 Found 50 hits with Last Name = 'forbes' and Initial = 'c'
Found 50 hits with Last Name = 'forbes' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM82122

(Vinylsulfone, 7)Show InChI InChI=1S/C15H14O3S2/c16-20(17,12-4-11-19)15-9-7-14(8-10-15)18-13-5-2-1-3-6-13/h1-10,12,19H,11H2/b12-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
| Assay Description
Inhibition assay using matrix metalloproteinase (MMP-2). |
Chem Biol Drug Des 74: 527-34 (2009)
Checked by Author
Article DOI: 10.1111/j.1747-0285.2009.00881.x
BindingDB Entry DOI: 10.7270/Q20P0XJV |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50335495
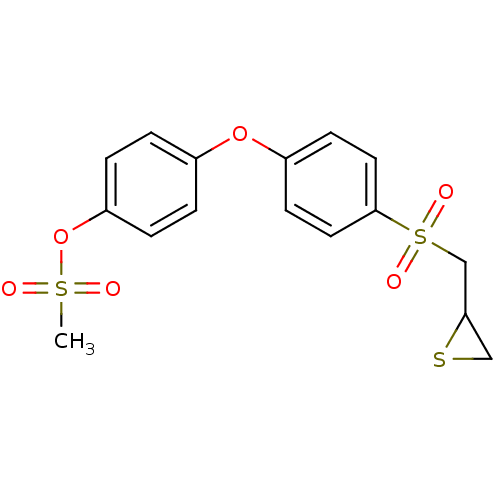
(4-(4-(thiiran-2-ylmethylsulfonyl)phenoxy)phenyl me...)Show SMILES CS(=O)(=O)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H16O6S3/c1-24(17,18)22-14-4-2-12(3-5-14)21-13-6-8-16(9-7-13)25(19,20)11-15-10-23-15/h2-9,15H,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP9 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50388914
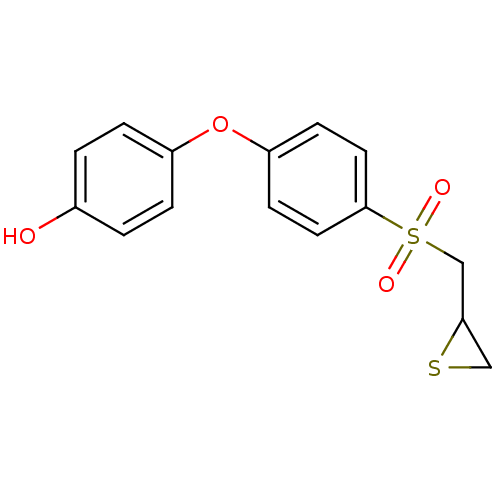
(CHEMBL2063274 | US10357546, p-OH SB-3CT)Show InChI InChI=1S/C15H14O4S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14,16H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50264809
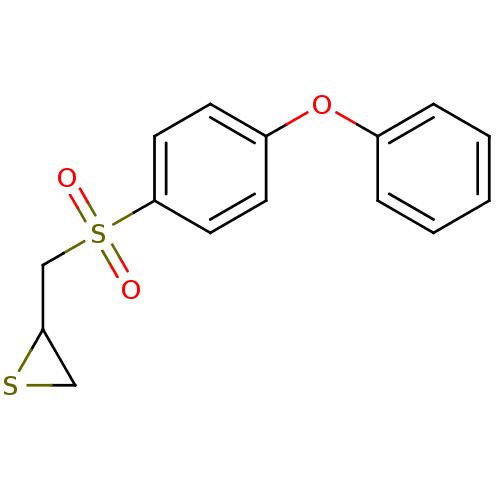
(2-((4-phenoxyphenylsulfonyl)methyl)thiirane | CHEM...)Show InChI InChI=1S/C15H14O3S2/c16-20(17,11-14-10-19-14)15-8-6-13(7-9-15)18-12-4-2-1-3-5-12/h1-9,14H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50335495

(4-(4-(thiiran-2-ylmethylsulfonyl)phenoxy)phenyl me...)Show SMILES CS(=O)(=O)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H16O6S3/c1-24(17,18)22-14-4-2-12(3-5-14)21-13-6-8-16(9-7-13)25(19,20)11-15-10-23-15/h2-9,15H,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50264809

