 Found 711 hits with Last Name = 'fries' and Initial = 'h'
Found 711 hits with Last Name = 'fries' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244020
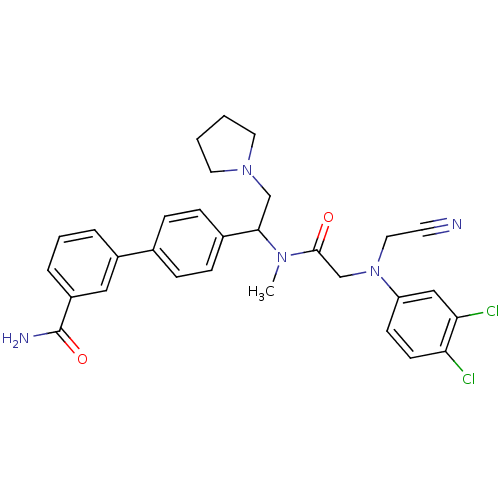
(4'-[1-({2-[Cyanomethyl-(3,4-dichloro-phenyl)-amino...)Show SMILES CN(C(CN1CCCC1)c1ccc(cc1)-c1cccc(c1)C(N)=O)C(=O)CN(CC#N)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C30H31Cl2N5O2/c1-35(29(38)20-37(16-13-33)25-11-12-26(31)27(32)18-25)28(19-36-14-2-3-15-36)22-9-7-21(8-10-22)23-5-4-6-24(17-23)30(34)39/h4-12,17-18,28H,2-3,14-16,19-20H2,1H3,(H2,34,39) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377220
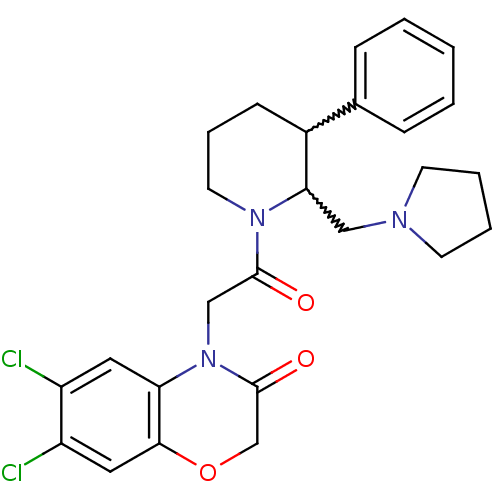
(CHEMBL255509)Show SMILES Clc1cc2OCC(=O)N(CC(=O)N3CCCC(C3CN3CCCC3)c3ccccc3)c2cc1Cl |w:17.18,16.25| Show InChI InChI=1S/C26H29Cl2N3O3/c27-20-13-22-24(14-21(20)28)34-17-26(33)31(22)16-25(32)30-12-6-9-19(18-7-2-1-3-8-18)23(30)15-29-10-4-5-11-29/h1-3,7-8,13-14,19,23H,4-6,9-12,15-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377218
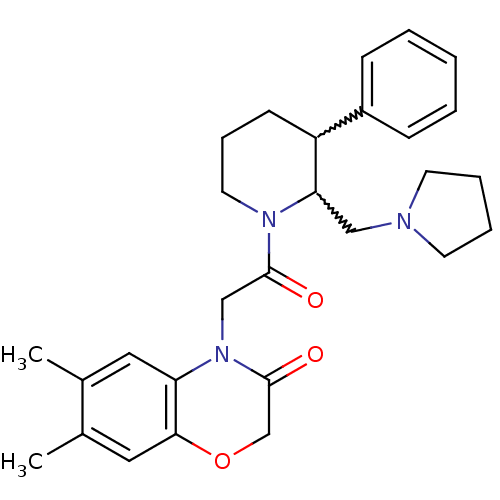
(CHEMBL257171)Show SMILES Cc1cc2OCC(=O)N(CC(=O)N3CCCC(C3CN3CCCC3)c3ccccc3)c2cc1C |w:17.18,16.25| Show InChI InChI=1S/C28H35N3O3/c1-20-15-24-26(16-21(20)2)34-19-28(33)31(24)18-27(32)30-14-8-11-23(22-9-4-3-5-10-22)25(30)17-29-12-6-7-13-29/h3-5,9-10,15-16,23,25H,6-8,11-14,17-19H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TNNI3K
(Homo sapiens (Human)) | BDBM50578225
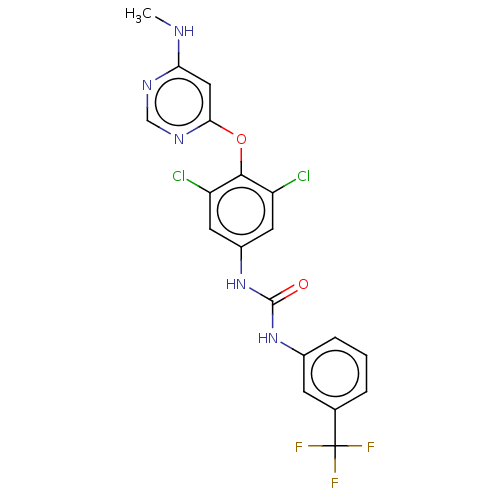
(CHEMBL4869303)Show SMILES CNc1cc(Oc2c(Cl)cc(NC(=O)Nc3cccc(c3)C(F)(F)F)cc2Cl)ncn1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to full length human His-MBP-TNNI3K assessed as off-rate constant in presence of rhodamine green labeled GW805818X by fluorescence c... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00700
BindingDB Entry DOI: 10.7270/Q23X8BG9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377217
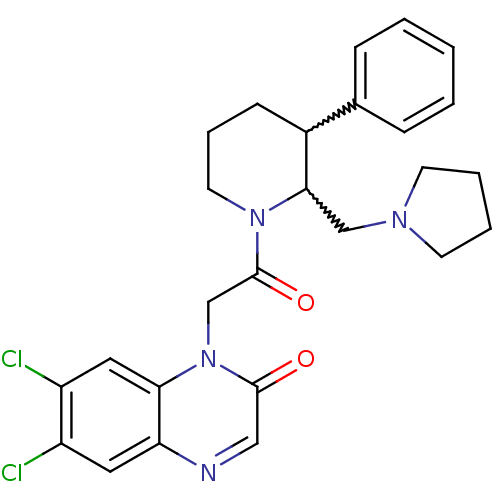
(CHEMBL256989)Show SMILES Clc1cc2ncc(=O)n(CC(=O)N3CCCC(C3CN3CCCC3)c3ccccc3)c2cc1Cl |w:17.18,16.25| Show InChI InChI=1S/C26H28Cl2N4O2/c27-20-13-22-23(14-21(20)28)32(25(33)15-29-22)17-26(34)31-12-6-9-19(18-7-2-1-3-8-18)24(31)16-30-10-4-5-11-30/h1-3,7-8,13-15,19,24H,4-6,9-12,16-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377215
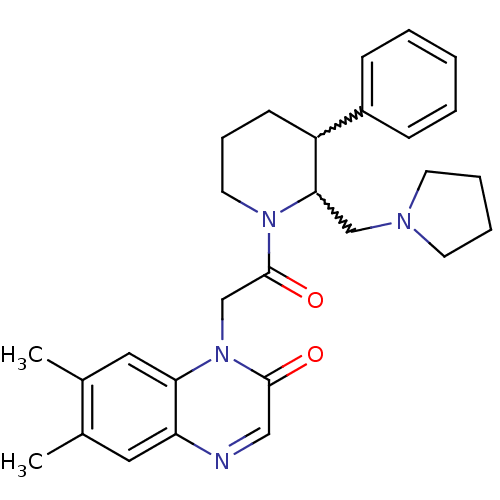
(CHEMBL257415)Show SMILES Cc1cc2ncc(=O)n(CC(=O)N3CCCC(C3CN3CCCC3)c3ccccc3)c2cc1C |w:17.18,16.25| Show InChI InChI=1S/C28H34N4O2/c1-20-15-24-25(16-21(20)2)32(27(33)17-29-24)19-28(34)31-14-8-11-23(22-9-4-3-5-10-22)26(31)18-30-12-6-7-13-30/h3-5,9-10,15-17,23,26H,6-8,11-14,18-19H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377227

