 Found 228 hits with Last Name = 'gordon' and Initial = 't'
Found 228 hits with Last Name = 'gordon' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of human sst2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 991-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D50NHN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of human sst5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 991-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D50NHN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of human sst3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 991-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D50NHN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of human sst1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 991-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D50NHN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of human sst4 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 991-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D50NHN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286344
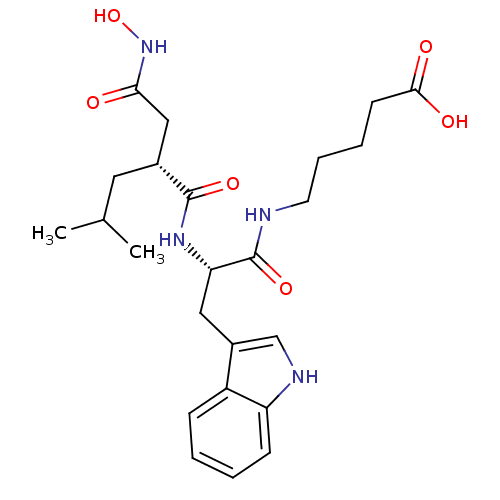
(5-[(S)-2-((R)-2-Hydroxycarbamoylmethyl-4-methyl-pe...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCCCCC(O)=O Show InChI InChI=1S/C24H34N4O6/c1-15(2)11-16(13-21(29)28-34)23(32)27-20(24(33)25-10-6-5-9-22(30)31)12-17-14-26-19-8-4-3-7-18(17)19/h3-4,7-8,14-16,20,26,34H,5-6,9-13H2,1-2H3,(H,25,33)(H,27,32)(H,28,29)(H,30,31)/t16-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286335
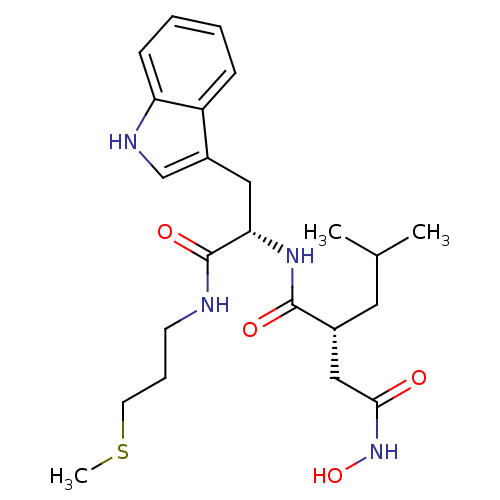
((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-(3-...)Show SMILES CSCCCNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO Show InChI InChI=1S/C23H34N4O4S/c1-15(2)11-16(13-21(28)27-31)22(29)26-20(23(30)24-9-6-10-32-3)12-17-14-25-19-8-5-4-7-18(17)19/h4-5,7-8,14-16,20,25,31H,6,9-13H2,1-3H3,(H,24,30)(H,26,29)(H,27,28)/t16-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286340
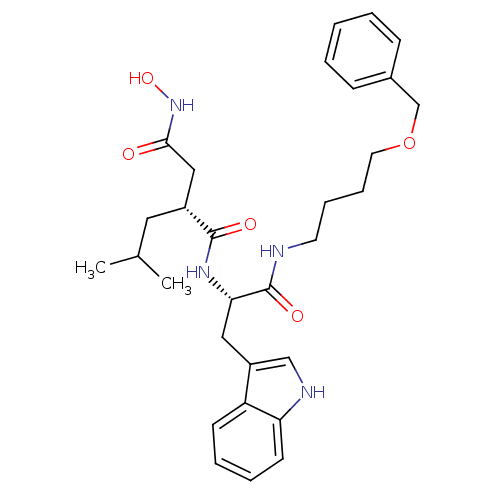
((R)-N*1*-[(S)-1-(4-Benzyloxy-butylcarbamoyl)-2-(1H...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCCCCOCc1ccccc1 Show InChI InChI=1S/C30H40N4O5/c1-21(2)16-23(18-28(35)34-38)29(36)33-27(17-24-19-32-26-13-7-6-12-25(24)26)30(37)31-14-8-9-15-39-20-22-10-4-3-5-11-22/h3-7,10-13,19,21,23,27,32,38H,8-9,14-18,20H2,1-2H3,(H,31,37)(H,33,36)(H,34,35)/t23-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286334
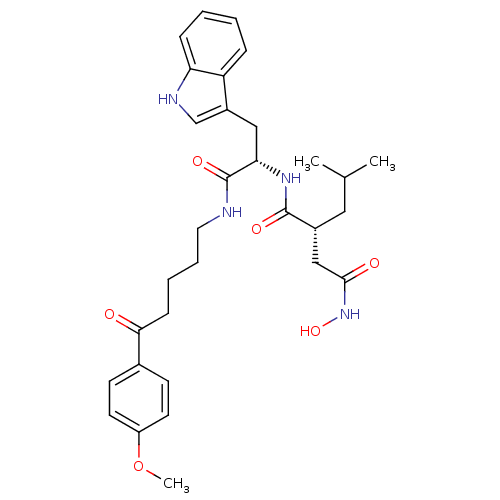
((R)-N*4*-Hydroxy-N*1*-{(S)-2-(1H-indol-3-yl)-1-[5-...)Show SMILES COc1ccc(cc1)C(=O)CCCCNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO Show InChI InChI=1S/C31H40N4O6/c1-20(2)16-22(18-29(37)35-40)30(38)34-27(17-23-19-33-26-9-5-4-8-25(23)26)31(39)32-15-7-6-10-28(36)21-11-13-24(41-3)14-12-21/h4-5,8-9,11-14,19-20,22,27,33,40H,6-7,10,15-18H2,1-3H3,(H,32,39)(H,34,38)(H,35,37)/t22-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286337
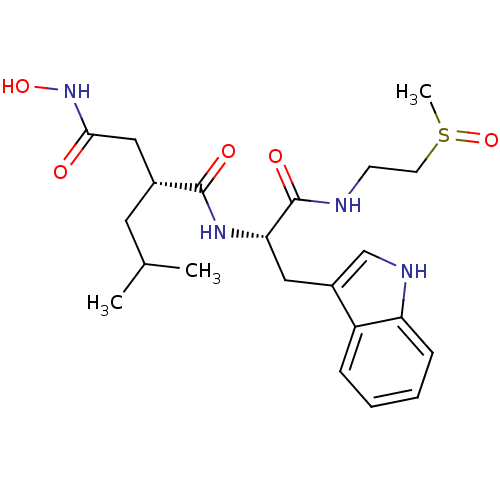
