 Found 167 hits with Last Name = 'green' and Initial = 'rc'
Found 167 hits with Last Name = 'green' and Initial = 'rc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50041291
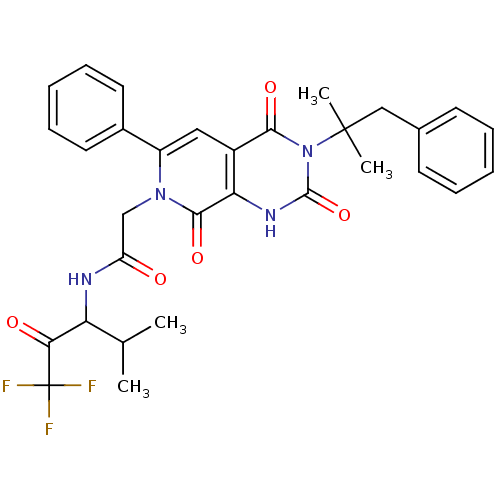
(2-[(R)-3-(1,1-Dimethyl-2-phenyl-ethyl)-2,4,8-triox...)Show SMILES CC(C)C(NC(=O)Cn1c(cc2c([nH]c(=O)n(c2=O)C(C)(C)Cc2ccccc2)c1=O)-c1ccccc1)C(=O)C(F)(F)F Show InChI InChI=1S/C31H31F3N4O5/c1-18(2)24(26(40)31(32,33)34)35-23(39)17-37-22(20-13-9-6-10-14-20)15-21-25(28(37)42)36-29(43)38(27(21)41)30(3,4)16-19-11-7-5-8-12-19/h5-15,18,24H,16-17H2,1-4H3,(H,35,39)(H,36,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition of Human Leukocyte Elastase (HLE) |
J Med Chem 37: 1259-61 (1994)
BindingDB Entry DOI: 10.7270/Q2251H7P |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50037348
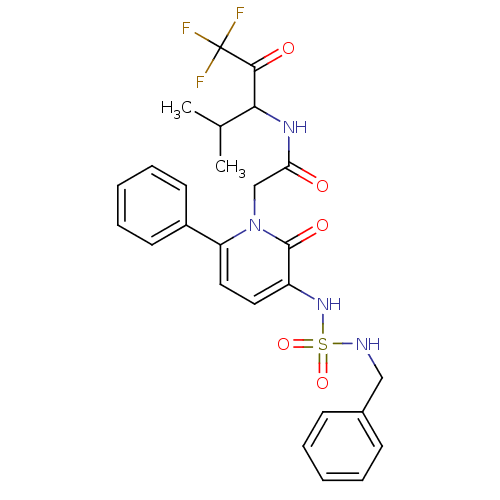
(1N-(3,3,3-trifluoro-1-isopropyl-2-oxopropyl)-2-(3-...)Show SMILES CC(C)C(NC(=O)Cn1c(ccc(NS(=O)(=O)NCc2ccccc2)c1=O)-c1ccccc1)C(=O)C(F)(F)F Show InChI InChI=1S/C26H27F3N4O5S/c1-17(2)23(24(35)26(27,28)29)31-22(34)16-33-21(19-11-7-4-8-12-19)14-13-20(25(33)36)32-39(37,38)30-15-18-9-5-3-6-10-18/h3-14,17,23,30,32H,15-16H2,1-2H3,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition of Human Leukocyte Elastase (HLE) |
J Med Chem 37: 1259-61 (1994)
BindingDB Entry DOI: 10.7270/Q2251H7P |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50301311
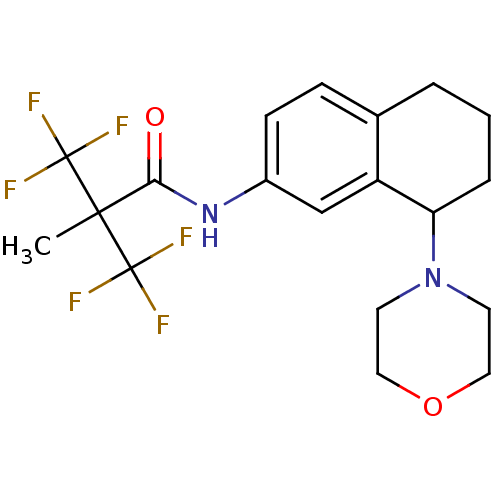
((+/-)3,3,3-trifluoro-2-methyl-N-(8-morpholino-5,6,...)Show SMILES CC(C(=O)Nc1ccc2CCCC(N3CCOCC3)c2c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C19H22F6N2O2/c1-17(18(20,21)22,19(23,24)25)16(28)26-13-6-5-12-3-2-4-15(14(12)11-13)27-7-9-29-10-8-27/h5-6,11,15H,2-4,7-10H2,1H3,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from human CB2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 5004-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.057
BindingDB Entry DOI: 10.7270/Q25M65S1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50301315
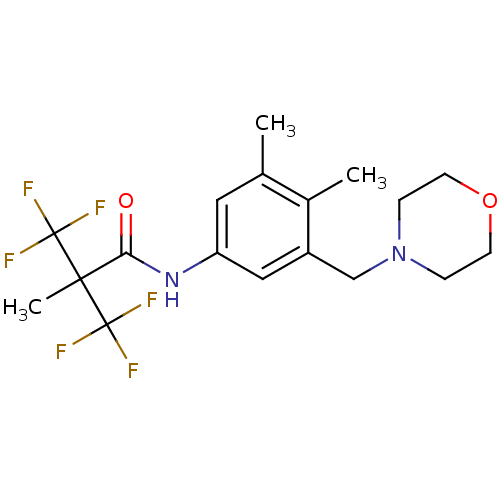
(CHEMBL568467 | N-(3,4-dimethyl-5-(morpholinomethyl...)Show SMILES Cc1cc(NC(=O)C(C)(C(F)(F)F)C(F)(F)F)cc(CN2CCOCC2)c1C Show InChI InChI=1S/C18H22F6N2O2/c1-11-8-14(9-13(12(11)2)10-26-4-6-28-7-5-26)25-15(27)16(3,17(19,20)21)18(22,23)24/h8-9H,4-7,10H2,1-3H3,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from human CB2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 5004-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.057
BindingDB Entry DOI: 10.7270/Q25M65S1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50377711
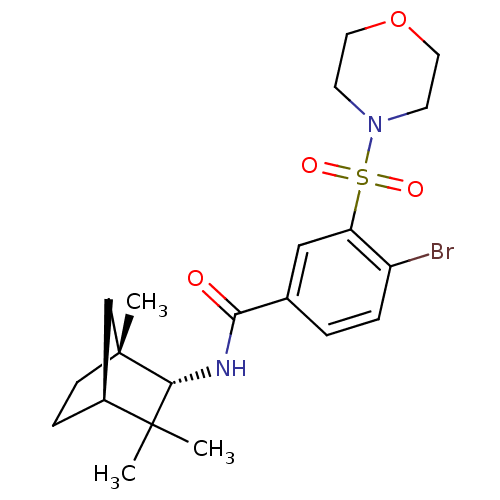
(CHEMBL256962)Show SMILES C[C@@]12CC[C@@H](C1)C(C)(C)[C@H]2NC(=O)c1ccc(Br)c(c1)S(=O)(=O)N1CCOCC1 |THB:10:9:3.2:5| Show InChI InChI=1S/C21H29BrN2O4S/c1-20(2)15-6-7-21(3,13-15)19(20)23-18(25)14-4-5-16(22)17(12-14)29(26,27)24-8-10-28-11-9-24/h4-5,12,15,19H,6-11,13H2,1-3H3,(H,23,25)/t15-,19+,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human cloned CB2 receptor |
Bioorg Med Chem Lett 18: 2830-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.006
BindingDB Entry DOI: 10.7270/Q2P2701M |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50277153
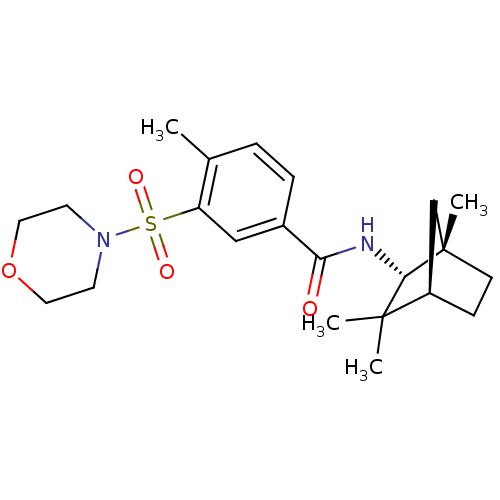
(4-methyl-3-(morpholinosulfonyl)-N-((1S,2R,4R)-1,3,...)Show SMILES Cc1ccc(cc1S(=O)(=O)N1CCOCC1)C(=O)N[C@@H]1[C@@]2(C)CC[C@H](C2)C1(C)C |r| Show InChI InChI=1S/C22H32N2O4S/c1-15-5-6-16(13-18(15)29(26,27)24-9-11-28-12-10-24)19(25)23-20-21(2,3)17-7-8-22(20,4)14-17/h5-6,13,17,20H,7-12,14H2,1-4H3,(H,23,25)/t17-,20+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from human CB2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 5004-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.057
BindingDB Entry DOI: 10.7270/Q25M65S1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50377707
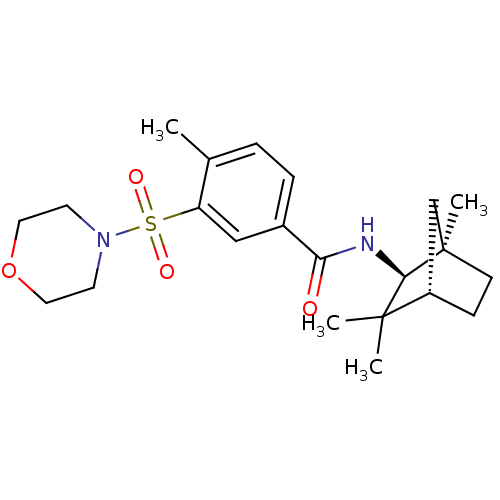
