

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11108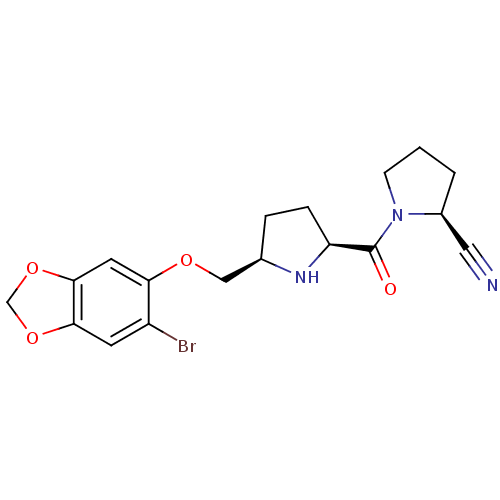 ((2S)-1-{[(2S,5R)-5-{[(6-bromo-2H-1,3-benzodioxol-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11103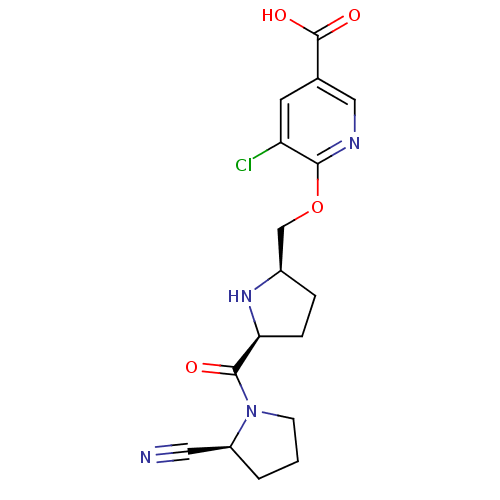 (2-cyanopyrrolidine 21aj | 5-chloro-6-{[(2R,5S)-5-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11087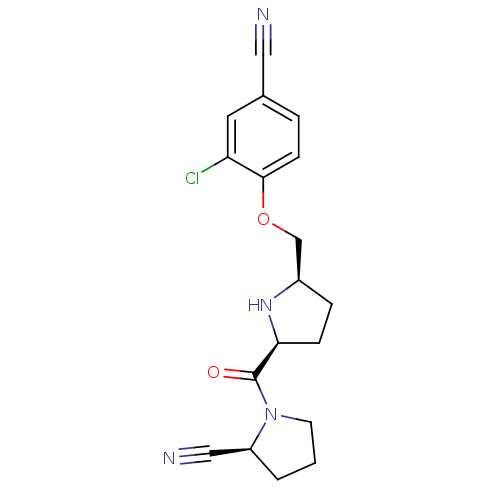 ((2S)-1-{[(2S,5R)-5-(2-chloro-4-cyanophenoxymethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11110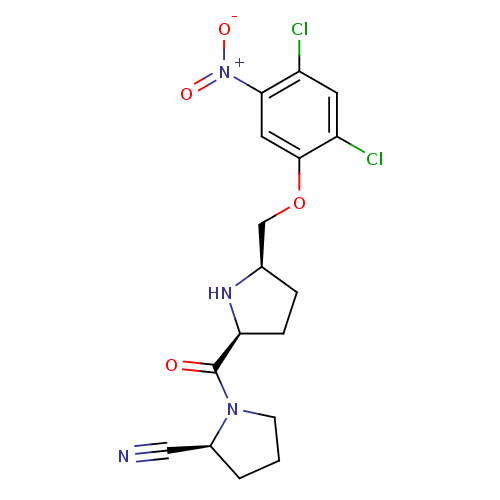 ((2S)-1-{[(2S,5R)-5-(2,4-dichloro-5-nitrophenoxymet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11101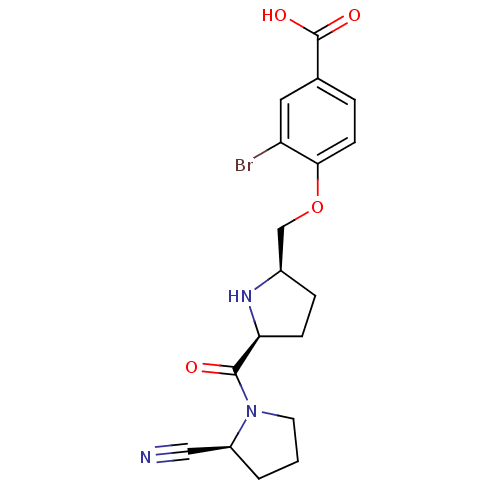 (2-cyanopyrrolidine 21ah | 3-bromo-4-{[(2R,5S)-5-{[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11112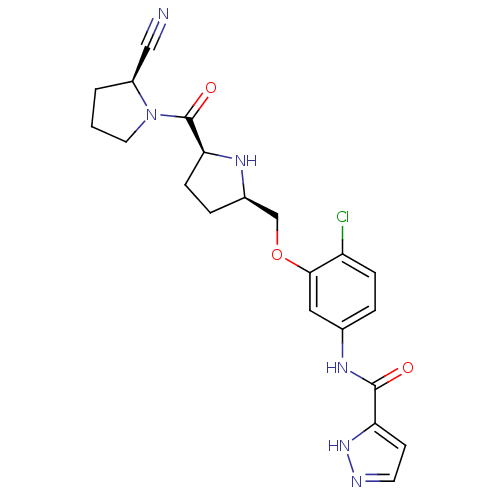 (2-cyanopyrrolidine 21as | N-(4-chloro-3-{[(2R,5S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11104 (2-cyanopyrrolidine 21ak | 6-chloro-5-{[(2R,5S)-5-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11107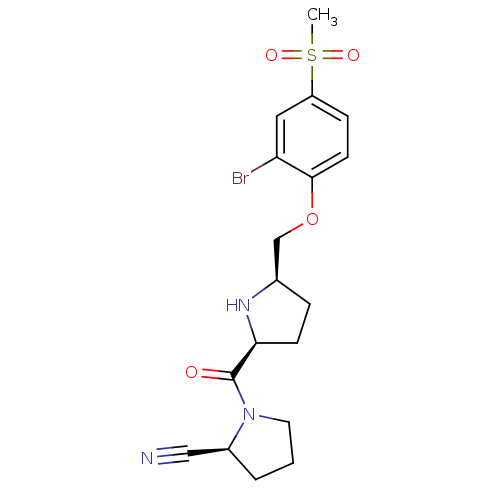 ((2S)-1-{[(2S,5R)-5-(2-bromo-4-methanesulfonylpheno...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11090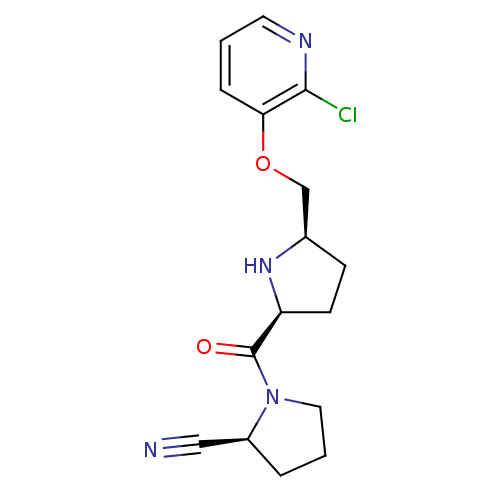 ((2S)-1-{[(2S,5R)-5-{[(2-chloropyridin-3-yl)oxy]met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11111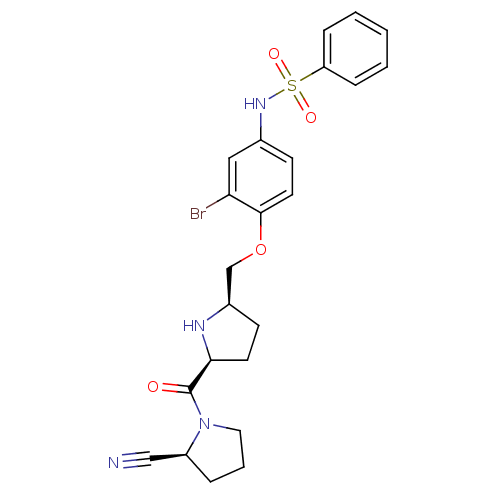 (2-cyanopyrrolidine 21ar | N-(3-bromo-4-{[(2R,5S)-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11100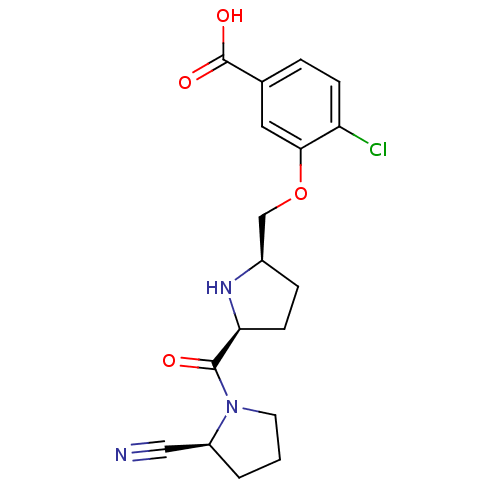 (2-cyanopyrrolidine 21ag | 4-chloro-3-{[(2R,5S)-5-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11085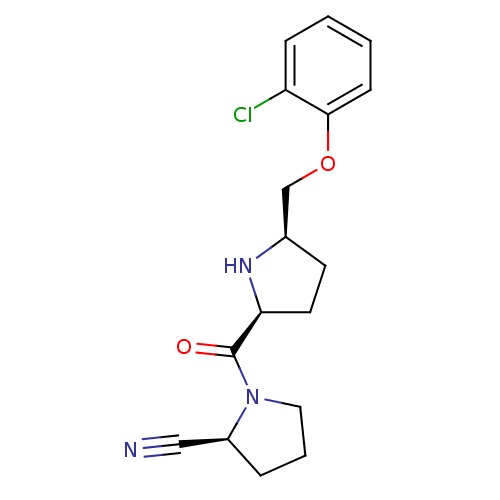 ((2S)-1-{[(2S,5R)-5-(2-chlorophenoxymethyl)pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11098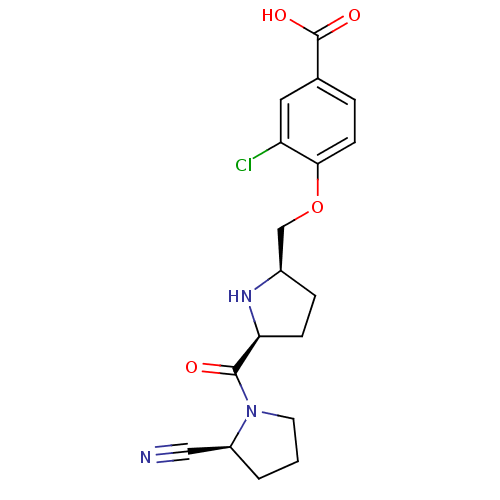 (2-cyanopyrrolidine 21ae | 3-chloro-4-{[(2R,5S)-5-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM11644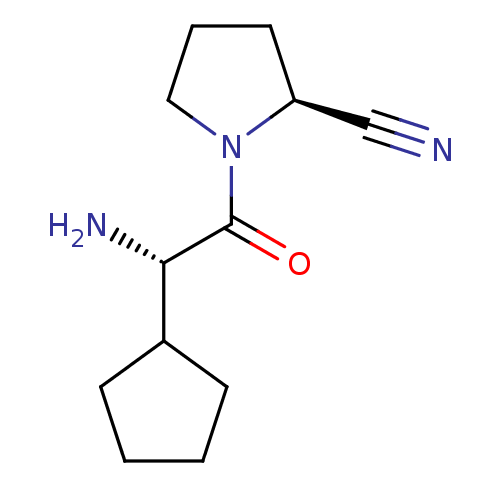 ((2S)-1-[(2S)-2-amino-2-cyclopentylacetyl]pyrrolidi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | Biochemistry 45: 7474-82 (2006) Article DOI: 10.1021/bi060184f BindingDB Entry DOI: 10.7270/Q2P26WCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11102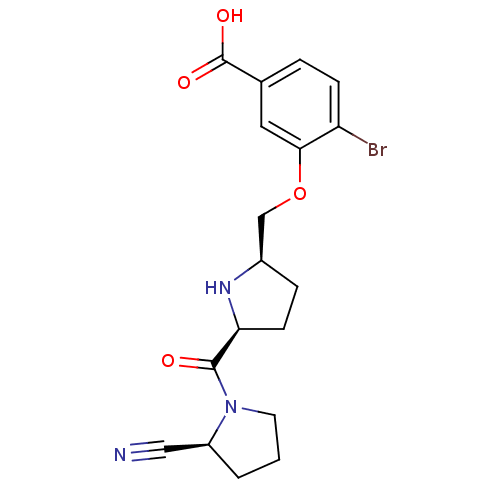 (2-cyanopyrrolidine 21ai | 4-bromo-3-{[(2R,5S)-5-{[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50315783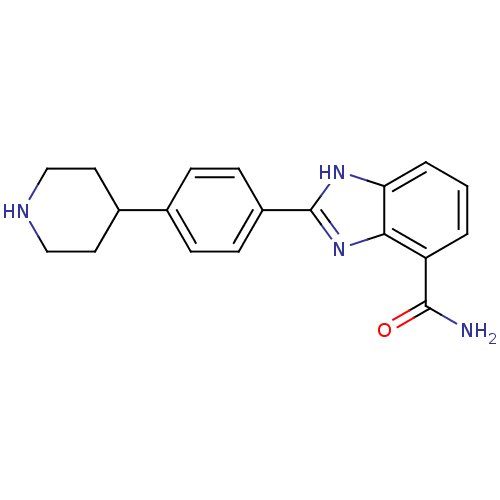 (2-(4-Piperidin-4-ylphenyl)-1H-benzimidazole-4-carb...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP2 by top count scintillation counting | J Med Chem 53: 3142-53 (2010) Article DOI: 10.1021/jm901775y BindingDB Entry DOI: 10.7270/Q2N879XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50300015 (2-(4-(Pyridin-3-yl)phenyl)-1H-benzo[d]imidazole-4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP1 using [3H]NAD+ by top count scintillation counting | J Med Chem 53: 3142-53 (2010) Article DOI: 10.1021/jm901775y BindingDB Entry DOI: 10.7270/Q2N879XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM15956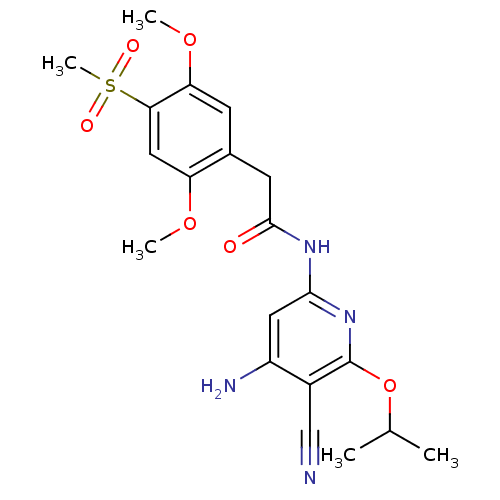 (Aminopyridine-Based Inhibitor 18b | N-(4-Amino-5-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Ser/Thr-kinase selectivity assays were performed using a radioactive