((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-(2-...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCCS(C)=O Show InChI InChI=1S/C22H32N4O5S/c1-14(2)10-15(12-20(27)26-30)21(28)25-19(22(29)23-8-9-32(3)31)11-16-13-24-18-7-5-4-6-17(16)18/h4-7,13-15,19,24,30H,8-12H2,1-3H3,(H,23,29)(H,25,28)(H,26,27)/t15-,19+,32?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286349
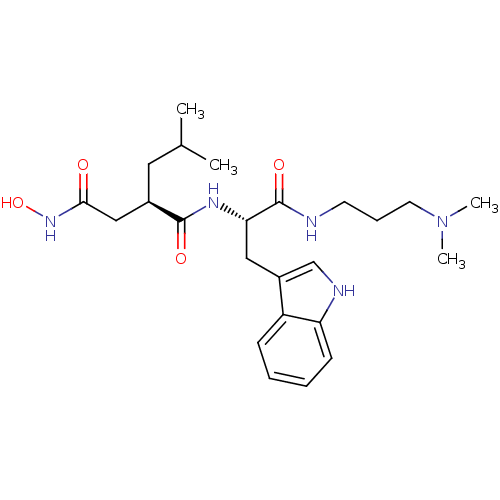
((R)-N*1*-[(S)-1-(3-Dimethylamino-propylcarbamoyl)-...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCCCN(C)C Show InChI InChI=1S/C24H37N5O4/c1-16(2)12-17(14-22(30)28-33)23(31)27-21(24(32)25-10-7-11-29(3)4)13-18-15-26-20-9-6-5-8-19(18)20/h5-6,8-9,15-17,21,26,33H,7,10-14H2,1-4H3,(H,25,32)(H,27,31)(H,28,30)/t17-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286350
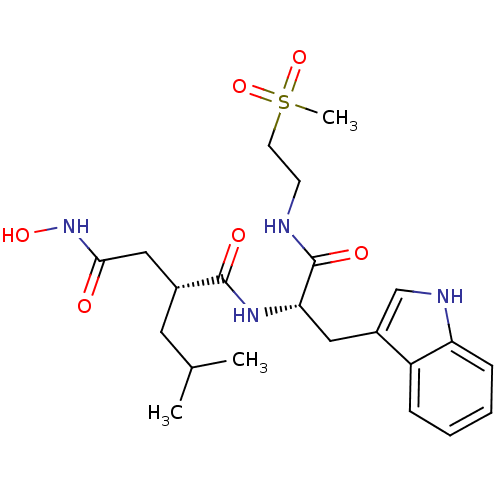
((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-(2-...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCCS(C)(=O)=O Show InChI InChI=1S/C22H32N4O6S/c1-14(2)10-15(12-20(27)26-30)21(28)25-19(22(29)23-8-9-33(3,31)32)11-16-13-24-18-7-5-4-6-17(16)18/h4-7,13-15,19,24,30H,8-12H2,1-3H3,(H,23,29)(H,25,28)(H,26,27)/t15-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286347
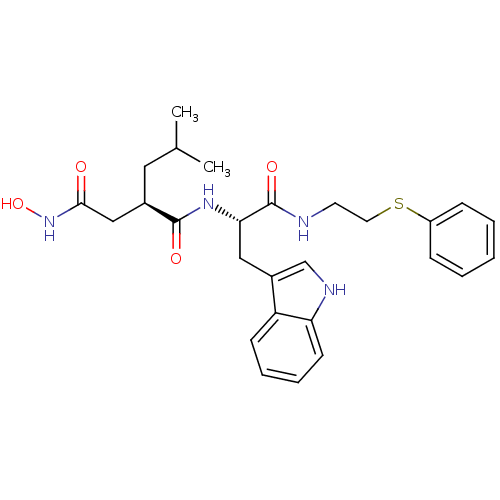
((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-(2-...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCCSc1ccccc1 Show InChI InChI=1S/C27H34N4O4S/c1-18(2)14-19(16-25(32)31-35)26(33)30-24(15-20-17-29-23-11-7-6-10-22(20)23)27(34)28-12-13-36-21-8-4-3-5-9-21/h3-11,17-19,24,29,35H,12-16H2,1-2H3,(H,28,34)(H,30,33)(H,31,32)/t19-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286345
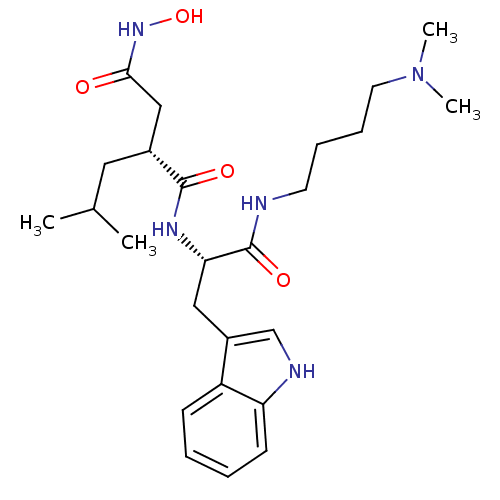
((R)-N*1*-[(S)-1-(4-Dimethylamino-butylcarbamoyl)-2...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCCCCN(C)C Show InChI InChI=1S/C25H39N5O4/c1-17(2)13-18(15-23(31)29-34)24(32)28-22(25(33)26-11-7-8-12-30(3)4)14-19-16-27-21-10-6-5-9-20(19)21/h5-6,9-10,16-18,22,27,34H,7-8,11-15H2,1-4H3,(H,26,33)(H,28,32)(H,29,31)/t18-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286339
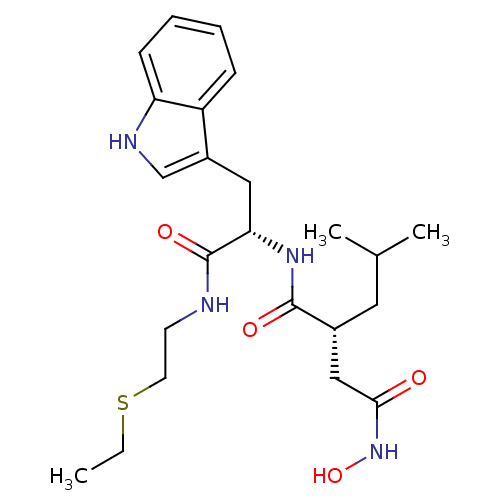
((R)-N*1*-[(S)-1-(2-Ethylsulfanyl-ethylcarbamoyl)-2...)Show SMILES CCSCCNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO Show InChI InChI=1S/C23H34N4O4S/c1-4-32-10-9-24-23(30)20(12-17-14-25-19-8-6-5-7-18(17)19)26-22(29)16(11-15(2)3)13-21(28)27-31/h5-8,14-16,20,25,31H,4,9-13H2,1-3H3,(H,24,30)(H,26,29)(H,27,28)/t16-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286343

((R)-N*1*-[(S)-1-(4-Dimethylaminomethyl-benzylcarba...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1ccc(CN(C)C)cc1 Show InChI InChI=1S/C29H39N5O4/c1-19(2)13-22(15-27(35)33-38)28(36)32-26(14-23-17-30-25-8-6-5-7-24(23)25)29(37)31-16-20-9-11-21(12-10-20)18-34(3)4/h5-12,17,19,22,26,30,38H,13-16,18H2,1-4H3,(H,31,37)(H,32,36)(H,33,35)/t22-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286346
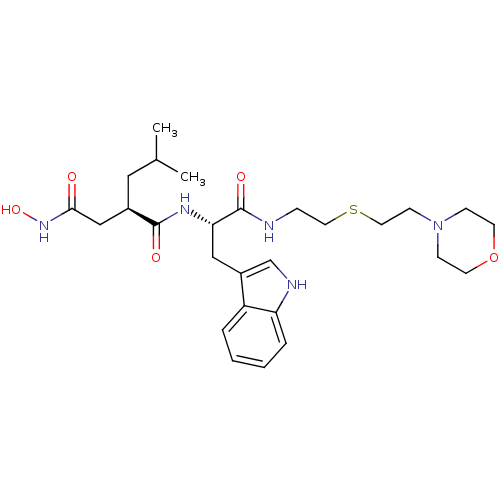