(CHEMBL255462)Show SMILES Clc1ccc2sc(=O)n(CC(=O)N3CCCC(C3CN3CCCC3)c3ccccc3)c2c1 |w:16.25,17.18| Show InChI InChI=1S/C25H28ClN3O2S/c26-19-10-11-23-21(15-19)29(25(31)32-23)17-24(30)28-14-6-9-20(18-7-2-1-3-8-18)22(28)16-27-12-4-5-13-27/h1-3,7-8,10-11,15,20,22H,4-6,9,12-14,16-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414549
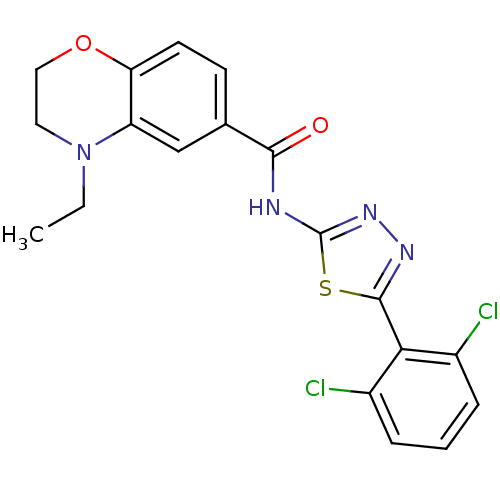
(CHEMBL563480)Show SMILES CCN1CCOc2ccc(cc12)C(=O)Nc1nnc(s1)-c1c(Cl)cccc1Cl Show InChI InChI=1S/C19H16Cl2N4O2S/c1-2-25-8-9-27-15-7-6-11(10-14(15)25)17(26)22-19-24-23-18(28-19)16-12(20)4-3-5-13(16)21/h3-7,10H,2,8-9H2,1H3,(H,22,24,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414547