FlashPlate-based assay platform. Substrate incorporated radioactivity was counte... | J Med Chem 49: 3563-80 (2006) Article DOI: 10.1021/jm060199b BindingDB Entry DOI: 10.7270/Q2P26WDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50315779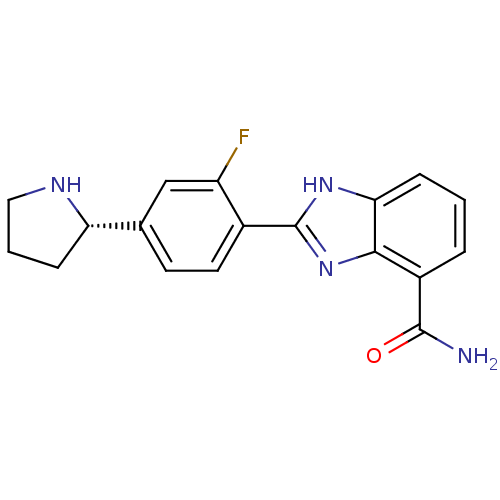 ((S)-2-(2-Fluoro-4-(pyrrolidin-2-yl)phenyl)-1H-benz...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP1 using [3H]NAD+ by top count scintillation counting | J Med Chem 53: 3142-53 (2010) Article DOI: 10.1021/jm901775y BindingDB Entry DOI: 10.7270/Q2N879XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11644 ((2S)-1-[(2S)-2-amino-2-cyclopentylacetyl]pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | Biochemistry 45: 7474-82 (2006) Article DOI: 10.1021/bi060184f BindingDB Entry DOI: 10.7270/Q2P26WCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50300014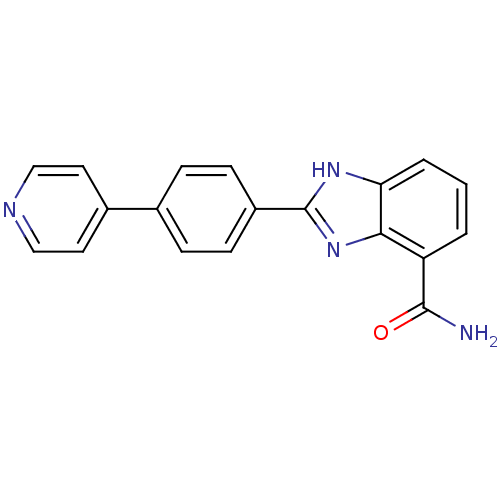 (2-(4-(Pyridin-4-yl)phenyl)-1H-benzimidazole-4-carb...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP2 by top count scintillation counting | J Med Chem 53: 3142-53 (2010) Article DOI: 10.1021/jm901775y BindingDB Entry DOI: 10.7270/Q2N879XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11105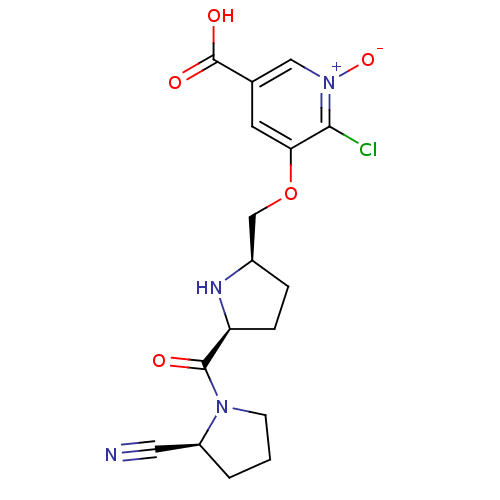 (2-cyanopyrrolidine 21al | 5-carboxy-2-chloro-3-{[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11106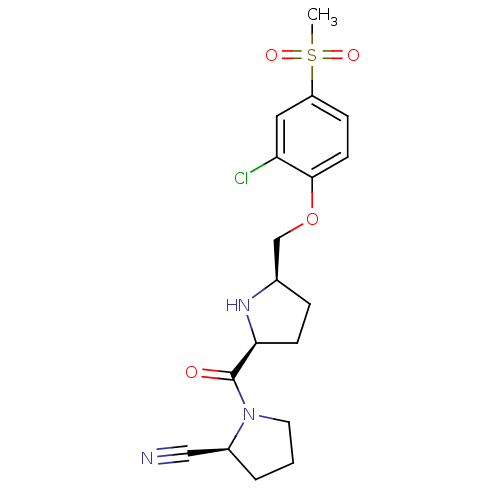 ((2S)-1-{[(2S,5R)-5-(2-chloro-4-methanesulfonylphen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50315779 ((S)-2-(2-Fluoro-4-(pyrrolidin-2-yl)phenyl)-1H-benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP2 by top count scintillation