((R)-N*4*-Hydroxy-N*1*-{(S)-2-(1H-indol-3-yl)-1-[2-...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCCSCCN1CCOCC1 Show InChI InChI=1S/C27H41N5O5S/c1-19(2)15-20(17-25(33)31-36)26(34)30-24(16-21-18-29-23-6-4-3-5-22(21)23)27(35)28-7-13-38-14-10-32-8-11-37-12-9-32/h3-6,18-20,24,29,36H,7-17H2,1-2H3,(H,28,35)(H,30,34)(H,31,33)/t20-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286342
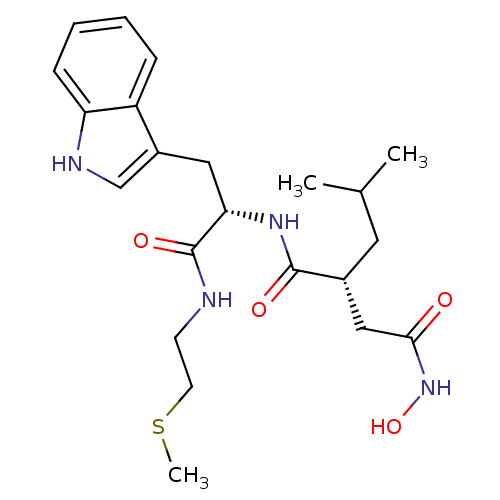
((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-(2-...)Show SMILES CSCCNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO Show InChI InChI=1S/C22H32N4O4S/c1-14(2)10-15(12-20(27)26-30)21(28)25-19(22(29)23-8-9-31-3)11-16-13-24-18-7-5-4-6-17(16)18/h4-7,13-15,19,24,30H,8-12H2,1-3H3,(H,23,29)(H,25,28)(H,26,27)/t15-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286348
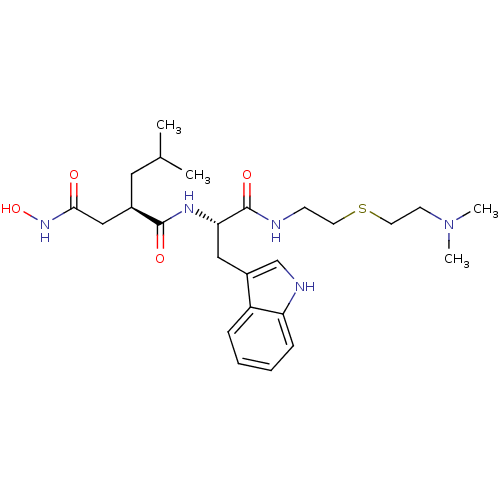
((R)-N*1*-[(S)-1-[2-(2-Dimethylamino-ethylsulfanyl)...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCCSCCN(C)C Show InChI InChI=1S/C25H39N5O4S/c1-17(2)13-18(15-23(31)29-34)24(32)28-22(25(33)26-9-11-35-12-10-30(3)4)14-19-16-27-21-8-6-5-7-20(19)21/h5-8,16-18,22,27,34H,9-15H2,1-4H3,(H,26,33)(H,28,32)(H,29,31)/t18-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286336
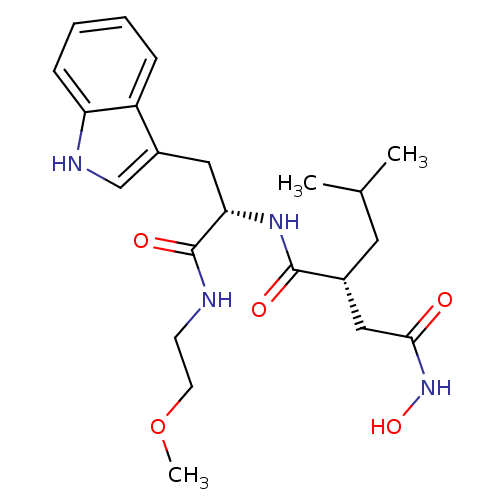
((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-(2-...)Show SMILES COCCNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO Show InChI InChI=1S/C22H32N4O5/c1-14(2)10-15(12-20(27)26-30)21(28)25-19(22(29)23-8-9-31-3)11-16-13-24-18-7-5-4-6-17(16)18/h4-7,13-15,19,24,30H,8-12H2,1-3H3,(H,23,29)(H,25,28)(H,26,27)/t15-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286338
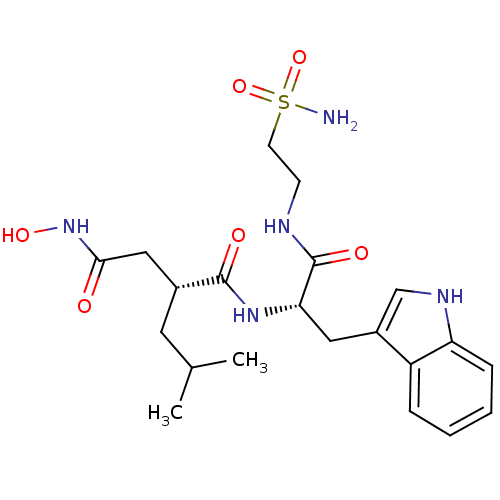
((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-(2-...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCCS(N)(=O)=O Show InChI InChI=1S/C21H31N5O6S/c1-13(2)9-14(11-19(27)26-30)20(28)25-18(21(29)23-7-8-33(22,31)32)10-15-12-24-17-6-4-3-5-16(15)17/h3-6,12-14,18,24,30H,7-11H2,1-2H3,(H,23,29)(H,25,28)(H,26,27)(H2,22,31,32)/t14-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50104969
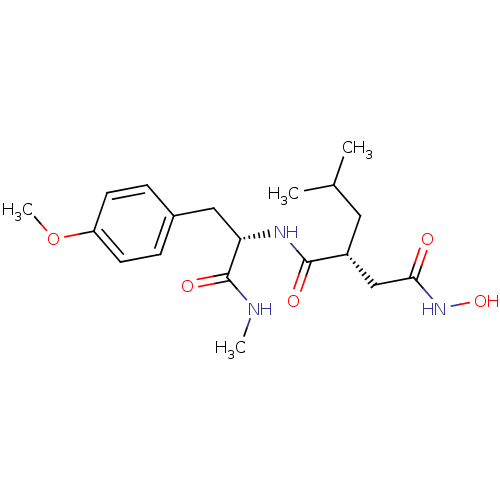
((R)-N*4*-Hydroxy-2-isobutyl-N*1*-[(S)-2-(4-methoxy...)Show SMILES CNC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)[C@H](CC(C)C)CC(=O)NO Show InChI InChI=1S/C19H29N3O5/c1-12(2)9-14(11-17(23)22-26)18(24)21-16(19(25)20-3)10-13-5-7-15(27-4)8-6-13/h5-8,12,14,16,26H,9-11H2,1-4H3,(H,20,25)(H,21,24)(H,22,23)/t14-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50286341
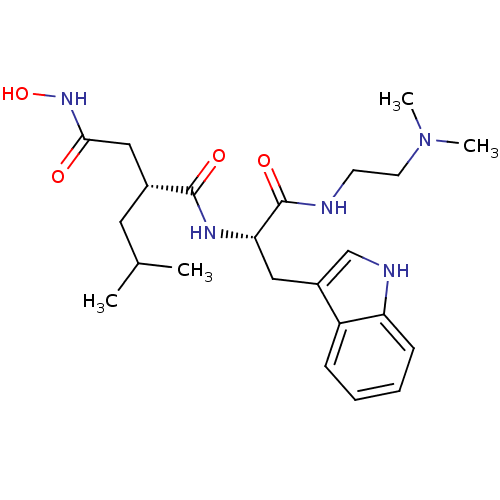
((R)-N*1*-[(S)-1-(2-Dimethylamino-ethylcarbamoyl)-2...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCCN(C)C Show InChI InChI=1S/C23H35N5O4/c1-15(2)11-16(13-21(29)27-32)22(30)26-20(23(31)24-9-10-28(3)4)12-17-14-25-19-8-6-5-7-18(17)19/h5-8,14-16,20,25,32H,9-13H2,1-4H3,(H,24,31)(H,26,30)(H,27,29)/t16-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human fidroblast collagenase (HFC) |
Bioorg Med Chem Lett 5: 337-342 (1995)
Article DOI: 10.1016/0960-894X(95)00031-N