(2-((4-phenoxyphenylsulfonyl)methyl)thiirane | CHEM...)Show InChI InChI=1S/C15H14O3S2/c16-20(17,11-14-10-19-14)15-8-6-13(7-9-15)18-12-4-2-1-3-5-12/h1-9,14H,10-11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP14 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50388911
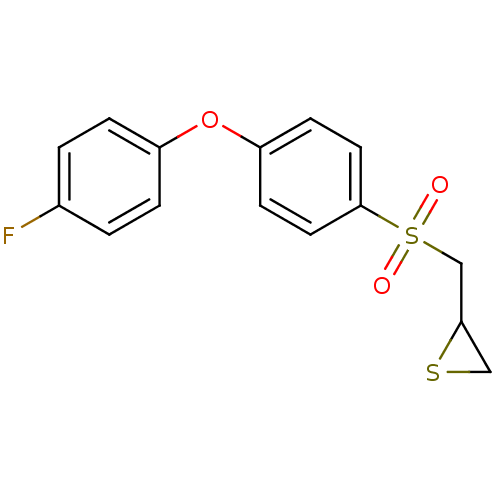
(CHEMBL2063270)Show InChI InChI=1S/C15H13FO3S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP9 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50388911

(CHEMBL2063270)Show InChI InChI=1S/C15H13FO3S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50264809

(2-((4-phenoxyphenylsulfonyl)methyl)thiirane | CHEM...)Show InChI InChI=1S/C15H14O3S2/c16-20(17,11-14-10-19-14)15-8-6-13(7-9-15)18-12-4-2-1-3-5-12/h1-9,14H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
| Assay Description
Inhibition assay using matrix metalloproteinase (MMP-2). |
Chem Biol Drug Des 74: 527-34 (2009)
Checked by Author
Article DOI: 10.1111/j.1747-0285.2009.00881.x
BindingDB Entry DOI: 10.7270/Q20P0XJV |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50388912
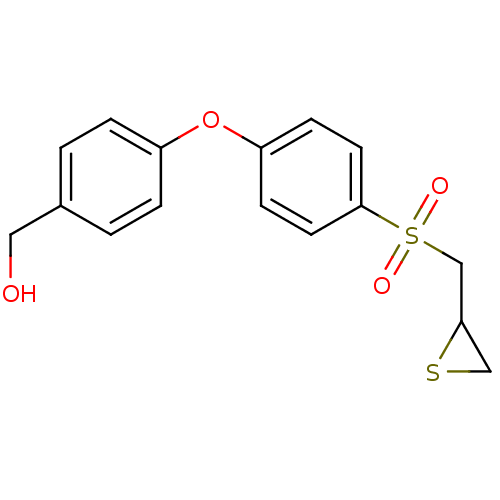
(CHEMBL2063273)Show InChI InChI=1S/C16H16O4S2/c17-9-12-1-3-13(4-2-12)20-14-5-7-16(8-6-14)22(18,19)11-15-10-21-15/h1-8,15,17H,9-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50388914

(CHEMBL2063274 | US10357546, p-OH SB-3CT)Show InChI InChI=1S/C15H14O4S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14,16H,9-10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP14 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50388917
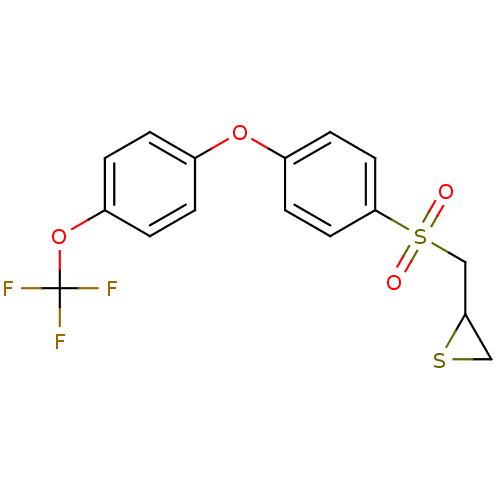
(CHEMBL2063277)Show SMILES FC(F)(F)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H13F3O4S2/c17-16(18,19)23-13-3-1-11(2-4-13)22-12-5-7-15(8-6-12)25(20,21)10-14-9-24-14/h1-8,14H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50388916
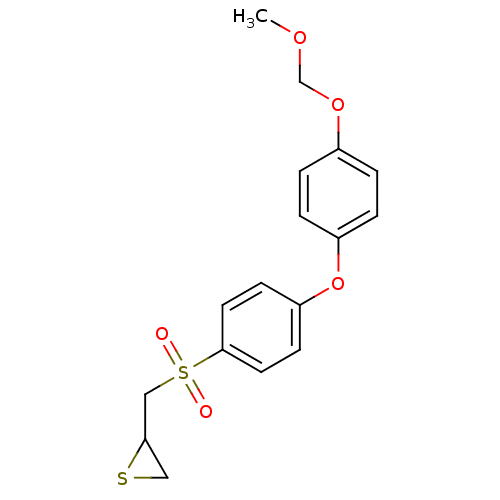
(CHEMBL2063276)Show InChI InChI=1S/C17H18O5S2/c1-20-12-21-13-2-4-14(5-3-13)22-15-6-8-17(9-7-15)24(18,19)11-16-10-23-16/h2-9,16H,10-12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP14 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50335495

