

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (RAT-Rattus norvegicus) | BDBM50110039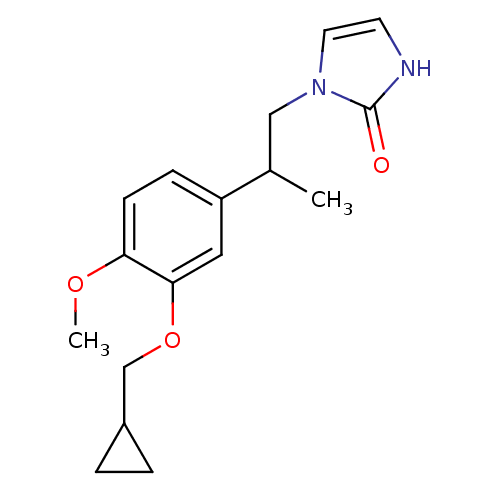 (1-[2-(3-Cyclopropylmethoxy-4-methoxy-phenyl)-propy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen-Cilag Curated by ChEMBL | Assay Description Displacement of [3H]rolipram from rat forebrain membrane | Bioorg Med Chem Lett 12: 653-8 (2002) BindingDB Entry DOI: 10.7270/Q2DV1KF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (RAT-Rattus norvegicus) | BDBM14361 ((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen-Cilag Curated by ChEMBL | Assay Description Inhibition of [3H]rolipram binding to Phosphodiesterase 4 of rat forebrain membrane | Bioorg Med Chem Lett 12: 653-8 (2002) BindingDB Entry DOI: 10.7270/Q2DV1KF6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (RAT-Rattus norvegicus) | BDBM14775 (3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen-Cilag Curated by ChEMBL | Assay Description Inhibition of [3H]rolipram binding to Phosphodiesterase 4 of rat forebrain membrane | Bioorg Med Chem Lett 12: 653-8 (2002) BindingDB Entry DOI: 10.7270/Q2DV1KF6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (RAT-Rattus