(CHEMBL558644)Show SMILES CN1CCOc2ccc(cc12)C(=O)Nc1nnc(s1)-c1c(Cl)cccc1Cl Show InChI InChI=1S/C18H14Cl2N4O2S/c1-24-7-8-26-14-6-5-10(9-13(14)24)16(25)21-18-23-22-17(27-18)15-11(19)3-2-4-12(15)20/h2-6,9H,7-8H2,1H3,(H,21,23,25) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377219
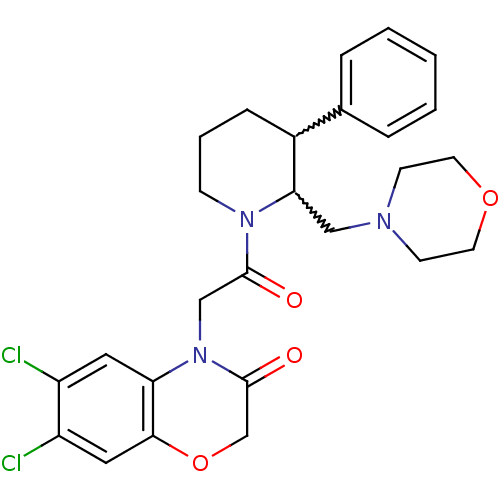
(CHEMBL402520)Show SMILES Clc1cc2OCC(=O)N(CC(=O)N3CCCC(C3CN3CCOCC3)c3ccccc3)c2cc1Cl |w:16.26,17.18| Show InChI InChI=1S/C26H29Cl2N3O4/c27-20-13-22-24(14-21(20)28)35-17-26(33)31(22)16-25(32)30-8-4-7-19(18-5-2-1-3-6-18)23(30)15-29-9-11-34-12-10-29/h1-3,5-6,13-14,19,23H,4,7-12,15-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377224
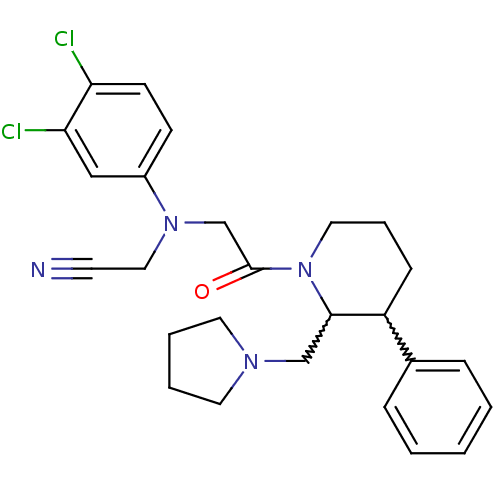
(CHEMBL257767)Show SMILES Clc1ccc(cc1Cl)N(CC#N)CC(=O)N1CCCC(C1CN1CCCC1)c1ccccc1 |w:19.29,20.22| Show InChI InChI=1S/C26H30Cl2N4O/c27-23-11-10-21(17-24(23)28)31(16-12-29)19-26(33)32-15-6-9-22(20-7-2-1-3-8-20)25(32)18-30-13-4-5-14-30/h1-3,7-8,10-11,17,22,25H,4-6,9,13-16,18-19H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377216
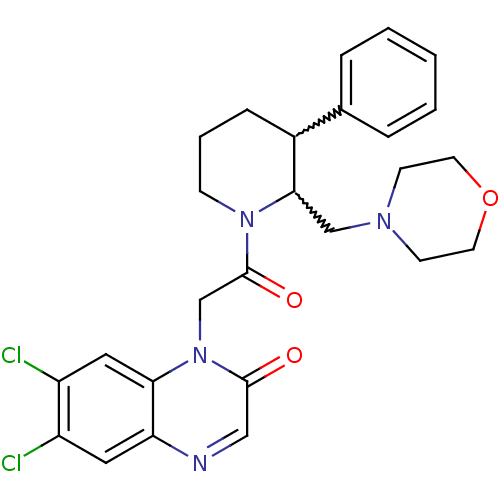
(CHEMBL256988)Show SMILES Clc1cc2ncc(=O)n(CC(=O)N3CCCC(C3CN3CCOCC3)c3ccccc3)c2cc1Cl |w:17.18,16.26| Show InChI InChI=1S/C26H28Cl2N4O3/c27-20-13-22-23(14-21(20)28)32(25(33)15-29-22)17-26(34)31-8-4-7-19(18-5-2-1-3-6-18)24(31)16-30-9-11-35-12-10-30/h1-3,5-6,13-15,19,24H,4,7-12,16-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244019
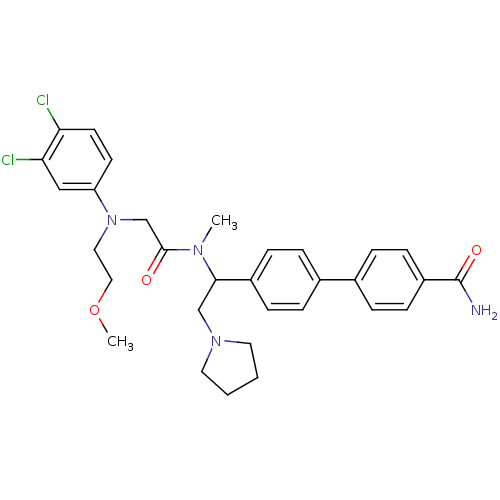
(4'-[1-({2-[(3,4-Dichloro-phenyl)-(2-methoxy-ethyl)...)Show SMILES COCCN(CC(=O)N(C)C(CN1CCCC1)c1ccc(cc1)-c1ccc(cc1)C(N)=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C31H36Cl2N4O3/c1-35(30(38)21-37(17-18-40-2)26-13-14-27(32)28(33)19-26)29(20-36-15-3-4-16-36)24-9-5-22(6-10-24)23-7-11-25(12-8-23)31(34)39/h5-14,19,29H,3-4,15-18,20-21H2,1-2H3,(H2,34,39) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244022
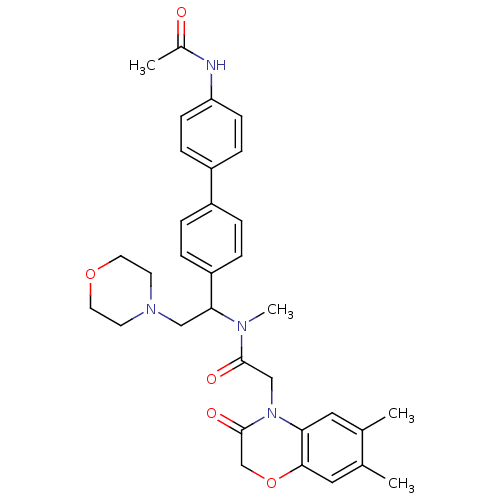
(CHEMBL449192 | N-[1-(4'-Acetylamino-biphenyl-4-yl)...)Show SMILES CN(C(CN1CCOCC1)c1ccc(cc1)-c1ccc(NC(C)=O)cc1)C(=O)CN1C(=O)COc2cc(C)c(C)cc12 Show InChI InChI=1S/C33H38N4O5/c1-22-17-29-31(18-23(22)2)42-21-33(40)37(29)20-32(39)35(4)30(19-36-13-15-41-16-14-36)27-7-5-25(6-8-27)26-9-11-28(12-10-26)34-24(3)38/h5-12,17-18,30H,13-16,19-21H2,1-4H3,(H,34,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50384444

(CHEMBL2035510)Show SMILES CC(C)C1(OC(=O)NC1=O)c1ccc([nH]c1=O)-c1ccc2cccc(F)c2c1 Show InChI InChI=1S/C21H17FN2O4/c1-11(2)21(19(26)24-20(27)28-21)15-8-9-17(23-18(15)25)13-7-6-12-4-3-5-16(22)14(12)10-13/h3-11H,1-2H3,(H,23,25)(H,24,26,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM50384444

