-8- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432826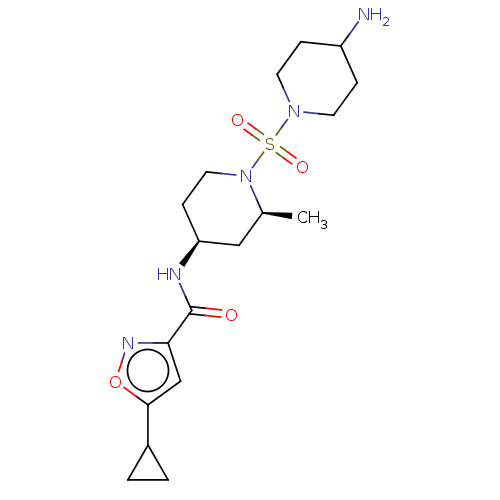 (N-((2S,4S)-1-((4-aminopiperidin-1- yl)sulfonyl)-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378446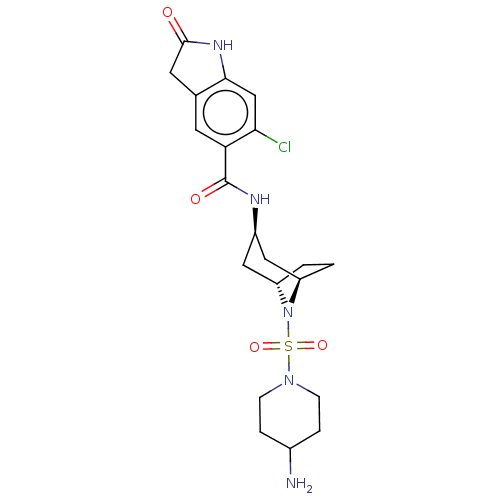 (N-((1R,3r,5S)-8-((4-aminopiperidin-1-yl)sulfonyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50586160 (CHEMBL5085993) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00448 BindingDB Entry DOI: 10.7270/Q2J10735 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433066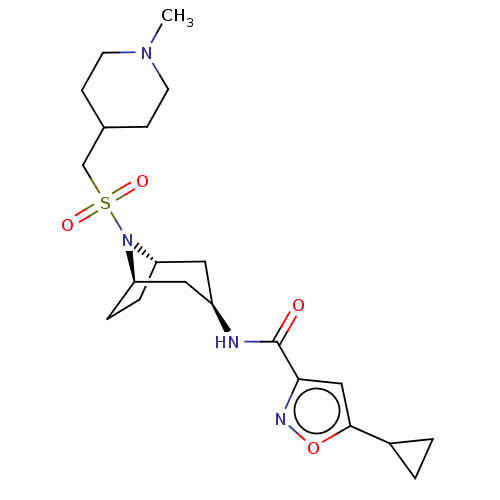 (5-cyclopropyl-N-((1R,3r,5S)-8-(((1- methylpiperidi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432827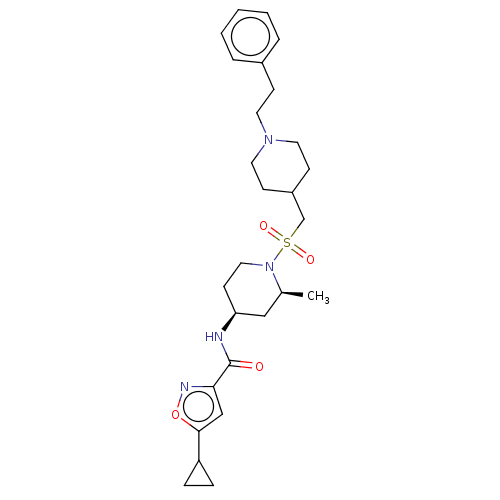 (5-cyclopropyl-N-((2S,4S)-2-methyl-1- (((1-phenethy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50586173 (CHEMBL5087318) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00448 