

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50128548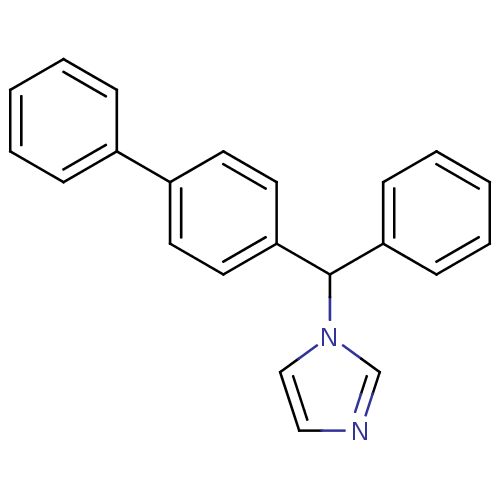 (1-(1-Biphenyl-4-yl-2-phenyl-methyl)-1H-imidazole |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 56.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro inhibition of human Cytochrome P450 17A1 activity | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM31774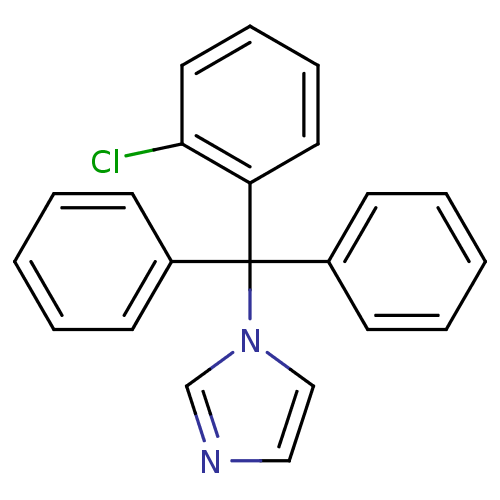 (CHEMBL104 | Canesten | Clotrimazole | Lotrimin | M...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 81.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro inhibition of human Cytochrome P450 17A1 activity | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM31772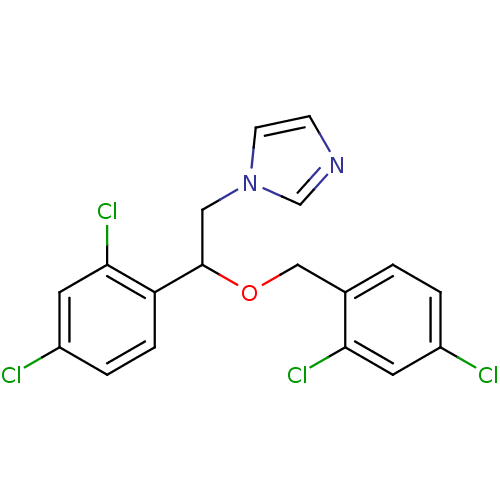 (1-[2-(2,4-dichlorobenzyl)oxy-2-(2,4-dichlorophenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 243 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro inhibition of human Cytochrome P450 17A1 activity | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM31773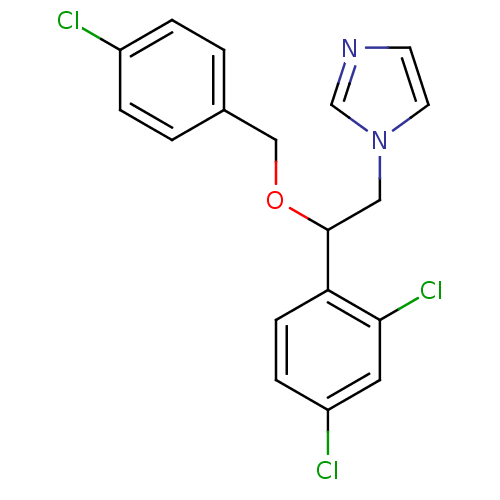 (ECONAZOLE | Econazole nitrate | Gyno-pevaryl | Pev...