(CHEMBL255474)Show SMILES Cc1ccc(cc1S(=O)(=O)N1CCOCC1)C(=O)N[C@H]1[C@]2(C)CC[C@@H](C2)C1(C)C |THB:18:19:23.22:25| Show InChI InChI=1S/C22H32N2O4S/c1-15-5-6-16(13-18(15)29(26,27)24-9-11-28-12-10-24)19(25)23-20-21(2,3)17-7-8-22(20,4)14-17/h5-6,13,17,20H,7-12,14H2,1-4H3,(H,23,25)/t17-,20+,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human cloned CB2 receptor |
Bioorg Med Chem Lett 18: 2830-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.006
BindingDB Entry DOI: 10.7270/Q2P2701M |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036127
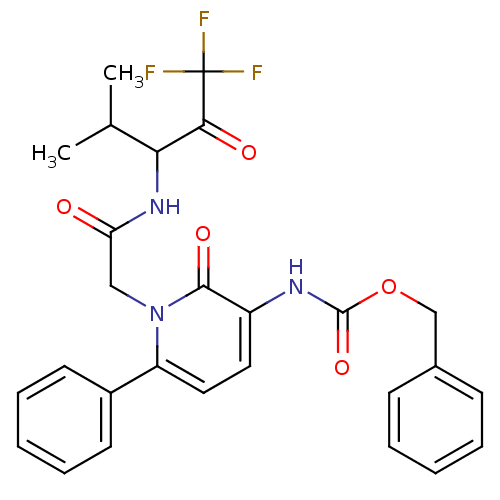
(CHEMBL11391 | {2-Oxo-6-phenyl-1-[(3,3,3-trifluoro-...)Show SMILES CC(C)C(NC(=O)Cn1c(ccc(NC(=O)OCc2ccccc2)c1=O)-c1ccccc1)C(=O)C(F)(F)F Show InChI InChI=1S/C27H26F3N3O5/c1-17(2)23(24(35)27(28,29)30)32-22(34)15-33-21(19-11-7-4-8-12-19)14-13-20(25(33)36)31-26(37)38-16-18-9-5-3-6-10-18/h3-14,17,23H,15-16H2,1-2H3,(H,31,37)(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition of Human Leukocyte Elastase (HLE) |
J Med Chem 37: 1259-61 (1994)
BindingDB Entry DOI: 10.7270/Q2251H7P |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50377706
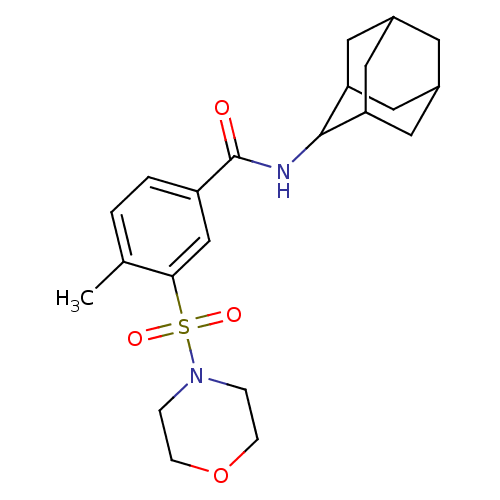
(CHEMBL256110)Show SMILES Cc1ccc(cc1S(=O)(=O)N1CCOCC1)C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:18:19:21:25.23.24,THB:23:22:19:25.24.26,23:24:21.22.28:19,26:24:21:28.27.19,26:27:21:25.23.24,(25.81,-25.08,;25.81,-26.62,;24.48,-27.39,;24.48,-28.93,;25.82,-29.71,;27.16,-28.93,;27.15,-27.38,;28.48,-26.61,;29.26,-27.94,;27.71,-25.28,;29.81,-25.83,;31.15,-26.59,;32.48,-25.81,;32.47,-24.27,;31.13,-23.51,;29.8,-24.29,;25.82,-31.25,;24.48,-32.01,;27.15,-32.02,;27.19,-33.56,;25.99,-34.46,;24.26,-34.26,;25.48,-35.21,;25.88,-36.67,;27.5,-36.72,;26.4,-35.81,;28.74,-35.82,;28.36,-34.36,;26.76,-34.26,)| Show InChI InChI=1S/C22H30N2O4S/c1-14-2-3-17(13-20(14)29(26,27)24-4-6-28-7-5-24)22(25)23-21-18-9-15-8-16(11-18)12-19(21)10-15/h2-3,13,15-16,18-19,21H,4-12H2,1H3,(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human cloned CB2 receptor |
Bioorg Med Chem Lett 18: 2830-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.006
BindingDB Entry DOI: 10.7270/Q2P2701M |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50037341
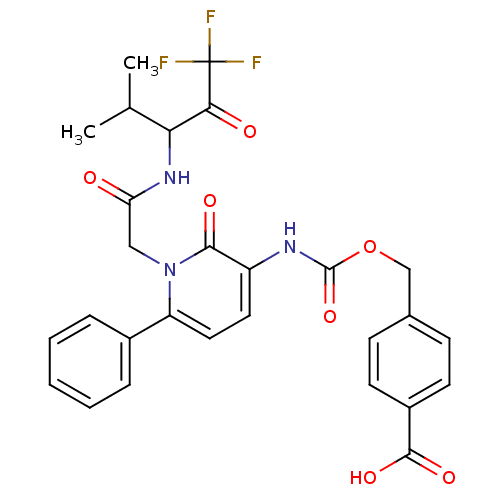
(4-{2-Oxo-6-phenyl-1-[(3,3,3-trifluoro-1-isopropyl-...)Show SMILES CC(C)C(NC(=O)Cn1c(ccc(NC(=O)OCc2ccc(cc2)C(O)=O)c1=O)-c1ccccc1)C(=O)C(F)(F)F Show InChI InChI=1S/C28H26F3N3O7/c1-16(2)23(24(36)28(29,30)31)33-22(35)14-34-21(18-6-4-3-5-7-18)13-12-20(25(34)37)32-27(40)41-15-17-8-10-19(11-9-17)26(38)39/h3-13,16,23H,14-15H2,1-2H3,(H,32,40)(H,33,35)(H,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition of Human Leukocyte Elastase (HLE) |
J Med Chem 37: 1259-61 (1994)
BindingDB Entry DOI: 10.7270/Q2251H7P |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50377705
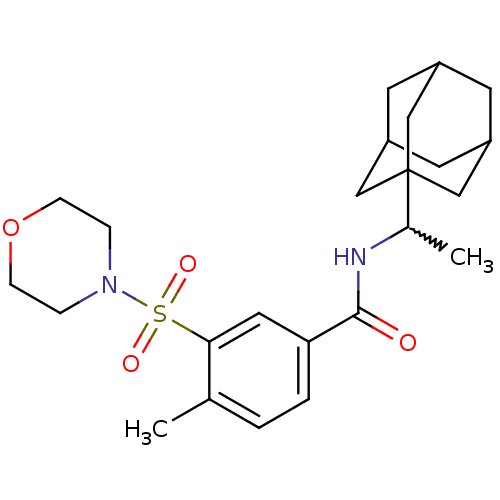
(CHEMBL442447)Show SMILES CC(NC(=O)c1ccc(C)c(c1)S(=O)(=O)N1CCOCC1)C12CC3CC(CC(C3)C1)C2 |w:1.0,TLB:24:25:29:22.23.28,28:23:30:29.27.26,28:27:30:22.23.24,THB:24:23:29:30.25.26| Show InChI InChI=1S/C24H34N2O4S/c1-16-3-4-21(12-22(16)31(28,29)26-5-7-30-8-6-26)23(27)25-17(2)24-13-18-9-19(14-24)11-20(10-18)15-24/h3-4,12,17-20H,5-11,13-15H2,1-2H3,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human cloned CB2 receptor |
Bioorg Med Chem Lett 18: 2830-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.006
BindingDB Entry DOI: 10.7270/Q2P2701M |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036112
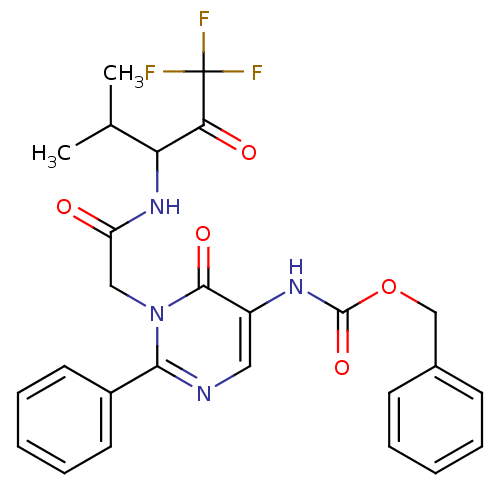
(CHEMBL11403 | {6-Oxo-2-phenyl-1-[(3,3,3-trifluoro-...)Show SMILES CC(C)C(NC(=O)Cn1c(ncc(NC(=O)OCc2ccccc2)c1=O)-c1ccccc1)C(=O)C(F)(F)F Show InChI InChI=1S/C26H25F3N4O5/c1-16(2)21(22(35)26(27,28)29)32-20(34)14-33-23(18-11-7-4-8-12-18)30-13-19(24(33)36)31-25(37)38-15-17-9-5-3-6-10-17/h3-13,16,21H,14-15H2,1-2H3,(H,31,37)(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition of Human Leukocyte Elastase (HLE) |
J Med Chem 37: 1259-61 (1994)
BindingDB Entry DOI: 10.7270/Q2251H7P |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50037375
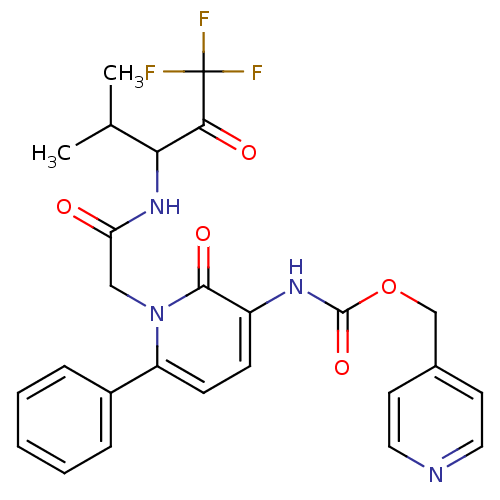