BindingDB Entry DOI: 10.7270/Q2J10735 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378447 (N-((2S,4S)-1-((4-aminopiperidin-1-yl)sulfonyl)-2-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432829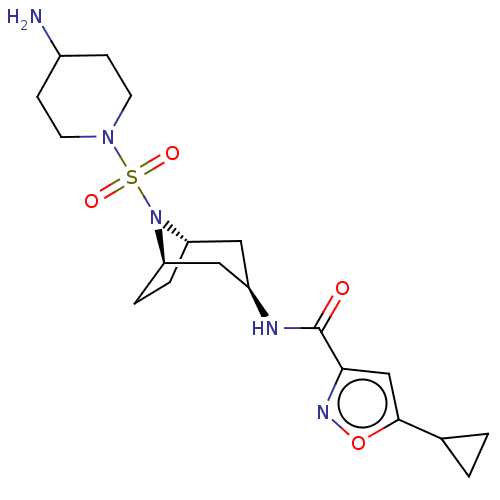 (N-((1R,3r,5S)-8-((4-aminopiperidin-1- yl)sulfonyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432828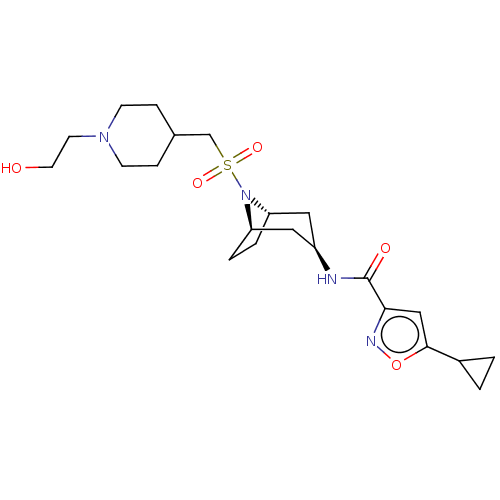 (5-cyclopropyl-N-((1R,3r,5S)-8-(((1-(2- hydroxyethy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433067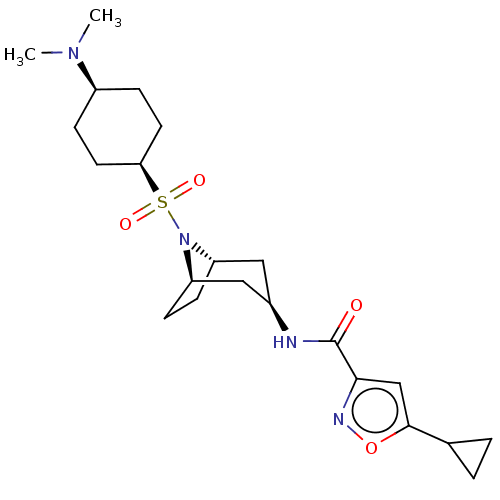 (5-cyclopropyl-N-((1R,3R,5S)-8-(((1s,4S)- 4-(dimeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378448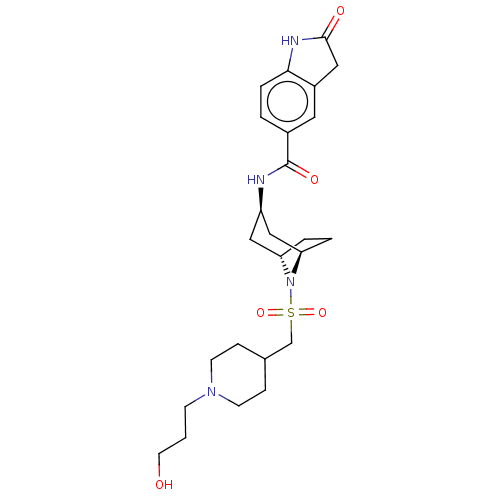 (N-((1R,3r,5S)-8-(((1-(3-hydroxypropyl)piperidin-4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433068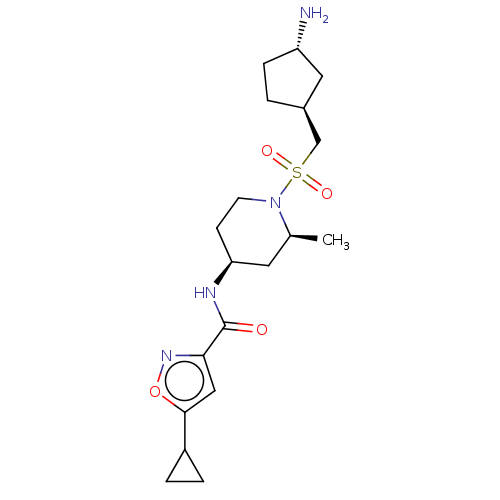 (N-((2S,4S)-1-((((1S,3S)-3- aminocyclopentyl)methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378449 (N-((2S,4S)-1-((4-aminopiperidin-1-yl)sulfonyl)-2-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432830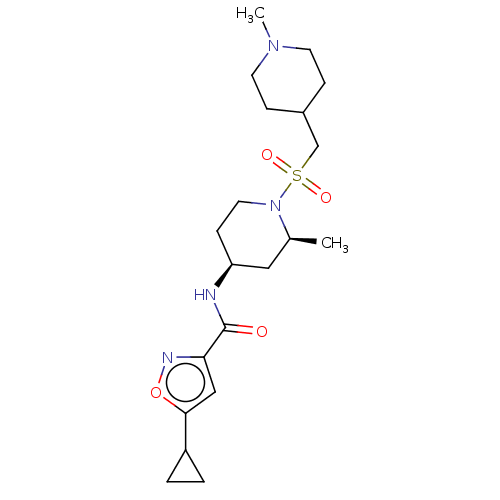 (5-cyclopropyl-N-((2S,4S)-2-methyl-1- (((1-methylpi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432823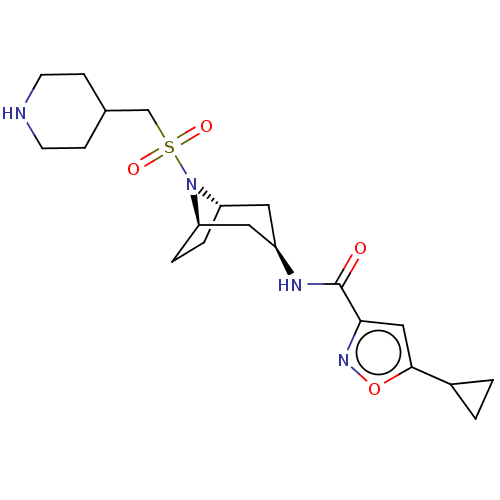 (5-cyclopropyl-N-((1R,3r,5S)-8- ((piperidin-4-ylmet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50193709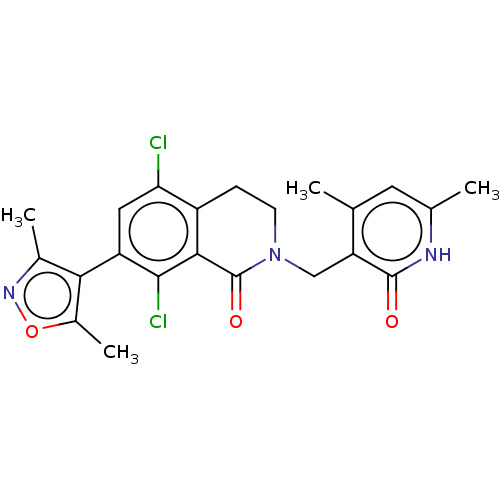 (CHEMBL3911017) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00448 BindingDB Entry DOI: 10.7270/Q2J10735 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433069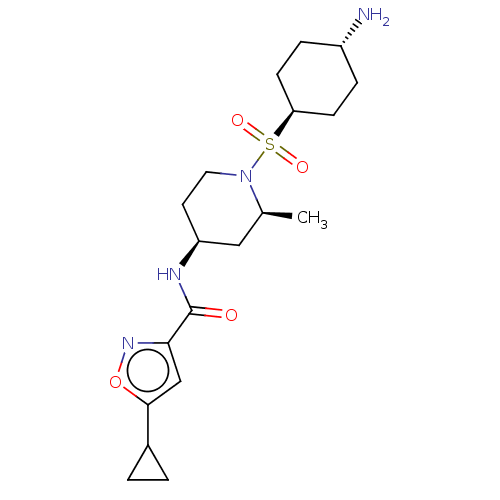 (N-((2S,4S)-1-(((1r,4S)-4- aminocyclohexyl)sulfonyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.03 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433070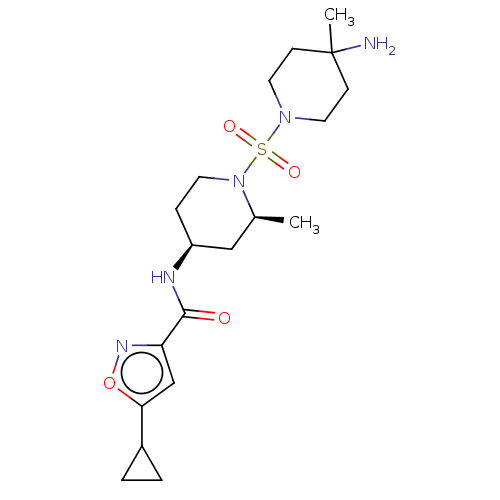 (N-((2S,4S)-1-((4-amino-4-methylpiperidin- l-yl)sul...