(CHEMBL2035510)Show SMILES CC(C)C1(OC(=O)NC1=O)c1ccc([nH]c1=O)-c1ccc2cccc(F)c2c1 Show InChI InChI=1S/C21H17FN2O4/c1-11(2)21(19(26)24-20(27)28-21)15-8-9-17(23-18(15)25)13-7-6-12-4-3-5-16(22)14(12)10-13/h3-11H,1-2H3,(H,23,25)(H,24,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414536
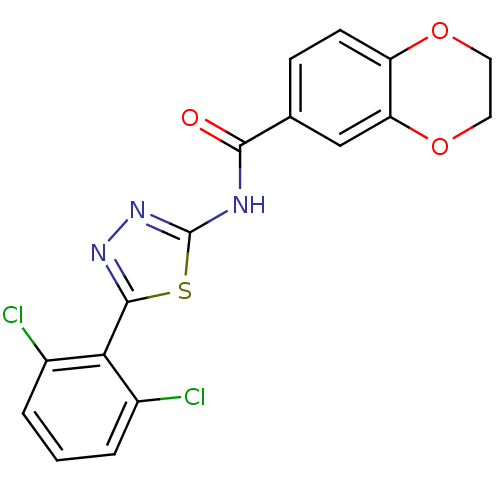
(CHEMBL551202)Show SMILES Clc1cccc(Cl)c1-c1nnc(NC(=O)c2ccc3OCCOc3c2)s1 Show InChI InChI=1S/C17H11Cl2N3O3S/c18-10-2-1-3-11(19)14(10)16-21-22-17(26-16)20-15(23)9-4-5-12-13(8-9)25-7-6-24-12/h1-5,8H,6-7H2,(H,20,22,23) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244021
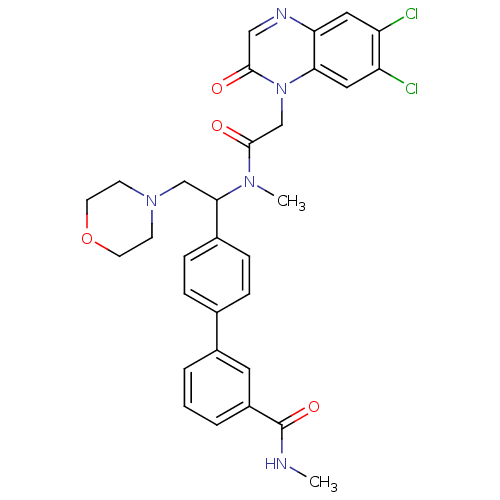
(4'-(1-{[2-(6,7-Dichloro-2-oxo-2H-quinoxalin-1-yl)-...)Show SMILES CNC(=O)c1cccc(c1)-c1ccc(cc1)C(CN1CCOCC1)N(C)C(=O)Cn1c2cc(Cl)c(Cl)cc2ncc1=O Show InChI InChI=1S/C31H31Cl2N5O4/c1-34-31(41)23-5-3-4-22(14-23)20-6-8-21(9-7-20)28(18-37-10-12-42-13-11-37)36(2)30(40)19-38-27-16-25(33)24(32)15-26(27)35-17-29(38)39/h3-9,14-17,28H,10-13,18-19H2,1-2H3,(H,34,41) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377229
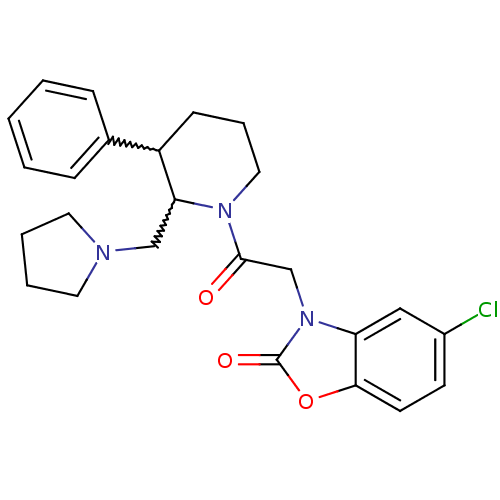
(CHEMBL257150)Show SMILES Clc1ccc2oc(=O)n(CC(=O)N3CCCC(C3CN3CCCC3)c3ccccc3)c2c1 |w:16.25,17.18| Show InChI InChI=1S/C25H28ClN3O3/c26-19-10-11-23-21(15-19)29(25(31)32-23)17-24(30)28-14-6-9-20(18-7-2-1-3-8-18)22(28)16-27-12-4-5-13-27/h1-3,7-8,10-11,15,20,22H,4-6,9,12-14,16-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377214
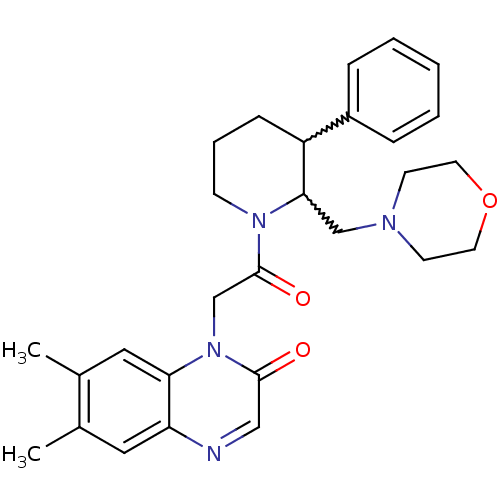
(CHEMBL256721)Show SMILES Cc1cc2ncc(=O)n(CC(=O)N3CCCC(C3CN3CCOCC3)c3ccccc3)c2cc1C |w:17.18,16.26| Show InChI InChI=1S/C28H34N4O3/c1-20-15-24-25(16-21(20)2)32(27(33)17-29-24)19-28(34)31-10-6-9-23(22-7-4-3-5-8-22)26(31)18-30-11-13-35-14-12-30/h3-5,7-8,15-17,23,26H,6,9-14,18-19H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244065
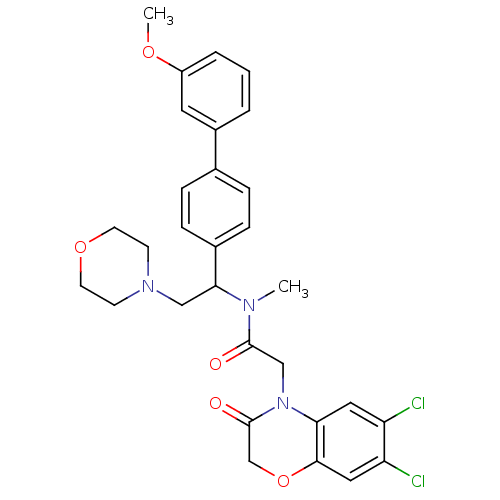
(2-(6,7-Dichloro-3-oxo-2,3-dihydro-benzo[1,4]oxazin...)Show SMILES COc1cccc(c1)-c1ccc(cc1)C(CN1CCOCC1)N(C)C(=O)CN1C(=O)COc2cc(Cl)c(Cl)cc12 Show InChI InChI=1S/C30H31Cl2N3O5/c1-33(29(36)18-35-26-15-24(31)25(32)16-28(26)40-19-30(35)37)27(17-34-10-12-39-13-11-34)21-8-6-20(7-9-21)22-4-3-5-23(14-22)38-2/h3-9,14-16,27H,10-13,17-19H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50384443