(CHEMBL275043 | {2-Oxo-6-phenyl-1-[(3,3,3-trifluoro...)Show SMILES CC(C)C(NC(=O)Cn1c(ccc(NC(=O)OCc2ccncc2)c1=O)-c1ccccc1)C(=O)C(F)(F)F Show InChI InChI=1S/C26H25F3N4O5/c1-16(2)22(23(35)26(27,28)29)32-21(34)14-33-20(18-6-4-3-5-7-18)9-8-19(24(33)36)31-25(37)38-15-17-10-12-30-13-11-17/h3-13,16,22H,14-15H2,1-2H3,(H,31,37)(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition of Human Leukocyte Elastase (HLE) |
J Med Chem 37: 1259-61 (1994)
BindingDB Entry DOI: 10.7270/Q2251H7P |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50377704
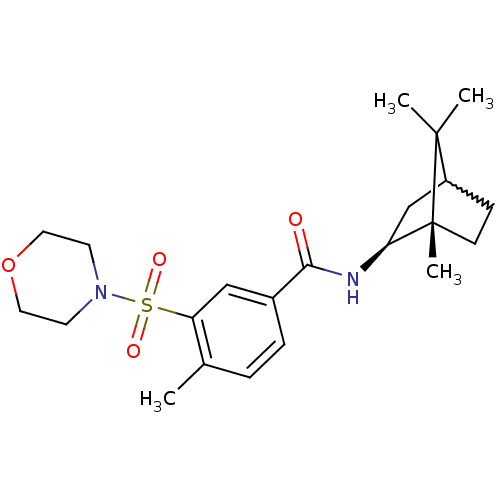
(CHEMBL255096)Show SMILES Cc1ccc(cc1S(=O)(=O)N1CCOCC1)C(=O)N[C@H]1CC2CC[C@@]1(C)C2(C)C |w:21.23,TLB:18:19:23.22:26| Show InChI InChI=1S/C22H32N2O4S/c1-15-5-6-16(13-18(15)29(26,27)24-9-11-28-12-10-24)20(25)23-19-14-17-7-8-22(19,4)21(17,2)3/h5-6,13,17,19H,7-12,14H2,1-4H3,(H,23,25)/t17?,19-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human cloned CB2 receptor |
Bioorg Med Chem Lett 18: 2830-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.006
BindingDB Entry DOI: 10.7270/Q2P2701M |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036103

(CHEMBL11098 | {2-(4-Fluoro-phenyl)-6-oxo-1-[(3,3,3...)Show SMILES CC(C)C(NC(=O)Cn1c(ncc(NC(=O)OCc2ccccc2)c1=O)-c1ccc(F)cc1)C(=O)C(F)(F)F Show InChI InChI=1S/C26H24F4N4O5/c1-15(2)21(22(36)26(28,29)30)33-20(35)13-34-23(17-8-10-18(27)11-9-17)31-12-19(24(34)37)32-25(38)39-14-16-6-4-3-5-7-16/h3-12,15,21H,13-14H2,1-2H3,(H,32,38)(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition of Human Leukocyte Elastase (HLE) |
J Med Chem 37: 1259-61 (1994)
BindingDB Entry DOI: 10.7270/Q2251H7P |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50301316
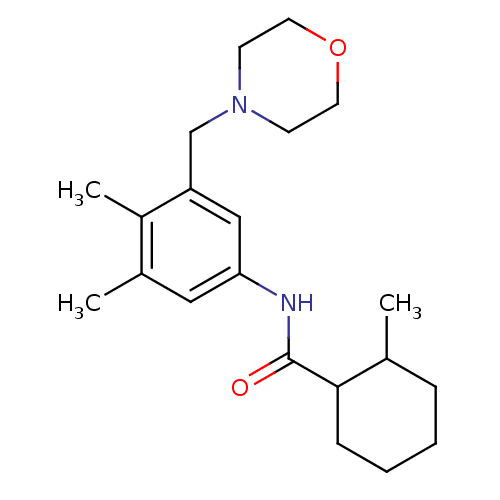
(CHEMBL568468 | N-(3,4-dimethyl-5-(morpholinomethyl...)Show InChI InChI=1S/C21H32N2O2/c1-15-6-4-5-7-20(15)21(24)22-19-12-16(2)17(3)18(13-19)14-23-8-10-25-11-9-23/h12-13,15,20H,4-11,14H2,1-3H3,(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from human CB2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 5004-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.057
BindingDB Entry DOI: 10.7270/Q25M65S1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50377710

(CHEMBL256753)Show SMILES CC1(C)[C@H]2CC[C@](C)(C2)[C@@H]1NC(=O)c1ccc(Br)c(c1)S(=O)(=O)N1CCCC1 |THB:10:9:4.5:8| Show InChI InChI=1S/C21H29BrN2O3S/c1-20(2)15-8-9-21(3,13-15)19(20)23-18(25)14-6-7-16(22)17(12-14)28(26,27)24-10-4-5-11-24/h6-7,12,15,19H,4-5,8-11,13H2,1-3H3,(H,23,25)/t15-,19+,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human cloned CB2 receptor |
Bioorg Med Chem Lett 18: 2830-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.006
BindingDB Entry DOI: 10.7270/Q2P2701M |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50301311

((+/-)3,3,3-trifluoro-2-methyl-N-(8-morpholino-5,6,...)Show SMILES CC(C(=O)Nc1ccc2CCCC(N3CCOCC3)c2c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C19H22F6N2O2/c1-17(18(20,21)22,19(23,24)25)16(28)26-13-6-5-12-3-2-4-15(14(12)11-13)27-7-9-29-10-8-27/h5-6,11,15H,2-4,7-10H2,1H3,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from human CB2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 5004-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.057
BindingDB Entry DOI: 10.7270/Q25M65S1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50301311

((+/-)3,3,3-trifluoro-2-methyl-N-(8-morpholino-5,6,...)Show SMILES CC(C(=O)Nc1ccc2CCCC(N3CCOCC3)c2c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C19H22F6N2O2/c1-17(18(20,21)22,19(23,24)25)16(28)26-13-6-5-12-3-2-4-15(14(12)11-13)27-7-9-29-10-8-27/h5-6,11,15H,2-4,7-10H2,1H3,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from human CB2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 5004-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.057
BindingDB Entry DOI: 10.7270/Q25M65S1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50377703
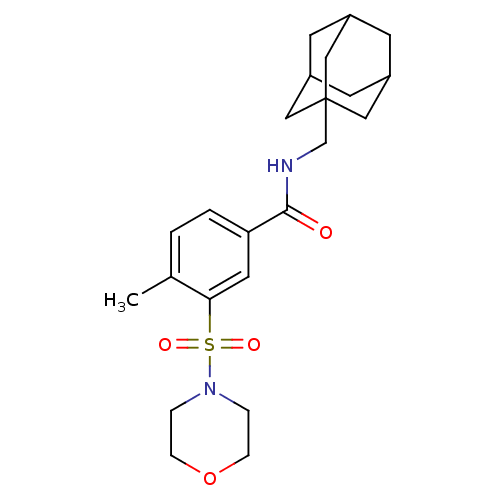