norvegicus) | BDBM50451480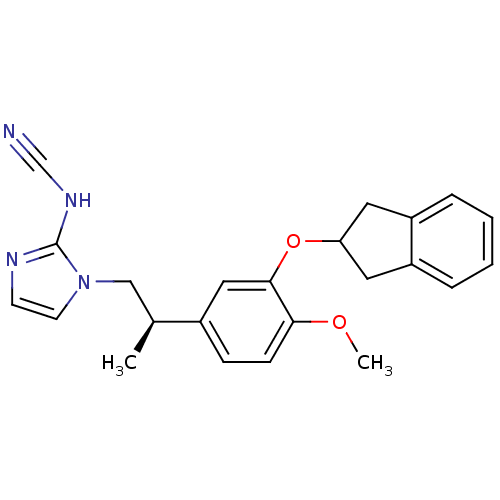 (CHEMBL2093042) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen-Cilag Curated by ChEMBL | Assay Description Displacement of [3H]rolipram from rat forebrain membrane | Bioorg Med Chem Lett 12: 653-8 (2002) BindingDB Entry DOI: 10.7270/Q2DV1KF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (RAT-Rattus norvegicus) | BDBM50110030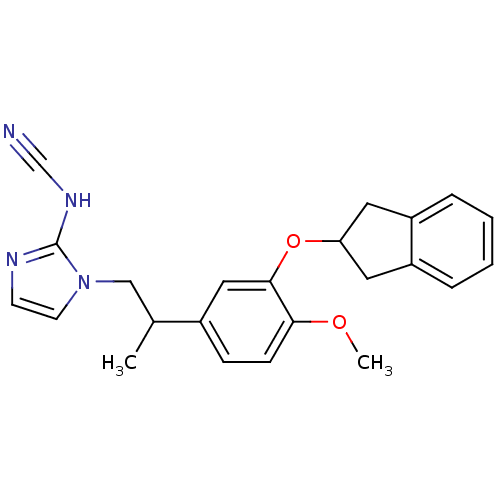 (1-{2-[3-(Indan-2-yloxy)-4-methoxy-phenyl]-propyl}-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen-Cilag Curated by ChEMBL | Assay Description Displacement of [3H]rolipram from rat forebrain membrane | Bioorg Med Chem Lett 12: 653-8 (2002) BindingDB Entry DOI: 10.7270/Q2DV1KF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (RAT-Rattus norvegicus) | BDBM50366802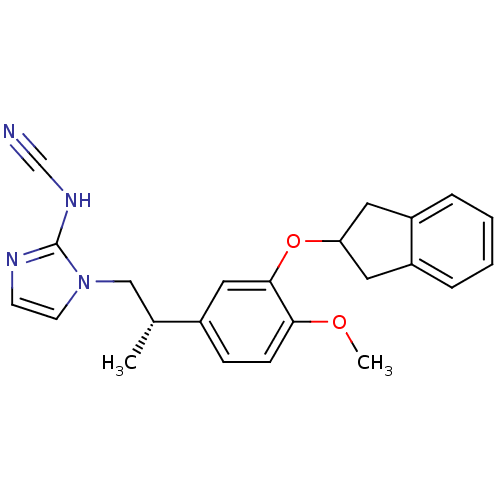 (CHEMBL1788264) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen-Cilag Curated by ChEMBL | Assay Description Displacement of [3H]rolipram from rat forebrain membrane | Bioorg Med Chem Lett 12: 653-8 (2002) BindingDB Entry DOI: 10.7270/Q2DV1KF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM14775 (3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen-Cilag Curated by ChEMBL | Assay Description Inhibitory activity against recombinant phosphodiesterase 4B (PDE4B) of human mononuclear lymphocytes | Bioorg Med Chem Lett 12: 653-8 (2002) BindingDB Entry DOI: 10.7270/Q2DV1KF6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128502 (US8796244, 81) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.447 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128624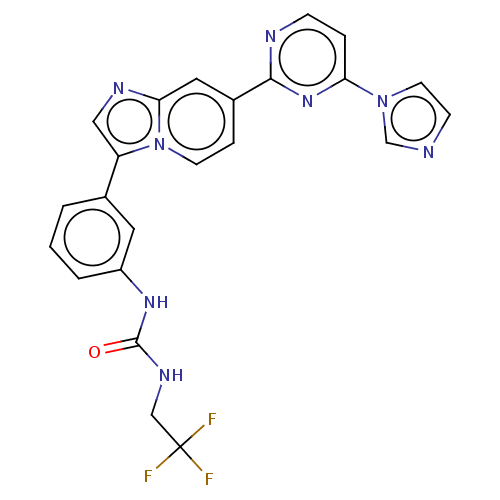 (US8796244, 213) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128551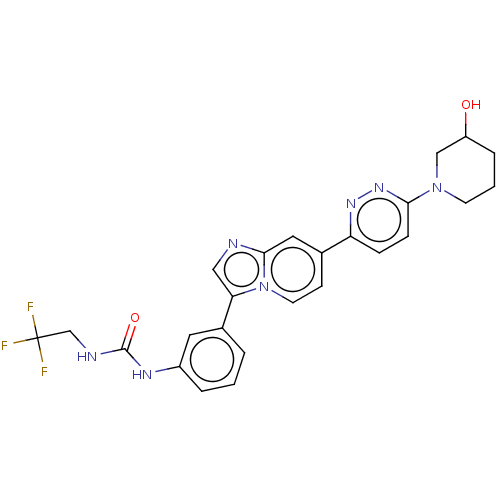 (US8796244, 133) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.484 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128659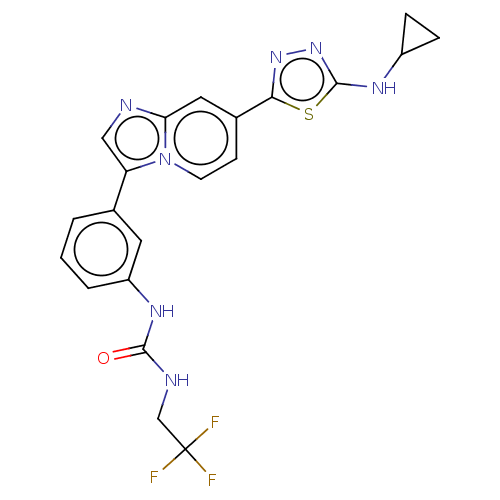 (US8796244, 250) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50127246 (8-[Amino-(4-chloro-phenyl)-(3-methyl-3H-imidazol-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of Geranylgeranylprotein transferase-I at 10 uM | Bioorg Med Chem Lett 13: 1543-7 (2003) BindingDB Entry DOI: 10.7270/Q2M61JNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50127246 (8-[Amino-(4-chloro-phenyl)-(3-methyl-3H-imidazol-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 1543-7 (2003) BindingDB Entry DOI: 10.7270/Q2M61JNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128516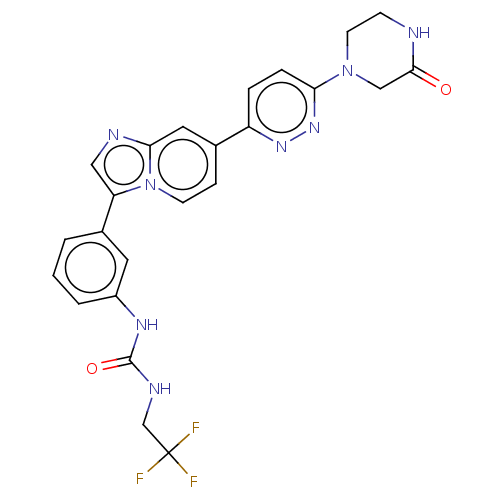 (US8796244, 95) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128496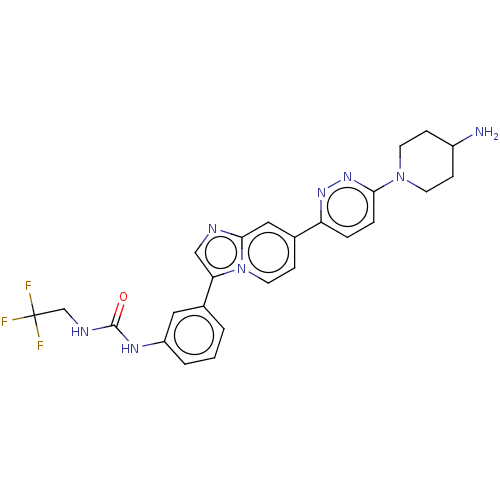 (US8796244, 75) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.535 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128668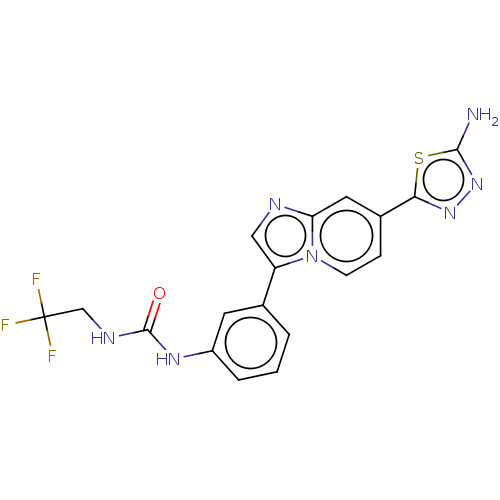 (US8796244, 261) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128468 (US8796244, 47) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.542 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128427 (US8796244, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.545 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128428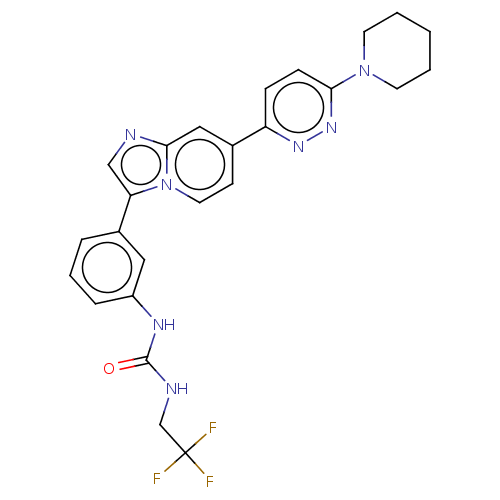 (US8796244, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128561 (US8796244, 145) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128488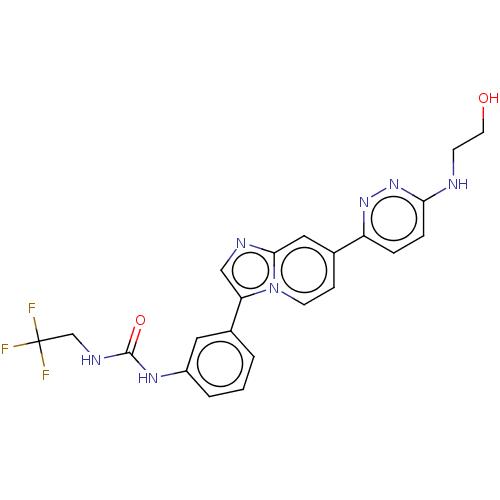 (US8796244, 67) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.569 