(4-(4-(thiiran-2-ylmethylsulfonyl)phenoxy)phenyl me...)Show SMILES CS(=O)(=O)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H16O6S3/c1-24(17,18)22-14-4-2-12(3-5-14)21-13-6-8-16(9-7-13)25(19,20)11-15-10-23-15/h2-9,15H,10-11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP14 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50388916

(CHEMBL2063276)Show InChI InChI=1S/C17H18O5S2/c1-20-12-21-13-2-4-14(5-3-13)22-15-6-8-17(9-7-15)24(18,19)11-16-10-23-16/h2-9,16H,10-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP9 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50388914

(CHEMBL2063274 | US10357546, p-OH SB-3CT)Show InChI InChI=1S/C15H14O4S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14,16H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP9 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50388916

(CHEMBL2063276)Show InChI InChI=1S/C17H18O5S2/c1-20-12-21-13-2-4-14(5-3-13)22-15-6-8-17(9-7-15)24(18,19)11-16-10-23-16/h2-9,16H,10-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50388910
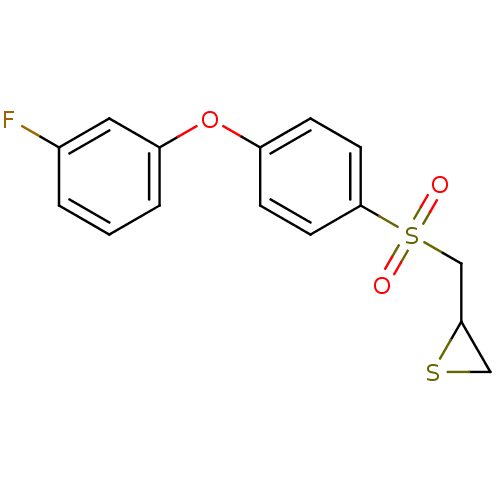
(CHEMBL2063271)Show InChI InChI=1S/C15H13FO3S2/c16-11-2-1-3-13(8-11)19-12-4-6-15(7-5-12)21(17,18)10-14-9-20-14/h1-8,14H,9-10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP14 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50388912

(CHEMBL2063273)Show InChI InChI=1S/C16H16O4S2/c17-9-12-1-3-13(4-2-12)20-14-5-7-16(8-6-14)22(18,19)11-15-10-21-15/h1-8,15,17H,9-11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 215 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP14 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50388915
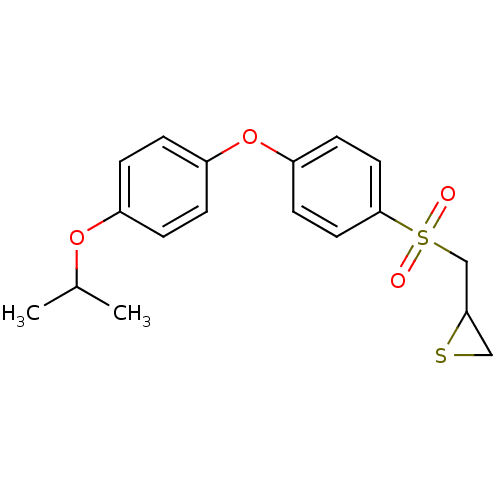
(CHEMBL2063275)Show InChI InChI=1S/C18H20O4S2/c1-13(2)21-14-3-5-15(6-4-14)22-16-7-9-18(10-8-16)24(19,20)12-17-11-23-17/h3-10,13,17H,11-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP14 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50388913
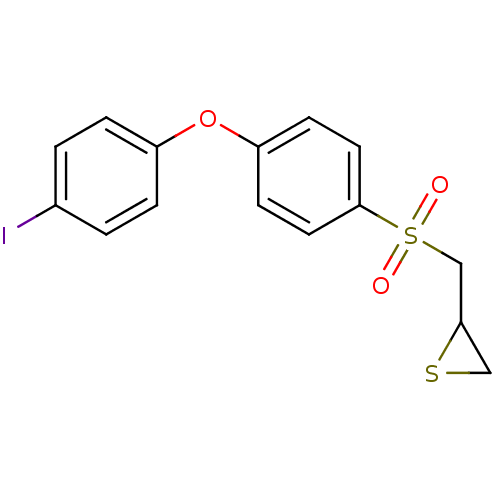
(CHEMBL2063272)Show InChI InChI=1S/C15H13IO3S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP9 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50388915