counting | J Med Chem 53: 3142-53 (2010) Article DOI: 10.1021/jm901775y BindingDB Entry DOI: 10.7270/Q2N879XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11109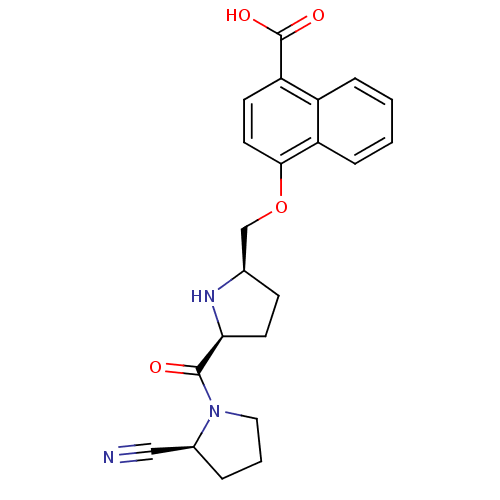 (2-cyanopyrrolidine 21ap | 4-{[(2R,5S)-5-{[(2S)-2-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11099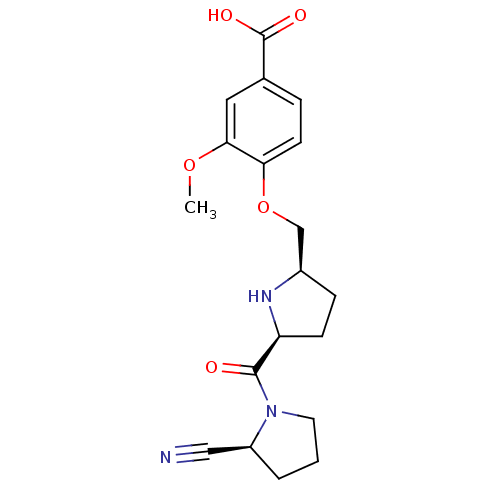 (2-cyanopyrrolidine 21af | 4-{[(2R,5S)-5-{[(2S)-2-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11088 ((2S)-1-{[(2S,5R)-5-(2,4-dichlorophenoxymethyl)pyrr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50315792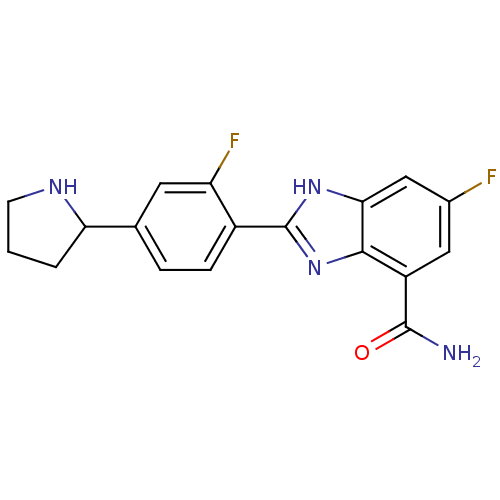 (6-Fluoro-2-(2-fluoro-4-pyrrolidin-2-ylphenyl)-1H-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP1 using [3H]NAD+ by top count scintillation counting | J Med Chem 53: 3142-53 (2010) Article DOI: 10.1021/jm901775y BindingDB Entry DOI: 10.7270/Q2N879XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50315780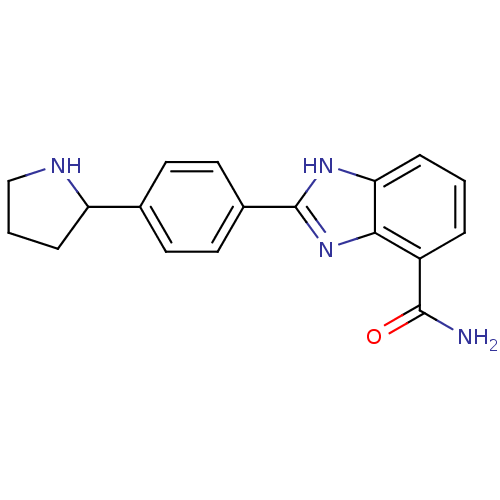 (2-(4-Pyrrolidin-2-ylphenyl)-1H-benzimidazole-4-car...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP2 by top count scintillation counting | J Med Chem 53: 3142-53 (2010) Article DOI: 10.1021/jm901775y BindingDB Entry DOI: 10.7270/Q2N879XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50315795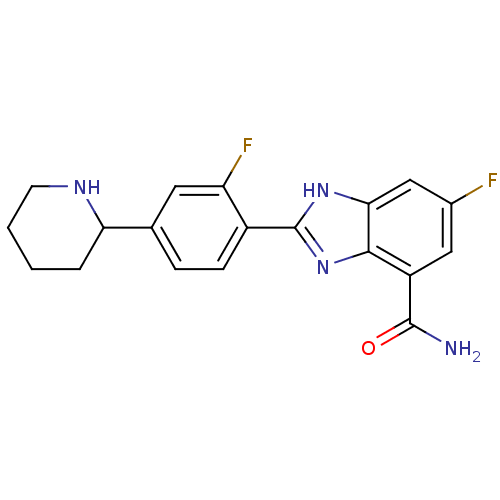 (6-Fluoro-2-(2-fluoro-4-piperidin-2-ylphenyl)-1H-be...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP1 using [3H]NAD+ by top count scintillation counting | J Med Chem 53: 3142-53 (2010) Article DOI: 10.1021/jm901775y BindingDB Entry DOI: 10.7270/Q2N879XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50315783 (2-(4-Piperidin-4-ylphenyl)-1H-benzimidazole-4-carb...