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 325 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro inhibition of human Cytochrome P450 17A1 activity | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50370218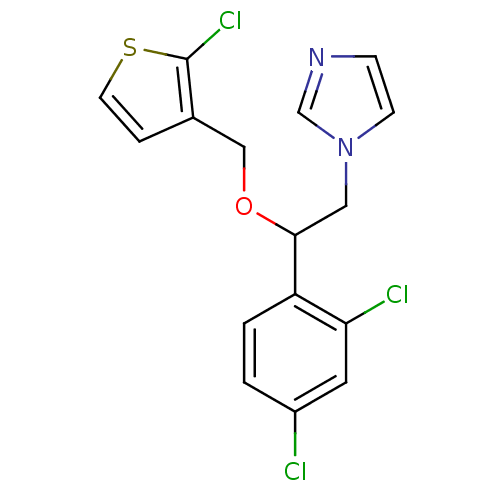 (TIOCONAZOLE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | 505 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro inhibition of human Cytochrome P450 17A1 activity | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50128554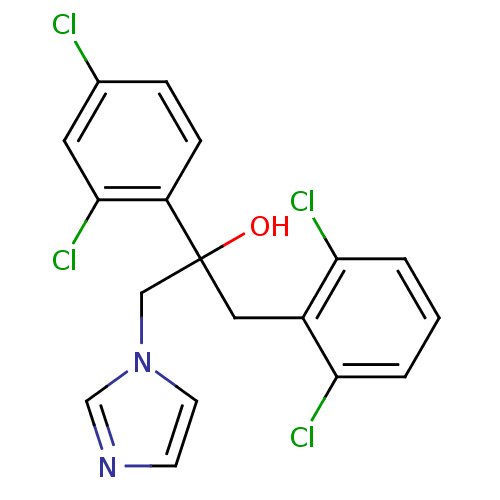 (1-(2,6-Dichloro-phenyl)-2-(2,4-dichloro-phenyl)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro inhibition of human Cytochrome P450 17A1 activity | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50097370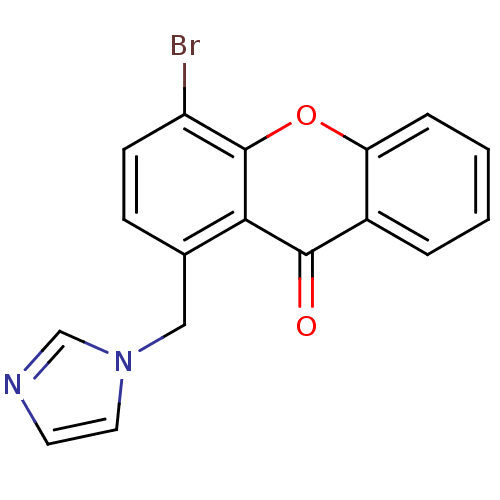 (1-((1H-imidazol-1-yl)methyl)-4-bromo-9H-xanthen-9-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro inhibition of human Cytochrome P450 17A1 activity | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50063477 ((3S,10R,13S)-17-Imidazol-1-yl-10,13-dimethyl-2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50215709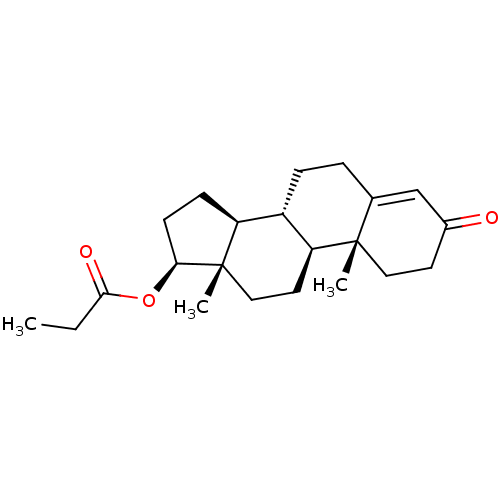 (CHEMBL1170 | Propionic acid (8R,9S,10R,13S,14S,17S...