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128425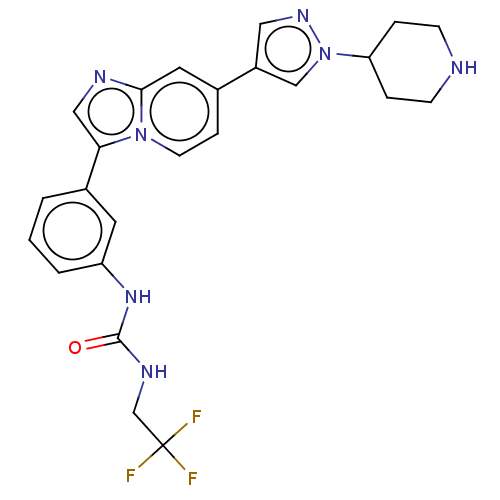 (US8796244, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128465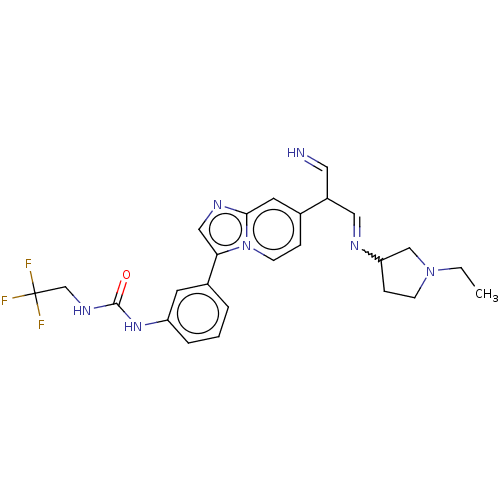 (US8796244, 44) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.582 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50136383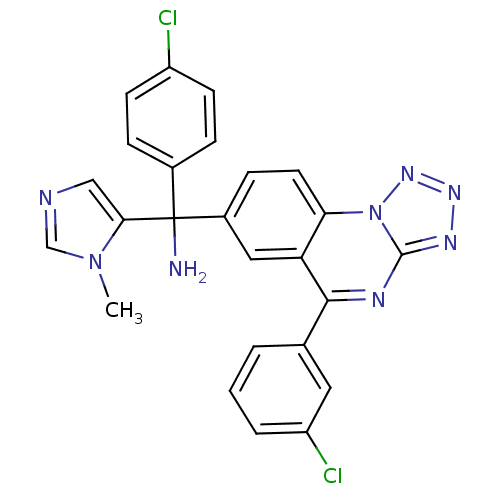 ((4-chlorophenyl)(5-(3-chlorophenyl)tetrazolo[1,5-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD) Curated by ChEMBL | Assay Description Inhibition of Geranylgeranylprotein transferase-I catalyzed incorporation of [3H]-GGPP into biotinYRASNRSCAIL peptide | Bioorg Med Chem Lett 13: 4365-9 (2003) BindingDB Entry DOI: 10.7270/Q21R6PX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50136383 ((4-chlorophenyl)(5-(3-chlorophenyl)tetrazolo[1,5-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD) Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 4365-9 (2003) BindingDB Entry DOI: 10.7270/Q21R6PX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128466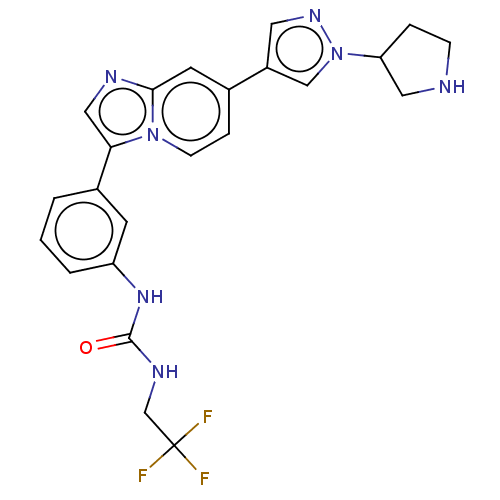 (US8796244, 45) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.603 