BindingDB Entry DOI: 10.7270/Q2P55NH1 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50099179
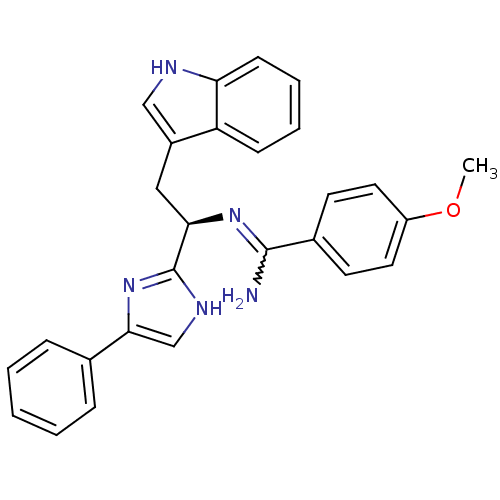
(CHEMBL368334 | N-[(R)-2-(1H-Indol-3-yl)-1-(4-pheny...)Show SMILES COc1ccc(cc1)C(N)=N[C@H](Cc1c[nH]c2ccccc12)c1nc(c[nH]1)-c1ccccc1 |w:8.9| Show InChI InChI=1S/C27H25N5O/c1-33-21-13-11-19(12-14-21)26(28)31-24(15-20-16-29-23-10-6-5-9-22(20)23)27-30-17-25(32-27)18-7-3-2-4-8-18/h2-14,16-17,24,29H,15H2,1H3,(H2,28,31)(H,30,32)/t24-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of human sst3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 991-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D50NHN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50099174
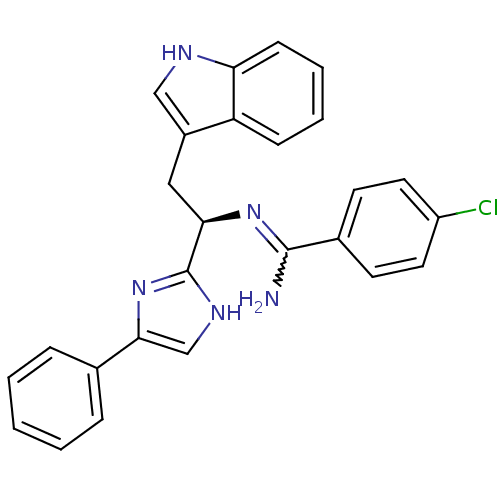
(4-Chloro-N-[(R)-2-(1H-indol-3-yl)-1-(4-phenyl-1H-i...)Show SMILES NC(=N[C@H](Cc1c[nH]c2ccccc12)c1nc(c[nH]1)-c1ccccc1)c1ccc(Cl)cc1 |w:1.0| Show InChI InChI=1S/C26H22ClN5/c27-20-12-10-18(11-13-20)25(28)31-23(14-19-15-29-22-9-5-4-8-21(19)22)26-30-16-24(32-26)17-6-2-1-3-7-17/h1-13,15-16,23,29H,14H2,(H2,28,31)(H,30,32)/t23-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of human sst3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 991-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D50NHN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibitory constant on human somatostatin receptor 5 |
Bioorg Med Chem Lett 11: 741-5 (2001)
BindingDB Entry DOI: 10.7270/Q2Z3205B |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50099172
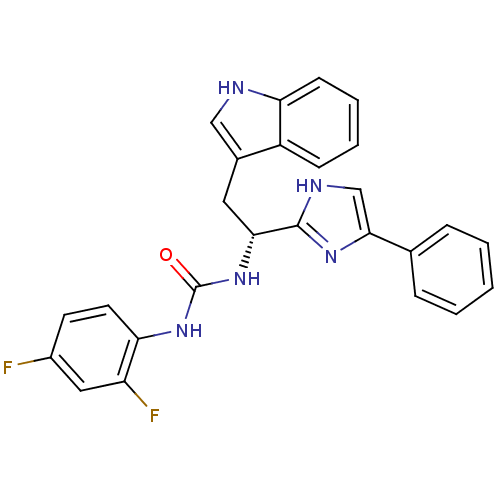
(1-(2,4-Difluoro-phenyl)-3-[(R)-2-(1H-indol-3-yl)-1...)Show SMILES Fc1ccc(NC(=O)N[C@H](Cc2c[nH]c3ccccc23)c2nc(c[nH]2)-c2ccccc2)c(F)c1 Show InChI InChI=1S/C26H21F2N5O/c27-18-10-11-22(20(28)13-18)32-26(34)33-23(12-17-14-29-21-9-5-4-8-19(17)21)25-30-15-24(31-25)16-6-2-1-3-7-16/h1-11,13-15,23,29H,12H2,(H,30,31)(H2,32,33,34)/t23-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of human sst3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 991-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D50NHN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50099173
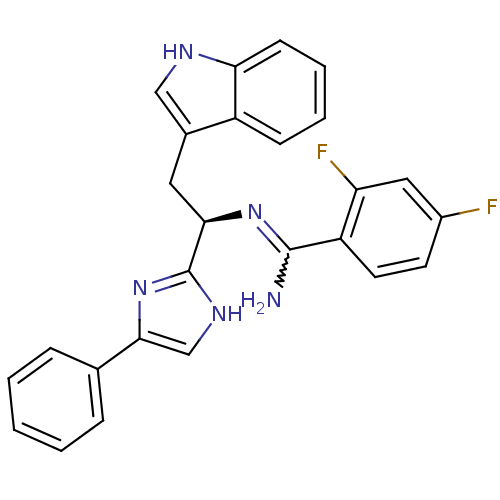
(2,4-Difluoro-N-[(R)-2-(1H-indol-3-yl)-1-(4-phenyl-...)Show SMILES NC(=N[C@H](Cc1c[nH]c2ccccc12)c1nc(c[nH]1)-c1ccccc1)c1ccc(F)cc1F |w:1.0| Show InChI InChI=1S/C26H21F2N5/c27-18-10-11-20(21(28)13-18)25(29)32-23(12-17-14-30-22-9-5-4-8-19(17)22)26-31-15-24(33-26)16-6-2-1-3-7-16/h1-11,13-15,23,30H,12H2,(H2,29,32)(H,31,33)/t23-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of human sst3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 991-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D50NHN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50099175
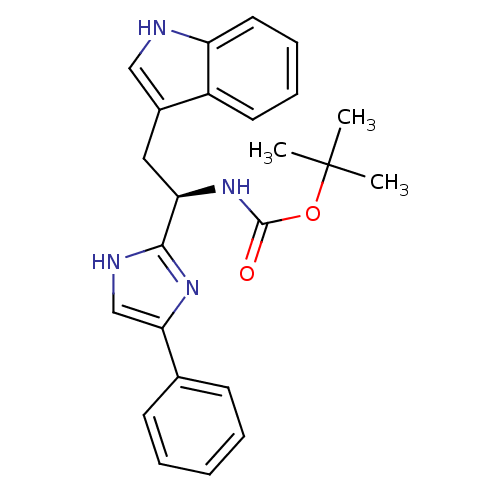