(CHEMBL258243)Show SMILES Cc1ccc(cc1S(=O)(=O)N1CCOCC1)C(=O)NCC12CC3CC(CC(C3)C1)C2 |TLB:23:24:28:21.22.27,27:22:29:28.26.25,27:26:29:21.22.23,THB:23:22:28:29.24.25| Show InChI InChI=1S/C23H32N2O4S/c1-16-2-3-20(11-21(16)30(27,28)25-4-6-29-7-5-25)22(26)24-15-23-12-17-8-18(13-23)10-19(9-17)14-23/h2-3,11,17-19H,4-10,12-15H2,1H3,(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human cloned CB2 receptor |
Bioorg Med Chem Lett 18: 2830-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.006
BindingDB Entry DOI: 10.7270/Q2P2701M |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50377709
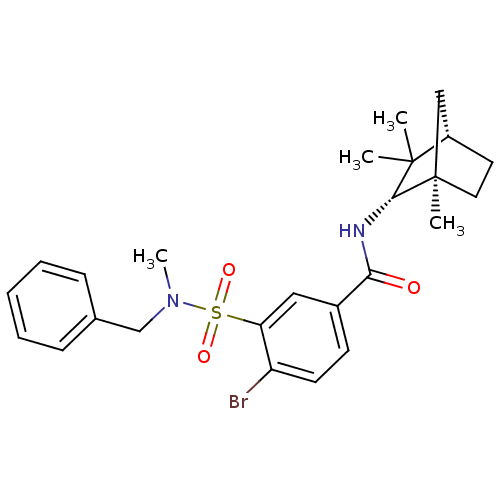
(CHEMBL256754)Show SMILES CN(Cc1ccccc1)S(=O)(=O)c1cc(ccc1Br)C(=O)N[C@@H]1C(C)(C)[C@H]2CC[C@]1(C)C2 |THB:21:22:27.28:31| Show InChI InChI=1S/C25H31BrN2O3S/c1-24(2)19-12-13-25(3,15-19)23(24)27-22(29)18-10-11-20(26)21(14-18)32(30,31)28(4)16-17-8-6-5-7-9-17/h5-11,14,19,23H,12-13,15-16H2,1-4H3,(H,27,29)/t19-,23+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human cloned CB2 receptor |
Bioorg Med Chem Lett 18: 2830-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.006
BindingDB Entry DOI: 10.7270/Q2P2701M |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50037100
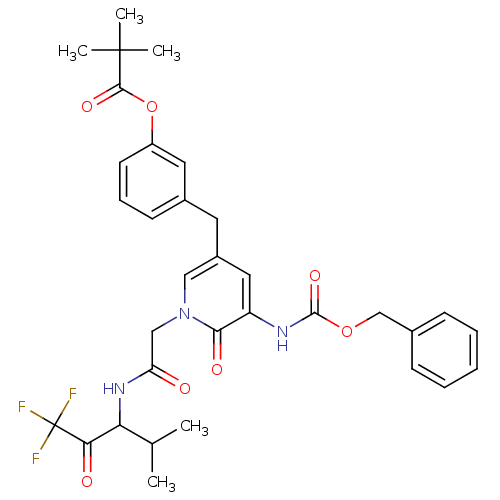
(2,2-Dimethyl-propionic acid 3-{5-benzyloxycarbonyl...)Show SMILES CC(C)C(NC(=O)Cn1cc(Cc2cccc(OC(=O)C(C)(C)C)c2)cc(NC(=O)OCc2ccccc2)c1=O)C(=O)C(F)(F)F Show InChI InChI=1S/C33H36F3N3O7/c1-20(2)27(28(41)33(34,35)36)38-26(40)18-39-17-23(14-22-12-9-13-24(15-22)46-30(43)32(3,4)5)16-25(29(39)42)37-31(44)45-19-21-10-7-6-8-11-21/h6-13,15-17,20,27H,14,18-19H2,1-5H3,(H,37,44)(H,38,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
A Business Unit of ZENECA Inc.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for ability to inhibit human leukocyte elastase activity |
J Med Chem 37: 3090-9 (1994)
BindingDB Entry DOI: 10.7270/Q2Z0375K |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036078
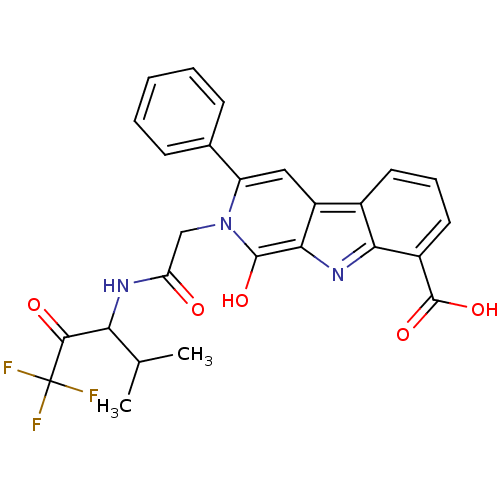
(1-Oxo-3-phenyl-2-[(3,3,3-trifluoro-1-isopropyl-2-o...)Show SMILES CC(C)C(NC(=O)Cn1c(O)c2nc3c(cccc3c2cc1-c1ccccc1)C(O)=O)C(=O)C(F)(F)F Show InChI InChI=1S/C26H22F3N3O5/c1-13(2)20(23(34)26(27,28)29)30-19(33)12-32-18(14-7-4-3-5-8-14)11-17-15-9-6-10-16(25(36)37)21(15)31-22(17)24(32)35/h3-11,13,20,35H,12H2,1-2H3,(H,30,33)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition of Human Leukocyte Elastase (HLE) |
J Med Chem 37: 1259-61 (1994)
BindingDB Entry DOI: 10.7270/Q2251H7P |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50377708
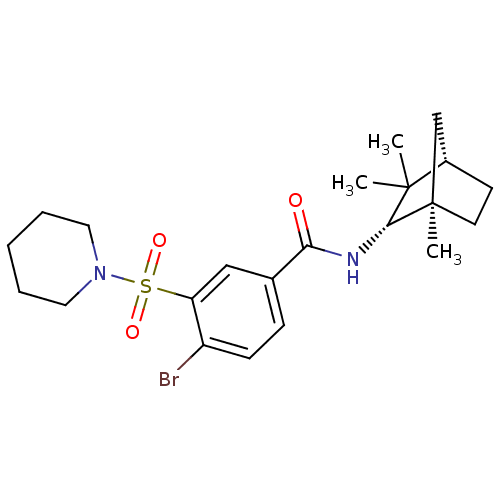
(CHEMBL256752)Show SMILES CC1(C)[C@H]2CC[C@](C)(C2)[C@@H]1NC(=O)c1ccc(Br)c(c1)S(=O)(=O)N1CCCCC1 |THB:10:9:4.5:8| Show InChI InChI=1S/C22H31BrN2O3S/c1-21(2)16-9-10-22(3,14-16)20(21)24-19(26)15-7-8-17(23)18(13-15)29(27,28)25-11-5-4-6-12-25/h7-8,13,16,20H,4-6,9-12,14H2,1-3H3,(H,24,26)/t16-,20+,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human cloned CB2 receptor |
Bioorg Med Chem Lett 18: 2830-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.006
BindingDB Entry DOI: 10.7270/Q2P2701M |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50301312
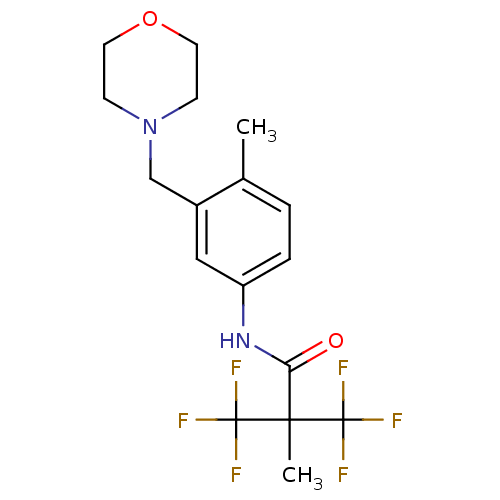
(3,3-difluoro-2-methyl-N-(4-methyl-3-(morpholinomet...)Show SMILES Cc1ccc(NC(=O)C(C)(C(F)(F)F)C(F)(F)F)cc1CN1CCOCC1 Show InChI InChI=1S/C17H20F6N2O2/c1-11-3-4-13(9-12(11)10-25-5-7-27-8-6-25)24-14(26)15(2,16(18,19)20)17(21,22)23/h3-4,9H,5-8,10H2,1-2H3,(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from human CB2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 5004-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.057
BindingDB Entry DOI: 10.7270/Q25M65S1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50301306
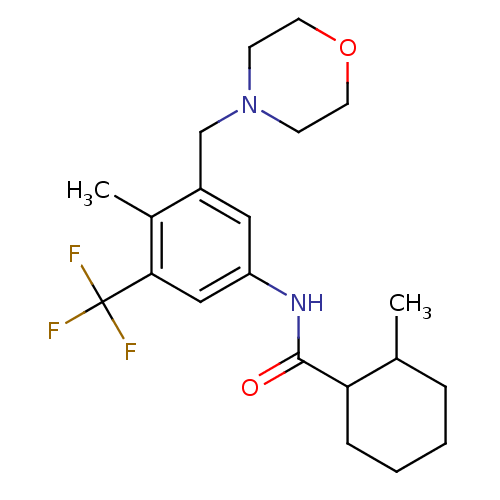
(2-methyl-N-(4-methyl-3-(morpholinomethyl)-5-(trifl...)Show SMILES CC1CCCCC1C(=O)Nc1cc(CN2CCOCC2)c(C)c(c1)C(F)(F)F Show InChI InChI=1S/C21H29F3N2O2/c1-14-5-3-4-6-18(14)20(27)25-17-11-16(13-26-7-9-28-10-8-26)15(2)19(12-17)21(22,23)24/h11-12,14,18H,3-10,13H2,1-2H3,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from human CB2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 5004-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.057