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50097363 (1-((1H-imidazol-1-yl)methyl)-4-nitro-9H-xanthen-9-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro inhibition of human Cytochrome P450 17A1 activity | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50063475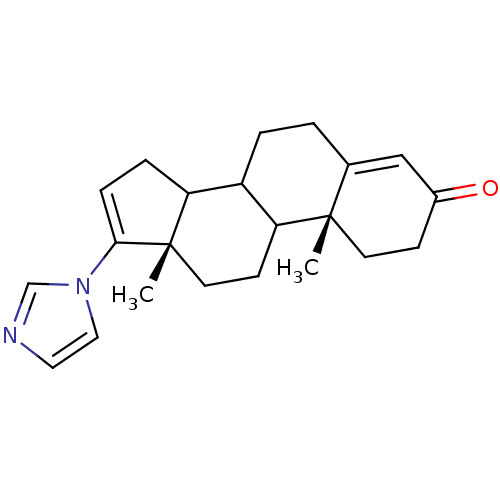 ((10R,13S)-17-Imidazol-1-yl-10,13-dimethyl-1,2,6,7,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50128550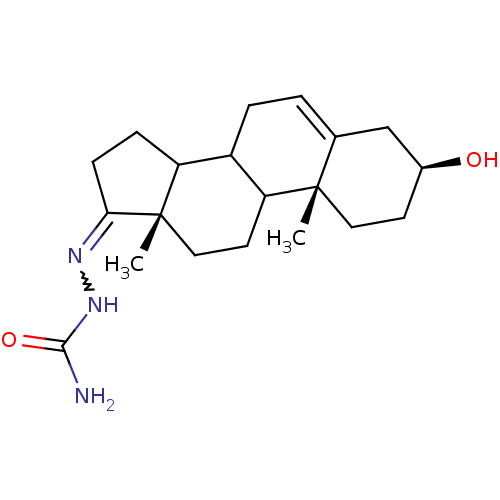 (CHEMBL77751 | NRB 03731) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 178 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50097365 (1-((1H-imidazol-1-yl)methyl)-9-oxo-9H-xanthene-4-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro inhibition of human Cytochrome P450 17A1 activity | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50128565 ((3S,10R,13S,17R)-10,13-Dimethyl-17-pyrimidin-4-yl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 225 