(CHEMBL175356 | [(R)-2-(1H-Indol-3-yl)-1-(4-phenyl-...)Show SMILES CC(C)(C)OC(=O)N[C@H](Cc1c[nH]c2ccccc12)c1nc(c[nH]1)-c1ccccc1 Show InChI InChI=1S/C24H26N4O2/c1-24(2,3)30-23(29)28-20(13-17-14-25-19-12-8-7-11-18(17)19)22-26-15-21(27-22)16-9-5-4-6-10-16/h4-12,14-15,20,25H,13H2,1-3H3,(H,26,27)(H,28,29)/t20-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of human sst3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 991-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D50NHN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50099170
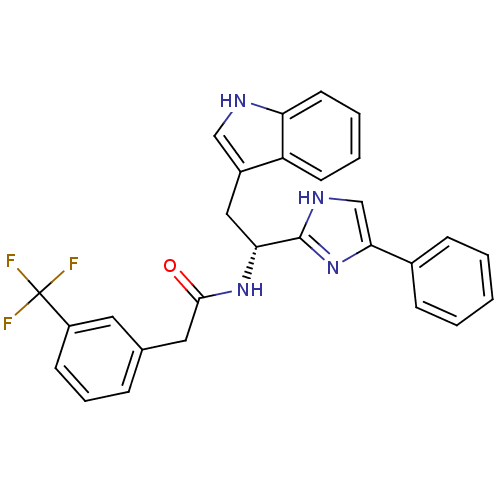
(CHEMBL368993 | N-[(R)-2-(1H-Indol-3-yl)-1-(4-pheny...)Show SMILES FC(F)(F)c1cccc(CC(=O)N[C@H](Cc2c[nH]c3ccccc23)c2nc(c[nH]2)-c2ccccc2)c1 Show InChI InChI=1S/C28H23F3N4O/c29-28(30,31)21-10-6-7-18(13-21)14-26(36)34-24(15-20-16-32-23-12-5-4-11-22(20)23)27-33-17-25(35-27)19-8-2-1-3-9-19/h1-13,16-17,24,32H,14-15H2,(H,33,35)(H,34,36)/t24-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of human sst3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 991-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D50NHN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50099182
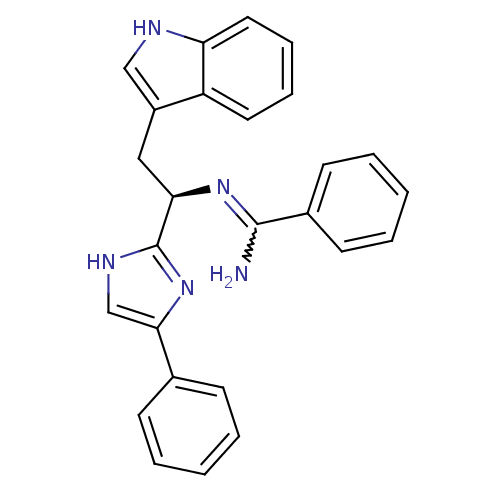
(CHEMBL174872 | N-[(R)-2-(1H-Indol-3-yl)-1-(4-pheny...)Show SMILES NC(=N[C@H](Cc1c[nH]c2ccccc12)c1nc(c[nH]1)-c1ccccc1)c1ccccc1 |w:1.0| Show InChI InChI=1S/C26H23N5/c27-25(19-11-5-2-6-12-19)30-23(15-20-16-28-22-14-8-7-13-21(20)22)26-29-17-24(31-26)18-9-3-1-4-10-18/h1-14,16-17,23,28H,15H2,(H2,27,30)(H,29,31)/t23-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of human sst3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 991-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D50NHN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50097789

(2-[8-(3-Amino-propyl)-6-benzo[b]thiophen-3-yl-imid...)Show SMILES CC(C)(C(=O)NC1CCCCC1)c1cn2cc(nc(CCCN)c2n1)-c1csc2ccccc12 Show InChI InChI=1S/C27H33N5OS/c1-27(2,26(33)29-18-9-4-3-5-10-18)24-16-32-15-22(30-21(12-8-14-28)25(32)31-24)20-17-34-23-13-7-6-11-19(20)23/h6-7,11,13,15-18H,3-5,8-10,12,14,28H2,1-2H3,(H,29,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibitory constant on human somatostatin receptor 5 |
Bioorg Med Chem Lett 11: 741-5 (2001)
BindingDB Entry DOI: 10.7270/Q2Z3205B |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50099180
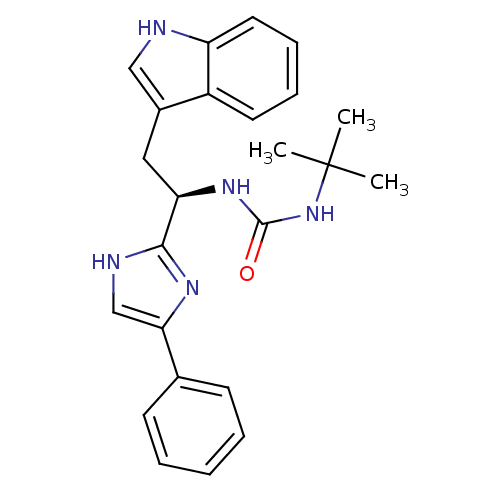
(1-tert-Butyl-3-[(R)-2-(1H-indol-3-yl)-1-(4-phenyl-...)Show SMILES CC(C)(C)NC(=O)N[C@H](Cc1c[nH]c2ccccc12)c1nc(c[nH]1)-c1ccccc1 Show InChI InChI=1S/C24H27N5O/c1-24(2,3)29-23(30)28-20(13-17-14-25-19-12-8-7-11-18(17)19)22-26-15-21(27-22)16-9-5-4-6-10-16/h4-12,14-15,20,25H,13H2,1-3H3,(H,26,27)(H2,28,29,30)/t20-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of human sst3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 991-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D50NHN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50099176
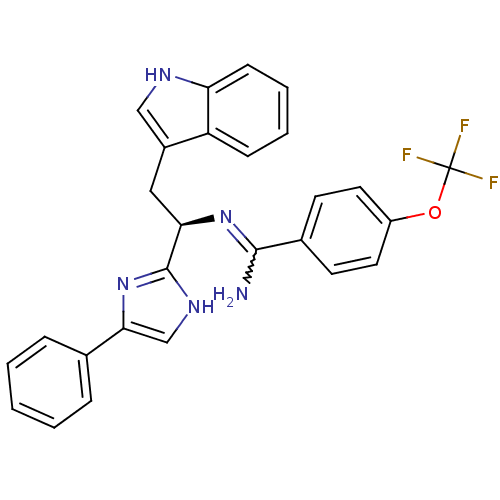