BindingDB Entry DOI: 10.7270/Q25M65S1 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50037342
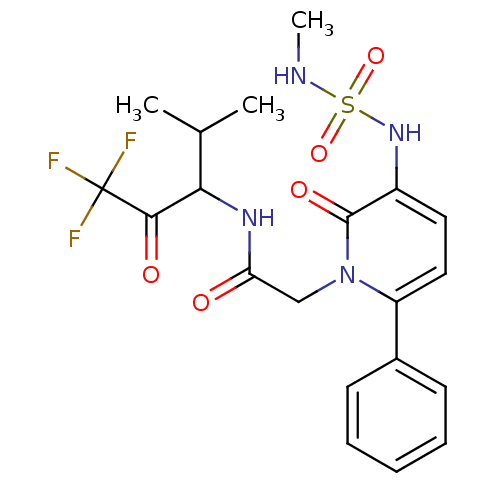
(1N-(3,3,3-trifluoro-1-isopropyl-2-oxopropyl)-2-(3-...)Show SMILES CNS(=O)(=O)Nc1ccc(-c2ccccc2)n(CC(=O)NC(C(C)C)C(=O)C(F)(F)F)c1=O Show InChI InChI=1S/C20H23F3N4O5S/c1-12(2)17(18(29)20(21,22)23)25-16(28)11-27-15(13-7-5-4-6-8-13)10-9-14(19(27)30)26-33(31,32)24-3/h4-10,12,17,24,26H,11H2,1-3H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition of Human Leukocyte Elastase (HLE) |
J Med Chem 37: 1259-61 (1994)
BindingDB Entry DOI: 10.7270/Q2251H7P |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50301317
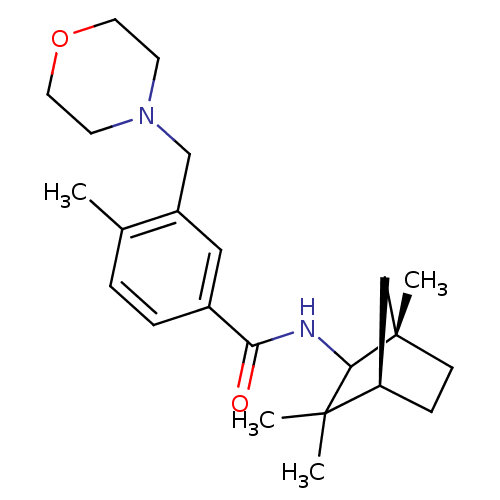
(4-methyl-3-(morpholinomethyl)-N-((1S,4R)-1,3,3-tri...)Show SMILES Cc1ccc(cc1CN1CCOCC1)C(=O)NC1[C@@]2(C)CC[C@H](C2)C1(C)C |r| Show InChI InChI=1S/C23H34N2O2/c1-16-5-6-17(13-18(16)15-25-9-11-27-12-10-25)20(26)24-21-22(2,3)19-7-8-23(21,4)14-19/h5-6,13,19,21H,7-12,14-15H2,1-4H3,(H,24,26)/t19-,21?,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from human CB2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 5004-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.057
BindingDB Entry DOI: 10.7270/Q25M65S1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50377702

(CHEMBL258244)Show SMILES Cc1ccc(cc1S(=O)(=O)N1CCOCC1)C(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:22:23:27:20.21.26,26:21:28:27.25.24,26:25:28:20.21.22,THB:22:21:27:28.23.24| Show InChI InChI=1S/C22H30N2O4S/c1-15-2-3-19(11-20(15)29(26,27)24-4-6-28-7-5-24)21(25)23-22-12-16-8-17(13-22)10-18(9-16)14-22/h2-3,11,16-18H,4-10,12-14H2,1H3,(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human cloned CB2 receptor |
Bioorg Med Chem Lett 18: 2830-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.006
BindingDB Entry DOI: 10.7270/Q2P2701M |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50037117
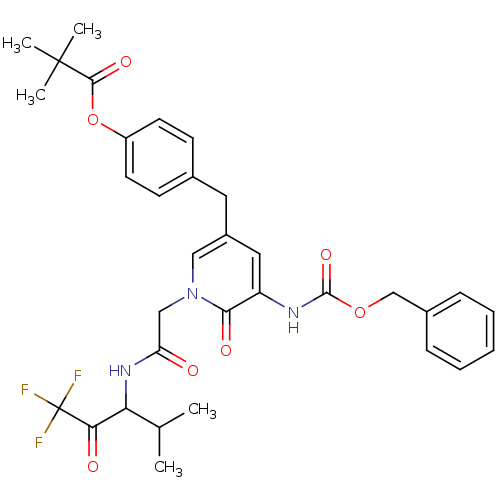
(2,2-Dimethyl-propionic acid 4-{5-benzyloxycarbonyl...)Show SMILES CC(C)C(NC(=O)Cn1cc(Cc2ccc(OC(=O)C(C)(C)C)cc2)cc(NC(=O)OCc2ccccc2)c1=O)C(=O)C(F)(F)F Show InChI InChI=1S/C33H36F3N3O7/c1-20(2)27(28(41)33(34,35)36)38-26(40)18-39-17-23(15-21-11-13-24(14-12-21)46-30(43)32(3,4)5)16-25(29(39)42)37-31(44)45-19-22-9-7-6-8-10-22/h6-14,16-17,20,27H,15,18-19H2,1-5H3,(H,37,44)(H,38,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
A Business Unit of ZENECA Inc.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for ability to inhibit human leukocyte elastase activity |
J Med Chem 37: 3090-9 (1994)
BindingDB Entry DOI: 10.7270/Q2Z0375K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50377706

(CHEMBL256110)Show SMILES Cc1ccc(cc1S(=O)(=O)N1CCOCC1)C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:18:19:21:25.23.24,THB:23:22:19:25.24.26,23:24:21.22.28:19,26:24:21:28.27.19,26:27:21:25.23.24,(25.81,-25.08,;25.81,-26.62,;24.48,-27.39,;24.48,-28.93,;25.82,-29.71,;27.16,-28.93,;27.15,-27.38,;28.48,-26.61,;29.26,-27.94,;27.71,-25.28,;29.81,-25.83,;31.15,-26.59,;32.48,-25.81,;32.47,-24.27,;31.13,-23.51,;29.8,-24.29,;25.82,-31.25,;24.48,-32.01,;27.15,-32.02,;27.19,-33.56,;25.99,-34.46,;24.26,-34.26,;25.48,-35.21,;25.88,-36.67,;27.5,-36.72,;26.4,-35.81,;28.74,-35.82,;28.36,-34.36,;26.76,-34.26,)| Show InChI InChI=1S/C22H30N2O4S/c1-14-2-3-17(13-20(14)29(26,27)24-4-6-28-7-5-24)22(25)23-21-18-9-15-8-16(11-18)12-19(21)10-15/h2-3,13,15-16,18-19,21H,4-12H2,1H3,(H,23,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human cloned CB1 receptor |
Bioorg Med Chem Lett 18: 2830-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.006
BindingDB Entry DOI: 10.7270/Q2P2701M |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50037111
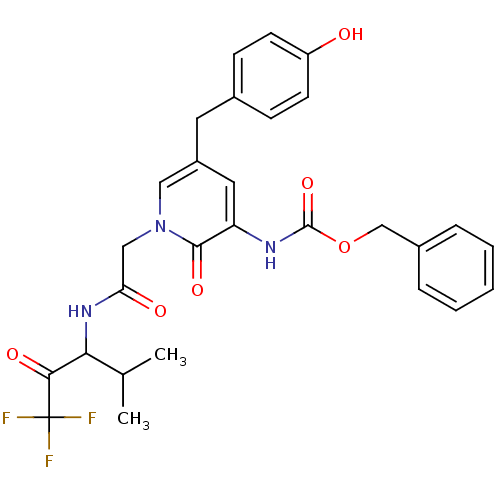