(CHEMBL2063275)Show InChI InChI=1S/C18H20O4S2/c1-13(2)21-14-3-5-15(6-4-14)22-16-7-9-18(10-8-16)24(19,20)12-17-11-23-17/h3-10,13,17H,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50264809

(2-((4-phenoxyphenylsulfonyl)methyl)thiirane | CHEM...)Show InChI InChI=1S/C15H14O3S2/c16-20(17,11-14-10-19-14)15-8-6-13(7-9-15)18-12-4-2-1-3-5-12/h1-9,14H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
| Assay Description
Inhibition assay using matrix metalloproteinase (MMP-2). |
Chem Biol Drug Des 74: 527-34 (2009)
Checked by Author
Article DOI: 10.1111/j.1747-0285.2009.00881.x
BindingDB Entry DOI: 10.7270/Q20P0XJV |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50388912

(CHEMBL2063273)Show InChI InChI=1S/C16H16O4S2/c17-9-12-1-3-13(4-2-12)20-14-5-7-16(8-6-14)22(18,19)11-15-10-21-15/h1-8,15,17H,9-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP9 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50388910

(CHEMBL2063271)Show InChI InChI=1S/C15H13FO3S2/c16-11-2-1-3-13(8-11)19-12-4-6-15(7-5-12)21(17,18)10-14-9-20-14/h1-8,14H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50388915

(CHEMBL2063275)Show InChI InChI=1S/C18H20O4S2/c1-13(2)21-14-3-5-15(6-4-14)22-16-7-9-18(10-8-16)24(19,20)12-17-11-23-17/h3-10,13,17H,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP9 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50335495

(4-(4-(thiiran-2-ylmethylsulfonyl)phenoxy)phenyl me...)Show SMILES CS(=O)(=O)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H16O6S3/c1-24(17,18)22-14-4-2-12(3-5-14)21-13-6-8-16(9-7-13)25(19,20)11-15-10-23-15/h2-9,15H,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 575 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP3 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50388911

(CHEMBL2063270)Show InChI InChI=1S/C15H13FO3S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14H,9-10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP14 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50388913

(CHEMBL2063272)Show InChI InChI=1S/C15H13IO3S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP2 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50264809

(2-((4-phenoxyphenylsulfonyl)methyl)thiirane | CHEM...)Show InChI InChI=1S/C15H14O3S2/c16-20(17,11-14-10-19-14)15-8-6-13(7-9-15)18-12-4-2-1-3-5-12/h1-9,14H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP9 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50388917

(CHEMBL2063277)Show SMILES FC(F)(F)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H13F3O4S2/c17-16(18,19)23-13-3-1-11(2-4-13)22-12-5-7-15(8-6-12)25(20,21)10-14-9-24-14/h1-8,14H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP9 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM82121

(Gama, gama-dimethyl derivative, 6)Show InChI InChI=1S/C17H18O3S2/c1-17(2)16(21-17)12-22(18,19)15-10-8-14(9-11-15)20-13-6-4-3-5-7-13/h3-11,16H,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
| Assay Description
Inhibition assay using matrix metalloproteinase (MMP-2). |
Chem Biol Drug Des 74: 527-34 (2009)
Checked by Author
Article DOI: 10.1111/j.1747-0285.2009.00881.x
BindingDB Entry DOI: 10.7270/Q20P0XJV |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM82119

(Sulfoxide, 4)Show InChI InChI=1S/C15H14O2S2/c16-19(11-14-10-18-14)15-8-6-13(7-9-15)17-12-4-2-1-3-5-12/h1-9,14H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
| Assay Description
Inhibition assay using matrix metalloproteinase (MMP-2). |
Chem Biol Drug Des 74: 527-34 (2009)
Checked by Author
Article DOI: 10.1111/j.1747-0285.2009.00881.x
BindingDB Entry DOI: 10.7270/Q20P0XJV |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50388914

(CHEMBL2063274 | US10357546, p-OH SB-3CT)Show InChI InChI=1S/C15H14O4S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14,16H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP3 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50388910

(CHEMBL2063271)Show InChI InChI=1S/C15H13FO3S2/c16-11-2-1-3-13(8-11)19-12-4-6-15(7-5-12)21(17,18)10-14-9-20-14/h1-8,14H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP9 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50388912