(CHEMBL176313 | N-[(R)-2-(1H-Indol-3-yl)-1-(4-pheny...)Show SMILES NC(=N[C@H](Cc1c[nH]c2ccccc12)c1nc(c[nH]1)-c1ccccc1)c1ccc(OC(F)(F)F)cc1 |w:1.0| Show InChI InChI=1S/C27H22F3N5O/c28-27(29,30)36-20-12-10-18(11-13-20)25(31)34-23(14-19-15-32-22-9-5-4-8-21(19)22)26-33-16-24(35-26)17-6-2-1-3-7-17/h1-13,15-16,23,32H,14H2,(H2,31,34)(H,33,35)/t23-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of human sst3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 991-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D50NHN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50097781
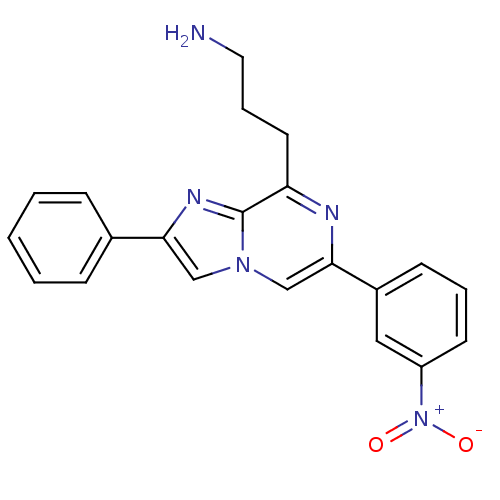
(3-[6-(3-Nitro-phenyl)-2-phenyl-imidazo[1,2-a]pyraz...)Show SMILES NCCCc1nc(cn2cc(nc12)-c1ccccc1)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C21H19N5O2/c22-11-5-10-18-21-24-19(15-6-2-1-3-7-15)13-25(21)14-20(23-18)16-8-4-9-17(12-16)26(27)28/h1-4,6-9,12-14H,5,10-11,22H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibitory constant on human somatostatin receptor 5 |
Bioorg Med Chem Lett 11: 741-5 (2001)
BindingDB Entry DOI: 10.7270/Q2Z3205B |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50099178
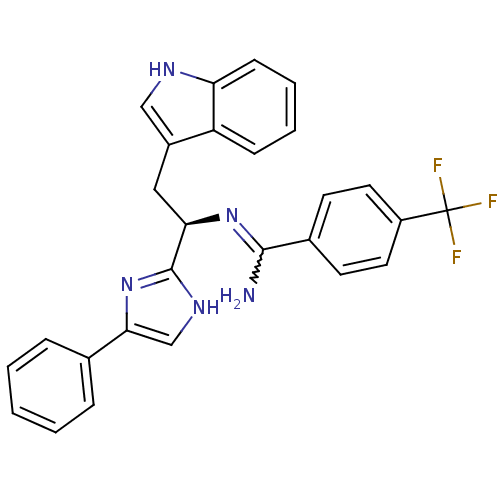
(CHEMBL367699 | N-[(R)-2-(1H-Indol-3-yl)-1-(4-pheny...)Show SMILES NC(=N[C@H](Cc1c[nH]c2ccccc12)c1nc(c[nH]1)-c1ccccc1)c1ccc(cc1)C(F)(F)F |w:1.0| Show InChI InChI=1S/C27H22F3N5/c28-27(29,30)20-12-10-18(11-13-20)25(31)34-23(14-19-15-32-22-9-5-4-8-21(19)22)26-33-16-24(35-26)17-6-2-1-3-7-17/h1-13,15-16,23,32H,14H2,(H2,31,34)(H,33,35)/t23-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of human sst3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 991-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D50NHN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50097784
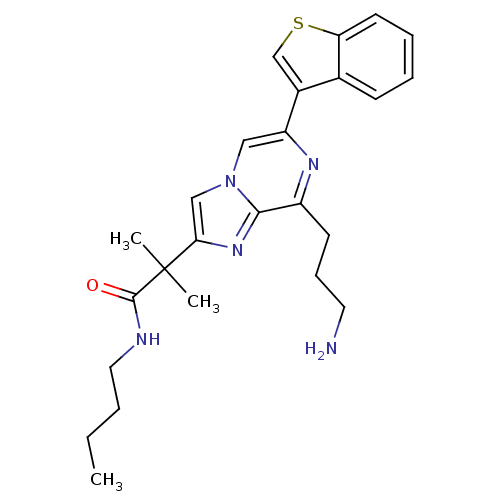
(2-[8-(3-Amino-propyl)-6-benzo[b]thiophen-3-yl-imid...)Show SMILES CCCCNC(=O)C(C)(C)c1cn2cc(nc(CCCN)c2n1)-c1csc2ccccc12 Show InChI InChI=1S/C25H31N5OS/c1-4-5-13-27-24(31)25(2,3)22-15-30-14-20(28-19(10-8-12-26)23(30)29-22)18-16-32-21-11-7-6-9-17(18)21/h6-7,9,11,14-16H,4-5,8,10,12-13,26H2,1-3H3,(H,27,31) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibitory constant on human somatostatin receptor 5 |
Bioorg Med Chem Lett 11: 741-5 (2001)
BindingDB Entry DOI: 10.7270/Q2Z3205B |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50097782
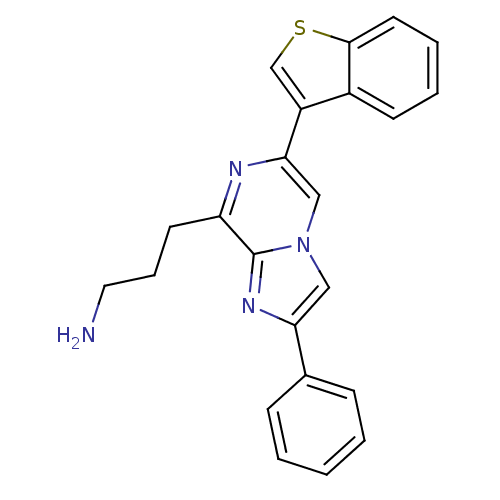
(3-(6-Benzo[b]thiophen-3-yl-2-phenyl-imidazo[1,2-a]...)Show SMILES NCCCc1nc(cn2cc(nc12)-c1ccccc1)-c1csc2ccccc12 Show InChI InChI=1S/C23H20N4S/c24-12-6-10-19-23-26-20(16-7-2-1-3-8-16)13-27(23)14-21(25-19)18-15-28-22-11-5-4-9-17(18)22/h1-5,7-9,11,13-15H,6,10,12,24H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibitory constant on human somatostatin receptor 5 |
Bioorg Med Chem Lett 11: 741-5 (2001)
BindingDB Entry DOI: 10.7270/Q2Z3205B |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50099174