(CHEMBL104303 | {5-(4-Hydroxy-benzyl)-2-oxo-1-[(3,3...)Show SMILES CC(C)C(NC(=O)Cn1cc(Cc2ccc(O)cc2)cc(NC(=O)OCc2ccccc2)c1=O)C(=O)C(F)(F)F Show InChI InChI=1S/C28H28F3N3O6/c1-17(2)24(25(37)28(29,30)31)33-23(36)15-34-14-20(12-18-8-10-21(35)11-9-18)13-22(26(34)38)32-27(39)40-16-19-6-4-3-5-7-19/h3-11,13-14,17,24,35H,12,15-16H2,1-2H3,(H,32,39)(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
A Business Unit of ZENECA Inc.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for ability to inhibit human leukocyte elastase activity |
J Med Chem 37: 3090-9 (1994)
BindingDB Entry DOI: 10.7270/Q2Z0375K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50239215
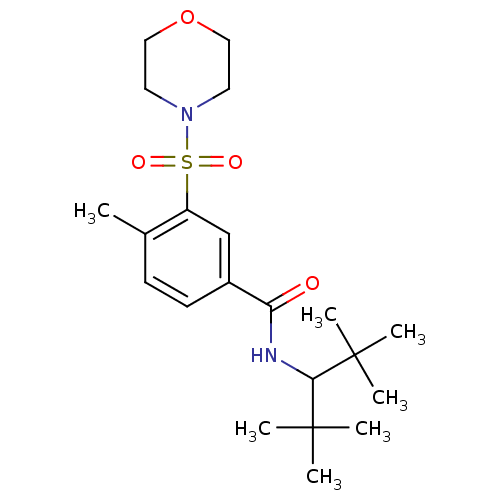
(4-methyl-3-(morpholinosulfonyl)-N-(2,2,4,4-tetrame...)Show SMILES Cc1ccc(cc1S(=O)(=O)N1CCOCC1)C(=O)NC(C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C21H34N2O4S/c1-15-8-9-16(18(24)22-19(20(2,3)4)21(5,6)7)14-17(15)28(25,26)23-10-12-27-13-11-23/h8-9,14,19H,10-13H2,1-7H3,(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human cloned CB2 receptor |
Bioorg Med Chem Lett 18: 2830-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.006
BindingDB Entry DOI: 10.7270/Q2P2701M |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50037102

(CHEMBL75936 | {2-Oxo-5-[3-(2,2,2-trifluoro-acetyla...)Show SMILES CC(C)C(NC(=O)Cn1cc(Cc2cccc(NC(=O)C(F)(F)F)c2)cc(NC(=O)OCc2ccccc2)c1=O)C(=O)C(F)(F)F Show InChI InChI=1S/C30H28F6N4O6/c1-17(2)24(25(42)29(31,32)33)39-23(41)15-40-14-20(11-19-9-6-10-21(12-19)37-27(44)30(34,35)36)13-22(26(40)43)38-28(45)46-16-18-7-4-3-5-8-18/h3-10,12-14,17,24H,11,15-16H2,1-2H3,(H,37,44)(H,38,45)(H,39,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
A Business Unit of ZENECA Inc.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for ability to inhibit human leukocyte elastase activity |
J Med Chem 37: 3090-9 (1994)
BindingDB Entry DOI: 10.7270/Q2Z0375K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50301307
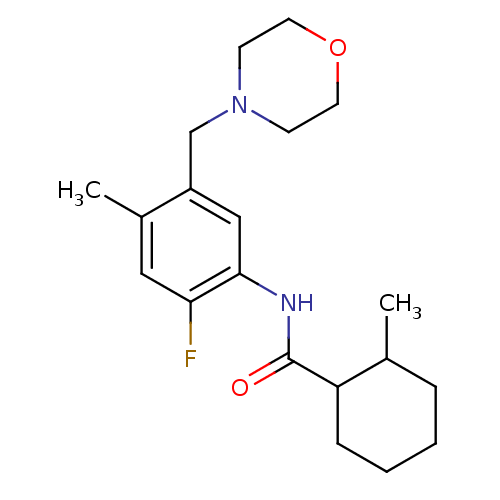
(CHEMBL577487 | N-(2-fluoro-4-methyl-5-(morpholinom...)Show InChI InChI=1S/C20H29FN2O2/c1-14-5-3-4-6-17(14)20(24)22-19-12-16(15(2)11-18(19)21)13-23-7-9-25-10-8-23/h11-12,14,17H,3-10,13H2,1-2H3,(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from human CB2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 5004-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.057
BindingDB Entry DOI: 10.7270/Q25M65S1 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036122
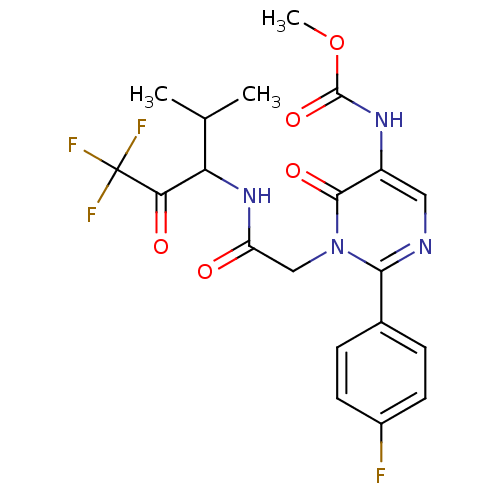
(CHEMBL11336 | {2-(4-Fluoro-phenyl)-6-oxo-1-[(3,3,3...)Show SMILES COC(=O)Nc1cnc(-c2ccc(F)cc2)n(CC(=O)NC(C(C)C)C(=O)C(F)(F)F)c1=O Show InChI InChI=1S/C20H20F4N4O5/c1-10(2)15(16(30)20(22,23)24)27-14(29)9-28-17(11-4-6-12(21)7-5-11)25-8-13(18(28)31)26-19(32)33-3/h4-8,10,15H,9H2,1-3H3,(H,26,32)(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
Inhibition of Human Leukocyte Elastase (HLE) |
J Med Chem 37: 1259-61 (1994)
BindingDB Entry DOI: 10.7270/Q2251H7P |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50037110
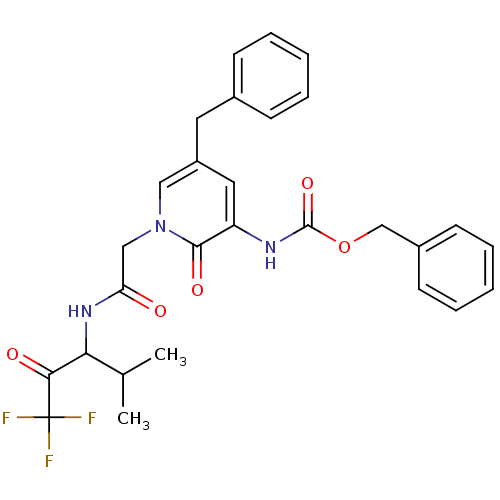
(CHEMBL107291 | {5-Benzyl-2-oxo-1-[(3,3,3-trifluoro...)Show SMILES CC(C)C(NC(=O)Cn1cc(Cc2ccccc2)cc(NC(=O)OCc2ccccc2)c1=O)C(=O)C(F)(F)F Show InChI InChI=1S/C28H28F3N3O5/c1-18(2)24(25(36)28(29,30)31)33-23(35)16-34-15-21(13-19-9-5-3-6-10-19)14-22(26(34)37)32-27(38)39-17-20-11-7-4-8-12-20/h3-12,14-15,18,24H,13,16-17H2,1-2H3,(H,32,38)(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
A Business Unit of ZENECA Inc.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for ability to inhibit human leukocyte elastase activity |
J Med Chem 37: 3090-9 (1994)
BindingDB Entry DOI: 10.7270/Q2Z0375K |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50037105
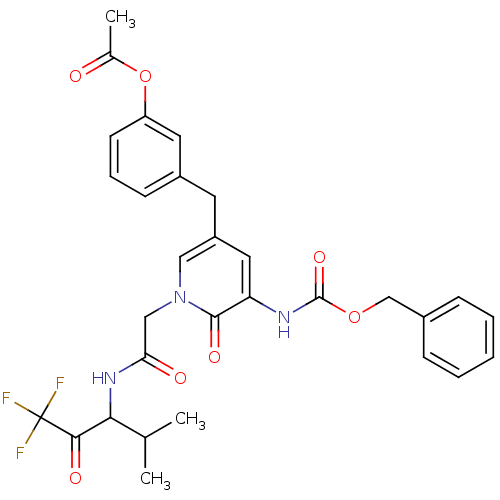
(Acetic acid 3-{5-benzyloxycarbonylamino-6-oxo-1-[(...)Show SMILES CC(C)C(NC(=O)Cn1cc(Cc2cccc(OC(C)=O)c2)cc(NC(=O)OCc2ccccc2)c1=O)C(=O)C(F)(F)F Show InChI InChI=1S/C30H30F3N3O7/c1-18(2)26(27(39)30(31,32)33)35-25(38)16-36-15-22(12-21-10-7-11-23(13-21)43-19(3)37)14-24(28(36)40)34-29(41)42-17-20-8-5-4-6-9-20/h4-11,13-15,18,26H,12,16-17H2,1-3H3,(H,34,41)(H,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