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50128564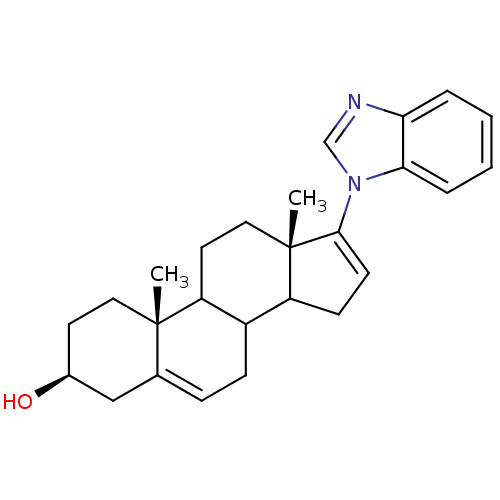 ((3S,10R,13S)-17-Benzoimidazol-1-yl-10,13-dimethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50409697 (CHEMBL2112282) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50063476 ((3S,10R,13S)-10,13-Dimethyl-17-[1,2,3]triazol-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 377 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50063479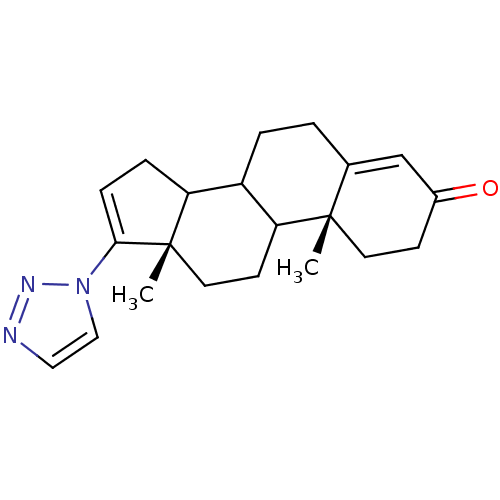 ((10R,13S)-10,13-Dimethyl-17-[1,2,3]triazol-1-yl-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 387 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50128563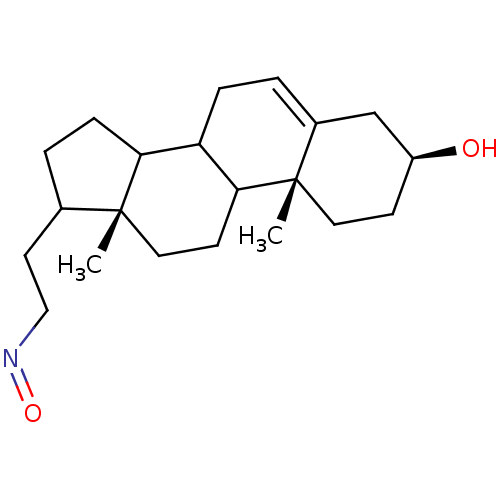 (((3S,10R,13R)-3-Hydroxy-10,13-dimethyl-2,3,4,7,8,9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50128543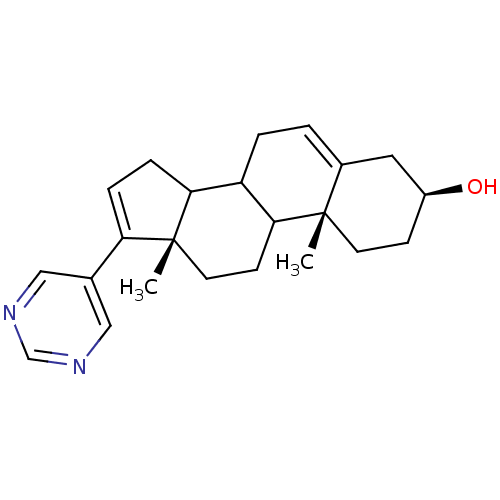 ((3S,10R,13S)-10,13-Dimethyl-17-pyrimidin-5-yl-2,3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50128551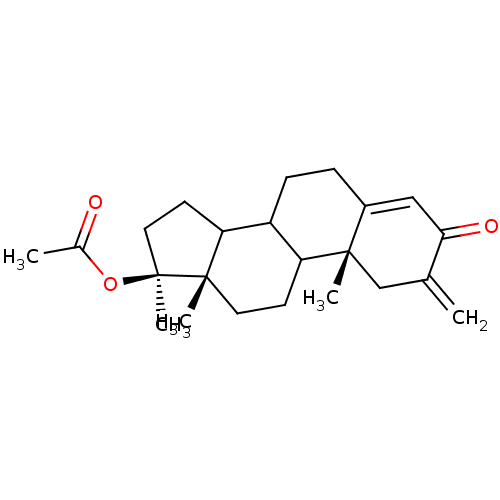 (Acetic acid (10R,13S,17S)-10,13,17-trimethyl-2-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 562 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50128556 ((R)-4-((3S,10R)-3-Hydroxy-10-methyl-2,3,4,7,8,9,10...