(4-Chloro-N-[(R)-2-(1H-indol-3-yl)-1-(4-phenyl-1H-i...)Show SMILES NC(=N[C@H](Cc1c[nH]c2ccccc12)c1nc(c[nH]1)-c1ccccc1)c1ccc(Cl)cc1 |w:1.0| Show InChI InChI=1S/C26H22ClN5/c27-20-12-10-18(11-13-20)25(28)31-23(14-19-15-29-22-9-5-4-8-21(19)22)26-30-16-24(32-26)17-6-2-1-3-7-17/h1-13,15-16,23,29H,14H2,(H2,28,31)(H,30,32)/t23-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 691 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of human sst5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 991-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D50NHN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50099177

(CHEMBL174490 | N-[(R)-2-(1H-Indol-3-yl)-1-(4-pheny...)Show SMILES CC(N)=N[C@H](Cc1c[nH]c2ccccc12)c1nc(c[nH]1)-c1ccccc1 |w:1.1| Show InChI InChI=1S/C21H21N5/c1-14(22)25-19(11-16-12-23-18-10-6-5-9-17(16)18)21-24-13-20(26-21)15-7-3-2-4-8-15/h2-10,12-13,19,23H,11H2,1H3,(H2,22,25)(H,24,26)/t19-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of human sst3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 991-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D50NHN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50219320
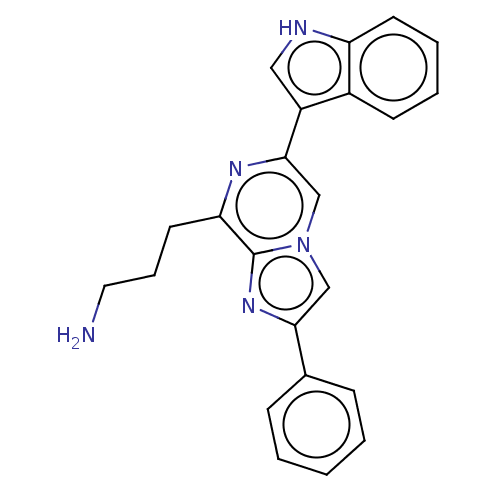
(CHEMBL2112935)Show SMILES NCCCc1nc(cn2cc(nc12)-c1ccccc1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C23H21N5/c24-12-6-11-20-23-27-21(16-7-2-1-3-8-16)14-28(23)15-22(26-20)18-13-25-19-10-5-4-9-17(18)19/h1-5,7-10,13-15,25H,6,11-12,24H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibitory constant on human somatostatin receptor 5 |
Bioorg Med Chem Lett 11: 741-5 (2001)
BindingDB Entry DOI: 10.7270/Q2Z3205B |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50099176

(CHEMBL176313 | N-[(R)-2-(1H-Indol-3-yl)-1-(4-pheny...)Show SMILES NC(=N[C@H](Cc1c[nH]c2ccccc12)c1nc(c[nH]1)-c1ccccc1)c1ccc(OC(F)(F)F)cc1 |w:1.0| Show InChI InChI=1S/C27H22F3N5O/c28-27(29,30)36-20-12-10-18(11-13-20)25(31)34-23(14-19-15-32-22-9-5-4-8-21(19)22)26-33-16-24(35-26)17-6-2-1-3-7-17/h1-13,15-16,23,32H,14H2,(H2,31,34)(H,33,35)/t23-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of human sst5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 991-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D50NHN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50099177

(CHEMBL174490 | N-[(R)-2-(1H-Indol-3-yl)-1-(4-pheny...)Show SMILES CC(N)=N[C@H](Cc1c[nH]c2ccccc12)c1nc(c[nH]1)-c1ccccc1 |w:1.1| Show InChI InChI=1S/C21H21N5/c1-14(22)25-19(11-16-12-23-18-10-6-5-9-17(16)18)21-24-13-20(26-21)15-7-3-2-4-8-15/h2-10,12-13,19,23H,11H2,1H3,(H2,22,25)(H,24,26)/t19-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of human sst5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 991-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D50NHN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50219321

(CHEMBL2112934)Show SMILES CC(C)(C(=O)NCCc1c[nH]c2ccccc12)c1cn2cc(Cc3ccccc3)nc(CCCN)c2n1 Show InChI InChI=1S/C30H34N6O/c1-30(2,29(37)32-16-14-22-18-33-25-12-7-6-11-24(22)25)27-20-36-19-23(17-21-9-4-3-5-10-21)34-26(13-8-15-31)28(36)35-27/h3-7,9-12,18-20,33H,8,13-17,31H2,1-2H3,(H,32,37) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibitory constant on human somatostatin receptor 5 |
Bioorg Med Chem Lett 11: 741-5 (2001)