A Business Unit of ZENECA Inc.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for ability to inhibit human leukocyte elastase activity |
J Med Chem 37: 3090-9 (1994)
BindingDB Entry DOI: 10.7270/Q2Z0375K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50301318
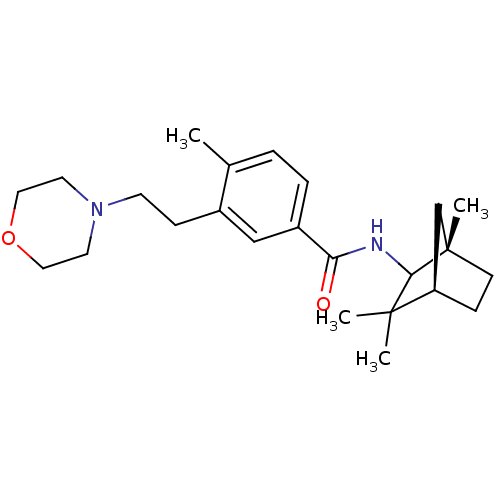
(4-methyl-3-(2-morpholinoethyl)-N-((1S,4R)-1,3,3-tr...)Show SMILES Cc1ccc(cc1CCN1CCOCC1)C(=O)NC1[C@@]2(C)CC[C@H](C2)C1(C)C |r| Show InChI InChI=1S/C24H36N2O2/c1-17-5-6-19(15-18(17)8-10-26-11-13-28-14-12-26)21(27)25-22-23(2,3)20-7-9-24(22,4)16-20/h5-6,15,20,22H,7-14,16H2,1-4H3,(H,25,27)/t20-,22?,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from human CB2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 5004-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.057
BindingDB Entry DOI: 10.7270/Q25M65S1 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50037113
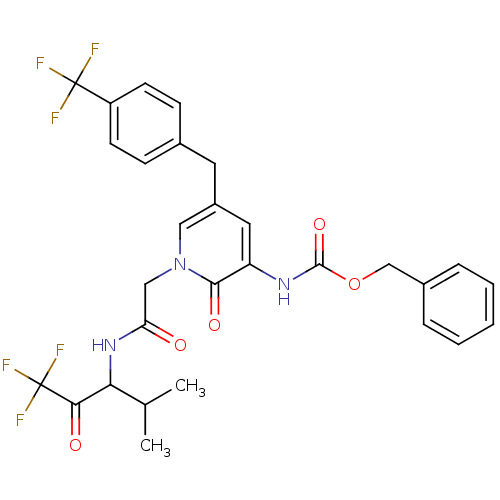
(CHEMBL104289 | [2-Oxo-1-[(3,3,3-trifluoro-1-isopro...)Show SMILES CC(C)C(NC(=O)Cn1cc(Cc2ccc(cc2)C(F)(F)F)cc(NC(=O)OCc2ccccc2)c1=O)C(=O)C(F)(F)F Show InChI InChI=1S/C29H27F6N3O5/c1-17(2)24(25(40)29(33,34)35)37-23(39)15-38-14-20(12-18-8-10-21(11-9-18)28(30,31)32)13-22(26(38)41)36-27(42)43-16-19-6-4-3-5-7-19/h3-11,13-14,17,24H,12,15-16H2,1-2H3,(H,36,42)(H,37,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
A Business Unit of ZENECA Inc.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for ability to inhibit human leukocyte elastase activity |
J Med Chem 37: 3090-9 (1994)
BindingDB Entry DOI: 10.7270/Q2Z0375K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50377701
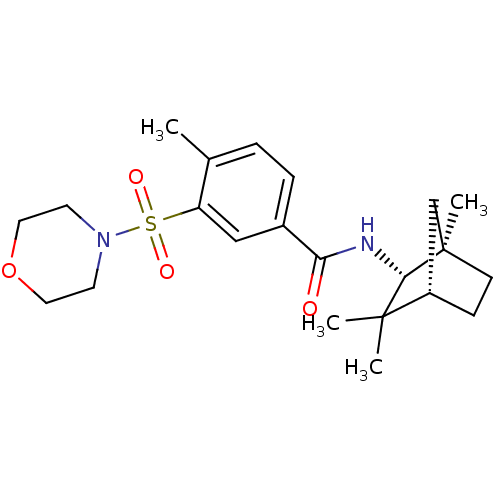
(CHEMBL404650)Show SMILES Cc1ccc(cc1S(=O)(=O)N1CCOCC1)C(=O)N[C@@H]1[C@]2(C)CC[C@@H](C2)C1(C)C |THB:18:19:23.22:25| Show InChI InChI=1S/C22H32N2O4S/c1-15-5-6-16(13-18(15)29(26,27)24-9-11-28-12-10-24)19(25)23-20-21(2,3)17-7-8-22(20,4)14-17/h5-6,13,17,20H,7-12,14H2,1-4H3,(H,23,25)/t17-,20-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human cloned CB2 receptor |
Bioorg Med Chem Lett 18: 2830-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.006
BindingDB Entry DOI: 10.7270/Q2P2701M |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50037116
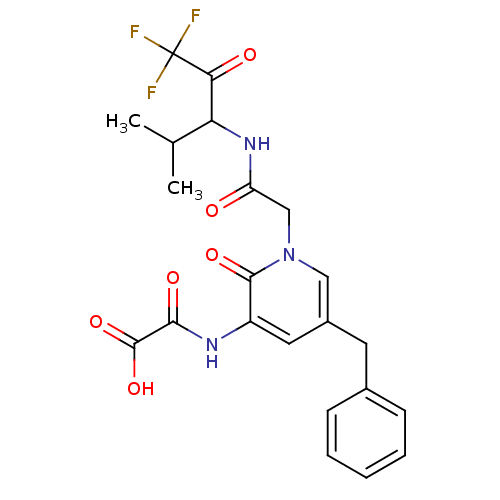
(CHEMBL107966 | N-{5-Benzyl-2-oxo-1-[(3,3,3-trifluo...)Show SMILES CC(C)C(NC(=O)Cn1cc(Cc2ccccc2)cc(NC(=O)C(O)=O)c1=O)C(=O)C(F)(F)F Show InChI InChI=1S/C22H22F3N3O6/c1-12(2)17(18(30)22(23,24)25)27-16(29)11-28-10-14(8-13-6-4-3-5-7-13)9-15(20(28)32)26-19(31)21(33)34/h3-7,9-10,12,17H,8,11H2,1-2H3,(H,26,31)(H,27,29)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
A Business Unit of ZENECA Inc.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for ability to inhibit human leukocyte elastase activity |
J Med Chem 37: 3090-9 (1994)
BindingDB Entry DOI: 10.7270/Q2Z0375K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50377704

(CHEMBL255096)Show SMILES Cc1ccc(cc1S(=O)(=O)N1CCOCC1)C(=O)N[C@H]1CC2CC[C@@]1(C)C2(C)C |w:21.23,TLB:18:19:23.22:26| Show InChI InChI=1S/C22H32N2O4S/c1-15-5-6-16(13-18(15)29(26,27)24-9-11-28-12-10-24)20(25)23-19-14-17-7-8-22(19,4)21(17,2)3/h5-6,13,17,19H,7-12,14H2,1-4H3,(H,23,25)/t17?,19-,22+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human cloned CB1 receptor |
Bioorg Med Chem Lett 18: 2830-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.006
BindingDB Entry DOI: 10.7270/Q2P2701M |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50037098
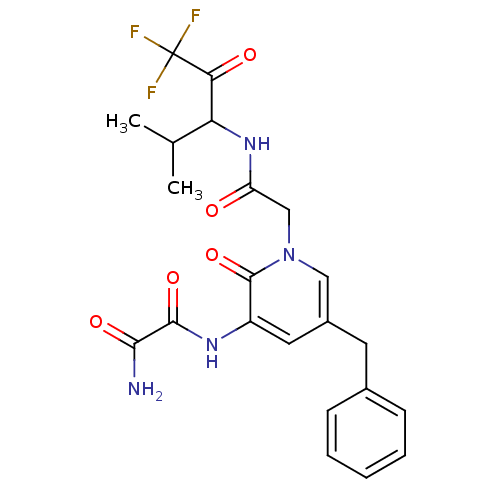
(CHEMBL104032 | N-{5-Benzyl-2-oxo-1-[(3,3,3-trifluo...)Show SMILES CC(C)C(NC(=O)Cn1cc(Cc2ccccc2)cc(NC(=O)C(N)=O)c1=O)C(=O)C(F)(F)F Show InChI InChI=1S/C22H23F3N4O5/c1-12(2)17(18(31)22(23,24)25)28-16(30)11-29-10-14(8-13-6-4-3-5-7-13)9-15(21(29)34)27-20(33)19(26)32/h3-7,9-10,12,17H,8,11H2,1-2H3,(H2,26,32)(H,27,33)(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