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 617 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50128546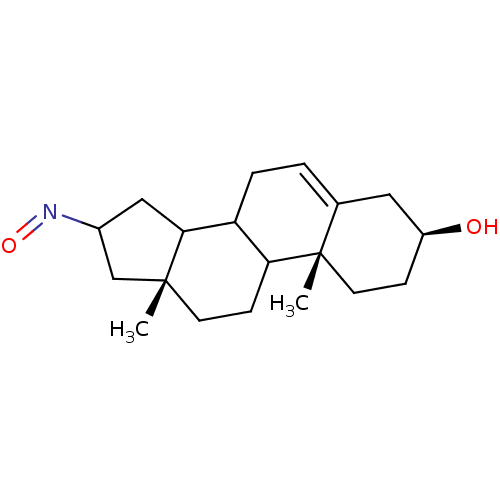 ((3S,10R,13R)-3-Hydroxy-10,13-dimethyl-1,2,3,4,7,8,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 628 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50128560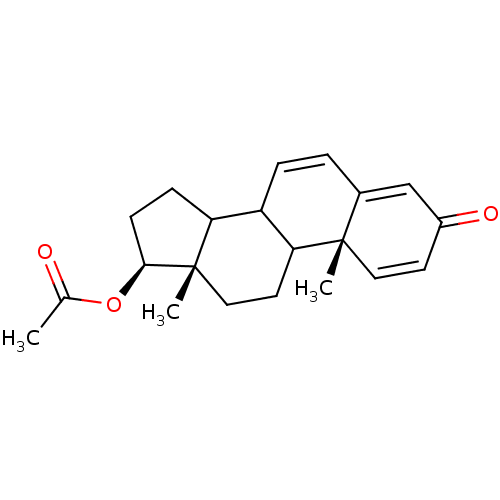 (Acetic acid (10R,13S,17S)-10,13-dimethyl-3-oxo-8,9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25458 ((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50063480 ((3S,10R,13S)-10,13-Dimethyl-17-[1,2,4]triazol-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50128544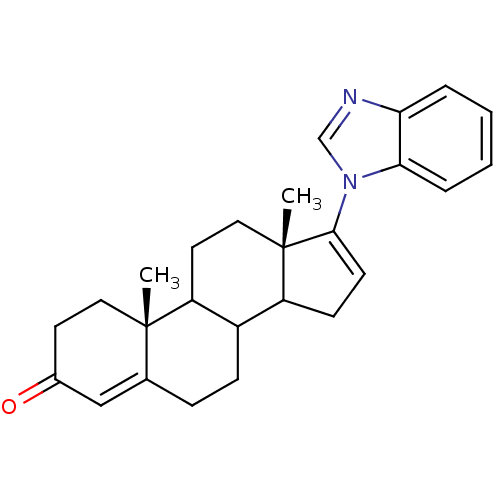 ((10R,13S)-17-Benzoimidazol-1-yl-10,13-dimethyl-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 915 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50061172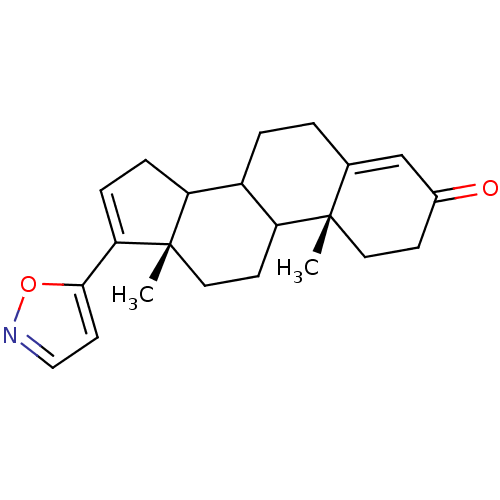 ((10R,13S)-17-Isoxazol-5-yl-10,13-dimethyl-1,2,6,7,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50063478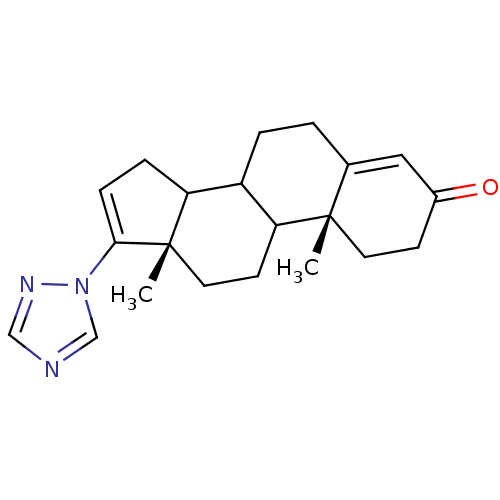 ((10R,13S)-10,13-Dimethyl-17-[1,2,4]triazol-1-yl-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50128552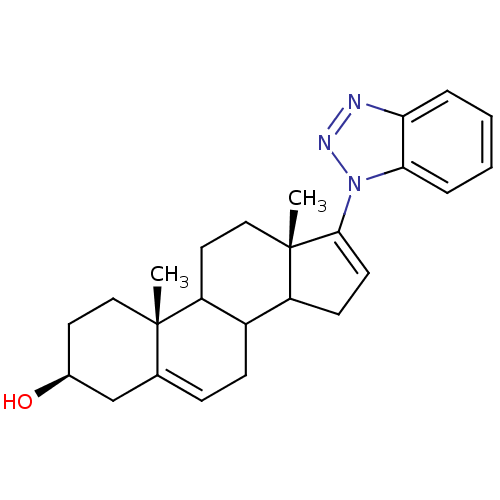 ((3S,10R,13S)-17-Benzotriazol-1-yl-10,13-dimethyl-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50128561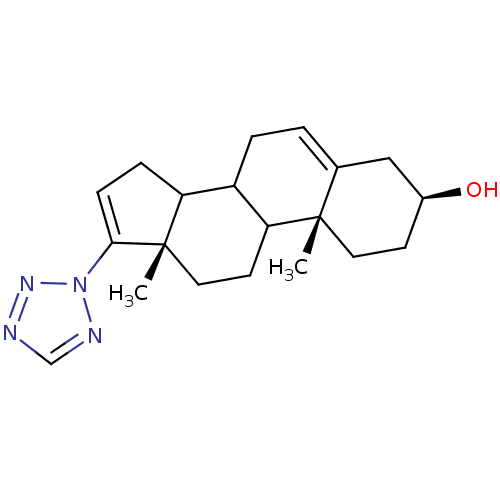 ((3S,10R,13S)-10,13-Dimethyl-17-tetrazol-2-yl-2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50409695 (CHEMBL2112280) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50128555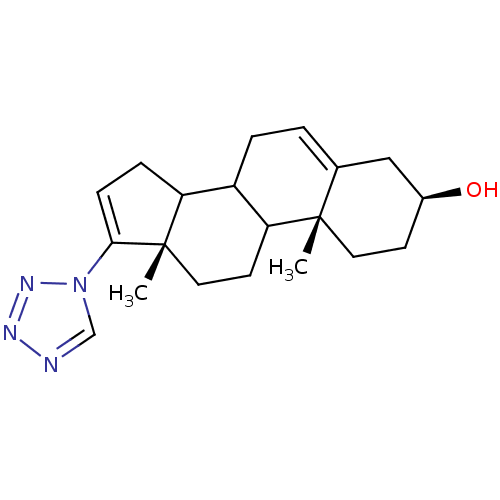 ((3S,10R,13S)-10,13-Dimethyl-17-tetrazol-1-yl-2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50128547 ((3S,10R,13S)-10,13-Dimethyl-17-[1,2,3]triazol-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50128542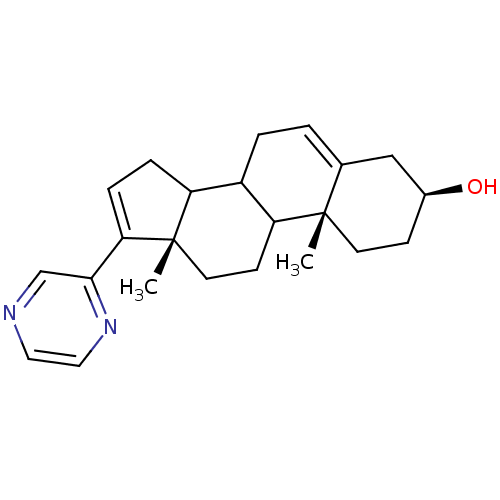 ((3S,10R,13S)-10,13-Dimethyl-17-pyrazin-2-yl-2,3,4,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50128557 ((3S,10R,13S)-17-Hydrazono-3-hydroxy-10,13-dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50409696 (CHEMBL2112281) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||