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128459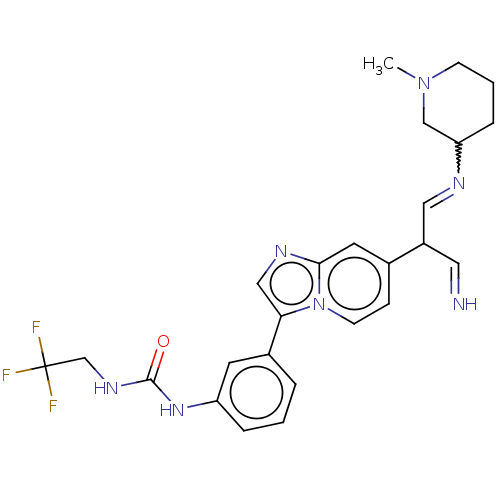 (US8796244, 38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128534 (US8796244, 113) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128461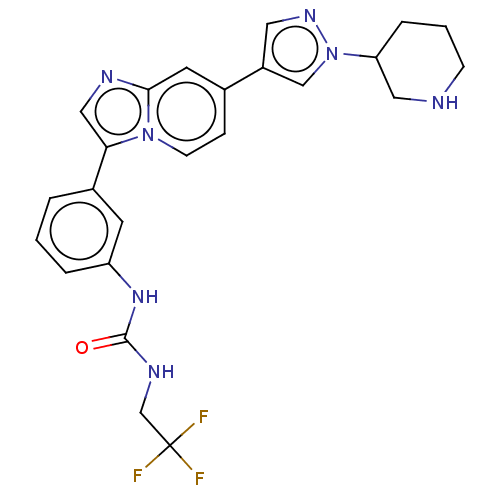 (US8796244, 40) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.612 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128453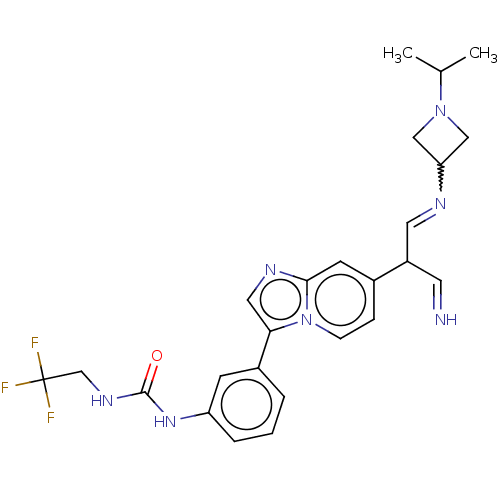 (US8796244, 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.613 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128436 (US8796244, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128725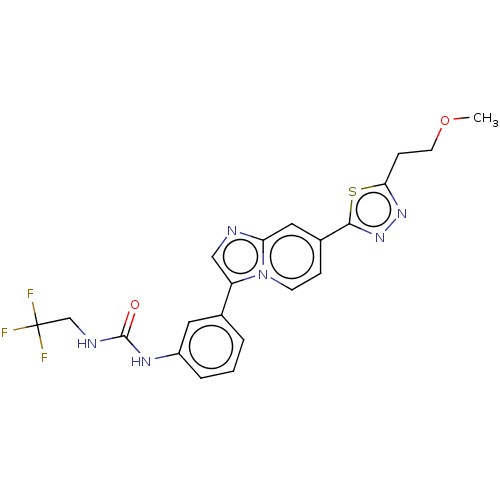 (US8796244, 319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.643 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128474 (US8796244, 53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.675 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128543 (US8796244, 122) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.704 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128443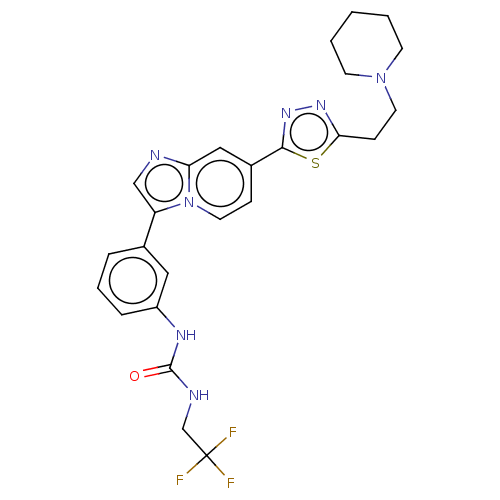 (US8796244, 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.704 