(CHEMBL1770317)Show SMILES CC(C)C1(OC(O)=NC1=O)c1ccc([nH]c1=O)-c1ccc2ccccc2c1 |c:6| Show InChI InChI=1S/C21H18N2O4/c1-12(2)21(19(25)23-20(26)27-21)16-9-10-17(22-18(16)24)15-8-7-13-5-3-4-6-14(13)11-15/h3-12H,1-2H3,(H,22,24)(H,23,25,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human EP3 |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414543
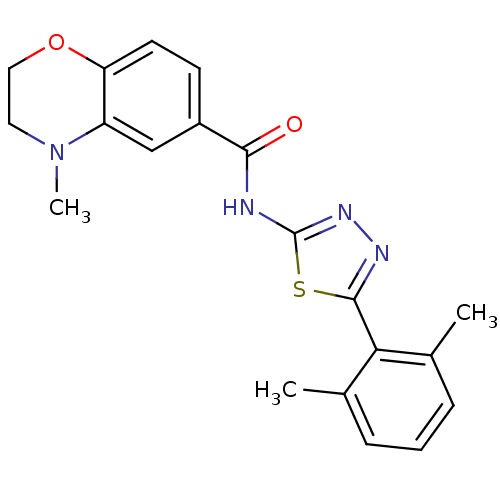
(CHEMBL550316)Show SMILES CN1CCOc2ccc(cc12)C(=O)Nc1nnc(s1)-c1c(C)cccc1C Show InChI InChI=1S/C20H20N4O2S/c1-12-5-4-6-13(2)17(12)19-22-23-20(27-19)21-18(25)14-7-8-16-15(11-14)24(3)9-10-26-16/h4-8,11H,9-10H2,1-3H3,(H,21,23,25) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50239135
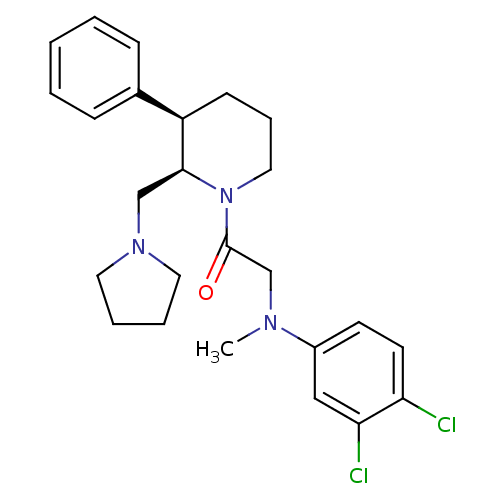
(2-((3,4-dichlorophenyl)(methyl)amino)-1-((2R,3R)-3...)Show SMILES CN(CC(=O)N1CCC[C@@H]([C@@H]1CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C25H31Cl2N3O/c1-28(20-11-12-22(26)23(27)16-20)18-25(31)30-15-7-10-21(19-8-3-2-4-9-19)24(30)17-29-13-5-6-14-29/h2-4,8-9,11-12,16,21,24H,5-7,10,13-15,17-18H2,1H3/t21-,24+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377223
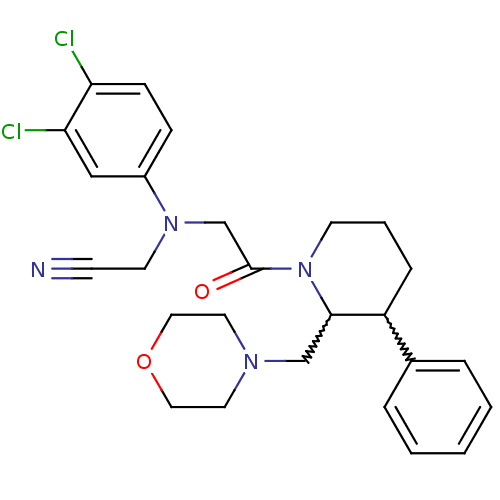
(CHEMBL258251)Show SMILES Clc1ccc(cc1Cl)N(CC#N)CC(=O)N1CCCC(C1CN1CCOCC1)c1ccccc1 |w:19.30,20.22| Show InChI InChI=1S/C26H30Cl2N4O2/c27-23-9-8-21(17-24(23)28)31(12-10-29)19-26(33)32-11-4-7-22(20-5-2-1-3-6-20)25(32)18-30-13-15-34-16-14-30/h1-3,5-6,8-9,17,22,25H,4,7,11-16,18-19H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377226

(CHEMBL255460)Show SMILES Clc1ccc2sc(=O)n(CC(=O)N3CCCC(C3CN3CCOCC3)c3ccccc3)c2c1 |w:16.26,17.18| Show InChI InChI=1S/C25H28ClN3O3S/c26-19-8-9-23-21(15-19)29(25(31)33-23)17-24(30)28-10-4-7-20(18-5-2-1-3-6-18)22(28)16-27-11-13-32-14-12-27/h1-3,5-6,8-9,15,20,22H,4,7,10-14,16-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244018
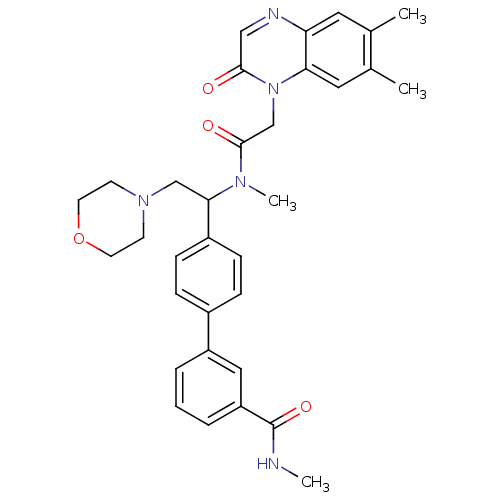
(4'-(1-{[2-(6,7-Dimethyl-2-oxo-2H-quinoxalin-1-yl)-...)Show SMILES CNC(=O)c1cccc(c1)-c1ccc(cc1)C(CN1CCOCC1)N(C)C(=O)Cn1c2cc(C)c(C)cc2ncc1=O Show InChI InChI=1S/C33H37N5O4/c1-22-16-28-29(17-23(22)2)38(31(39)19-35-28)21-32(40)36(4)30(20-37-12-14-42-15-13-37)25-10-8-24(9-11-25)26-6-5-7-27(18-26)33(41)34-3/h5-11,16-19,30H,12-15,20-21H2,1-4H3,(H,34,41) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50483809
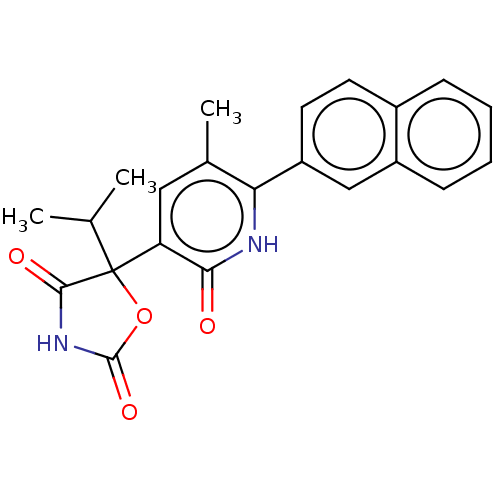
(CHEMBL1770337)Show SMILES CC(C)C1(OC(=O)NC1=O)c1cc(C)c([nH]c1=O)-c1ccc2ccccc2c1 Show InChI InChI=1S/C22H20N2O4/c1-12(2)22(20(26)24-21(27)28-22)17-10-13(3)18(23-19(17)25)16-9-8-14-6-4-5-7-15(14)11-16/h4-12H,1-3H3,(H,23,25)(H,24,26,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... |
Bioorg Med Chem Lett 21: 2806-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.107
BindingDB Entry DOI: 10.7270/Q2NZ8BGM |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM50414549