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.04 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433071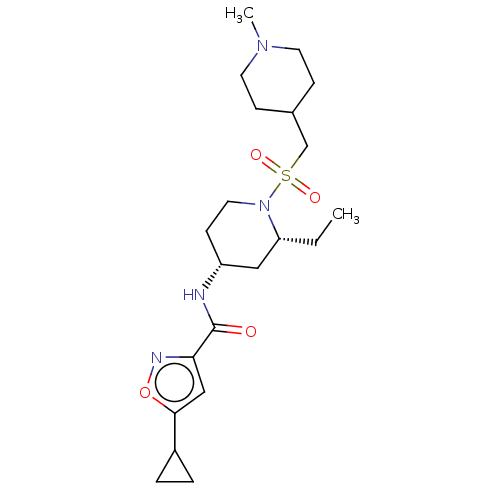 (5-cyclopropyl-N-((2R,4R)-2-ethyl-1-(((1- methylpip...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.07 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378450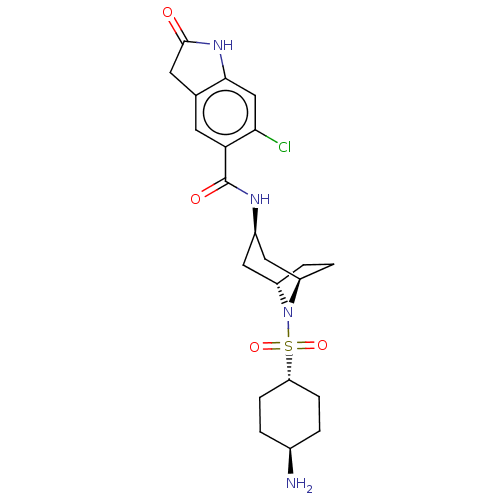 (N-((1R,3R,5S)-8-(((1r,4R)-4-aminocyclohexyl)sulfon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432831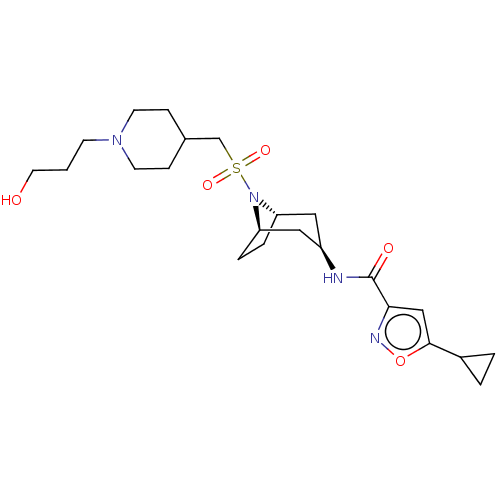 (5-cyclopropyl-N-((1R,3r,5S)-8-(((1-(3- hydroxyprop...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433072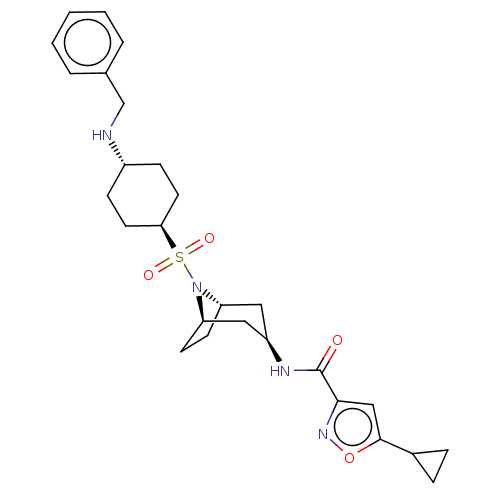 (N-((1R,3R,5S)-8-(((1r,4R)-4- (benzylamino)cyclohex...