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128550 (US8796244, 132) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128503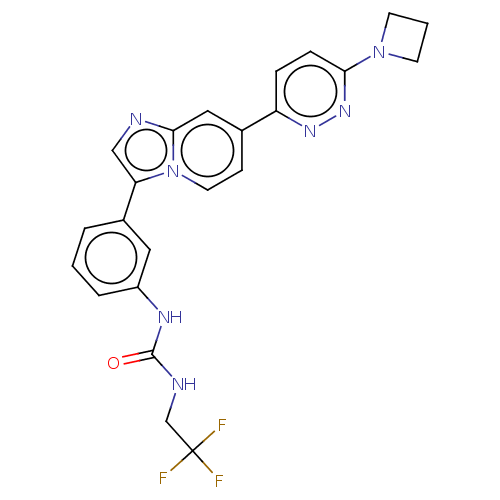 (US8796244, 82) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.715 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128497 (US8796244, 76) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128491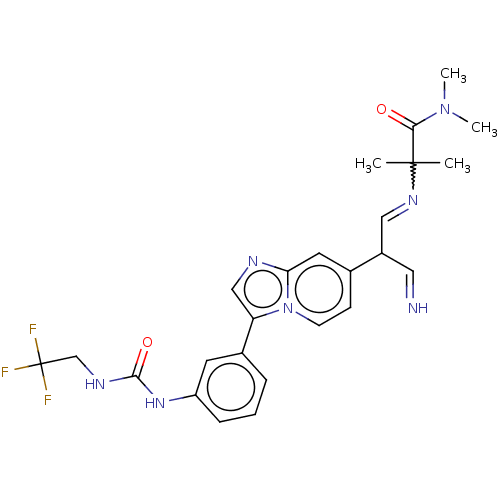 (US8796244, 70) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128545 (US8796244, 124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.789 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50126335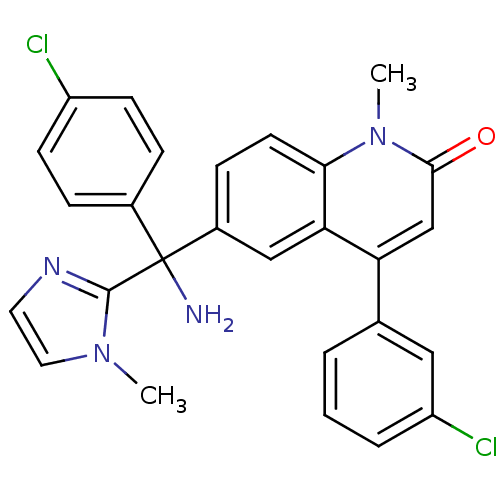 (6-[(R)-Amino-(4-chloro-phenyl)-(3-methyl-3H-imidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | MMDB PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase | Bioorg Med Chem Lett 13: 1543-7 (2003) BindingDB Entry DOI: 10.7270/Q2M61JNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128458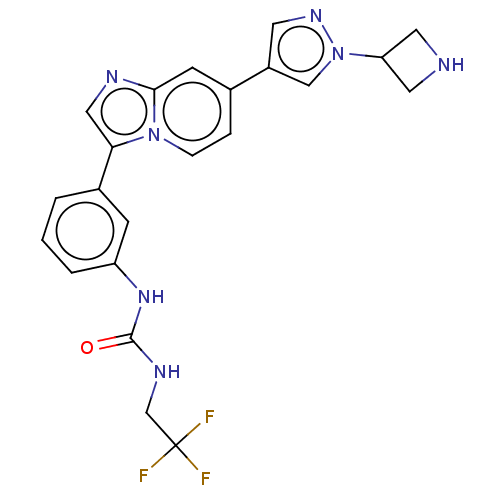 (US8796244, 37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.807 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128457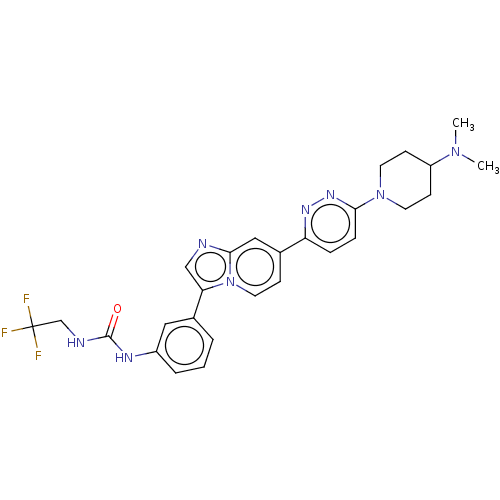 (US8796244, 36) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128422 (US8796244, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128653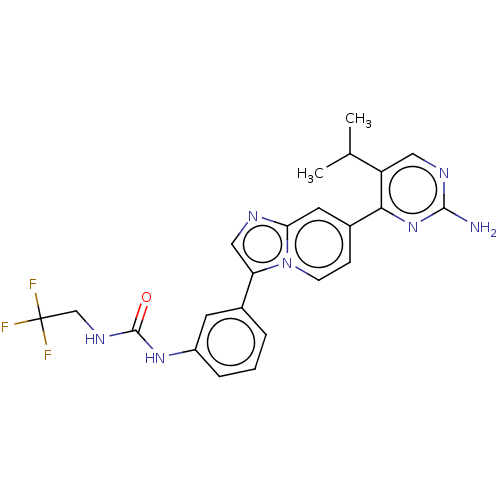 (US8796244, 242) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128512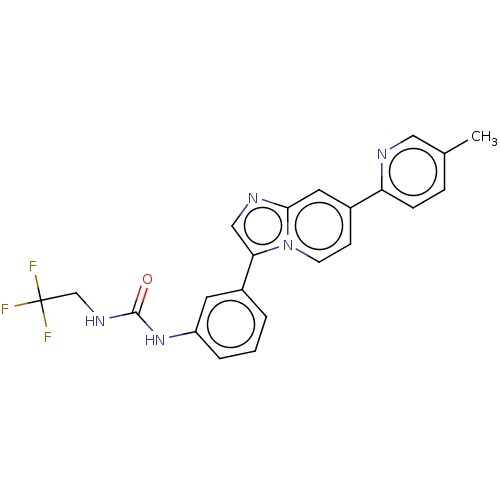 (US8796244, 91) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128423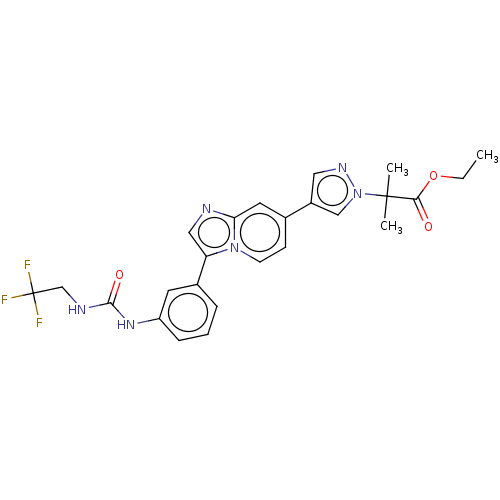 (US8796244, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128646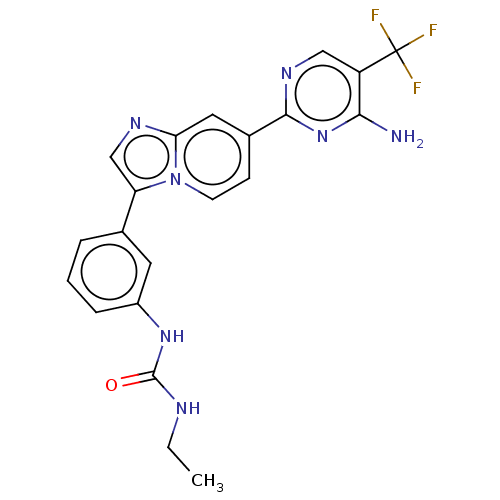 (US8796244, 235) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128501 (US8796244, 80) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM128532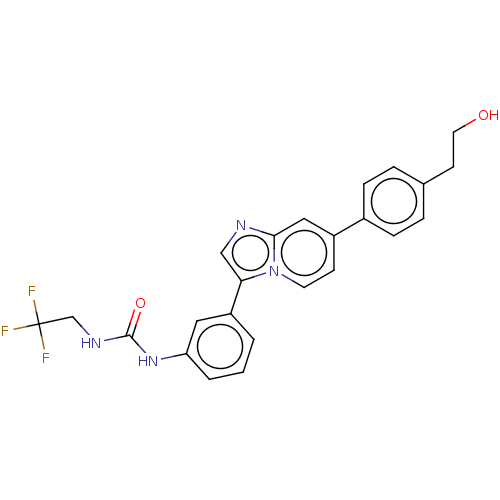 (US8796244, 111) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Astex Therapeutics Ltd US Patent | Assay Description Enzymes (from Upstate) were prepared at 2× final concentration in 1× kinase assay buffer (Table 1). Enzymes were then incubated with test compounds, ... | US Patent US8796244 (2014) BindingDB Entry DOI: 10.7270/Q20C4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 721 total ) | Next | Last >> |