(CHEMBL563480)Show SMILES CCN1CCOc2ccc(cc12)C(=O)Nc1nnc(s1)-c1c(Cl)cccc1Cl Show InChI InChI=1S/C19H16Cl2N4O2S/c1-2-25-8-9-27-15-7-6-11(10-14(15)25)17(26)22-19-24-23-18(28-19)16-12(20)4-3-5-13(16)21/h3-7,10H,2,8-9H2,1H3,(H,22,24,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of rat EP3 receptor |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50417191

(CHEMBL1272136)Show SMILES CCc1cccc(NC(=O)Nc2cccn(Cc3c(F)cccc3Cl)c2=O)c1 Show InChI InChI=1S/C21H19ClFN3O2/c1-2-14-6-3-7-15(12-14)24-21(28)25-19-10-5-11-26(20(19)27)13-16-17(22)8-4-9-18(16)23/h3-12H,2,13H2,1H3,(H2,24,25,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced intracellular calcium mobilization by ... |
Bioorg Med Chem Lett 20: 6744-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.137
BindingDB Entry DOI: 10.7270/Q25M65Z8 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50384445
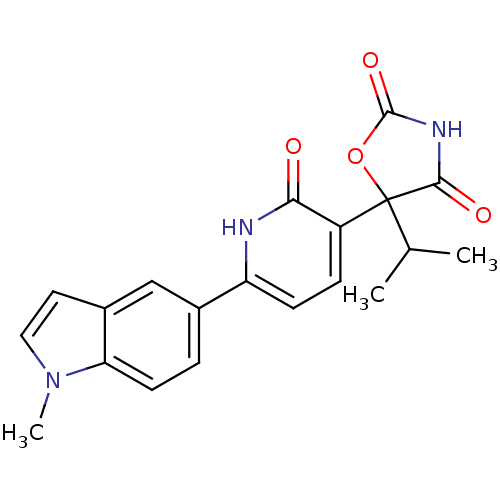
(CHEMBL2035508)Show SMILES CC(C)C1(OC(=O)NC1=O)c1ccc([nH]c1=O)-c1ccc2n(C)ccc2c1 Show InChI InChI=1S/C20H19N3O4/c1-11(2)20(18(25)22-19(26)27-20)14-5-6-15(21-17(14)24)12-4-7-16-13(10-12)8-9-23(16)3/h4-11H,1-3H3,(H,21,24)(H,22,25,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50483823
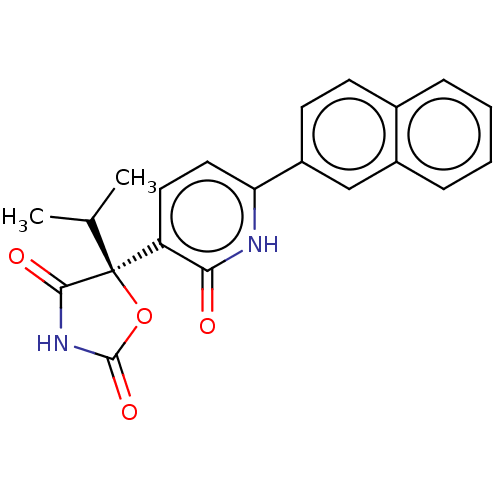
(CHEMBL1770341)Show SMILES CC(C)[C@]1(OC(=O)NC1=O)c1ccc([nH]c1=O)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C21H18N2O4/c1-12(2)21(19(25)23-20(26)27-21)16-9-10-17(22-18(16)24)15-8-7-13-5-3-4-6-14(13)11-15/h3-12H,1-2H3,(H,22,24)(H,23,25,26)/t21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... |
Bioorg Med Chem Lett 21: 2806-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.107
BindingDB Entry DOI: 10.7270/Q2NZ8BGM |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50483821
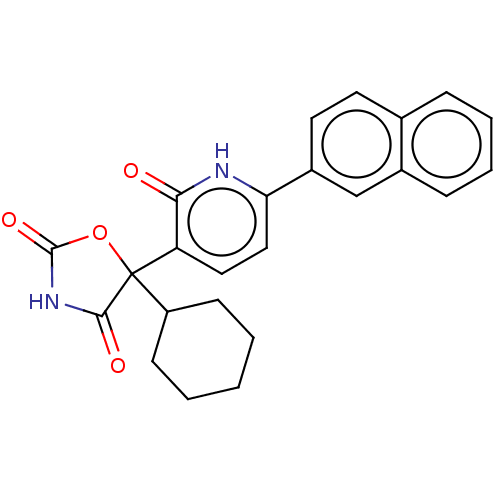
(CHEMBL1770320)Show SMILES O=C1NC(=O)C(O1)(C1CCCCC1)c1ccc([nH]c1=O)-c1ccc2ccccc2c1 Show InChI InChI=1S/C24H22N2O4/c27-21-19(24(18-8-2-1-3-9-18)22(28)26-23(29)30-24)12-13-20(25-21)17-11-10-15-6-4-5-7-16(15)14-17/h4-7,10-14,18H,1-3,8-9H2,(H,25,27)(H,26,28,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... |
Bioorg Med Chem Lett 21: 2806-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.107
BindingDB Entry DOI: 10.7270/Q2NZ8BGM |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50483814

(CHEMBL1770319)Show SMILES O=C1NC(=O)C(O1)(C1CCCC1)c1ccc([nH]c1=O)-c1ccc2ccccc2c1 Show InChI InChI=1S/C23H20N2O4/c26-20-18(23(17-7-3-4-8-17)21(27)25-22(28)29-23)11-12-19(24-20)16-10-9-14-5-1-2-6-15(14)13-16/h1-2,5-6,9-13,17H,3-4,7-8H2,(H,24,26)(H,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... |
Bioorg Med Chem Lett 21: 2806-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.107
BindingDB Entry DOI: 10.7270/Q2NZ8BGM |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM50384445

(CHEMBL2035508)Show SMILES CC(C)C1(OC(=O)NC1=O)c1ccc([nH]c1=O)-c1ccc2n(C)ccc2c1 Show InChI InChI=1S/C20H19N3O4/c1-11(2)20(18(25)22-19(26)27-20)14-5-6-15(21-17(14)24)12-4-7-16-13(10-12)8-9-23(16)3/h4-11H,1-3H3,(H,21,24)(H,22,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414548