A Business Unit of ZENECA Inc.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for ability to inhibit human leukocyte elastase activity |
J Med Chem 37: 3090-9 (1994)
BindingDB Entry DOI: 10.7270/Q2Z0375K |
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM50036127

(CHEMBL11391 | {2-Oxo-6-phenyl-1-[(3,3,3-trifluoro-...)Show SMILES CC(C)C(NC(=O)Cn1c(ccc(NC(=O)OCc2ccccc2)c1=O)-c1ccccc1)C(=O)C(F)(F)F Show InChI InChI=1S/C27H26F3N3O5/c1-17(2)23(24(35)27(28,29)30)32-22(34)15-33-21(19-11-7-4-8-12-19)14-13-20(25(33)36)31-26(37)38-16-18-9-5-3-6-10-18/h3-14,17,23H,15-16H2,1-2H3,(H,31,37)(H,32,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Group
Curated by ChEMBL
| Assay Description
The compound was evaluated for the binding affinity against bovine pancreatic chymotrypsinogen |
J Med Chem 37: 1259-61 (1994)
BindingDB Entry DOI: 10.7270/Q2251H7P |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50301319
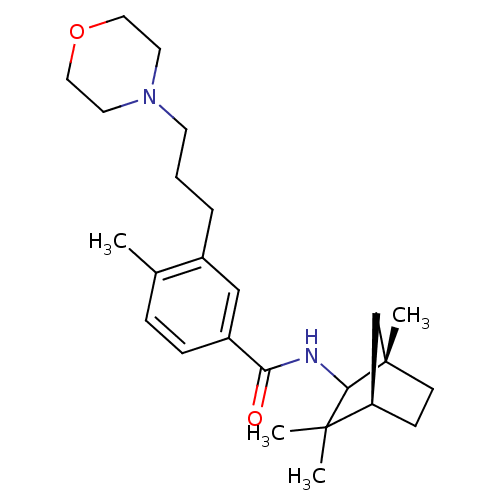
(4-methyl-3-(3-morpholinopropyl)-N-((1S,4R)-1,3,3-t...)Show SMILES Cc1ccc(cc1CCCN1CCOCC1)C(=O)NC1[C@@]2(C)CC[C@H](C2)C1(C)C |r| Show InChI InChI=1S/C25H38N2O2/c1-18-7-8-20(16-19(18)6-5-11-27-12-14-29-15-13-27)22(28)26-23-24(2,3)21-9-10-25(23,4)17-21/h7-8,16,21,23H,5-6,9-15,17H2,1-4H3,(H,26,28)/t21-,23?,25+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from human CB2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 5004-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.057
BindingDB Entry DOI: 10.7270/Q25M65S1 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50037118
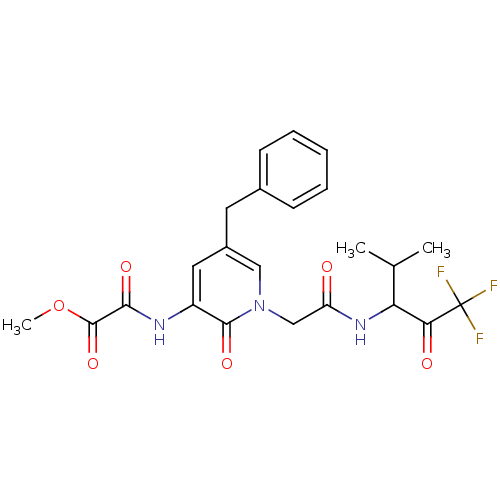
(CHEMBL106793 | N-{5-Benzyl-2-oxo-1-[(3,3,3-trifluo...)Show SMILES COC(=O)C(=O)Nc1cc(Cc2ccccc2)cn(CC(=O)NC(C(C)C)C(=O)C(F)(F)F)c1=O Show InChI InChI=1S/C23H24F3N3O6/c1-13(2)18(19(31)23(24,25)26)28-17(30)12-29-11-15(9-14-7-5-4-6-8-14)10-16(21(29)33)27-20(32)22(34)35-3/h4-8,10-11,13,18H,9,12H2,1-3H3,(H,27,32)(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
A Business Unit of ZENECA Inc.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for ability to inhibit human leukocyte elastase activity |
J Med Chem 37: 3090-9 (1994)
BindingDB Entry DOI: 10.7270/Q2Z0375K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50301313

(2-methyl-N-(4-methyl-3-(morpholinomethyl)phenyl)cy...)Show InChI InChI=1S/C20H30N2O2/c1-15-7-8-18(13-17(15)14-22-9-11-24-12-10-22)21-20(23)19-6-4-3-5-16(19)2/h7-8,13,16,19H,3-6,9-12,14H2,1-2H3,(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from human CB2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 5004-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.057
BindingDB Entry DOI: 10.7270/Q25M65S1 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50037099
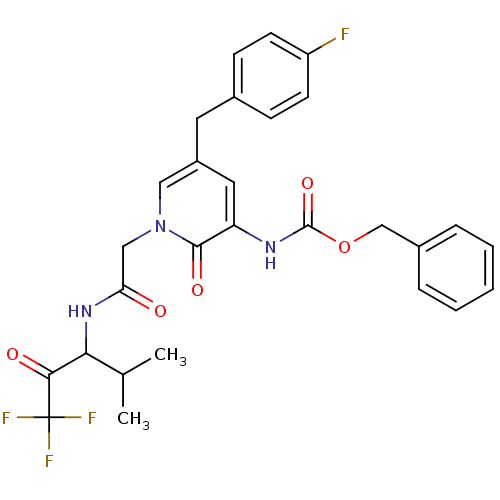
(CHEMBL104557 | {5-(4-Fluoro-benzyl)-2-oxo-1-[(3,3,...)Show SMILES CC(C)C(NC(=O)Cn1cc(Cc2ccc(F)cc2)cc(NC(=O)OCc2ccccc2)c1=O)C(=O)C(F)(F)F Show InChI InChI=1S/C28H27F4N3O5/c1-17(2)24(25(37)28(30,31)32)34-23(36)15-35-14-20(12-18-8-10-21(29)11-9-18)13-22(26(35)38)33-27(39)40-16-19-6-4-3-5-7-19/h3-11,13-14,17,24H,12,15-16H2,1-2H3,(H,33,39)(H,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
A Business Unit of ZENECA Inc.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for ability to inhibit human leukocyte elastase activity |
J Med Chem 37: 3090-9 (1994)
BindingDB Entry DOI: 10.7270/Q2Z0375K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50377705

(CHEMBL442447)Show SMILES CC(NC(=O)c1ccc(C)c(c1)S(=O)(=O)N1CCOCC1)C12CC3CC(CC(C3)C1)C2 |w:1.0,TLB:24:25:29:22.23.28,28:23:30:29.27.26,28:27:30:22.23.24,THB:24:23:29:30.25.26| Show InChI InChI=1S/C24H34N2O4S/c1-16-3-4-21(12-22(16)31(28,29)26-5-7-30-8-6-26)23(27)25-17(2)24-13-18-9-19(14-24)11-20(10-18)15-24/h3-4,12,17-20H,5-11,13-15H2,1-2H3,(H,25,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human cloned CB1 receptor |
Bioorg Med Chem Lett 18: 2830-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.006
BindingDB Entry DOI: 10.7270/Q2P2701M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data