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.12 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433073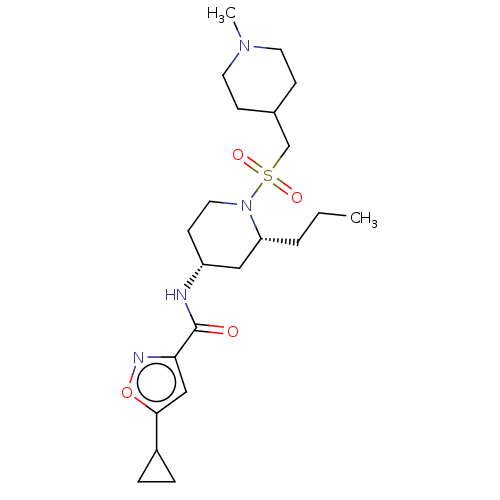 (5-cyclopropyl-N-((2R,4R)-1-(((1- methylpiperidin-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.14 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433074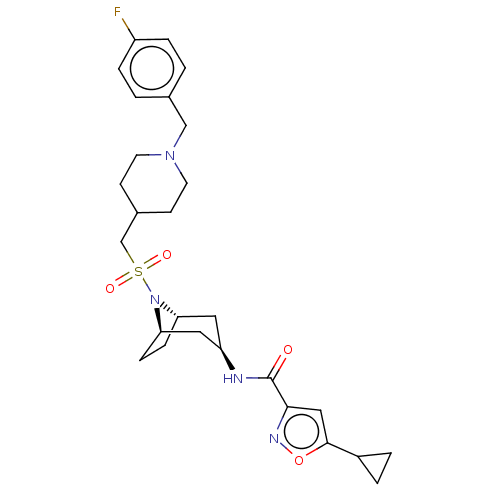 (5-cyclopropyl-N-((1R,3r,5S)-8-(((1-(4- fluorobenzy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432832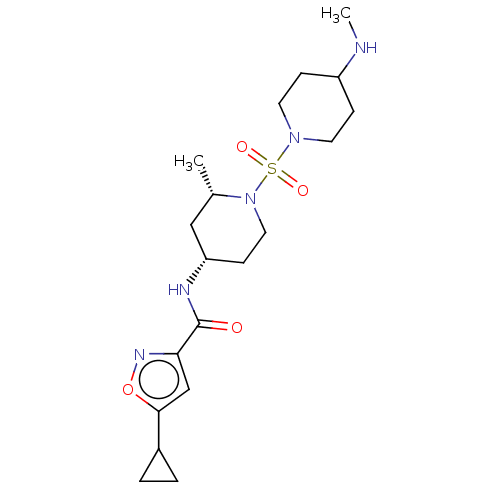 (5-cyclopropyl-N-((2S,4S)-2-methyl-1-((4- (methylam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433075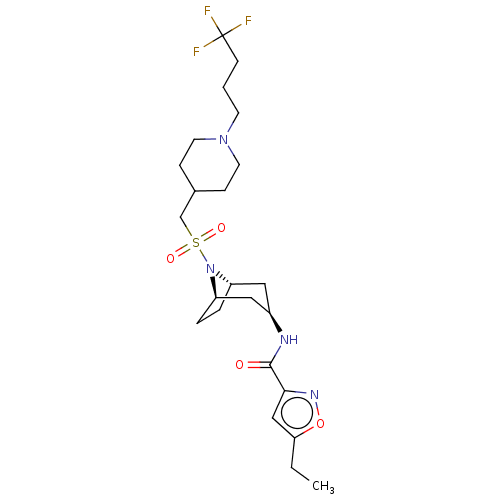 (5-ethyl-N-((1R,3r,5S)-8-(((1-(4,4,4- trifluorobuty...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM378746 (N-(1-((1-(4-chlorobenzyl)-1H-pyrazol-4-yl)methyl)a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 20 mM Bicine (pH=7.6), 1 mM TCEP, 0.005% Bovine Skin Gelatin, and 0.002% Tween20, prepared on... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432833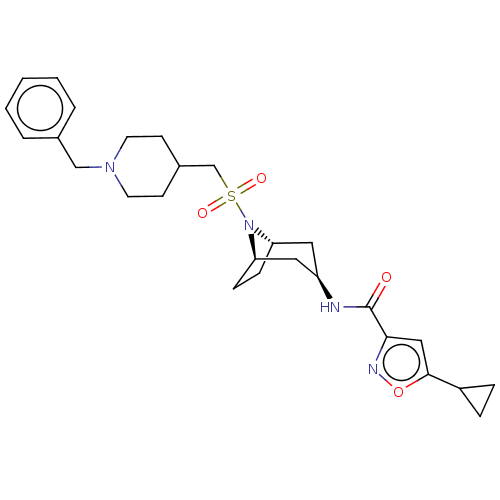 (N-((1R,3r,5S)-8-(((1-benzylpiperidin-4- yl)methyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433076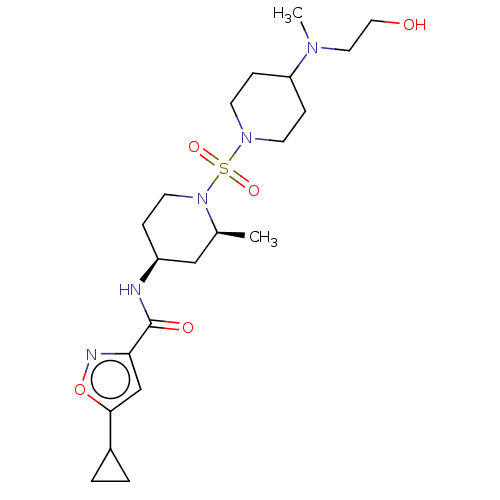 (5-cyclopropyl-N-((2S,4S)-1-((4-((2- hydroxyethyl)(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.21 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433077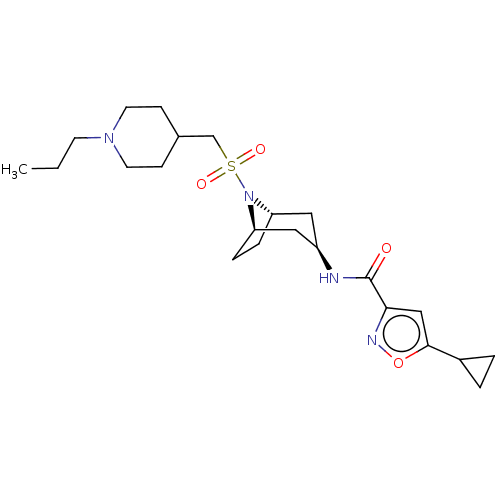 (5-cyclopropyl-N-((1R,3r,5S)-8-(((1- propylpiperidi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.22 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433078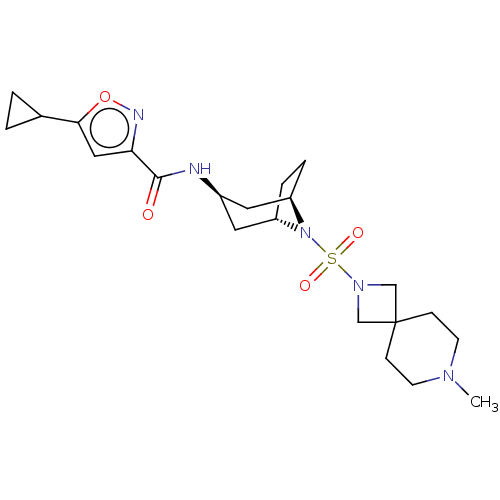 (5-cyclopropyl-N-((1R,3r,5S)-8-((7-methyl- 2,7-diaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.23 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433079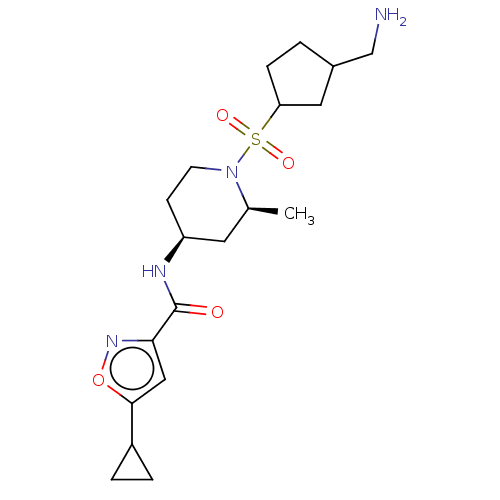 (N-((2S,4S)-1-((3- (aminomethyl)cyclopentyl)sulfony...