(CHEMBL550055)Show SMILES Clc1cccc(Cl)c1-c1nnc(NC(=O)c2ccc3OCCNc3c2)s1 Show InChI InChI=1S/C17H12Cl2N4O2S/c18-10-2-1-3-11(19)14(10)16-22-23-17(26-16)21-15(24)9-4-5-13-12(8-9)20-6-7-25-13/h1-5,8,20H,6-7H2,(H,21,23,24) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377222
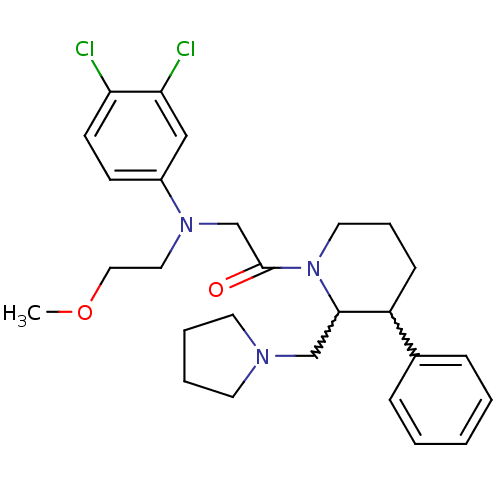
(CHEMBL256937)Show SMILES COCCN(CC(=O)N1CCCC(C1CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 |w:12.21,13.14| Show InChI InChI=1S/C27H35Cl2N3O2/c1-34-17-16-31(22-11-12-24(28)25(29)18-22)20-27(33)32-15-7-10-23(21-8-3-2-4-9-21)26(32)19-30-13-5-6-14-30/h2-4,8-9,11-12,18,23,26H,5-7,10,13-17,19-20H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM50384443

(CHEMBL1770317)Show SMILES CC(C)C1(OC(O)=NC1=O)c1ccc([nH]c1=O)-c1ccc2ccccc2c1 |c:6| Show InChI InChI=1S/C21H18N2O4/c1-12(2)21(19(25)23-20(26)27-21)16-9-10-17(22-18(16)24)15-8-7-13-5-3-4-6-14(13)11-15/h3-12H,1-2H3,(H,22,24)(H,23,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50384443

(CHEMBL1770317)Show SMILES CC(C)C1(OC(O)=NC1=O)c1ccc([nH]c1=O)-c1ccc2ccccc2c1 |c:6| Show InChI InChI=1S/C21H18N2O4/c1-12(2)21(19(25)23-20(26)27-21)16-9-10-17(22-18(16)24)15-8-7-13-5-3-4-6-14(13)11-15/h3-12H,1-2H3,(H,22,24)(H,23,25,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50384443

(CHEMBL1770317)Show SMILES CC(C)C1(OC(O)=NC1=O)c1ccc([nH]c1=O)-c1ccc2ccccc2c1 |c:6| Show InChI InChI=1S/C21H18N2O4/c1-12(2)21(19(25)23-20(26)27-21)16-9-10-17(22-18(16)24)15-8-7-13-5-3-4-6-14(13)11-15/h3-12H,1-2H3,(H,22,24)(H,23,25,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... |
Bioorg Med Chem Lett 21: 2806-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.107
BindingDB Entry DOI: 10.7270/Q2NZ8BGM |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414533
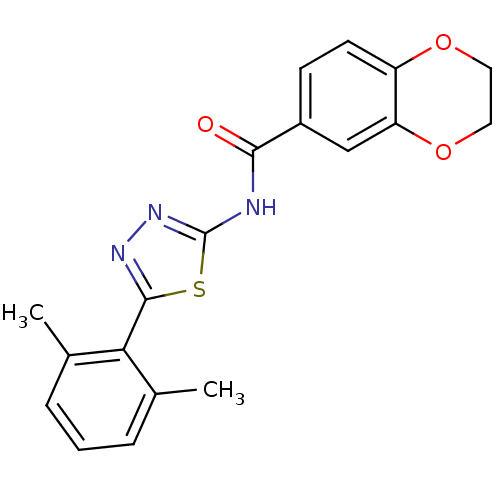
(CHEMBL549437)Show InChI InChI=1S/C19H17N3O3S/c1-11-4-3-5-12(2)16(11)18-21-22-19(26-18)20-17(23)13-6-7-14-15(10-13)25-9-8-24-14/h3-7,10H,8-9H2,1-2H3,(H,20,22,23) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414537

(CHEMBL554297)Show SMILES COc1cc(Cl)c(-c2nnc(NC(=O)c3ccc4OCCOc4c3)s2)c(Cl)c1 Show InChI InChI=1S/C18H13Cl2N3O4S/c1-25-10-7-11(19)15(12(20)8-10)17-22-23-18(28-17)21-16(24)9-2-3-13-14(6-9)27-5-4-26-13/h2-3,6-8H,4-5H2,1H3,(H,21,23,24) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM50384446

(CHEMBL2035509)Show SMILES CC(C)C1(OC(=O)NC1=O)c1ccc([nH]c1=O)-c1ccc(C)c(C)c1 Show InChI InChI=1S/C19H20N2O4/c1-10(2)19(17(23)21-18(24)25-19)14-7-8-15(20-16(14)22)13-6-5-11(3)12(4)9-13/h5-10H,1-4H3,(H,20,22)(H,21,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50243919
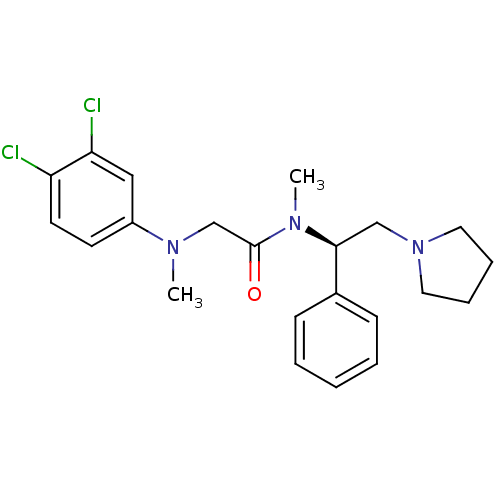
((R)-2-((3,4-dichlorophenyl)(methyl)amino)-N-methyl...)Show SMILES CN(CC(=O)N(C)[C@@H](CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C22H27Cl2N3O/c1-25(18-10-11-19(23)20(24)14-18)16-22(28)26(2)21(15-27-12-6-7-13-27)17-8-4-3-5-9-17/h3-5,8-11,14,21H,6-7,12-13,15-16H2,1-2H3/t21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50243970
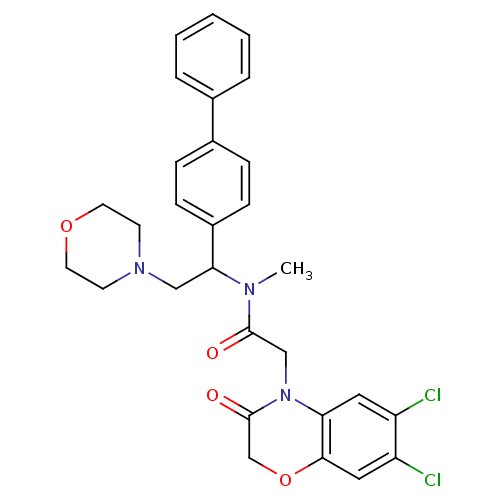
((+/-)N-(1-Biphenyl-4-yl-2-morpholin-4-yl-ethyl)-2-...)Show SMILES CN(C(CN1CCOCC1)c1ccc(cc1)-c1ccccc1)C(=O)CN1C(=O)COc2cc(Cl)c(Cl)cc12 Show InChI InChI=1S/C29H29Cl2N3O4/c1-32(28(35)18-34-25-15-23(30)24(31)16-27(25)38-19-29(34)36)26(17-33-11-13-37-14-12-33)22-9-7-21(8-10-22)20-5-3-2-4-6-20/h2-10,15-16,26H,11-14,17-19H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TNNI3K
(Mus musculus) | BDBM50578225