(CHEMBL2063273)Show InChI InChI=1S/C16H16O4S2/c17-9-12-1-3-13(4-2-12)20-14-5-7-16(8-6-14)22(18,19)11-15-10-21-15/h1-8,15,17H,9-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP3 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50388917

(CHEMBL2063277)Show SMILES FC(F)(F)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H13F3O4S2/c17-16(18,19)23-13-3-1-11(2-4-13)22-12-5-7-15(8-6-12)25(20,21)10-14-9-24-14/h1-8,14H,9-10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP14 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50388916

(CHEMBL2063276)Show InChI InChI=1S/C17H18O5S2/c1-20-12-21-13-2-4-14(5-3-13)22-15-6-8-17(9-7-15)24(18,19)11-16-10-23-16/h2-9,16H,10-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP3 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50264809

(2-((4-phenoxyphenylsulfonyl)methyl)thiirane | CHEM...)Show InChI InChI=1S/C15H14O3S2/c16-20(17,11-14-10-19-14)15-8-6-13(7-9-15)18-12-4-2-1-3-5-12/h1-9,14H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP3 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50388915

(CHEMBL2063275)Show InChI InChI=1S/C18H20O4S2/c1-13(2)21-14-3-5-15(6-4-14)22-16-7-9-18(10-8-16)24(19,20)12-17-11-23-17/h3-10,13,17H,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP3 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50335495

(4-(4-(thiiran-2-ylmethylsulfonyl)phenoxy)phenyl me...)Show SMILES CS(=O)(=O)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H16O6S3/c1-24(17,18)22-14-4-2-12(3-5-14)21-13-6-8-16(9-7-13)25(19,20)11-15-10-23-15/h2-9,15H,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP7 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50388910

(CHEMBL2063271)Show InChI InChI=1S/C15H13FO3S2/c16-11-2-1-3-13(8-11)19-12-4-6-15(7-5-12)21(17,18)10-14-9-20-14/h1-8,14H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP3 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50388914

(CHEMBL2063274 | US10357546, p-OH SB-3CT)Show InChI InChI=1S/C15H14O4S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14,16H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP7 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM82120

(Alpha, alpha-dimethyl derivative, 5)Show InChI InChI=1S/C17H18O3S2/c1-17(2,16-12-21-16)22(18,19)15-10-8-14(9-11-15)20-13-6-4-3-5-7-13/h3-11,16H,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
| Assay Description
Inhibition assay using matrix metalloproteinase (MMP-2). |
Chem Biol Drug Des 74: 527-34 (2009)
Checked by Author
Article DOI: 10.1111/j.1747-0285.2009.00881.x
BindingDB Entry DOI: 10.7270/Q20P0XJV |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50388912

(CHEMBL2063273)Show InChI InChI=1S/C16H16O4S2/c17-9-12-1-3-13(4-2-12)20-14-5-7-16(8-6-14)22(18,19)11-15-10-21-15/h1-8,15,17H,9-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP7 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50264809

(2-((4-phenoxyphenylsulfonyl)methyl)thiirane | CHEM...)Show InChI InChI=1S/C15H14O3S2/c16-20(17,11-14-10-19-14)15-8-6-13(7-9-15)18-12-4-2-1-3-5-12/h1-9,14H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP7 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50388914

(CHEMBL2063274 | US10357546, p-OH SB-3CT)Show InChI InChI=1S/C15H14O4S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14,16H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP1 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50335495

(4-(4-(thiiran-2-ylmethylsulfonyl)phenoxy)phenyl me...)Show SMILES CS(=O)(=O)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H16O6S3/c1-24(17,18)22-14-4-2-12(3-5-14)21-13-6-8-16(9-7-13)25(19,20)11-15-10-23-15/h2-9,15H,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP1 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50388912

(CHEMBL2063273)Show InChI InChI=1S/C16H16O4S2/c17-9-12-1-3-13(4-2-12)20-14-5-7-16(8-6-14)22(18,19)11-15-10-21-15/h1-8,15,17H,9-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP1 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50264809

(2-((4-phenoxyphenylsulfonyl)methyl)thiirane | CHEM...)Show InChI InChI=1S/C15H14O3S2/c16-20(17,11-14-10-19-14)15-8-6-13(7-9-15)18-12-4-2-1-3-5-12/h1-9,14H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of human MMP1 using fluorogenic substrate by Dixon plot analysis |
ACS Med Chem Lett 3: 490-495 (2012)
Article DOI: 10.1021/ml300050b
BindingDB Entry DOI: 10.7270/Q2T43V4Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data