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.24 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433080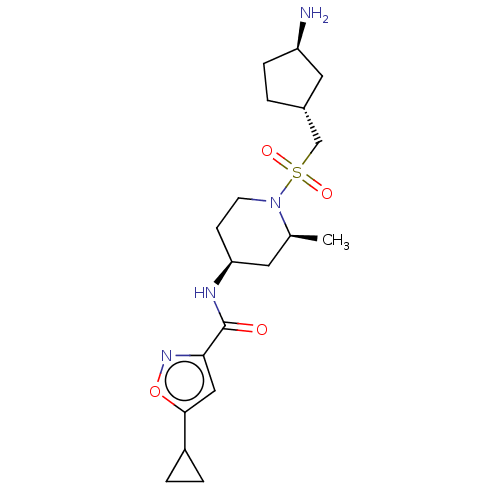 (N-((2S,4S)-1-((((1R,3R)-3- aminocyclopentyl)methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.29 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432837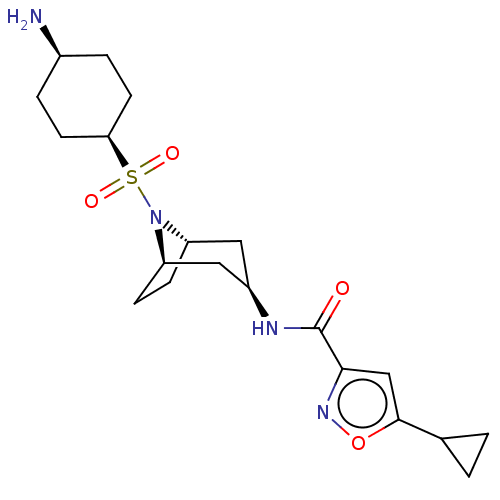 (N-((1R,3R,5S)-8-(((1s,4S)-4- aminocyclohexyl)sulfo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432836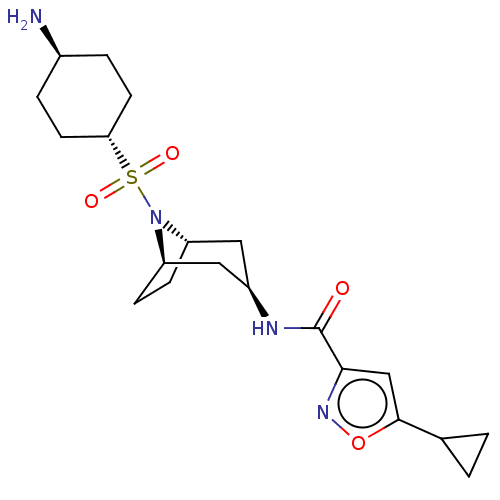 (N-((1R,3R,5S)-8-(((1r,4R)-4- aminocyclohexyl)sulfo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432835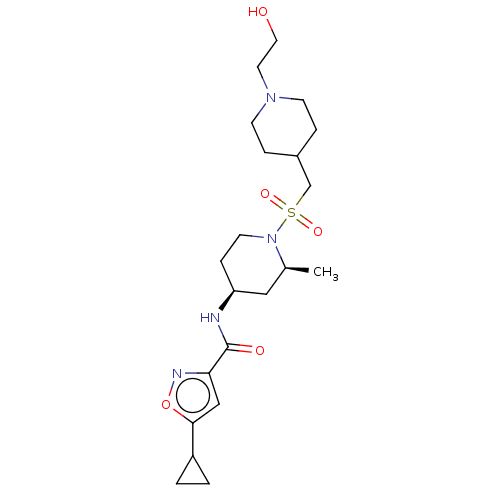 (5-cyclopropyl-N-((2S,4S)-1-(((1-(2- hydroxyethyl)p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432834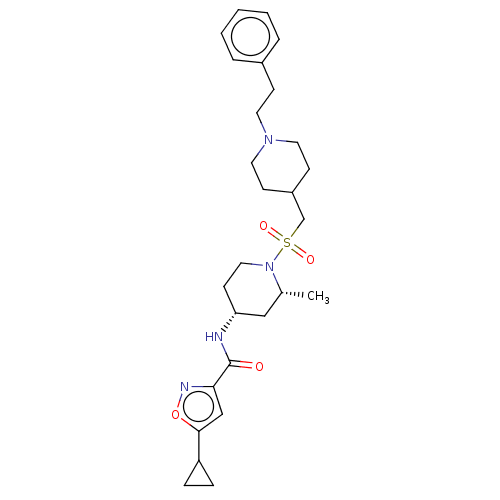 (5-cyclopropyl-N-((2R,4R)-2-methyl-1- (((1-phenethy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50586158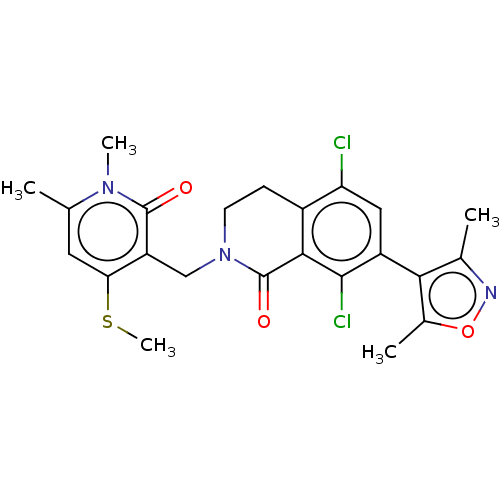 (CHEMBL5088217) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EZH2 (unknown origin) using 3H-SAM as substrate incubated for 1 hour by filter binding method | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00448 BindingDB Entry DOI: 10.7270/Q2J10735 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433081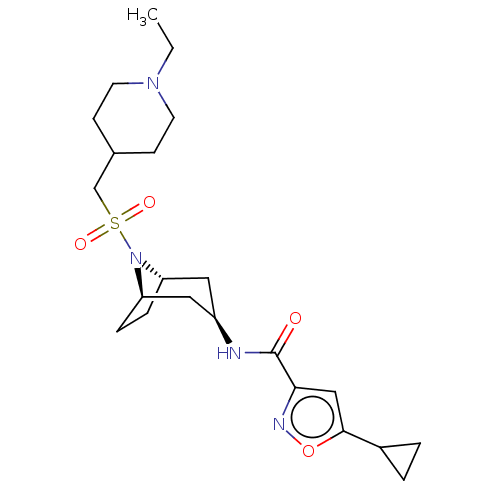 (5-cyclopropyl-N-((1R,3r,5S)-8-(((1- ethylpiperidin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.36 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433082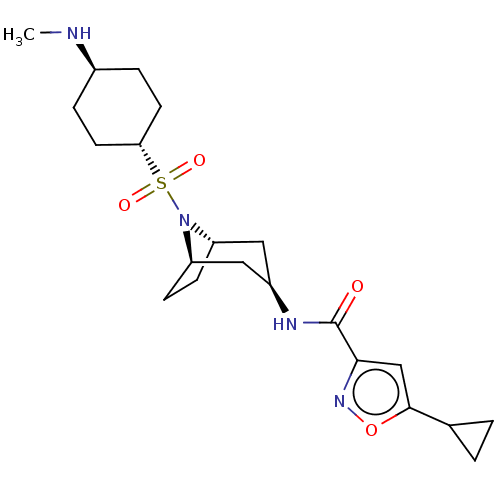 (5-cyclopropyl-N-((1R,3R,5S)-8-(((1r,4R)- 4-(methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.37 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2684 total ) | Next | Last >> |