(CHEMBL4869303)Show SMILES CNc1cc(Oc2c(Cl)cc(NC(=O)Nc3cccc(c3)C(F)(F)F)cc2Cl)ncn1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to full length mouse His-MBP-TNNI3K assessed as off-rate constant in presence of rhodamine green labeled GW805818X by fluorescence c... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00700
BindingDB Entry DOI: 10.7270/Q23X8BG9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50243868
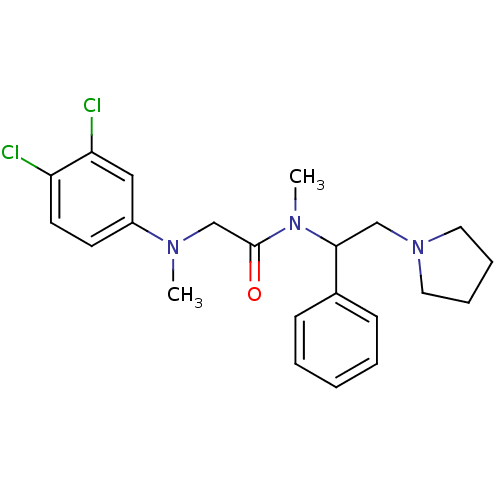
((+/-)-2-((3,4-dichlorophenyl)(methyl)amino)-N-meth...)Show SMILES CN(CC(=O)N(C)C(CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C22H27Cl2N3O/c1-25(18-10-11-19(23)20(24)14-18)16-22(28)26(2)21(15-27-12-6-7-13-27)17-8-4-3-5-9-17/h3-5,8-11,14,21H,6-7,12-13,15-16H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50414544
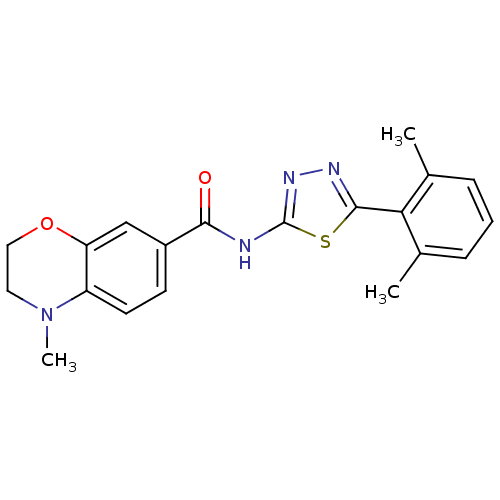
(CHEMBL562329)Show SMILES CN1CCOc2cc(ccc12)C(=O)Nc1nnc(s1)-c1c(C)cccc1C Show InChI InChI=1S/C20H20N4O2S/c1-12-5-4-6-13(2)17(12)19-22-23-20(27-19)21-18(25)14-7-8-15-16(11-14)26-10-9-24(15)3/h4-8,11H,9-10H2,1-3H3,(H,21,23,25) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in cells assessed as mobilization of intracellular calcium by FLIPR assay |
Bioorg Med Chem Lett 19: 4292-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.074
BindingDB Entry DOI: 10.7270/Q2SQ90DS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50483827

(CHEMBL1770322)Show SMILES O=C1NC(=O)C(Cc2ccccc2)(O1)c1ccc([nH]c1=O)-c1ccc2ccccc2c1 Show InChI InChI=1S/C25H18N2O4/c28-22-20(25(23(29)27-24(30)31-25)15-16-6-2-1-3-7-16)12-13-21(26-22)19-11-10-17-8-4-5-9-18(17)14-19/h1-14H,15H2,(H,26,28)(H,27,29,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... |
Bioorg Med Chem Lett 21: 2806-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.107
BindingDB Entry DOI: 10.7270/Q2NZ8BGM |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50243971
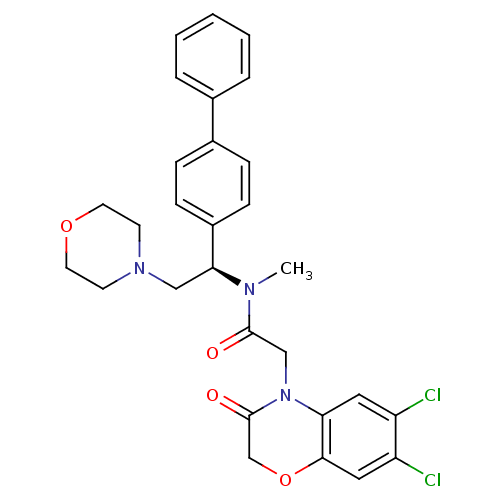
(CHEMBL453075 | N-((R)-1-Biphenyl-4-yl-2-morpholin-...)Show SMILES CN([C@@H](CN1CCOCC1)c1ccc(cc1)-c1ccccc1)C(=O)CN1C(=O)COc2cc(Cl)c(Cl)cc12 |r| Show InChI InChI=1S/C29H29Cl2N3O4/c1-32(28(35)18-34-25-15-23(30)24(31)16-27(25)38-19-29(34)36)26(17-33-11-13-37-14-12-33)22-9-7-21(8-10-22)20-5-3-2-4-6-20/h2-10,15-16,26H,11-14,17-19H2,1H3/t26-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data