BindingDB Entry DOI: 10.7270/Q2Z3205B |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50219321

(CHEMBL2112934)Show SMILES CC(C)(C(=O)NCCc1c[nH]c2ccccc12)c1cn2cc(Cc3ccccc3)nc(CCCN)c2n1 Show InChI InChI=1S/C30H34N6O/c1-30(2,29(37)32-16-14-22-18-33-25-12-7-6-11-24(22)25)27-20-36-19-23(17-21-9-4-3-5-10-21)34-26(13-8-15-31)28(36)35-27/h3-7,9-12,18-20,33H,8,13-17,31H2,1-2H3,(H,32,37) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibitory constant on human somatostatin receptor type 3 |
Bioorg Med Chem Lett 11: 741-5 (2001)
BindingDB Entry DOI: 10.7270/Q2Z3205B |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50099169
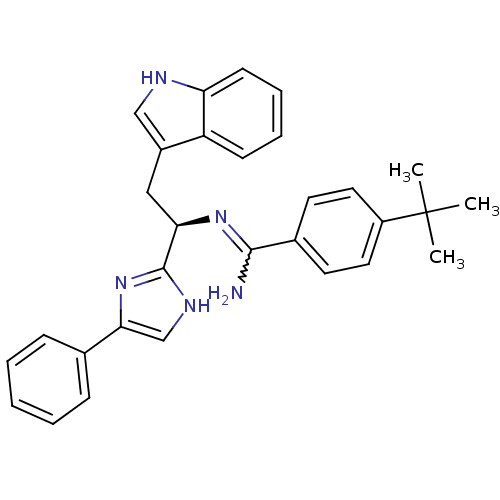
(4-tert-Butyl-N-[(R)-2-(1H-indol-3-yl)-1-(4-phenyl-...)Show SMILES CC(C)(C)c1ccc(cc1)C(N)=N[C@H](Cc1c[nH]c2ccccc12)c1nc(c[nH]1)-c1ccccc1 |w:10.11| Show InChI InChI=1S/C30H31N5/c1-30(2,3)23-15-13-21(14-16-23)28(31)34-26(17-22-18-32-25-12-8-7-11-24(22)25)29-33-19-27(35-29)20-9-5-4-6-10-20/h4-16,18-19,26,32H,17H2,1-3H3,(H2,31,34)(H,33,35)/t26-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of human sst3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 991-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D50NHN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM50099181
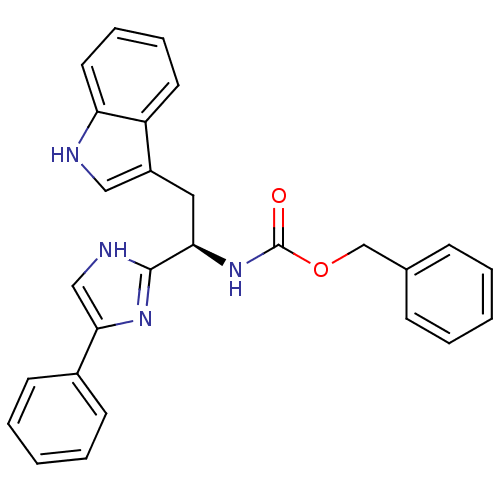
(CHEMBL177337 | [(R)-2-(1H-Indol-3-yl)-1-(4-phenyl-...)Show SMILES O=C(N[C@H](Cc1c[nH]c2ccccc12)c1nc(c[nH]1)-c1ccccc1)OCc1ccccc1 Show InChI InChI=1S/C27H24N4O2/c32-27(33-18-19-9-3-1-4-10-19)31-24(15-21-16-28-23-14-8-7-13-22(21)23)26-29-17-25(30-26)20-11-5-2-6-12-20/h1-14,16-17,24,28H,15,18H2,(H,29,30)(H,31,32)/t24-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of human sst1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 991-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D50NHN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM50099182

(CHEMBL174872 | N-[(R)-2-(1H-Indol-3-yl)-1-(4-pheny...)Show SMILES NC(=N[C@H](Cc1c[nH]c2ccccc12)c1nc(c[nH]1)-c1ccccc1)c1ccccc1 |w:1.0| Show InChI InChI=1S/C26H23N5/c27-25(19-11-5-2-6-12-19)30-23(15-20-16-28-22-14-8-7-13-21(20)22)26-29-17-24(31-26)18-9-3-1-4-10-18/h1-14,16-17,23,28H,15H2,(H2,27,30)(H,29,31)/t23-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of human sst1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 991-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D50NHN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM50099173

(2,4-Difluoro-N-[(R)-2-(1H-indol-3-yl)-1-(4-phenyl-...)Show SMILES NC(=N[C@H](Cc1c[nH]c2ccccc12)c1nc(c[nH]1)-c1ccccc1)c1ccc(F)cc1F |w:1.0| Show InChI InChI=1S/C26H21F2N5/c27-18-10-11-20(21(28)13-18)25(29)32-23(12-17-14-30-22-9-5-4-8-19(17)22)26-31-15-24(33-26)16-6-2-1-3-7-16/h1-11,13-15,23,30H,12H2,(H2,29,32)(H,31,33)/t23-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of human sst1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 991-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D50NHN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data