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP1 using [3H]NAD+ by top count scintillation counting | J Med Chem 53: 3142-53 (2010) Article DOI: 10.1021/jm901775y BindingDB Entry DOI: 10.7270/Q2N879XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11645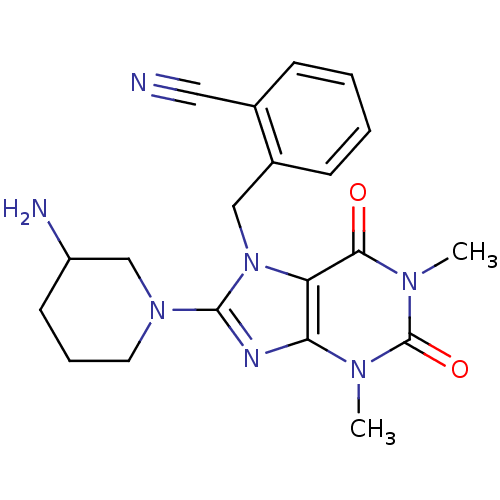 (2-{[8-(3-aminopiperidin-1-yl)-1,3-dimethyl-2,6-dio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2 | -49.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | Biochemistry 45: 7474-82 (2006) Article DOI: 10.1021/bi060184f BindingDB Entry DOI: 10.7270/Q2P26WCG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50315790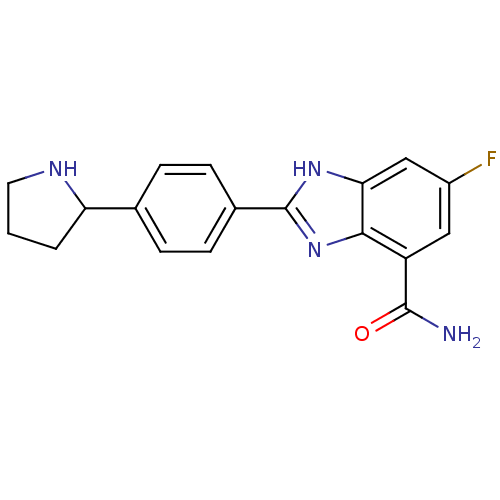 (6-Fluoro-2-(4-pyrrolidin-2-ylphenyl)-1H-benzimidaz...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP1 using [3H]NAD+ by top count scintillation counting | J Med Chem 53: 3142-53 (2010) Article DOI: 10.1021/jm901775y BindingDB Entry DOI: 10.7270/Q2N879XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM15908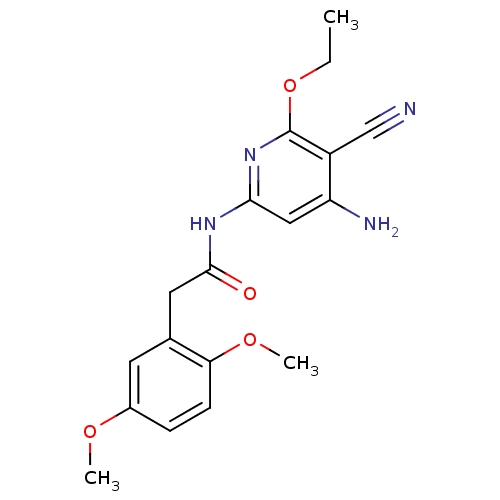 (Aminopyridine-Based Inhibitor 6o | N-(4-Amino-5-cy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Ser/Thr-kinase selectivity assays were performed using a radioactive FlashPlate-based assay platform. Substrate incorporated radioactivity was counte... | J Med Chem 49: 3563-80 (2006) Article DOI: 10.1021/jm060199b BindingDB Entry DOI: 10.7270/Q2P26WDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50300016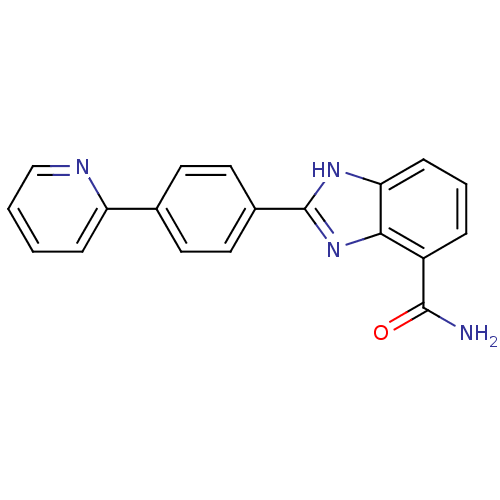 (2-(4-(Pyridin-2-yl)phenyl)-1H-benzo[d]imidazole-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP2 by top count scintillation counting | J Med Chem 53: 3142-53 (2010) Article DOI: 10.1021/jm901775y BindingDB Entry DOI: 10.7270/Q2N879XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50300016 (2-(4-(Pyridin-2-yl)phenyl)-1H-benzo[d]imidazole-4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP1 using [3H]NAD+ by top count scintillation counting | J Med Chem 53: 3142-53 (2010) Article DOI: 10.1021/jm901775y BindingDB Entry DOI: 10.7270/Q2N879XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11097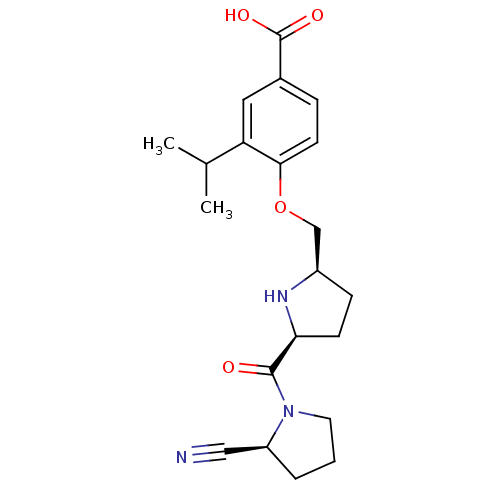 (2-cyanopyrrolidine 21ad | 4-{[(2R,5S)-5-{[(2S)-2-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50300014 (2-(4-(Pyridin-4-yl)phenyl)-1H-benzimidazole-4-carb...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP1 using [3H]NAD+ by top count scintillation counting | J Med Chem 53: 3142-53 (2010) Article DOI: 10.1021/jm901775y BindingDB Entry DOI: 10.7270/Q2N879XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11092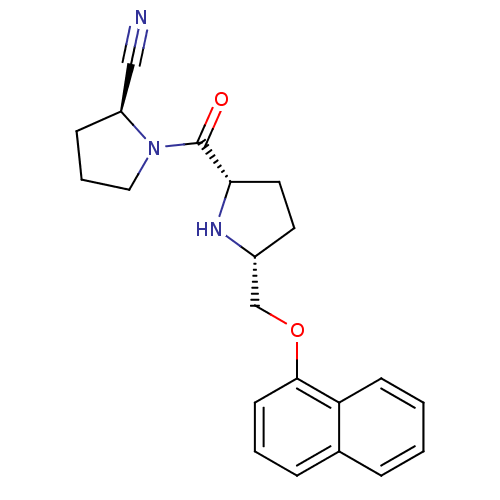 ((2S)-1-{[(2S,5R)-5-[(naphthalen-1-yloxy)methyl]pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11091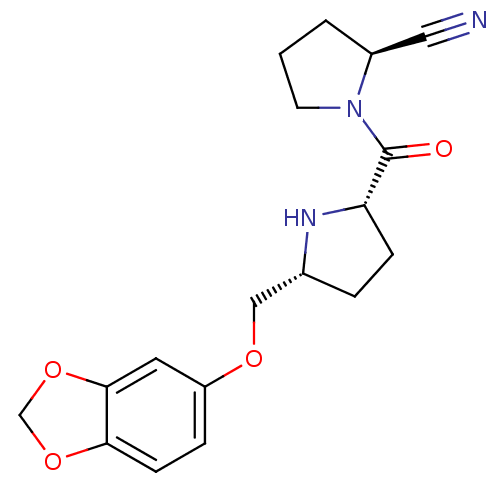 ((2S)-1-{[(2S,5R)-5-[(2H-1,3-benzodioxol-5-yloxy)me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50315778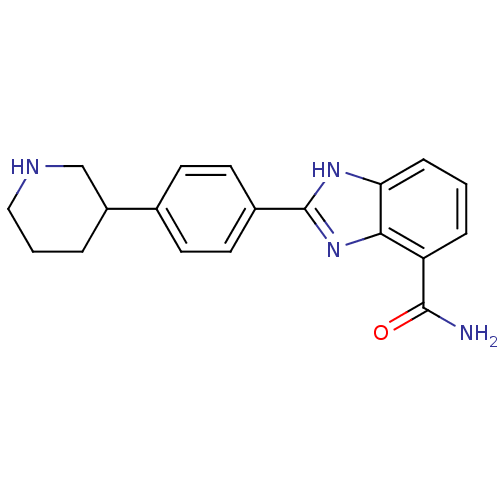 (2-(4-Piperidin-3-ylphenyl)-1H-benzimidazole-4-carb...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP2 by top count scintillation counting | J Med Chem 53: 3142-53 (2010) Article DOI: 10.1021/jm901775y BindingDB Entry DOI: 10.7270/Q2N879XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50315793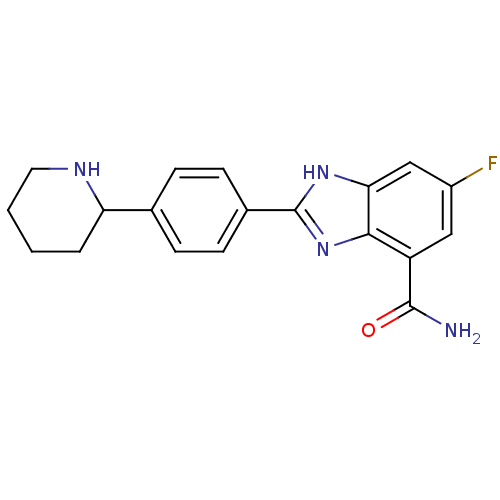 (6-Fluoro-2-(4-piperidin-2-ylphenyl)-1H-benzimidazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP1 using [3H]NAD+ by top count scintillation counting | J Med Chem 53: 3142-53 (2010) Article DOI: 10.1021/jm901775y BindingDB Entry DOI: 10.7270/Q2N879XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50315779 ((S)-2-(2-Fluoro-4-(pyrrolidin-2-yl)phenyl)-1H-benz...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP1 using [3H]NAD+ by top count scintillation counting | J Med Chem 53: 3142-53 (2010) Article DOI: 10.1021/jm901775y BindingDB Entry DOI: 10.7270/Q2N879XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27122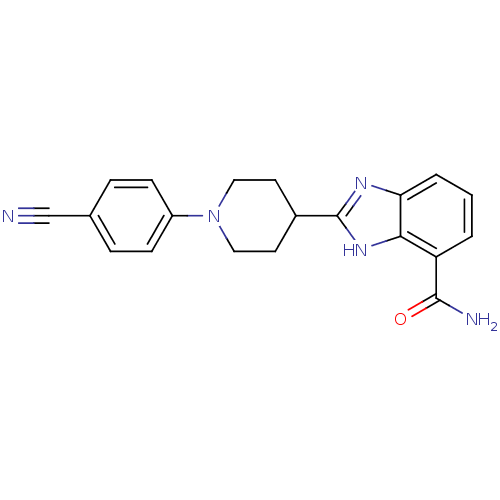 (2-[1-(4-cyanophenyl)piperidin-4-yl]-1H-1,3-benzodi...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description PARP assays utilizing SPA bead-based detection were carried out in 96-well plates. After reaction was terminated, the reaction mixtures were transfer... | Bioorg Med Chem 16: 6965-75 (2008) Article DOI: 10.1016/j.bmc.2008.05.044 BindingDB Entry DOI: 10.7270/Q2JD4V3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50315802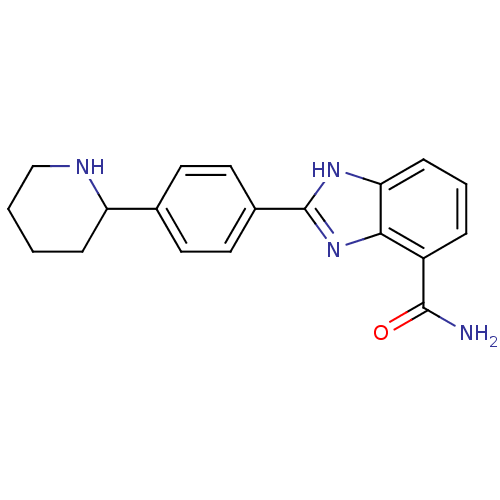 (2-(4-Piperidin-2-ylphenyl)-1H-benzimidazole-4-carb...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP2 by top count scintillation counting | J Med Chem 53: 3142-53 (2010) Article DOI: 10.1021/jm901775y BindingDB Entry DOI: 10.7270/Q2N879XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50315779 ((S)-2-(2-Fluoro-4-(pyrrolidin-2-yl)phenyl)-1H-benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP2 by top count scintillation counting | J Med Chem 53: 3142-53 (2010) Article DOI: 10.1021/jm901775y BindingDB Entry DOI: 10.7270/Q2N879XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50315778 (2-(4-Piperidin-3-ylphenyl)-1H-benzimidazole-4-carb...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP1 using [3H]NAD+ by top count scintillation counting | J Med Chem 53: 3142-53 (2010) Article DOI: 10.1021/jm901775y BindingDB Entry DOI: 10.7270/Q2N879XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50315799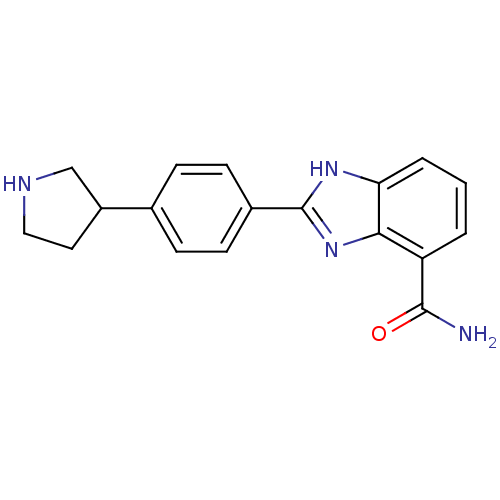 (3-(4-Pyrrolidin-2-ylphenyl)-1H-benzimidazole-4-car...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP1 using [3H]NAD+ by top count scintillation counting | J Med Chem 53: 3142-53 (2010) Article DOI: 10.1021/jm901775y BindingDB Entry DOI: 10.7270/Q2N879XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50315780 (2-(4-Pyrrolidin-2-ylphenyl)-1H-benzimidazole-4-car...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP1 using [3H]NAD+ by top count scintillation counting | J Med Chem 53: 3142-53 (2010) Article DOI: 10.1021/jm901775y BindingDB Entry DOI: 10.7270/Q2N879XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM15976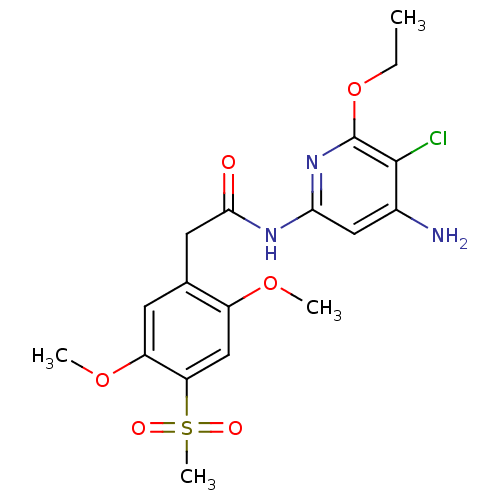 (Aminopyridine-Based Inhibitor 35 | N-(4-Amino-5-ch...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Ser/Thr-kinase selectivity assays were performed using a radioactive FlashPlate-based assay platform. Substrate incorporated radioactivity was counte... | J Med Chem 49: 3563-80 (2006) Article DOI: 10.1021/jm060199b BindingDB Entry DOI: 10.7270/Q2P26WDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 523 total ) | Next | Last >> |